Processing of Porous-Core Materials for Bone Implant Applications: A Permeability and Mechanical Strength Analysis
Abstract
1. Introduction
2. Materials and Methods
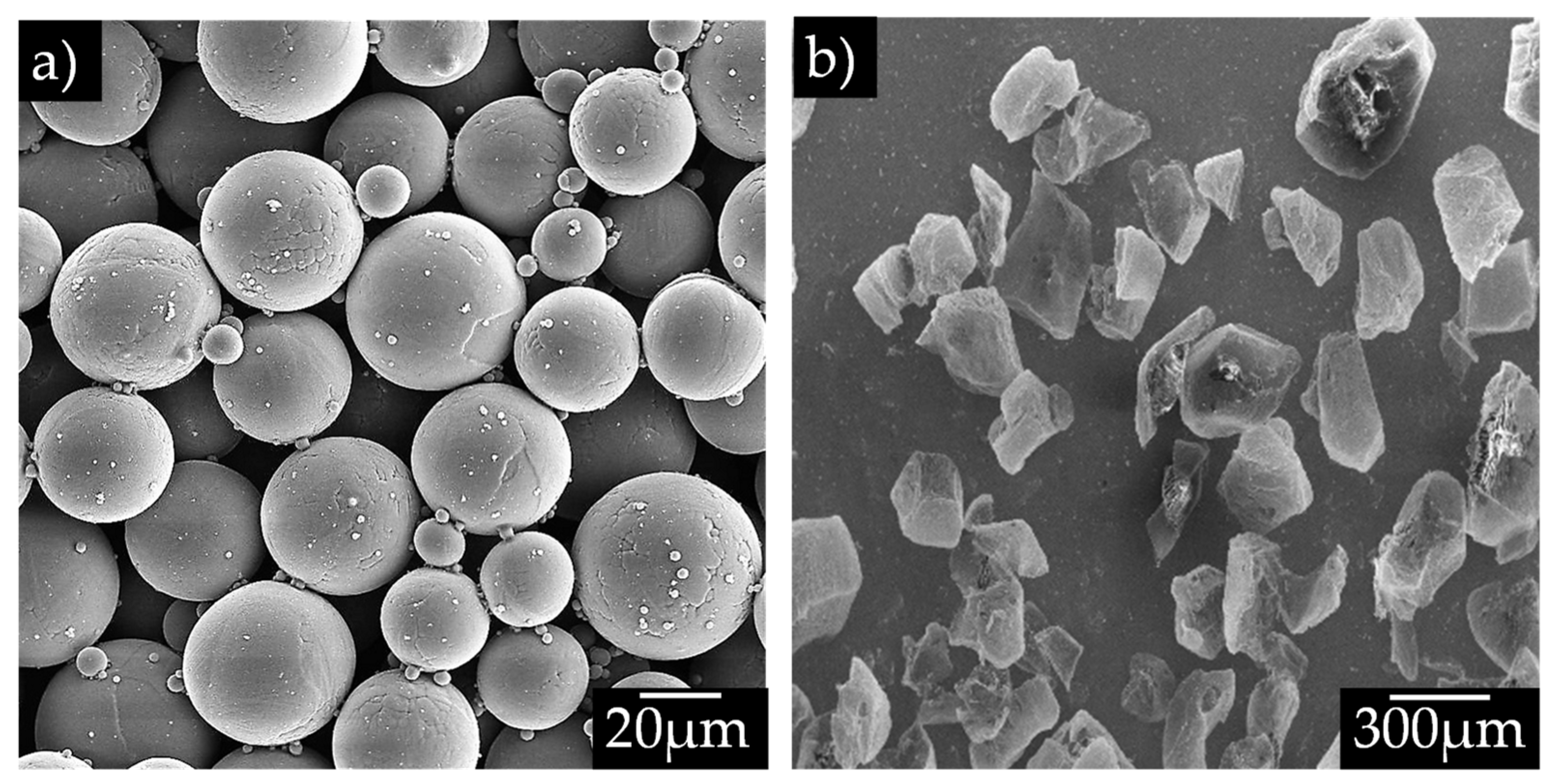
2.1. Materials
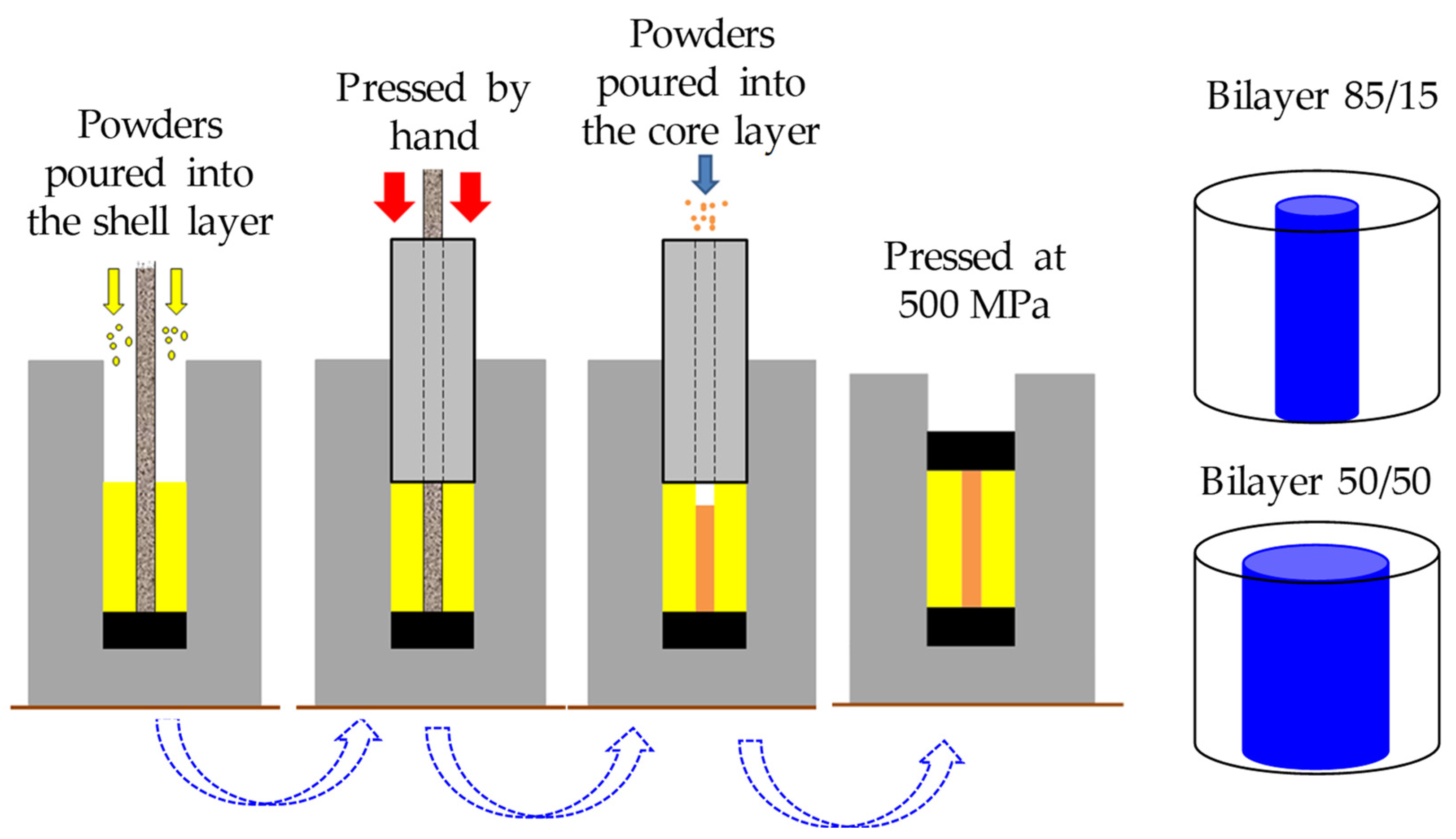
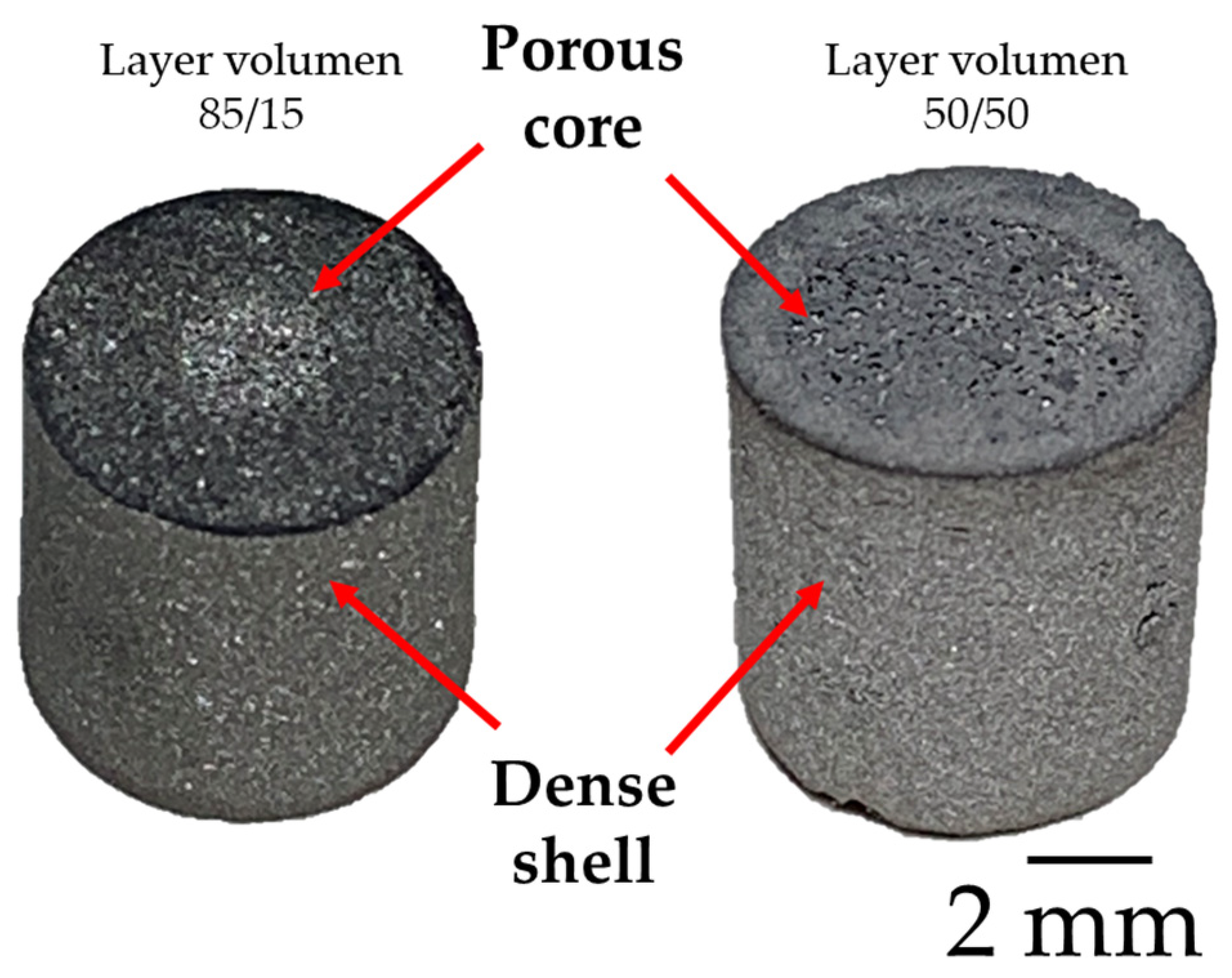
2.2. Sample Preparation
2.3. Microstructure Characterization and Mechanical Evaluation
3. Results and Discussion
3.1. Dilatometry Analysis
3.2. Tomography Analysis
3.3. Mechanical Strength Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Xu, S.; Zhou, S.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y.M. Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: A review. Biomaterials 2016, 83, 127–141. [Google Scholar] [CrossRef]
- Murr, L.E.; Gaytan, S.M.; Martinez, E.; Medina, F.; Wicker, R.B. Next generation orthopaedic implants by additive manufacturing using electron beam melting. Int. J. Biomater. 2012, 2012, 245727. [Google Scholar] [CrossRef]
- Chen, Q.Z.; Thompson, I.D.; Boccaccini, A.R. 45S5 Bioglass®-derived glass–ceramic scaffolds for bone tissue engineering. Biomaterials 2006, 27, 2414–2425. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Mitra, I.; Avila, J.D.; Upadhyayula, M.; Bose, S. Porous metal implants: Processing, properties, and challenges. Int. J. Extreme Manuf. 2023, 5, 032014. [Google Scholar] [CrossRef]
- Russell Levive, B.; Spoere, S.; Poggie, R.A.; Della Valle, C.J.; Jacobs, J.J. Experimental and clinical performance of porous tantalum in orthopaedic surgery. J. Biomater. 2006, 27, 4671–4681. [Google Scholar] [CrossRef]
- España, F.A.; Balla, V.K.; Bose, S.; Bandyopadhyay, A. Design and fabrication of CoCrMo alloy based novel structures for load bearing implants using laser engineered net shaping. Mater. Sci. Eng. C 2010, 30, 50–57. [Google Scholar] [CrossRef]
