Aluminum Co-Deposition via DC Magnetron Sputtering for Enhanced Pitting Resistance of Copper–Nickel Alloys
Abstract
1. Introduction
2. Materials and Method
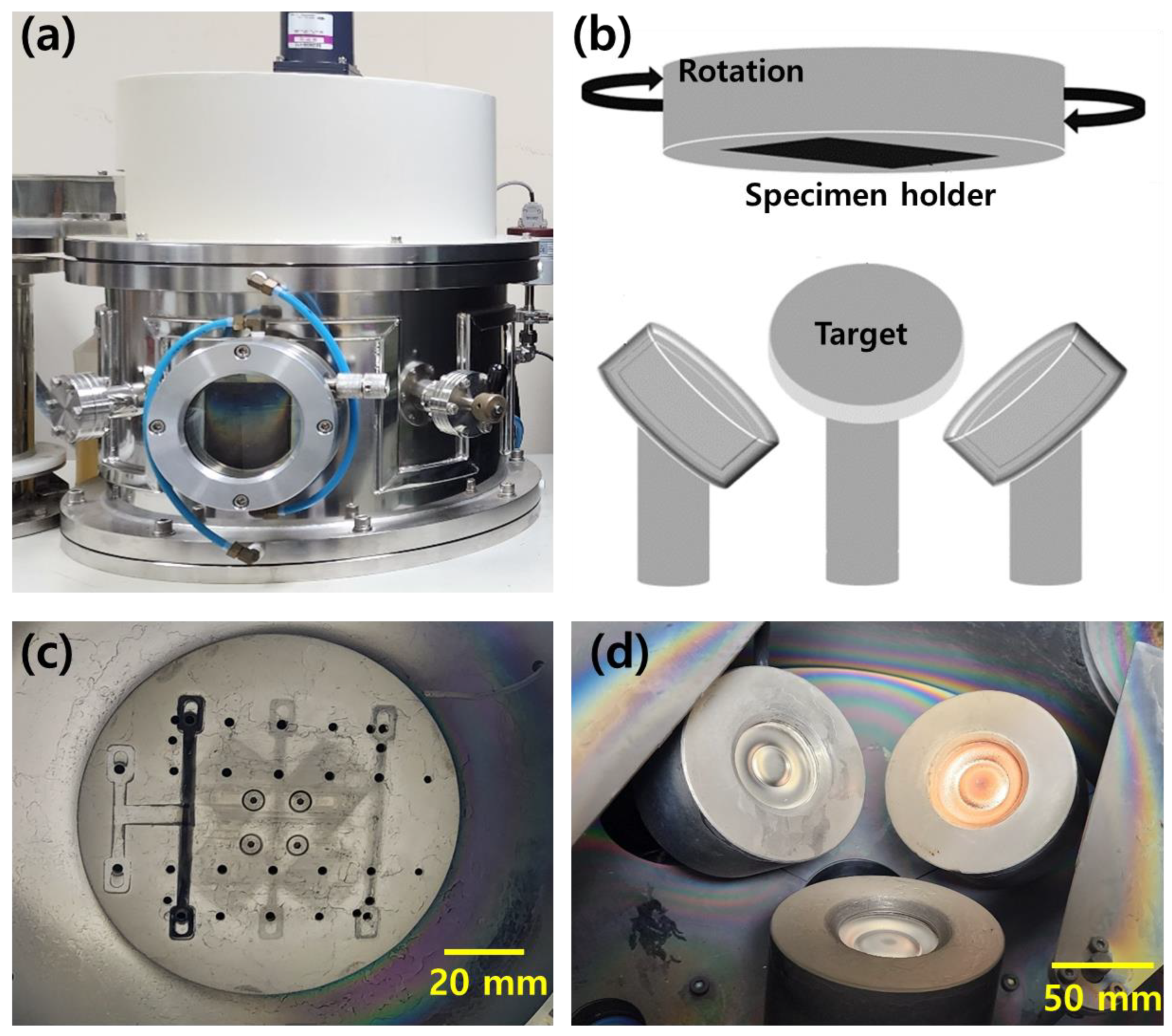
