Progress in Corrosion Protection Research for Supercritical CO2 Transportation Pipelines
Abstract
1. Introduction
2. SC-CO2 Corrosion
2.1. Overview of SC-CO2 Corrosion
- (1)
- The initial step is the combination of water and CO2 to form carbonic acid (H2CO3) and subsequently partial homogenous dissociation in two steps to form bicarbonate and carbonate ions.
- (2)
- In the next stage of reactions, the cathodic reaction can occur either by direct reduction of hydrogen ions, or the reduction of carbonic acid or carbonate ions.
- (3)
- The next stage is the anodic dissolution of iron.
- (4)
- FeCO3 precipitation then occurs via Fe2+ through a one-stage reaction with carbonates, or a two-stage reaction with bicarbonates.
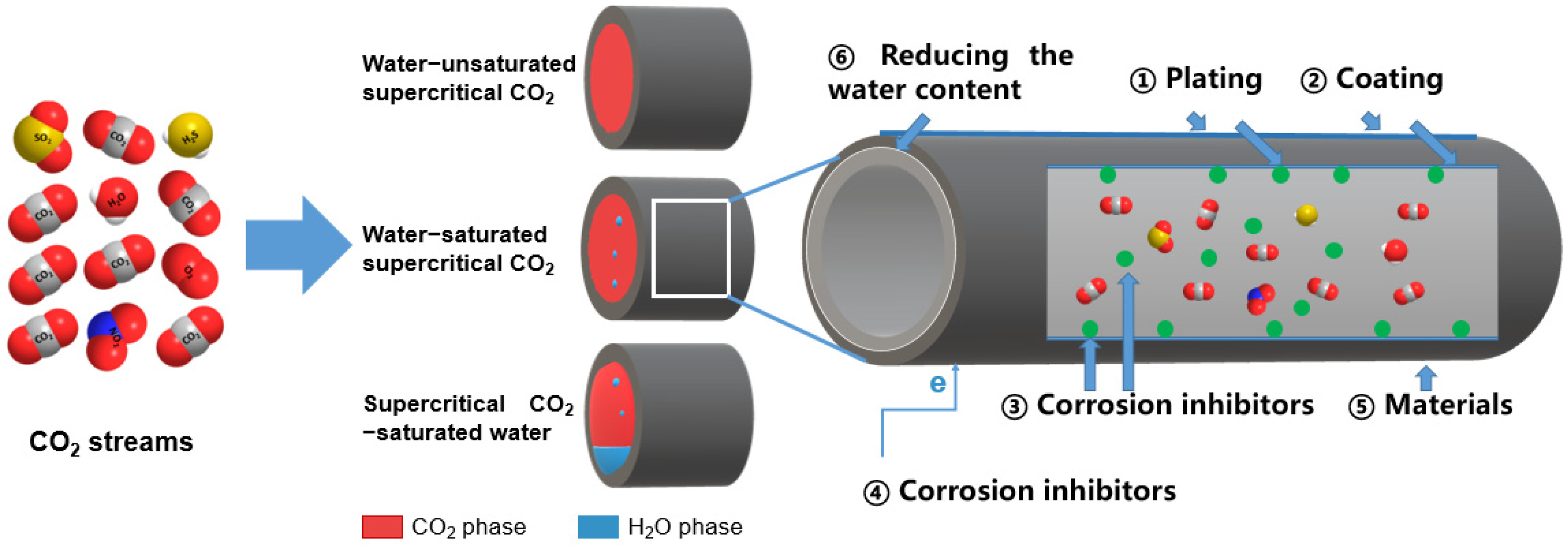
2.1.1. Corrosion in Water-Unsaturated SC-CO2
2.1.2. Corrosion in Water-Saturated SC-CO2
2.1.3. Corrosion in (SC-CO2)-Saturated Water
| Type | Author | Material | Temperature/K | Pressure/Mpa | Test Time/h | Corrosion Rate/mm/y |
|---|---|---|---|---|---|---|
| Water-unsaturated SC-CO2 | Hua et al. [31] | X65 steel | 323.15 | 8.0 | 48 | 0.015 |
| Water-saturated SC-CO2 | Hua et al. [31] | X65 steel | 323.15 | 8.0 | 24 | <0.1 |
| 48 | <0.03 | |||||
| Li et al. [33] | X80 steel | 333.15 | 8.0 | 140 | 0.716 | |
| Hua et al. [34] | X65 steel | 303.15 | 8.0 | 48 | 0.1 | |
| (SC-CO2)-saturated water | Hua et al. [31] | X65 steel | 323.15 | 8.0 | 6.5 | 10.8 |
| 96 | 4.1 | |||||
| Zhang et al. [37] | X65 steel | 353.15 | 9.5 | 7 | 20.6 | |
| 96 | 7.35 |
2.2. Effect Factors
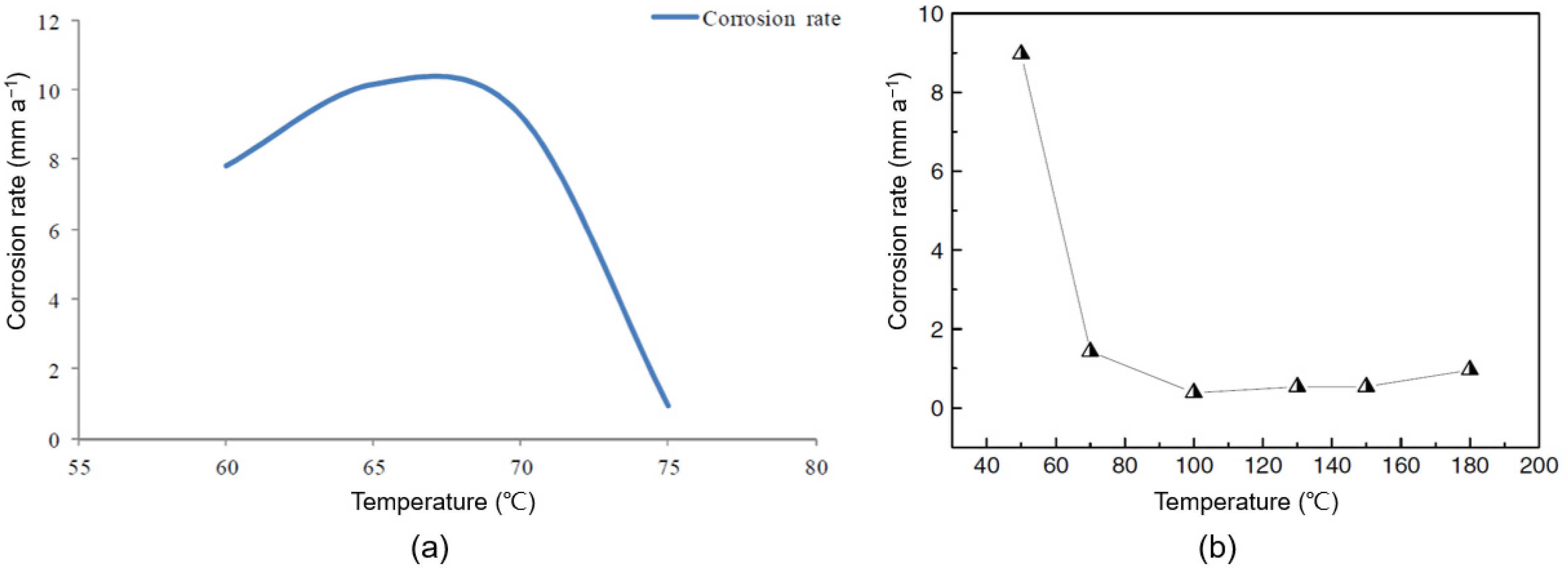
2.2.1. Temperature
2.2.2. CO2 Pressure
2.2.3. Gas Impurities
2.2.4. Materials
2.2.5. Flow Rate
3. Common Corrosion Protection Methods for SC-CO2 Transport Pipelines
3.1. Plating
3.2. Coatings
3.2.1. Corrosion-Resistant Coatings
3.2.2. Ceramic Coatings
| Corrosion Inhibitors | Matrix | Substrate | Thickness/um | Corrosion Environment | Immersion Time/Days | |
|---|---|---|---|---|---|---|
| CeO2 loaded with benzotriazole [104] | epoxy resin | Q235 | 30 ± 2 | 0.5 mol/L NaCl solution | 120 | |