- Bansiddhi, A.; Sargeant, T.D.; Stupp, S.I.; Dunand, D.C. Porous NiTi for bone implants: A review. Acta Biomater. 2008, 4, 773–782. [Google Scholar] [CrossRef]
- Ballarre, J.; Manjubala, I.; Schreiner, W.H.; Orellano, J.C.; Fratzl, P.; Ceré, S. Improving the osteointegration and bone–implant interface by incorporation of bioactive particles in sol–gel coatings of stainless-steel implants. Acta Biomater. 2010, 6, 1601–1609. [Google Scholar] [CrossRef]
- Shirazi, S.F.S.; Gharehkhani, S.; Mehrali, M.; Yarmand, H.; Metselaar, H.S.C.; Kadri, N.A.; Osman, N.A.A. A review on powder-based additive manufacturing for tissue engineering: Selective laser sintering and inkjet 3D printing. Sci. Technol. Adv. 2015, 16, 033502. [Google Scholar] [CrossRef]
- Dumas, M.; Terriault, P.; Brailovski, V. Modelling and characterization of a porosity graded lattice structure for additively manufactured biomaterials. Mater. Des. 2017, 121, 383–392. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, L.; Liu, L.; Lv, L.; Gao, L.; Liu, N.; Wang, X.; Ye, J. Mechanical behavior of a titanium alloy scaffold mimicking trabecular structure. J. Orthop. Surg. Res. 2020, 15, 40. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, X.; Chen, X.; He, Y.; Cheng, L.; Huo, M.; Yin, J.; Hao, F.; Chen, S.; Wang, P.; et al. Additive manufacturing of structural materials. Mater. Sci. Eng. R Rep. 2021, 145, 100596. [Google Scholar] [CrossRef]
- Arifvianto, B.; Zhou, J. Fabrication of metallic biomedical scaffolds with the space holder method: A review. Materials 2014, 7, 3588–3622. [Google Scholar] [CrossRef]
- Reig, L.; Amigó, V.; Busquets, D.J.; Calero, J.A. Development of porous Ti6Al4V samples by microsphere sintering. Mater. Process. Technol. 2012, 212, 3–7. [Google Scholar] [CrossRef]
- Torres, Y.; Rodríguez, J.A.; Arias, S.; Echeverry, M.; Robledo, S.; Amigo, V.; Pavón, J.J. Processing, characterization and biological testing of porous titanium obtained by space-holder technique. J. Mater. Sci. 2012, 47, 6565–6576. [Google Scholar] [CrossRef]
- Zhang, E.; Zhao, X.; Hu, J.; Wang, R.; Fu, S.; Qin, G. Antibacterial metals and alloys for potential biomedical implants. Bioact. Mater. 2021, 6, 2569–2612. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Cui, Y.-W.; Zhang, L.-C. Recent Development in Beta Titanium Alloys for Biomedical Applications. Metals 2020, 10, 1139. [Google Scholar] [CrossRef]
- Cabezas-Villa, J.L.; Olmos, L.; Bouvard, D.; Lemus-Ruiz, J.; Jiménez, O. Processing and properties of highly porous Ti6Al4V mimicking human bones. Mater. Res. 2018, 33, 650–661. [Google Scholar] [CrossRef]
- Anene, F.A.; Aiza Jaafar, C.N.; Zainol, I.; Azmah Hanim, M.A.; Suraya, M.T. Biomedical materials: A review of titanium based alloys. Proc. Inst. Mech. Eng. C J. Mech. Eng. Sci. 2021, 235, 3792–3805. [Google Scholar] [CrossRef]
- Jakubowicz, J.; Adamek, G.; Dewidar, M. Titanium foam made with saccharose as a space holder. J. Porous Mater. 2013, 20, 1137–1141. [Google Scholar] [CrossRef]
- Bahraminasab, M.; Sahari, B.B.; Edwards, K.L.; Farahmand, F.; Arumugam, M.; Hong, T.S. Aseptic loosening of femoral components–a review of current and future trends in materials used. Mater. Des. 2012, 42, 459–470. [Google Scholar] [CrossRef]
- Gibson, L.J.; Ashby, M.F. Cellular Solids: Structure and Properties, 2nd ed.; Cambridge University Press: Cambridge, UK, 1999; p. 175. [Google Scholar]
- Phani, K.K.; Niyogi, S.K. Young’s modulus of porous brittle solids. J. Mater. Sci. 1987, 22, 257–263. [Google Scholar] [CrossRef]