| CeO2 PANI [105] | epoxy resin | Carbon steel | 80 ± 5 | 3.5 wt% NaCl solution | 20 | |
| Triethanolamine Polyethylenimine [106] | epoxy resin | DC01 | 23.7 ± 1.8 | 0.5 mol/L NaCl solution | 24 | |
| Pr(NO3)3 benzimidazole [107] | silane | Mild steel | - | 3.5 wt% NaCl solution | 24 | |
| Tannic Acid Modified Cerium-Montmorillonites [108] | WPU | Q235 | 100 ± 0.5 | 3.5 wt% NaCl solution | 50 | |
| PS-PVP@rGO (N-BPG) [92] | fluorocarbon resin | Q235 | 35 ± 2 | 3.5 wt% NaCl soluti | 7 |
3.2.3. Others
3.3. Corrosion Inhibitors
3.3.1. Organic Corrosion Inhibitors
- (1)
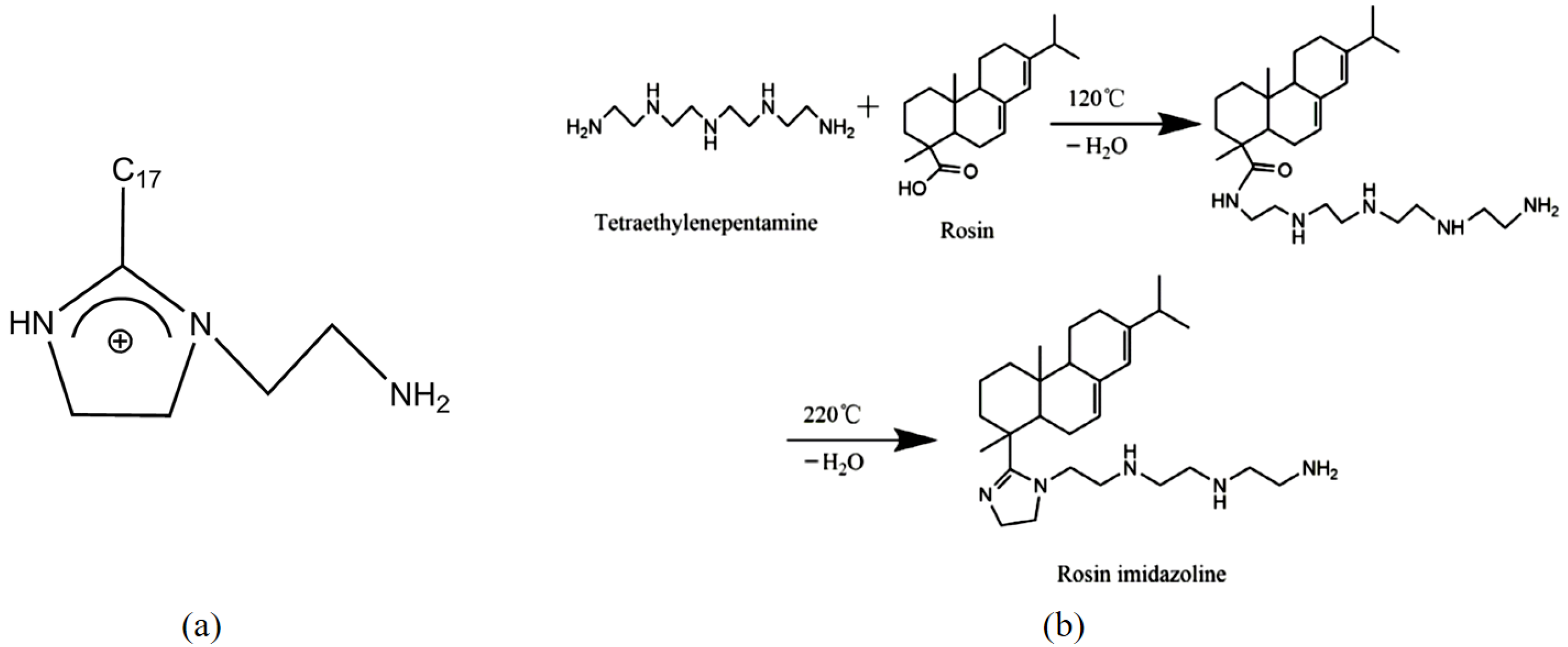
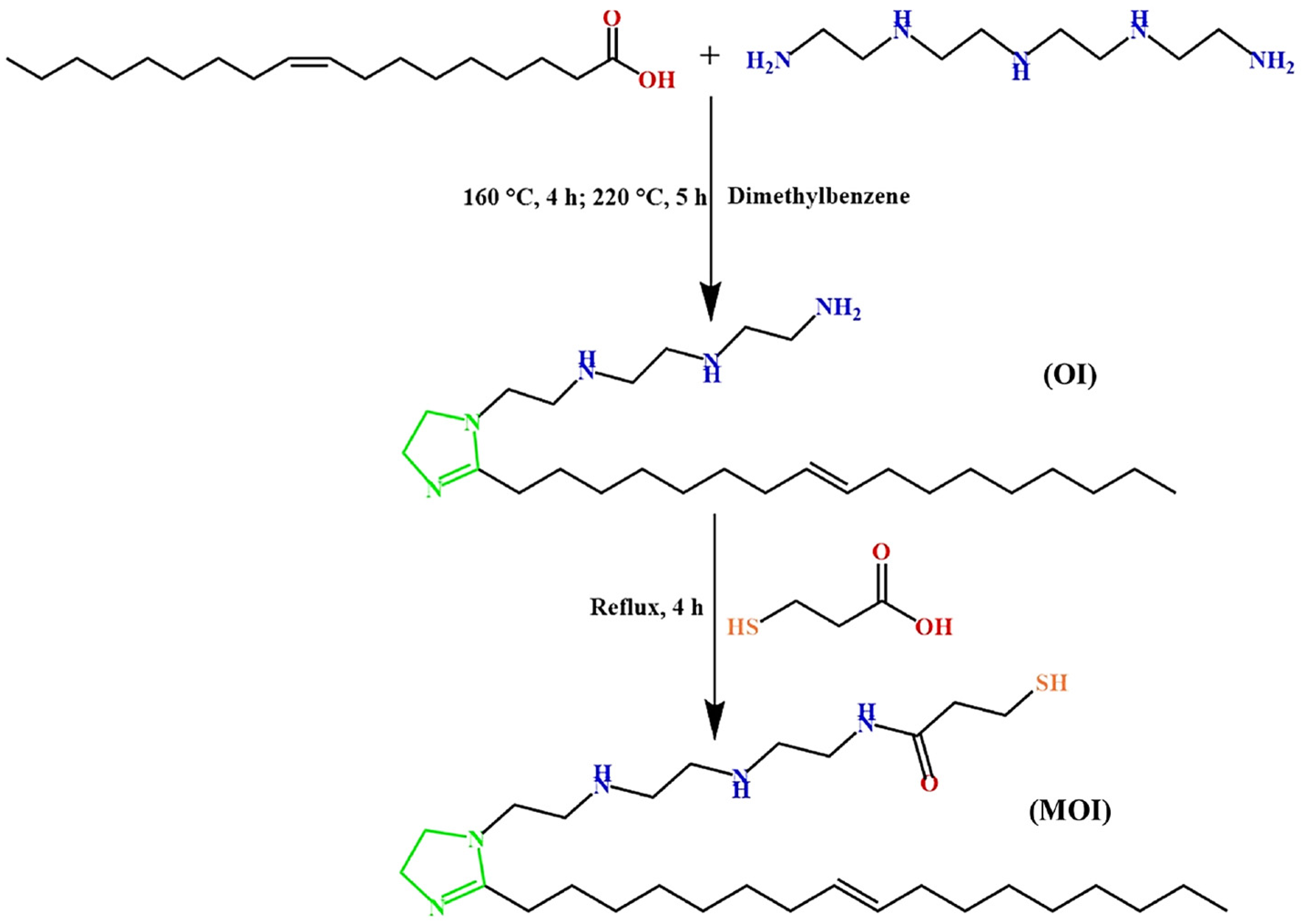
- Imidazoline and its derivative class corrosion inhibitors
- (2)
- Amine corrosion inhibitors
- (3)
- Thiol-based corrosion inhibitors
- (4)
- Ester-based corrosion inhibitors
- (5)
- Ecofriendly inhibitors
- (6)
- Others
3.3.2. Inorganic Corrosion Inhibitors
3.3.3. Composite Corrosion Inhibitors
3.4. Cathodic Protection Method
3.5. Corrosion-Resistant Materials
3.6. Reducing Water Content at the Pipeline Inlet
4. Challenges
4.1. Challenges in Corrosion Protection of SC-CO2 Transport Pipelines
4.1.1. Complex Corrosion Mechanisms
4.1.2. Difficulty in Selecting Pipeline Materials
4.1.3. Internal Wall Corrosion Issues
4.1.4. Construction and Maintenance Costs of SC-CO2 Transport Pipelines
4.2. Challenges Faced by Corrosion Inhibitors for SC-CO2 Transport Pipeline Protection
4.2.1. Development of Self-Adaptable Corrosion Inhibitors
4.2.2. Stability of Corrosion Inhibitors
4.2.3. Development of Low-Cost Corrosion Inhibitors
4.2.4. Development of Eco-Friendly Corrosion Inhibitors
5. Conclusions and Outlook
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Annual Report on Carbon Dioxide Capture, Utilization and Storage (CCUS) in China (2021)—Research on CCUS Path in China; Ministry of Ecology and Environment of the People’s Republic of China, Institute of Rock and Soil Mechanics, Chinese Academy of Sciences: Beijing, China, 2021.