- Nielsen, L.F. Elasticity and damping of porous materials and impregnated materials. J. Am. Ceram. Soc. 1984, 67, 93–98. [Google Scholar] [CrossRef]
- Breme, J.; Wadewitz, V. Comparison of titanium-tantalum and titanium-niobium alloys for application as dental implants . Int. J. Oral Maxillofac. Implant. 1989, 4, 113–118. [Google Scholar]
- Woodard, J.R.; Hilldore, A.J.; Lan, S.K.; Park, C.J.; Morgan, A.W.; Eurell, J.A.C.; Clark, S.G.; Wheeler, M.B.; Jamison, R.D.; Johnson, A.J.W. The mechanical properties and osteoconductivity of hydroxyapatite bone scaffolds with multi-scale porosity. Biomaterials 2007, 28, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, M.; Fujibayashi, S.; Neo, M.; Suzuki, J.; Kokubo, T.; Nakamura, T. Mechanical properties and osteoconductivity of porous bioactive titanium. Biomaterials 2005, 26, 6014–6023. [Google Scholar] [CrossRef] [PubMed]
- Dabrowski, B.; Swieszkowski, W.; Godlinski, D.; Kurzydlowski, K.J. Highly porous titanium scaffolds for orthopaedic applications. ACS Appl. Biomater. B 2010, 95, 53–61. [Google Scholar] [CrossRef]
- Wang, C.A.; Le, H.; Huang, Y. Rapid assembly processes of ordered inorganic/organic nanocomposites. In Biomimetics: Learning from Nature; Books on Demand: Norderstedt, Germany, 2010; pp. 1–24. [Google Scholar]
- Kienapfel, H.; Sprey, C.; Wilke, A.; Griss, P. Implant fixation by bone ingrowth. J. Arthroplast. 1999, 14, 355–368. [Google Scholar] [CrossRef]
- Singh, R.; Lee, P.D.; Lindley, T.C.; Dashwood, R.J.; Ferrie, E.; Imwinkelried, T. Characterization of the structure and permeability of titanium foams for spinal fusion devices. Acta Biomater. 2009, 5, 477–487. [Google Scholar] [CrossRef]
- Varley, M.C.; Neelakantan, S.; Clyne, T.W.; Dean, J.; Brooks, R.A.; Markaki, A.E. Cell structure, stiffness and permeability of freeze-dried collagen scaffolds in dry and hydrated states. Acta Biomater. 2016, 33, 166–175. [Google Scholar] [CrossRef]
- Olmos, L.; Bouvard, D.; Cabezas-Villa, J.L.; Lemus-Ruiz, J.; Jiménez, O.; Arteaga, D. Analysis of compression and permeability behavior of porous Ti6Al4V by computed microtomography. Met. Mater. Int. 2019, 25, 669–682. [Google Scholar] [CrossRef]
- Nauman, E.A.; Fong, K.E.; Keaveny, T.M. Dependence of intertrabecular permeability on flow direction and anatomic site. Ann. Biomed. Eng. 1999, 27, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.C.; Kuo, C.N.; Wu, T.H.; Liu, T.Y.; Chen, Y.W.; Guo, X.H.; Huang, J.C. Empirical rule for predicting mechanical properties of Ti-6Al-4V bone implants with radial-gradient porosity bionic structures. Mater. Today Commun. 2021, 27, 102346. [Google Scholar] [CrossRef]
- Davoodi, E.; Montazerian, H.; Esmaeilizadeh, R.; Darabi, A.C.; Rashidi, A.; Kadkhodapour, J.; Ahed, H.; Hoorfar, M.; Milani, A.S.; Weiss, P.S.; et al. Additively manufactured gradient porous Ti–6Al–4V hip replacement implants embedded with cell-laden gelatin methacryloyl hydrogels. ACS Appl. Mater. Interfaces 2021, 13, 22110–22123. [Google Scholar] [CrossRef] [PubMed]
- Koju, N.; Niraula, S.; Fotovvati, B. Additively Manufactured Porous Ti6Al4V for Bone Implants: A Review. Metals 2022, 12, 687. [Google Scholar] [CrossRef]
- Li, X.; Xiong, Y.Z.; Zhang, H.; Gao, R.N. Development of functionally graded porous titanium/silk fibroin composite scaffold for bone repair. Mater. Lett. 2021, 282, 128670. [Google Scholar] [CrossRef]