- Wang, P.; Shi, B.; Li, N.; Kang, R.; Li, Y.; Wang, G.; Yang, L. CCUS development in China and forecast its contribution to emission reduction. Sci. Rep. 2023, 13, 17811. [Google Scholar] [CrossRef]
- Jung, S.-H.; Kim, H.; Kang, Y.; Jeong, E. Analysis of Korea’s Green Technology Policy and Investment Trends for the Realization of Carbon Neutrality: Focusing on CCUS Technology. Processes 2022, 10, 501. [Google Scholar] [CrossRef]
- Che, X.; Yi, X.; Dai, Z.; Zhang, Z.; Zhang, Y. Application and Development Countermeasures of CCUS Technology in China’s Petroleum Industry. Atmosphere 2022, 13, 1757. [Google Scholar] [CrossRef]
- Duval-Dachary, S.; Lorne, D.; Beauchet, S.; Salou, T.; Hélias, A. Life cycle assessment of carbon capture and utilisation as a negative emission technology: Recommendations and case study. Int. J. Life Cycle Assess. 2024, 1–13. [Google Scholar] [CrossRef]
- Bang, A.; Moreno, D.; Lund, H.; Nielsen, S. Regional CCUS strategies in the context of a fully decarbonized society. J. Clean. Prod. 2024, 477, 143882. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef]
- Leonzio, G.; Bogle, I.D.L.; Ugo Foscolo, P. Life cycle assessment of a carbon capture utilization and storage supply chain in Italy and Germany: Comparison between carbon dioxide storage and utilization systems. Sustain. Energy Technol. Assess. 2023, 55, 102743. [Google Scholar] [CrossRef]
- Hammond, G.P.; Akwe, S.S.O.; Williams, S. Techno-economic appraisal of fossil-fuelled power generation systems with carbon dioxide capture and storage. Energy 2011, 36, 975–984. [Google Scholar] [CrossRef]
- Nocito, F.; Dibenedetto, A. Atmospheric CO2 mitigation technologies: Carbon capture utilization and storage. Curr. Opin. Green Sustain. Chem. 2020, 21, 34–43. [Google Scholar] [CrossRef]
- Herzog, H.J. Scaling up carbon dioxide capture and storage: From megatons to gigatons. Energy Econ. 2011, 33, 597–604. [Google Scholar] [CrossRef]
- Jiang, K.; Ashworth, P. The development of Carbon Capture Utilization and Storage (CCUS) research in China: A bibliometric perspective. Renew. Sustain. Energy Rev. 2021, 138, 110521. [Google Scholar] [CrossRef]
- Gao, W.; Liang, S.; Wang, R.; Jiang, Q.; Zhang, Y.; Zheng, Q.; Xie, B.; Toe, C.Y.; Zhu, X.; Wang, J.; et al. Industrial carbon dioxide capture and utilization: State of the art and future challenges. Chem. Soc. Rev. 2020, 49, 8584–8686. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, W.; Wang, Y.; Shen, J.; Wang, Y.; Geng, Z.; Wang, Q.; Zhu, T. Progress of CCUS technology in the iron and steel industry and the suggestion of the integrated application schemes for China. Chem. Eng. J. 2022, 450, 138438. [Google Scholar] [CrossRef]
- Cozier, M. Supporting the development of CCUS in the UK. Greenh. Gases Sci. Technol. 2018, 8, 799–802. [Google Scholar] [CrossRef]
- Wesche, J.; Germán, S.; Gonçalves, L.; Jödicke, I.; López-Asensio, S.; Prades, A.; Preuß, S.; Algado, C.O.; Dütschke, E. CCUS or no CCUS? Societal support for policy frameworks and stakeholder perceptions in France, Spain, and Poland. Greenh. Gases Sci. Technol. 2023, 13, 48–66. [Google Scholar] [CrossRef]
- Sun, L.; Chen, W. Development and application of a multi-stage CCUS source–sink matching model. Appl. Energy 2017, 185, 1424–1432. [Google Scholar] [CrossRef]
- Liu, J.; Feng, G.; Zhao, P. Application and Optimization of CCUS Technology in Shale Gas Production and Storage. Energies 2023, 16, 5483. [Google Scholar] [CrossRef]
- Zhdaneev, O.V.; Zaitsev, A.V. Flow measurement for CCUS applications. Sustain. Energy Fuels 2023, 7, 5249–5258. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, X.; Xia, Y. Dynamic Response Analysis of Corroded Pipelines Containing SCCO2 under Rockfall Impact. Processes 2024, 12, 2201. [Google Scholar] [CrossRef]
- Wang, W.; Guang, Y.; Liu, W.; Shen, K.; Huffman, M.; Wang, Q. Experimental investigation of stress corrosion on supercritical CO2 transportation pipelines against leakage for CCUS applications. Energy Rep. 2023, 9, 266–276. [Google Scholar] [CrossRef]
- Farh, H.M.H.; Ben Seghier, M.E.A.; Zayed, T. A comprehensive review of corrosion protection and control techniques for metallic pipelines. Eng. Fail. Anal. 2023, 143, 106885. [Google Scholar] [CrossRef]
- Liu, E.; Lu, X.; Wang, D. A Systematic Review of Carbon Capture, Utilization and Storage: Status, Progress and Challenges. Energies 2023, 16, 2865. [Google Scholar] [CrossRef]
- Hasan, M.M.F.; Zantye, M.S.; Kazi, M.-K. Challenges and opportunities in carbon capture, utilization and storage: A process systems engineering perspective. Comput. Chem. Eng. 2022, 166, 107925. [Google Scholar] [CrossRef]
- Sun, H.; Wang, H.; Zeng, Y.; Liu, J. Corrosion challenges in supercritical CO2 transportation, storage, and utilization—A review. Renew. Sustain. Energy Rev. 2023, 179, 113292. [Google Scholar] [CrossRef]
- Zhou, Y.; Xie, F.; Wang, D.; Wang, Y.; Wu, M. Carbon capture, utilization and storage (CCUS) pipeline steel corrosion failure analysis: A review. Eng. Fail. Anal. 2024, 155, 107745. [Google Scholar] [CrossRef]
- Cole, I.S.; Corrigan, P.; Sim, S.; Birbilis, N. Corrosion of pipelines used for CO2 transport in CCS: Is it a real problem? Int. J. Greenh. Gas Control. 2011, 5, 749–756. [Google Scholar] [CrossRef]
- Zhu, Z.; Cheng, Y.; Xiao, B.; Khan, H.I.; Xu, H.; Zhang, N. Corrosion behavior of ferritic and ferritic-martensitic steels in supercritical carbon dioxide. Energy 2019, 175, 1075–1084. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.; Ma, H.; Huo, Z.; Wang, Y. Experimental Investigation on Corrosion Behavior of X80 Pipeline Steel under Carbon Dioxide Aqueous Conditions. ACS Omega 2022, 7, 6142–6150. [Google Scholar] [CrossRef]
- Nikolai, P.; Rabiyat, B.; Aslan, A.; Ilmutdin, A. Supercritical CO2: Properties and Technological Applications—A Review. J. Therm. Sci. 2019, 28, 394–430. [Google Scholar] [CrossRef]
- Hua, Y.; Barker, R.; Neville, A. Comparison of corrosion behaviour for X-65 carbon steel in supercritical CO2-saturated water and water-saturated/unsaturated supercritical CO2. J. Supercrit. Fluids 2015, 97, 224–237. [Google Scholar] [CrossRef]
- Cui, G.; Yang, Z.; Liu, J.; Li, Z. A comprehensive review of metal corrosion in a supercritical CO2 environment. Int. J. Greenh. Gas Control. 2019, 90, 102814. [Google Scholar] [CrossRef]
- Li, C.; Xiang, Y.; Song, C.; Ji, Z. Assessing the corrosion product scale formation characteristics of X80 steel in supercritical CO2-H2O binary systems with flue gas and NaCl impurities relevant to CCUS technology. J. Supercrit. Fluids 2019, 146, 107–119. [Google Scholar] [CrossRef]