- Wang, S.; Liu, L.; Li, K.; Zhu, L.; Chen, J.; Hao, Y. Pore functionally graded Ti6Al4V scaffolds for bone tissue engineering application. Mater. Des. 2019, 168, 107643. [Google Scholar] [CrossRef]
- Xiong, Y.Z.; Gao, R.N.; Zhang, H.; Dong, L.L.; Li, J.T.; Li, X. Rationally designed functionally graded porous Ti6Al4V scaffolds with high strength and toughness built via selective laser melting for load-bearing orthopedic applications. J. Mech. Behav. Biomed. Mater. 2020, 104, 103673. [Google Scholar] [CrossRef]
- Yu, G.; Li, Z.; Li, S.; Zhang, Q.; Hua, Y.; Liu, H.; Zhao, X.; Dhaidhai, D.T.; Li, W.; Wang, X. The select of internal architecture for porous Ti alloy scaffold: A compromise between mechanical properties and permeability. Mater. Des. 2020, 192, 108754. [Google Scholar] [CrossRef]
- Dewidar, M.M.; Lim, J.K. Properties of solid core and porous surface Ti–6Al–4V implants manufactured by powder metallurgy. J. Alloys Compd. 2008, 454, 442–446. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, H.J.; Hong, S.H.; Kim, J.T.; Lee, W.H.; Park, J.M.; Kim, K.B. Characterization and deformation behavior of Ti hybrid compacts with solid-to-porous gradient structure. Mater. Des. 2014, 60, 66–71. [Google Scholar] [CrossRef]
- Ahmadi, S.; Sadrnezhaad, S.K. A novel method for production of foamy core@ compact shell Ti6Al4V bone-like composite. J. Alloys Compd. 2016, 656, 416–422. [Google Scholar] [CrossRef]
- Olmos, L.; Mihalcea, E.; Vergara-Hernández, H.J.; Bouvard, D.; Jimenez, O.; Chávez, J.; Camacho, N.; Macías, R. Design of architectured Ti6Al4V-based materials for biomedical applications fabricated via powder metallurgy. Mater. Today Commun. 2021, 29, 102937. [Google Scholar] [CrossRef]
- ASTM D695-02; Standard Test Method for Compressive Properties of Rigid Plastics. ASTM: West Conshohocken, PA, USA, 2002.
- Okuma, G.; Kadowaki, D.; Shinoda, Y.; Akatsu, T.; Guillon, O.; Wakai, F. Determination of the size of representative volume element for viscous sintering. JCS 2016, 124, 421–425. [Google Scholar] [CrossRef]
- Trueba, P.; Chicardi, E.; Rodríguez-Ortiz, J.A.; Torres, Y. Development and implementation of a sequential compaction device to obtain radial graded porosity cylinders. J. Manuf. Process. 2020, 50, 142–153. [Google Scholar] [CrossRef]
- Cocks, A.C. Constitutive modelling of powder compaction and sintering. Prog. Mater. Sci. 2001, 46, 201–222. [Google Scholar] [CrossRef]
- Parthasarathy, J.; Starly, B.; Raman, S.; Christensen, A. Mechanical evaluation of porous titanium (Ti6Al4V) structures with electron beam melting (EBM). Mech. Behav. Biomed. Mater. 2010, 3, 249–259. [Google Scholar] [CrossRef]
- Sallica-Leva, E.; Jardini, A.L.; Fogagnolo, J.B. Microstructure and mechanical behavior of porous Ti–6Al–4V parts obtained by selective laser melting. Mech. Behav. Biomed. Mater. 2013, 26, 98–108. [Google Scholar] [CrossRef]
- Coffigniez, M.; Gremillard, L.; Balvay, S.; Lachambre, J.; Adrien, J.; Boulnat, X. Direct-ink writing of strong and biocompatible titanium scaffolds with bimodal interconnected porosity. Addit. Manuf. 2021, 39, 101859. [Google Scholar] [CrossRef]
- Itälä, A.I.; Ylänen, H.O.; Ekholm, C.; Karlsson, K.H.; Aro, H.T. Pore diameter of more than 100 μm is not requisite for bone ingrowth in rabbits. J. Biomed. Mater. Res. 2001, 58, 679–683. [Google Scholar] [CrossRef]
- Taniguchi, N.; Fujibayashi, S.; Takemoto, M.; Sasaki, K.; Otsuki, B.; Nakamura, T.; Matsuda, T.; Kokubo, T.; Matsuda, S. Effect of pore size on bone ingrowth into porous titanium implants fabricated by additive manufacturing: An in vivo experiment. Mater. Sci. Eng. C 2016, 59, 690–701. [Google Scholar] [CrossRef]
- Bohner, M.; Baroud, G.; Bernstein, A.; Doebelin, N.; Galea, L.; Hesse, B.; Heuberger, R.; Meille, S.; Michel, P.; von Rechenberg, B.; et al. Characterization and distribution of mechanically competent mineralized tissue in micropores of β-tricalcium phosphate bone substitutes. Today Commun. 2017, 20, 106–115. [Google Scholar] [CrossRef]
- Kaviany, M. Principles of Heat Transfer in Porous Media; Springer: New York, NY, USA, 1995. [Google Scholar]
- Katz, A.J.; Thompson, A.H. Quantitative prediction of permeability in porous rock. Phys. Rev. B 1986, 34, 8179. [Google Scholar] [CrossRef]
- Torres, Y.; Lascano, S.; Bris, J.; Pavón, J.; Rodriguez, J.A. Development of porous titanium for biomedical applications: A comparison between loose sintering and space-holder techniques. Mater. Sci. Eng. C 2014, 37, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Gryko, A.; Prochor, P.; Sajewicz, E. Finite element analysis of the influence of porosity and pore geometry on mechanical properties of orthopaedic scaffolds. J. Mech. Behav. Biomed. Mater. 2022, 132, 105275. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Xu, D.S.; Yang, R.; Li, D.; Wu, W.T.; Guo, Z.X. Theoretical study of the effects of alloying elements on the strength and modulus of β-type bio-titanium alloys. Mater. Sci. Eng. A 1999, 260, 269–274. [Google Scholar] [CrossRef]