- Hua, Y.; Jonnalagadda, R.; Zhang, L.; Neville, A.; Barker, R. Assessment of general and localized corrosion behavior of X65 and 13Cr steels in water-saturated supercritical CO2 environments with SO2/O2. Int. J. Greenh. Gas Control. 2017, 64, 126–136. [Google Scholar] [CrossRef]
- Xiang, Y.; Xu, M.; Choi, Y.-S. State-of-the-art Overview of Pipeline Steel Corrosion in Impure Dense CO2 for CCS Transportation: Mechanisms and Models. Corros. Eng. Sci. Technol. 2017, 52, 485–509. [Google Scholar] [CrossRef]
- Barker, R.; Burkle, D.; Charpentier, T.; Thompson, H.; Neville, A. A review of iron carbonate (FeCO3) formation in the oil and gas industry. Corros. Sci. 2018, 142, 312–341. [Google Scholar] [CrossRef]
- Zhang, Y.; Pang, X.; Qu, S.; Li, X.; Gao, K. Discussion of the CO2 corrosion mechanism between low partial pressure and supercritical condition. Corros. Sci. 2012, 59, 186–197. [Google Scholar] [CrossRef]
- Li, M.; Gross, A.; Taylor, B.; Zhang, H.; Liu, J. Effects of Cl-ion and temperature variations on steel corrosion in supercritical CO2 saturated aqueous environments. Process Saf. Environ. Prot. 2024, 187, 1446–1453. [Google Scholar] [CrossRef]
- Guo, H.; Wang, Y.; Tan, L.; Lu, Z.; Bai, L. Corrosion behaviors of iron in a supercritical CO2 environment: A molecular dynamics study. J. Mater. Sci. 2023, 58, 14758–14772. [Google Scholar] [CrossRef]
- de Azevedo Rodrigues, T.R.S.; Marcolino, J.B.; de Moraes, M.K.; Lopes, N.F.; da Costa, E.M. Influence of CO2 subcritical and supercritical pressures on the protective properties of corrosion product scales formed on X65 steel. J. Supercrit. Fluids 2024, 206, 106184. [Google Scholar] [CrossRef]
- Li, C.; Xiang, Y.; Wang, R.; Yuan, J.; Xu, Y.; Li, W.; Zheng, Z. Exploring the influence of flue gas impurities on the electrochemical corrosion mechanism of X80 steel in a supercritical CO2-saturated aqueous environment. Corros. Sci. 2023, 211, 110899. [Google Scholar] [CrossRef]
- Sarrade, S.; Féron, D.; Rouillard, F.; Perrin, S.; Robin, R.; Ruiz, J.-C.; Turc, H.-A. Overview on corrosion in supercritical fluids. J. Supercrit. Fluids 2017, 120, 335–344. [Google Scholar] [CrossRef]
- Choi, Y.-S.; Nesic, S.; Young, D. Effect of Impurities on the Corrosion Behavior of CO2 Transmission Pipeline Steel in Supercritical CO2−Water Environments. Environ. Sci. Technol. 2011, 45, 3818. [Google Scholar] [CrossRef]
- Sui, P.; Sun, J.; Hua, Y.; Liu, H.; Zhou, M.; Zhang, Y.; Liu, J.; Wang, Y. Effect of temperature and pressure on corrosion behavior of X65 carbon steel in water-saturated CO2 transport environments mixed with H2S. Int. J. Greenh. Gas Control. 2018, 73, 60–69. [Google Scholar] [CrossRef]
- Sun, W.; Nešić, S.; Woollam, R.C. The effect of temperature and ionic strength on iron carbonate (FeCO3) solubility limit. Corros. Sci. 2009, 51, 1273–1276. [Google Scholar] [CrossRef]
- Hua, Y.; Barker, R.; Neville, A. Effect of temperature on the critical water content for general and localised corrosion of X65 carbon steel in the transport of supercritical CO2. Int. J. Greenh. Gas Control. 2014, 31, 48–60. [Google Scholar] [CrossRef]
- Yin, Z.F.; Feng, Y.R.; Zhao, W.Z.; Bai, Z.Q.; Lin, G.F. Effect of temperature on CO2 corrosion of carbon steel. Surf. Interface Anal. 2009, 41, 517–523. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Y.; Cheng, Y.; Zuo, X.; Wang, Y.; Yuan, X.; Huang, H. Effect of Temperature on the Corrosion Behavior and Corrosion Resistance of Copper–Aluminum Laminated Composite Plate. Materials 2022, 15, 1621. [Google Scholar] [CrossRef]
- Konovalova, V. The effect of temperature on the corrosion rate of iron-carbon alloys. Mater. Today Proc. 2021, 38, 1326–1329. [Google Scholar] [CrossRef]
- Peng, S.; Zeng, Z. An experimental study on the internal corrosion of a subsea multiphase pipeline. Petroleum 2015, 1, 75–81. [Google Scholar] [CrossRef]
- Lin, G.; Zheng, M.; Bai, Z.; Zhao, X. Effect of Temperature and Pressure on the Morphology of Carbon Dioxide Corrosion Scales. Corrosion 2006, 62, 501–507. [Google Scholar] [CrossRef]
- Jiang, D.; Zhong, S.; Xiao, W.; Liu, D.; Wu, M.; Liu, S. Structural, mechanical, electronic, and thermodynamic properties of pure tungsten metal under different pressures: A first-principles study. Int. J. Quantum Chem. 2020, 120, e26231. [Google Scholar] [CrossRef]
- Chen, X.; Li, C.; Ming, N.; He, C. Effects of temperature on the corrosion behaviour of X70 steel in CO2-Containing formation water. J. Nat. Gas Sci. Eng. 2021, 88, 103815. [Google Scholar] [CrossRef]
- Honarvar Nazari, M.; Allahkaram, S.R.; Kermani, M.B. The effects of temperature and pH on the characteristics of corrosion product in CO2 corrosion of grade X70 steel. Mater. Des. 2010, 31, 3559–3563. [Google Scholar] [CrossRef]
- Xu, M.; Li, W.; Zhou, Y.; Yang, X.; Wang, Z.; Li, Z. Effect of pressure on corrosion behavior of X60, X65, X70, and X80 carbon steels in water-unsaturated supercritical CO2 environments. Int. J. Greenh. Gas Control. 2016, 51, 357–368. [Google Scholar] [CrossRef]
- Mahaffey, J.; Adam, D.; Brittan, A.; Anderson, M.; Sridharan, K. Corrosion of Alloy Haynes 230 in High Temperature Supercritical Carbon Dioxide with Oxygen Impurity Additions. Oxid. Met. 2016, 86, 567–580. [Google Scholar] [CrossRef]
- Cen, H.; Cao, J.; Chen, Z.; Guo, X. 2-Mercaptobenzothiazole as a corrosion inhibitor for carbon steel in supercritical CO2-H2O condition. Appl. Surf. Sci. 2019, 476, 422–434. [Google Scholar] [CrossRef]
- Wei, L.; Pang, X.; Gao, K. Effect of small amount of H2S on the corrosion behavior of carbon steel in the dynamic supercritical CO2 environments. Corros. Sci. 2016, 103, 132–144. [Google Scholar] [CrossRef]
- Sun, C.; Ding, T.; Sun, J.; Lin, X.; Zhao, W.; Chen, H. Insights into the effect of H2S on the corrosion behavior of N80 steel in supercritical CO2 environment. J. Mater. Res. Technol. 2023, 26, 5462–5477. [Google Scholar] [CrossRef]
- Liu, J.; Yao, D.; Chen, K.; Wang, C.; Sun, C.; Pan, H.; Meng, F.; Chen, B.; Wang, L. Effect of H2O Content on the Corrosion Behavior of X52 Steel in Supercritical CO2 Streams Containing O2, H2S, SO2 and NO2 Impurities. Energies 2023, 16, 6119. [Google Scholar] [CrossRef]
- Sun, C.; Yan, X.; Sun, J.; Pang, J.; Zhao, W.; Lin, X. Unraveling the effect of O2, NO2 and SO2 impurities on the stress corrosion behavior of X65 steel in water-saturated supercritical CO2 streams. Corros. Sci. 2022, 209, 110729. [Google Scholar] [CrossRef]
- Li, K.; Zhu, Z.; Xiao, B.; Luo, J.-L.; Zhang, N. State of the art overview material degradation in high-temperature supercritical CO2 environments. Prog. Mater. Sci. 2023, 136, 101107. [Google Scholar] [CrossRef]
- Sun, C.; Wang, Y.; Sun, J.; Lin, X.; Li, X.; Liu, H.; Cheng, X. Effect of impurity on the corrosion behavior of X65 steel in water-saturated supercritical CO2 system. J. Supercrit. Fluids 2016, 116, 70–82. [Google Scholar] [CrossRef]
- Li, Z.; Niu, W.; Zhao, Y.; Xu, Y.; Liu, Y.; Guo, F.; Sun, C. Effect of O2, SO2 and NO2 Impurities on Corrosion Behavior of X52 Steel in Supercritical CO2 Transport Environments. Corros. Prot. 2023, 44, 60–67, 73. [Google Scholar]
- Liu, Y.; Xiao, G.; Wang, M.; Guo, Q.; Wang, Z.; Wu, Y.; Xu, H.; Chen, D. Corrosion behavior of heat-resistant steel T91 in high-temperature supercritical carbon dioxide with impurity O2, SO2 or H2S. J. Supercrit. Fluids 2023, 198, 105936. [Google Scholar] [CrossRef]
- Sun, C.; Sun, J.; Wang, Y.; Lin, X.; Li, X.; Cheng, X.; Liu, H. Synergistic effect of O2, H2S and SO2 impurities on the corrosion behavior of X65 steel in water-saturated supercritical CO2 system. Corros. Sci. 2016, 107, 193–203. [Google Scholar] [CrossRef]
- Sun, J.; Sun, C.; Zhang, G.; Li, X.; Zhao, W.; Jiang, T.; Liu, H.; Cheng, X.; Wang, Y. Effect of O2 and H2S impurities on the corrosion behavior of X65 steel in water-saturated supercritical CO2 system. Corros. Sci. 2016, 107, 31–40. [Google Scholar] [CrossRef]
- Tang, S.; Zhu, C.; Cui, G.; Xing, X.; Mu, J.; Li, Z. Analysis of internal corrosion of supercritical CO2 pipeline. Corros. Rev. 2021, 39, 219–241. [Google Scholar] [CrossRef]
- He, Y.; Peng, C.; Feng, Y.; Wang, R.; Zhong, J. Effects of alloying elements on the microstructure and corrosion behavior of Mg–Li–Al–Y alloys. J. Alloys Compd. 2020, 834, 154344. [Google Scholar] [CrossRef]
- Jin, Z.; An, F.; Li, S.; Cui, Z.; Lu, W.; Lu, S. Effect of annealing temperature on the microstructure evolution and corrosion behavior of Carbon-interstitial FeMnCoCrNi high-entropy alloys. Corros. Sci. 2024, 228, 111813. [Google Scholar] [CrossRef]
- Yu, L.; Lu, Z.; Xian, J.; Chen, X.; Peng, S.; Li, X.; Li, H. Effects of Al content on microstructure and tensile properties of Ni-based ODS superalloys. J. Alloys Compd. 2023, 941, 168965. [Google Scholar] [CrossRef]
- Rodionova, I.G.; Feoktistova, M.V.; Baklanova, O.N.; Amezhnov, A.V.; D’yakonov, D.L. Effect of Chemical Composition and Microstructure Parameters on Carbon and Low-Alloy Steel Corrosion Resistance. Metallurgist 2018, 61, 770–776. [Google Scholar] [CrossRef]
- Sun, C.; Liu, S.; Li, J.; Zeng, H.; Luo, J.-L. Insights into the Interfacial Process in Electroless Ni–P Coating on Supercritical CO2 Transport Pipeline as Relevant to Carbon Capture and Storage. ACS Appl. Mater. Interfaces 2019, 11, 16243–16251. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Leng, X.; Zhao, L.; Bai, B.; Wang, X.; Chen, H. Review on the Corrosion Behaviour of Nickel-Based Alloys in Supercritical Carbon Dioxide under High Temperature and Pressure. Crystals 2023, 13, 725. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, Z.; Jia, H.; Gao, R.; Ran, M.; Zeng, X.; Lozano-Perez, S.; Zheng, W.; Zhou, Z. The effect of surface grinding and Si addition on the corrosion of Fe-12Cr ODS steels in supercritical CO2. Corros. Sci. 2023, 224, 111533. [Google Scholar] [CrossRef]
- Saarimaa, V.; Kaleva, A.; Ismailov, A.; Laihinen, T.; Virtanen, M.; Levänen, E.; Väisänen, P. Corrosion product formation on zinc-coated steel in wet supercritical carbon dioxide. Arab. J. Chem. 2022, 15, 103636. [Google Scholar] [CrossRef]
- Firouzdor, V.; Sridharan, K.; Cao, G.; Anderson, M.; Allen, T.R. Corrosion of a stainless steel and nickel-based alloys in high temperature supercritical carbon dioxide environment. Corros. Sci. 2013, 69, 281–291. [Google Scholar] [CrossRef]
- Zhang, G.A.; Liu, D.; Li, Y.Z.; Guo, X.P. Corrosion behaviour of N80 carbon steel in formation water under dynamic supercritical CO2 condition. Corros. Sci. 2017, 120, 107–120. [Google Scholar] [CrossRef]
- Wei, L.; Pang, X.; Gao, K. Corrosion of low alloy steel and stainless steel in supercritical CO2/H2O/H2S systems. Corros. Sci. 2016, 111, 637–648. [Google Scholar] [CrossRef]
- Mao, X.; Xie, J.; Fan, W.; Song, W.; Zhao, M. Corrosion Behavior and Material Selection of Tubing for a H2S Containing Gas Well. Corros. Prot. 2013, 34, 946–949. [Google Scholar]
- Helleis, R.; Maia, G.A.R.; de Castro, E.G.; Berbel, L.O.; Costa, I.; Banczek, E.d.P. Niobium- and titanium-based coating for the protection of carbon steel SAE 1020 against corrosion. Anti-Corros. Methods Mater. 2022, 69, 426–433. [Google Scholar] [CrossRef]
- Meng, Z.; Shi, Y.; Qiao, Z.; Yang, J.; Wang, L. Study on microstructure and corrosion resistance of Ti-doped nickel-based alloy coatings. J. Solid State Electrochem. 2022, 26, 1677–1686. [Google Scholar] [CrossRef]
- Brittan, A.M.; Mahaffey, J.T.; Colgan, N.E.; Elbakhshwan, M.; Anderson, M.H. Carburization resistance of cu-coated stainless steel in supercritical carbon dioxide environments. Corros. Sci. 2020, 169, 108639. [Google Scholar] [CrossRef]
- Devendra, B.K.; Praveen, B.M.; Tripathi, V.S.; Nagaraju, G.; Nagaraju, D.H.; Nayana, K.O. Highly corrosion resistant platinum-rhodium alloy coating and its photocatalytic activity. Inorg. Chem. Commun. 2021, 134, 109065. [Google Scholar] [CrossRef]
- Zuo, Y.; Li, S.; Zhao, X.; Tang, Y. Preparation and performance of a Pd-Co gradient coating on stainless steel. Surf. Coat. Technol. 2017, 330, 249–254. [Google Scholar] [CrossRef]
- Huang, D.; Xu, Z.; Jia, X.; Yu, H.; He, Y.; Dong, Z.; Li, S.; Zhang, H. Uniform Microstructure and Excellent Corrosion Resistance of HVOF-Sprayed CoCrNi Medium-Entropy Alloy Coating in Fluoride Ion Environment. Met. Mater. Int. 2024, 30, 61–76. [Google Scholar] [CrossRef]
- Wang, J.; Lei, W.; Deng, Y.; Xue, Z.; Qian, H.; Liu, W.; Li, X. Effect of current density on microstructure and corrosion resistance of Ni-graphene oxide composite coating electrodeposited under supercritical carbon dioxide. Surf. Coat. Technol. 2019, 358, 765–774. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, K.; Fu, H.; Yang, X.; Lin, J. Effect of NbC Addition on the Microstructure and Wear Resistance of Laser Cladding Nickel-Based Alloy Coatings. J. Mater. Eng. Perform. 2023, 1–15. [Google Scholar] [CrossRef]
- Sim, S.; Cole, I.S.; Choi, Y.S.; Birbilis, N. A review of the protection strategies against internal corrosion for the safe transport of supercritical CO2 via steel pipelines for CCS purposes. Int. J. Greenh. Gas Control. 2014, 29, 185–199. [Google Scholar] [CrossRef]
- Hu, C.; Li, T.; Yin, H.; Hu, L.; Tang, J.; Ren, K. Preparation and corrosion protection of three different acids doped polyaniline/epoxy resin composite coatings on carbon steel. Colloids Surf. A Physicochem. Eng. Asp. 2021, 612, 126069. [Google Scholar] [CrossRef]