- Kováčik, J. Correlation between Young’s modulus and porosity in porous materials. J. Mater. Sci. Lett. 1999, 18, 1007–1010. [Google Scholar] [CrossRef]








| Sample | Relative Density (D) | Relative Density (R-M) | Ratio DR-M/D |
|---|---|---|---|
| Ti6Al4V P0 | 0.9633 ± 8 × 10−3 | -- | |
| Ti6Al4V P30 | 0.5436 ± 1 × 10−2 | -- | |
| Ti6Al4V P40 | 0.3972 ± 2 × 10−2 | -- | |
| Ti6Al4V P50 | 0.3378 ± 2 × 10−2 | -- | |
| Bilayer 85/15 P30 | 0.7706 ± 3 × 10−2 | 0.9043 | 1.17 |
| Bilayer 85/15 P40 | 0.7544 ± 4 × 10−2 | 0.8837 | 1.17 |
| Bilayer 85/15 P50 | 0.7238 ± 3 × 10−2 | 0.8753 | 1.20 |
| Bilayer 50/50 P30 | 0.6557 ± 6 × 10−2 | 0.7532 | 1.14 |
| Bilayer 50/50 P40 | 0.6026 ± 4 × 10−2 | 0.6800 | 1.12 |
| Bilayer 50/50 P50 | 0.5827 ± 5 × 10−2 | 0.6502 | 1.11 |
| Volume Fraction of Pore Formers (%) | Pore Volume Fraction (%) | Median Pore Size (d50 µm) | Median Strut Size (d50 µm) | Permeability (m2 ×10−10) | Tortuosity |
|---|---|---|---|---|---|
| 30 | 32.32 ± 1 | 174.10 | 97.12 | 0.19 ± 8 × 10−2 | 1.82 |
| 40 | 42.50 ± 2 | 168.36 | 82.26 | 0.47 ± 9 × 10−2 | 1.58 |
| 50 | 57.26 ± 2 | 184.79 | 61.27 | 1.36 ± 1 × 10−1 | 1.37 |
| Sample | E (GPa) | ER-M (GPa) | ER-M/E | σy (MPa) | σyR-M (MPa) | σyR-M/σy | σy/E (10−3) |
|---|---|---|---|---|---|---|---|
| Ti6Al4V P0 | 83.7 ± 3.01 | -- | 846.5 | 10.11 | |||
| Ti6Al4V P30 | 4.7 ± 0.16 | -- | 58 | 12.34 | |||
| Ti6Al4V P40 | 1.6 ± 0.05 | -- | 31.3 | 19.56 | |||
| Ti6Al4V P50 | 0.32 ± 0.01 | -- | 9.7 | 30.31 | |||
| Bilayer 85/15 P30 | 40.8 ± 1.47 | 72.59 | 1.77 | 643.9 | 735.61 | 1.14 | 15.78 |
| Bilayer 85/15 P40 | 33.7 ± 1.21 | 44.15 | 2.15 | 445.5 | 451.81 | 1.01 | 21.73 |
| Bilayer 85/15 P50 | 27.8 ± 1.00 | 72.15 | 2.14 | 615.5 | 731.86 | 1.18 | 18.26 |
| Bilayer 50/50 P30 | 20.5 ± 0.73 | 42.60 | 2.87 | 399.8 | 438.44 | 1.09 | 27.01 |
| Bilayer 50/50 P40 | 14.8 ± 0.53 | 71.97 | 2.58 | 608.5 | 728.82 | 1.19 | 21.88 |
| Bilayer 50/50 P50 | 6.83 ± 0.24 | 42.60 | 6.23 | 299 | 438.44 | 1.46 | 43.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macías, R.; Olmos, L.; Garnica, P.; Alanis, I.; Bouvard, D.; Chávez, J.; Jiménez, O.; Márquez-Beltrán, C.; Cabezas-Vila, J.L. Processing of Porous-Core Materials for Bone Implant Applications: A Permeability and Mechanical Strength Analysis. Coatings 2024, 14, 65. https://doi.org/10.3390/coatings14010065
Macías R, Olmos L, Garnica P, Alanis I, Bouvard D, Chávez J, Jiménez O, Márquez-Beltrán C, Cabezas-Vila JL. Processing of Porous-Core Materials for Bone Implant Applications: A Permeability and Mechanical Strength Analysis. Coatings. 2024; 14(1):65. https://doi.org/10.3390/coatings14010065
Chicago/Turabian StyleMacías, Rogelio, Luis Olmos, Pedro Garnica, Ivon Alanis, Didier Bouvard, Jorge Chávez, Omar Jiménez, César Márquez-Beltrán, and Jose L. Cabezas-Vila. 2024. "Processing of Porous-Core Materials for Bone Implant Applications: A Permeability and Mechanical Strength Analysis" Coatings 14, no. 1: 65. https://doi.org/10.3390/coatings14010065
APA StyleMacías, R., Olmos, L., Garnica, P., Alanis, I., Bouvard, D., Chávez, J., Jiménez, O., Márquez-Beltrán, C., & Cabezas-Vila, J. L. (2024). Processing of Porous-Core Materials for Bone Implant Applications: A Permeability and Mechanical Strength Analysis. Coatings, 14(1), 65. https://doi.org/10.3390/coatings14010065