- Salzano de Luna, M. Recent Trends in Waterborne and Bio-Based Polyurethane Coatings for Corrosion Protection. Adv. Mater. Interfaces 2022, 9, 2101775. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Q.; Wang, G.; Liang, J.; Liu, Y.; Zhang, S.; Liu, Y.; Chen, Q.; Zhao, G.; Liu, Y. Impressive reinforcement on the anti-corrosion ability of the commercial fluorocarbon paint via nitrogen-doped super-easily-dispersed graphene. Diam. Relat. Mater. 2023, 136, 109923. [Google Scholar] [CrossRef]
- Verma, A.; Bandyopadhyay, R.; Tiwary, C.S.; Kanti Das, B.; Bhattacharya, J. Engineering defect concentration and morphology of green graphene for the development of hard and anticorrosive coating on carbon steel. Corros. Sci. 2023, 224, 111523. [Google Scholar] [CrossRef]
- Avchukir, K.; Burkitbayeva, B.D. Conductive Polymer/SiO2 Composite as an Anticorrosive Coating Against Carbon Dioxide Corrosion of Mild Steel. A Simulation Study. Eurasian Chem.-Technol. J. 2020, 22, 295. [Google Scholar] [CrossRef]
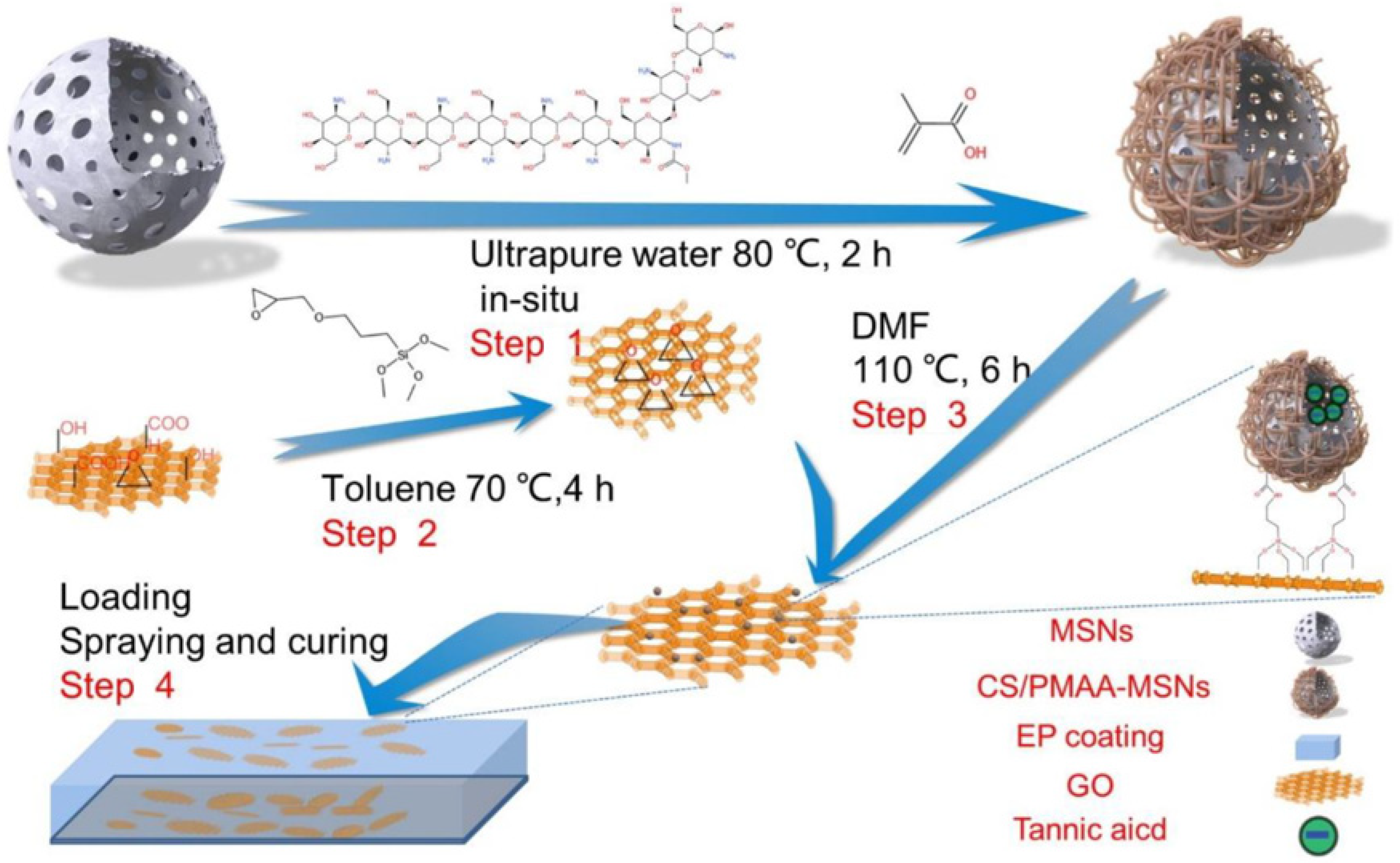
- Liu, Z.; Zhang, B.; Yu, H.; Zhang, Z.; Jiang, W.; Ma, Z. A Smart Anticorrosive Epoxy Coating Based on Graphene Oxide/Functional Mesoporous Silica Nanoparticles for Controlled Release of Corrosion Inhibitors. Coatings 2022, 12, 1749. [Google Scholar] [CrossRef]
- Cao, Y.; He, J.; Wu, J.; Wang, X.; Lu, W.; Lin, J.; Xu, Y.; Chen, G.; Zeng, B.; Dai, L. A Smart Anticorrosive Epoxy Coating Based on Environmental-Stimuli-Responsive Copolymer Assemblies for Controlled Release of Corrosion Inhibitors. Macromol. Mater. Eng. 2022, 307, 2100983. [Google Scholar] [CrossRef]
- Alagi, P.; Ghorpade, R.; Choi, Y.J.; Patil, U.; Kim, I.; Baik, J.H.; Hong, S.C. Carbon Dioxide-Based Polyols as Sustainable Feedstock of Thermoplastic Polyurethane for Corrosion-Resistant Metal Coating. ACS Sustain. Chem. Eng. 2017, 5, 3871–3881. [Google Scholar] [CrossRef]
- Li, Y.; Wu, C.; Xue, M.; Cai, J.; Huang, Y.; Yang, H. Preparation of Sol-Gel Derived Anticorrosive Coating on Q235 Carbon Steel Substrate with Long-Term Corrosion Prevention Durability. Materials 2019, 12, 1960. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, X.; Liu, H.; Han, F.; Liu, S. Graphene modified phosphate-based metal/ceramic composite coating for corrosion protection in the high-temperature marine environment. Ceram. Int. 2022, 48, 25858–25871. [Google Scholar] [CrossRef]
- Ammar, A.U.; Shahid, M.; Ahmed, M.K.; Khan, M.; Khalid, A.; Khan, Z.A. Electrochemical Study of Polymer and Ceramic-Based Nanocomposite Coatings for Corrosion Protection of Cast Iron Pipeline. Materials 2018, 11, 332. [Google Scholar] [CrossRef]
- Bura, R.; Kumar, B.; Prasad, R.M. Polymer-derived SiOC ceramic coating for corrosion protection. Int. J. Appl. Ceram. Technol. 2024, 21, 900–909. [Google Scholar] [CrossRef]
- Pietrzyk, B.; Miszczak, S. Functional Ceramic Coatings. Coatings 2021, 11, 130. [Google Scholar] [CrossRef]
- Mashtalyar, D.V.; Nadaraia, K.V.; Belov, E.A.; Imshinetskiy, I.M.; Sinebrukhov, S.L.; Gnedenkov, S.V. Features of Composite Layers Created Using an Aqueous Suspension of a Fluoropolymer. Polymers 2022, 14, 4667. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gu, C.; Wen, Z.; Hou, B. Improvement of active corrosion protection of carbon steel by water-based epoxy coating with smart CeO2 nanocontainers. Prog. Org. Coat. 2018, 115, 195–204. [Google Scholar] [CrossRef]
- Lei, Y.; Qiu, Z.; Tan, N.; Du, H.; Li, D.; Liu, J.; Liu, T.; Zhang, W.; Chang, X. Polyaniline/CeO2 nanocomposites as corrosion inhibitors for improving the corrosive performance of epoxy coating on carbon steel in 3.5% NaCl solution. Prog. Org. Coat. 2020, 139, 105430. [Google Scholar] [CrossRef]
- Raj, R.; Morozov, Y.; Calado, L.M.; Taryba, M.G.; Kahraman, R.; Shakoor, R.A.; Montemor, M.F. Calcium carbonate particles loaded with triethanolamine and polyethylenimine for enhanced corrosion protection of epoxy coated steel. Corros. Sci. 2020, 167, 108548. [Google Scholar] [CrossRef]
- Samiee, R.; Ramezanzadeh, B.; Mahdavian, M.; Alibakhshi, E. Assessment of the smart self-healing corrosion protection properties of a water-base hybrid organo-silane film combined with non-toxic organic/inorganic environmentally friendly corrosion inhibitors on mild steel. J. Clean. Prod. 2019, 220, 340–356. [Google Scholar] [CrossRef]
- Li, S.; Xu, Y.; Xiang, F.; Liu, P.; Wang, H.; Wei, W.; Dong, S. Enhanced corrosion resistance of self-healing waterborne polyurethane coating based on tannic acid modified cerium-montmorillonites composite fillers. Prog. Org. Coat. 2023, 178, 107454. [Google Scholar] [CrossRef]
- Majidi, R.; Danaee, I.; Vrsalović, L.; Zarei, D. Development of a smart anticorrosion epoxy coating containing a pH-sensitive GO/MOF nanocarrier loaded with 2-mercaptobenzothiazole corrosion inhibitor. Mater. Chem. Phys. 2023, 308, 128291. [Google Scholar] [CrossRef]
- Yadav, A.; Kumar, R.; Choudhary, H.K.; Sahoo, B. Graphene-oxide coating for corrosion protection of iron particles in saline water. Carbon 2018, 140, 477–487. [Google Scholar] [CrossRef]
- Xue, Z.; Lei, W.; Wang, Y.; Qian, H.; Li, Q. Effect of pulse duty cycle on mechanical properties and microstructure of nickel-graphene composite coating produced by pulse electrodeposition under supercritical carbon dioxide. Surf. Coat. Technol. 2017, 325, 417–428. [Google Scholar] [CrossRef]
- Nam, N.D.; Bui, Q.V.; Mathesh, M.; Tan, M.Y.J.; Forsyth, M. A study of 4-carboxyphenylboronic acid as a corrosion inhibitor for steel in carbon dioxide containing environments. Corros. Sci. 2013, 76, 257–266. [Google Scholar] [CrossRef]
- Zhao, Q.; Guo, J.; Cui, G.; Han, T.; Wu, Y. Chitosan derivatives as green corrosion inhibitors for P110 steel in a carbon dioxide environment. Colloids Surf. B Biointerfaces 2020, 194, 111150. [Google Scholar] [CrossRef] [PubMed]
- Usman, B.J.; Ali, S.A. Carbon Dioxide Corrosion Inhibitors: A review. Arab. J. Sci. Eng. 2018, 43, 1–22. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Quraishi, M.A.; Sorour, A.A.; Verma, C. A review on corrosion inhibitors for high-pressure supercritical CO2 environment: Challenges and opportunities. J. Pet. Sci. Eng. 2022, 215, 110695. [Google Scholar] [CrossRef]
- Wang, X.; Yang, J.; Chen, X. 2-Benzylsulfanyl-1H-benzimidazole and its mixture as highly efficient corrosion inhibitors for carbon steel under dynamic supercritical CO2 flow conditions. Corros. Sci. 2024, 235, 112170. [Google Scholar] [CrossRef]
- Wei, L.; Chen, Z.; Guo, X. Inhibition Behavior of an Imidazoline Inhibitor for Carbon Steel in a Supercritical CO2/H2O System. J. Electrochem. Soc. 2017, 164, C602. [Google Scholar] [CrossRef]
- Shamsa, A.; Barker, R.; Hua, Y.; Barmatov, E.; Hughes, T.L.; Neville, A. Performance evaluation of an imidazoline corrosion inhibitor in a CO2-saturated environment with emphasis on localised corrosion. Corros. Sci. 2020, 176, 108916. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, W.; Zhang, C.; Zhao, J. A Novel Imidazoline Derivative Used as an Effective Corrosion Inhibitor for Carbon Steel in a CO2/H2S Environment. Int. J. Electrochem. Sci. 2019, 14, 8579–8594. [Google Scholar] [CrossRef]
- Jevremović, I.; Singer, M.; Nešić, S.; Mišković-Stanković, V. Electrochemistry of carbon dioxide corrosion mitigation using tall oil diethylenetriamine imidazoline as corrosion inhibitor for mild steel. Mater. Corros. 2016, 67, 756–768. [Google Scholar] [CrossRef]
- Geng, S.; Hu, J.; Yu, J.; Zhang, C.; Wang, H.; Zhong, X. Rosin imidazoline as an eco-friendly corrosion inhibitor for the carbon steel in CO2-containing solution and its synergistic effect with thiourea. J. Mol. Struct. 2022, 1250, 131778. [Google Scholar] [CrossRef]
- Cuoq, F.; Benguigui, J.; Geijselaers, C.; Lampert, F. Linking Thermoelectric Effect and Adsorption of Film Forming Amine as a Corrosion Inhibitor for Industrial Systems. Ind. Eng. Chem. Res. 2020, 59, 8492–8495. [Google Scholar] [CrossRef]
- Biswal, J.; Pant, H.J.; Sharma, S.C.; Gupta, A.K. Investigation of effect of an amine inhibitor on corrosion of carbon steel by using thin layer activation technique. J. Radioanal. Nucl. Chem. 2020, 323, 1339–1345. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Hou, B.S.; Li, Y.Y.; Lei, Y.; Wang, X.; Liu, H.F.; Zhang, G.A. Two amino acid derivatives as high efficient green inhibitors for the corrosion of carbon steel in CO2-saturated formation water. Corros. Sci. 2021, 189, 109596. [Google Scholar] [CrossRef]
- Abbas, M.A.; Arafa, E.I.; Gad, E.S.; Bedair, M.A.; El-Azabawy, O.E.; Al-Shafey, H.I. Performance assessment by experimental and Theoretical approaches of newly synthetized benzyl amide derivatives as corrosion inhibitors for carbon steel in 1.0 M hydrochloric acid environment. Inorg. Chem. Commun. 2022, 143, 109758. [Google Scholar] [CrossRef]
- Verma, C.; Alrefaee, S.H.; Rhee, K.Y.; Quraishi, M.A.; Ebenso, E.E. Thiol (-SH) substituent as functional motif for effective corrosion protection: A review on current advancements and future directions. J. Mol. Liq. 2021, 324, 115111. [Google Scholar] [CrossRef]
- Tan, B.; Zhang, S.; Qiang, Y.; Li, W.; Liu, H.; Xu, C.; Chen, S. Insight into the corrosion inhibition of copper in sulfuric acid via two environmentally friendly food spices: Combining experimental and theoretical methods. J. Mol. Liq. 2019, 286, 110891. [Google Scholar] [CrossRef]
- Wang, X.; Yang, J.; Chen, X.; Yan, K. Exploring the Effectiveness of Natural Food Flavors in Protecting Carbon Steel against CO2–Induced Corrosion. ACS Omega 2023, 8, 31305–31317. [Google Scholar] [CrossRef]
- Belarbi, Z.; Vu, T.N.; Farelas, F.; Young, D.; Singer, M.; Nešić, S. Thiols as Volatile Corrosion Inhibitors for Top-of-the-Line Corrosion. Corrosion 2017, 73, 892–899. [Google Scholar] [CrossRef]
- Xu, F.-L.; Yang, J.-D.; Qiu, R.; Hou, J.; Zheng, J.-Y.; Zhang, J.-W.; Wang, L.; Sun, Z.-Y.; Lin, C.-G. Thiol self-assemble layer as inhibitor to protect B10 from seawater corrosion. Prog. Org. Coat. 2016, 97, 82–90. [Google Scholar] [CrossRef]
- Zheng, Z.; Hu, J.; Eliaz, N.; Zhou, L.; Yuan, X.; Zhong, X. Mercaptopropionic acid-modified oleic imidazoline as a highly efficient corrosion inhibitor for carbon steel in CO2-saturated formation water. Corros. Sci. 2022, 194, 109930. [Google Scholar] [CrossRef]
- Mobin, M.; Aslam, R. Ester-Based Pyridinium Gemini Surfactants as Novel Inhibitors for Mild Steel Corrosion in 1 M HCl Solution. Tenside Surfactants Deterg. 2017, 54, 486–499. [Google Scholar] [CrossRef]
- Popoola, L.T. Organic green corrosion inhibitors (OGCIs): A critical review. Corros. Rev. 2019, 37, 71–102. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Sorour, A.A.; Saji, V.S.; Quraishi, M.A. Green corrosion inhibitors based on biomacromolecules and macrocycles: A review. Sustain. Chem. Pharm. 2023, 36, 101295. [Google Scholar] [CrossRef]
- Sivakumar, P.R.; Srikanth, A.P. Green corrosion inhibitor: A comparative study. Sādhanā 2020, 45, 56. [Google Scholar] [CrossRef]
- Sun, A.; Cui, G.; Liu, Q. Capsule corrosion inhibitor loaded with hyperbranched chitosan: Carbon dioxide corrosion protection for downhole pipelines in oil fields. Colloids Surf. A Physicochem. Eng. Asp. 2023, 664, 131106. [Google Scholar] [CrossRef]
- He, H.; Shi, J.; Yu, S.; Yang, J.; Xu, K.; He, C.; Li, X. Exploring green and efficient zero-dimensional carbon-based inhibitors for carbon steel: From performance to mechanism. Constr. Build. Mater. 2024, 411, 134334. [Google Scholar] [CrossRef]
- Gabris, M.A.; Ping, J. Carbon nanomaterial-based nanogenerators for harvesting energy from environment. Nano Energy 2021, 90, 106494. [Google Scholar] [CrossRef]
- Bagheri, A.R.; Aramesh, N.; Bilal, M.; Xiao, J.; Kim, H.-W.; Yan, B. Carbon nanomaterials as emerging nanotherapeutic platforms to tackle the rising tide of cancer—A review. Bioorganic Med. Chem. 2021, 51, 116493. [Google Scholar] [CrossRef]
- Su, Y.; Yang, X.; Zhang, Q.; Sun, J.; Liu, Z. Carbon nanomaterials for highly stable Zn anode: Recent progress and future outlook. J. Electroanal. Chem. 2022, 904, 115883. [Google Scholar] [CrossRef]
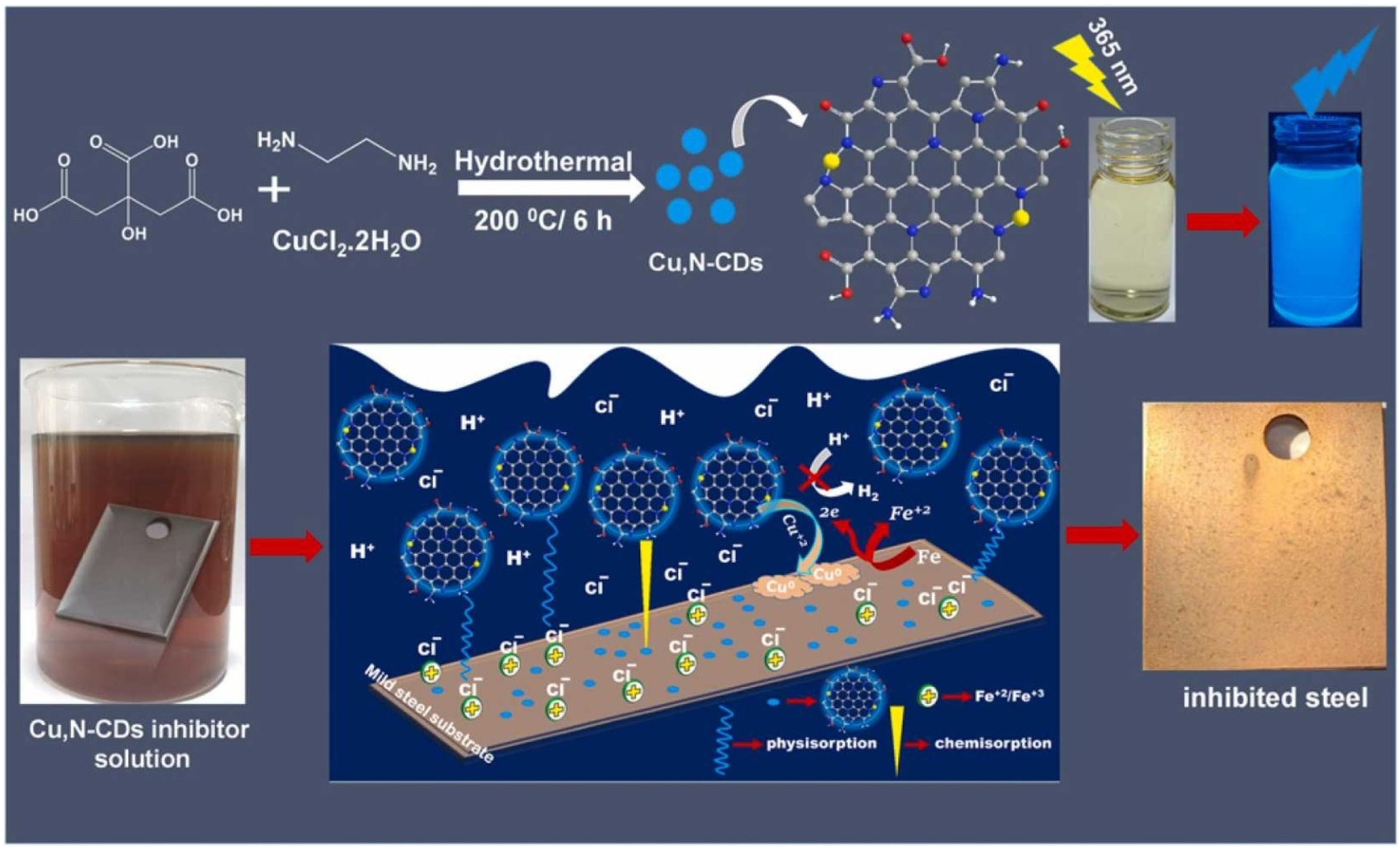
- Padhan, S.; Rout, T.K.; Nair, U.G. N-doped and Cu,N-doped carbon dots as corrosion inhibitor for mild steel corrosion in acid medium. Colloids Surf. A Physicochem. Eng. Asp. 2022, 653, 129905. [Google Scholar] [CrossRef]
- Saraswat, V.; Yadav, M. Improved corrosion resistant performance of mild steel under acid environment by novel carbon dots as green corrosion inhibitor. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127172. [Google Scholar] [CrossRef]
- Shirazi, Z.; Golikand, A.N.; Keshavarz, M.H. A new nanocomposite based on poly (o-anthranilic acid), graphene oxide and functionalized carbon nanotube as an efficient corrosion inhibitor for stainless steel in severe environmental corrosion. Compos. Commun. 2020, 22, 100467. [Google Scholar] [CrossRef]
- Yu, J.; Yan, H.; Yang, M.; Ma, Y.; Fan, X.; Zhu, M. Polyphenol-reduced graphene oxide toward high-performance corrosion inhibitor. Surf. Topogr. Metrol. Prop. 2019, 7, 025010. [Google Scholar] [CrossRef]
- Li, J.; Lv, J.; Fu, L.; Tang, M.; Wu, X. New Ecofriendly Nitrogen-Doped Carbon Quantum Dots as Effective Corrosion Inhibitor for Saturated CO2 3% NaCl Solution. Russ. J. Appl. Chem. 2020, 93, 380–392. [Google Scholar] [CrossRef]
- Zeng, S.; Zhang, F.; Liu, Y.; Ouyang, S.; Ye, Y.W.; Chen, H. Synthesis of Ce, N co–doped carbon dots as green and effective corrosion inhibitor for copper in acid environment. J. Taiwan Inst. Chem. Eng. 2022, 141, 104608. [Google Scholar] [CrossRef]
- Cen, H.; Cao, J.; Chen, Z. Functionalized carbon nanotubes as a novel inhibitor to enhance the anticorrosion performance of carbon steel in CO2-saturated NaCl solution. Corros. Sci. 2020, 177, 109011. [Google Scholar] [CrossRef]
- Hou, B.S.; Zhang, Q.H.; Li, Y.Y.; Zhu, G.Y.; Liu, H.F.; Zhang, G.A. A pyrimidine derivative as a high efficiency inhibitor for the corrosion of carbon steel in oilfield produced water under supercritical CO2 conditions. Corros. Sci. 2020, 164, 108334. [Google Scholar] [CrossRef]
- Wang, C.; Chen, J.; Hu, B.; Liu, Z.; Wang, C.; Han, J.; Su, M.; Li, Y.; Li, C. Modified chitosan-oligosaccharide and sodium silicate as efficient sustainable inhibitor for carbon steel against chloride-induced corrosion. J. Clean. Prod. 2019, 238, 117823. [Google Scholar] [CrossRef]
- Bian-li, Q.; Jun-qi, L.; Chao-yi, C. Synergy corrosion effect of thiosulfate and sulfide on Q235 steel in sodium aluminate solution. Mater. Res. Express 2019, 6, 025607. [Google Scholar] [CrossRef]
- Ma, I.A.W.; Ammar, S.; Kumar, S.S.A.; Ramesh, K.; Ramesh, S. A concise review on corrosion inhibitors: Types, mechanisms and electrochemical evaluation studies. J. Coat. Technol. Res. 2022, 19, 241–268. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Nguyen, A.S.; Tran, B.A.; Vu, K.O.; Tran, D.L.; Phan, T.T.; Scharnagl, N.; Zheludkevich, M.L.; To, T.X.H. Molybdate intercalated hydrotalcite/graphene oxide composite as corrosion inhibitor for carbon steel. Surf. Coat. Technol. 2020, 399, 126165. [Google Scholar] [CrossRef]
- Yan, T.; Xu, L.-C.; Zeng, Z.-X.; Pan, W.-G. Mechanism and anti-corrosion measures of carbon dioxide corrosion in CCUS: A review. iScience 2024, 27, 108594. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Tang, J.; Wang, Y.; Wang, H.; Zuo, Y. Study on Synergistic Corrosion Inhibition Effect between Calcium Lignosulfonate (CLS) and Inorganic Inhibitors on Q235 Carbon Steel in Alkaline Environment with Cl−. Molecules 2020, 25, 4200. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wu, T.; Zeng, F.; Chen, K.; Li, L.; Qu, Q. Streptococcus mutans soluble extracellular polymeric substances and sodium molybdate as mixed corrosion inhibitor for X70 steel. J. Mater. Sci. 2023, 58, 2915–2934. [Google Scholar] [CrossRef]
- Chen, M.; Xue, M.; Huang, C.; Chen, Y.; Wu, J.; Xu, H.; Zhang, X. Study on the corrosion inhibition performance of imidazoline composite inhibitor. Asia-Pac. J. Chem. Eng. 2022, 17, e2671. [Google Scholar] [CrossRef]
- Guo, R.; Li, W.; Wang, X.; Lv, Y.; Chen, M.; Chen, Z.; Liu, Z.; Han, G.-C. Antimicrobial corrosion study of the epoxy coating with the graphene oxide supported Schiff base quaternary ammonium salt additives. Mater. Today Commun. 2023, 35, 105517. [Google Scholar] [CrossRef]
- Fakir, S.H.; Chaitanya Kumar, K.; Ali, H.; Rawat, J.; Haq Farooqi, I. Synthesis and evaluation of green corrosion inhibitor from rice husk to mitigate corrosion of carbon steel in NaCl environment. Mater. Today Proc. 2023, 82, 29–37. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, L.; Han, J.; Zhu, C. Properties of sodium molybdate-based compound corrosion inhibitor for hot-dip galvanized steel in marine environment. Corros. Rev. 2023, 41, 225–235. [Google Scholar] [CrossRef]
- Ackland, B.G.; Dylejko, K.P. Critical questions and answers about cathodic protection. Corros. Eng. Sci. Technol. 2019, 54, 688–697. [Google Scholar] [CrossRef]
- Yan, Y.; Deng, H.; Xiao, W.; Cao, X.; Hou, X. Research Progress on Anti-corrosion Technologies for Supercritical CO2 Pipeline. Corros. Sci. Prot. Technol. 2019, 31, 436–442. [Google Scholar]
- Lu, H.; Ma, X.; Huang, K.; Fu, L.; Azimi, M. Carbon dioxide transport via pipelines: A systematic review. J. Clean. Prod. 2020, 266, 121994. [Google Scholar] [CrossRef]
- Zhao, Q.; Huang, X.; Zhan, Z.; Zhou, S.; Liu, J.; Zhu, P.; Wei, L.; Li, X.; Li, C.; Xie, Y. Effect of alloying elements (Mn, Ti, and Mo) on the corrosion behavior of FeCoNiCr-based high entropy alloy in supercritical water. Corros. Sci. 2023, 220, 111291. [Google Scholar] [CrossRef]
- Parks, C.J. Corrosion of Candidate High Temperature Alloys in Supercritical Carbon Dioxide. Ph.D. Thesis, Carleton University, Ottawa, ON, Canada, 2013. [Google Scholar]
- Xiao, B.; Zhang, N.; Li, K.; Zhu, Z.; Zhang, T.; Zhou, M. Corrosion behaviour of Ni-based alloy Inconel 740H in supercritical carbon dioxide at 650–700 °C. Corros. Eng. Sci. Technol. 2023, 58, 180–189. [Google Scholar] [CrossRef]
- Farelas, F.; Choi, Y.S.; Nešić, S. Corrosion Behavior of API 5L X65 Carbon Steel Under Supercritical and Liquid Carbon Dioxide Phases in the Presence of Water and Sulfur Dioxide. Corrosion 2012, 69, 243–250. [Google Scholar] [CrossRef]
- Xiang, Y.; Wang, Z.; Yang, X.; Li, Z.; Ni, W. The upper limit of moisture content for supercritical CO2 pipeline transport. J. Supercrit. Fluids 2012, 67, 14–21. [Google Scholar] [CrossRef]
- Vitali, M.; Corvaro, F.; Marchetti, B.; Terenzi, A. Thermodynamic challenges for CO2 pipelines design: A critical review on the effects of impurities, water content, and low temperature. Int. J. Greenh. Gas Control. 2022, 114, 103605. [Google Scholar] [CrossRef]


 , X60 steel;
, X60 steel;  , X65 steel;
, X65 steel;  , X70 steel;
, X70 steel;  , X80 steel [55].
, X80 steel [55].
 , X60 steel;
, X60 steel;  , X65 steel;
, X65 steel;  , X70 steel;
, X70 steel;  , X80 steel [55].
, X80 steel [55].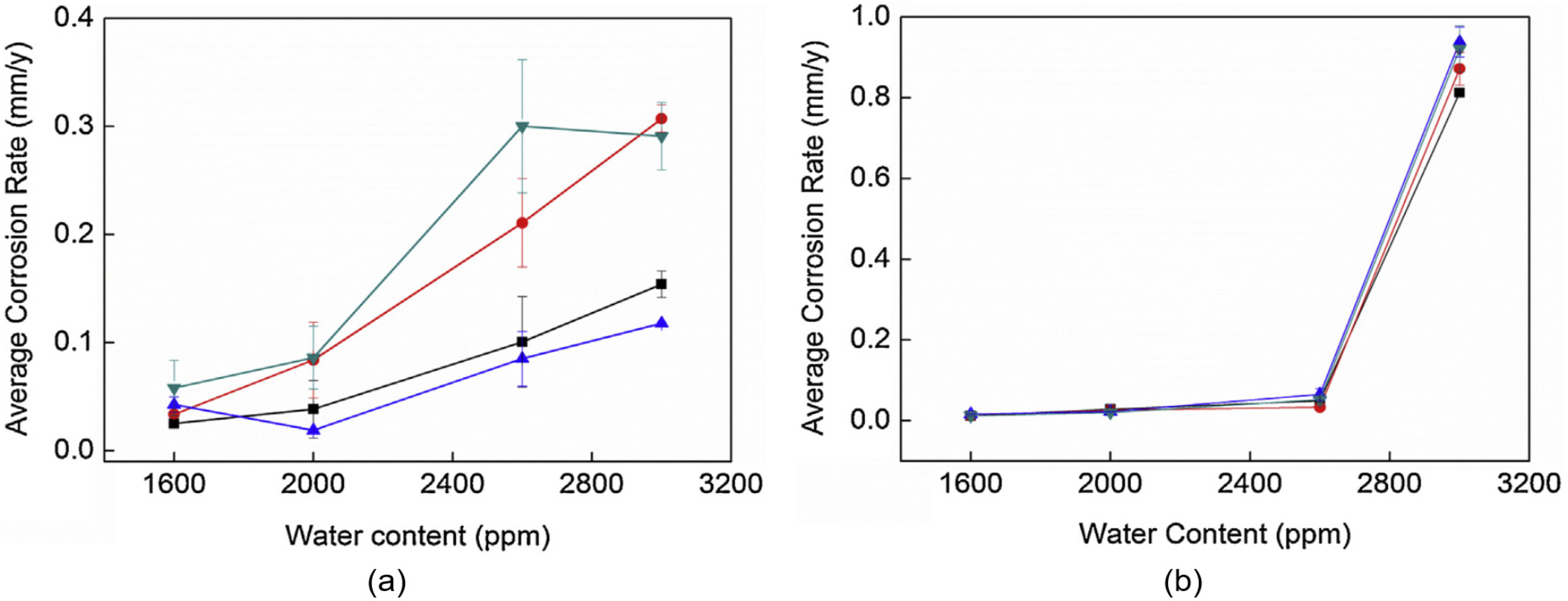

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Wang, J.; Li, X.; Huang, J.; Chen, H.; Bi, J.; Liu, S.; Lu, G.; Song, K.; Guo, S. Progress in Corrosion Protection Research for Supercritical CO2 Transportation Pipelines. Coatings 2024, 14, 1378. https://doi.org/10.3390/coatings14111378
Zhao C, Wang J, Li X, Huang J, Chen H, Bi J, Liu S, Lu G, Song K, Guo S. Progress in Corrosion Protection Research for Supercritical CO2 Transportation Pipelines. Coatings. 2024; 14(11):1378. https://doi.org/10.3390/coatings14111378
Chicago/Turabian StyleZhao, Cailing, Jianming Wang, Xin Li, Jinzhen Huang, Huikai Chen, Jinye Bi, Sawen Liu, Guoqiang Lu, Kun Song, and Shengjun Guo. 2024. "Progress in Corrosion Protection Research for Supercritical CO2 Transportation Pipelines" Coatings 14, no. 11: 1378. https://doi.org/10.3390/coatings14111378
APA StyleZhao, C., Wang, J., Li, X., Huang, J., Chen, H., Bi, J., Liu, S., Lu, G., Song, K., & Guo, S. (2024). Progress in Corrosion Protection Research for Supercritical CO2 Transportation Pipelines. Coatings, 14(11), 1378. https://doi.org/10.3390/coatings14111378