2.1. Preparation of Cu–Ni and Cu–Ni–Al Thin Films
2.2. Materials Characterization
2.3. Corrosion Evaluation
3. Results and Discussion
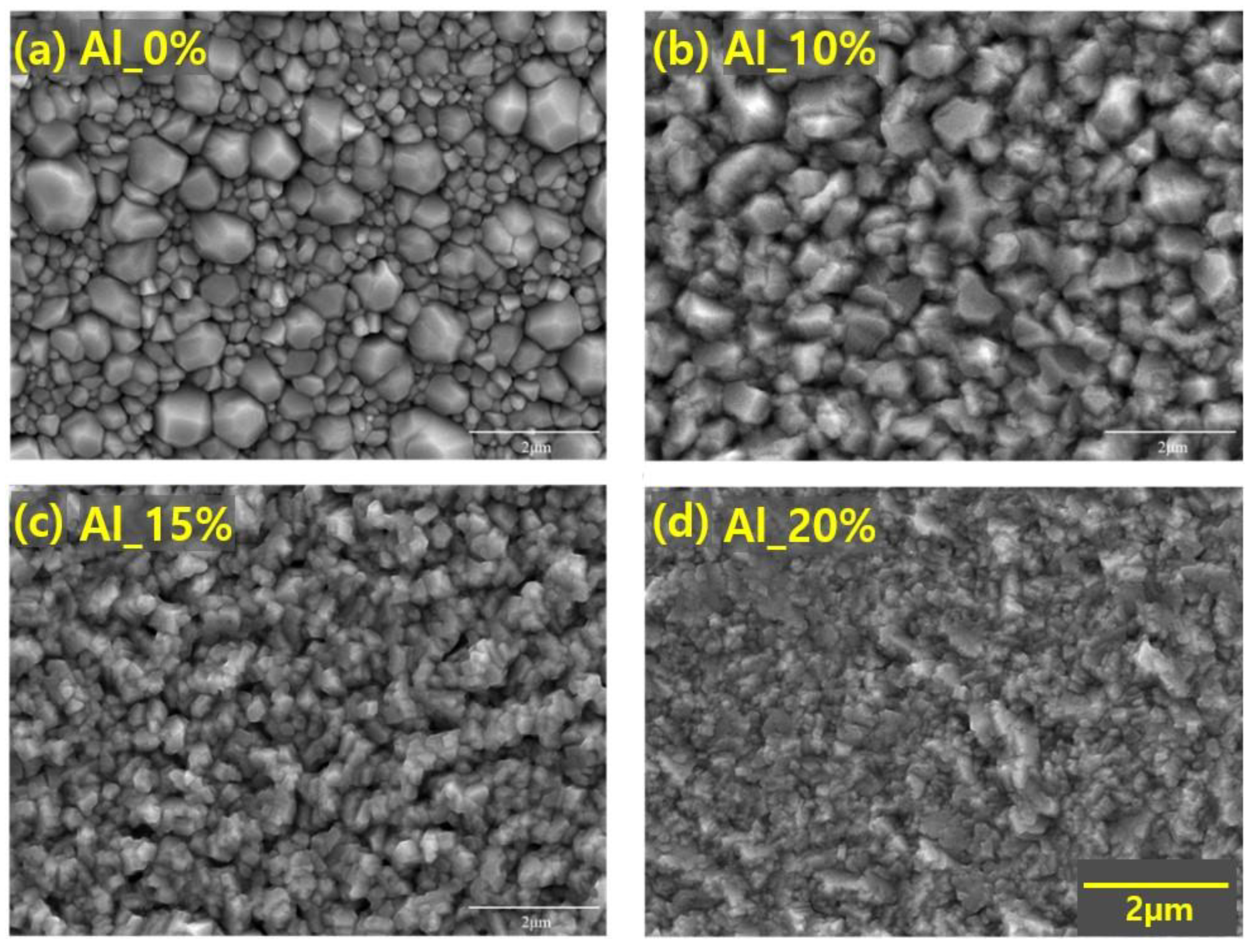
3.1. Surface Analysis
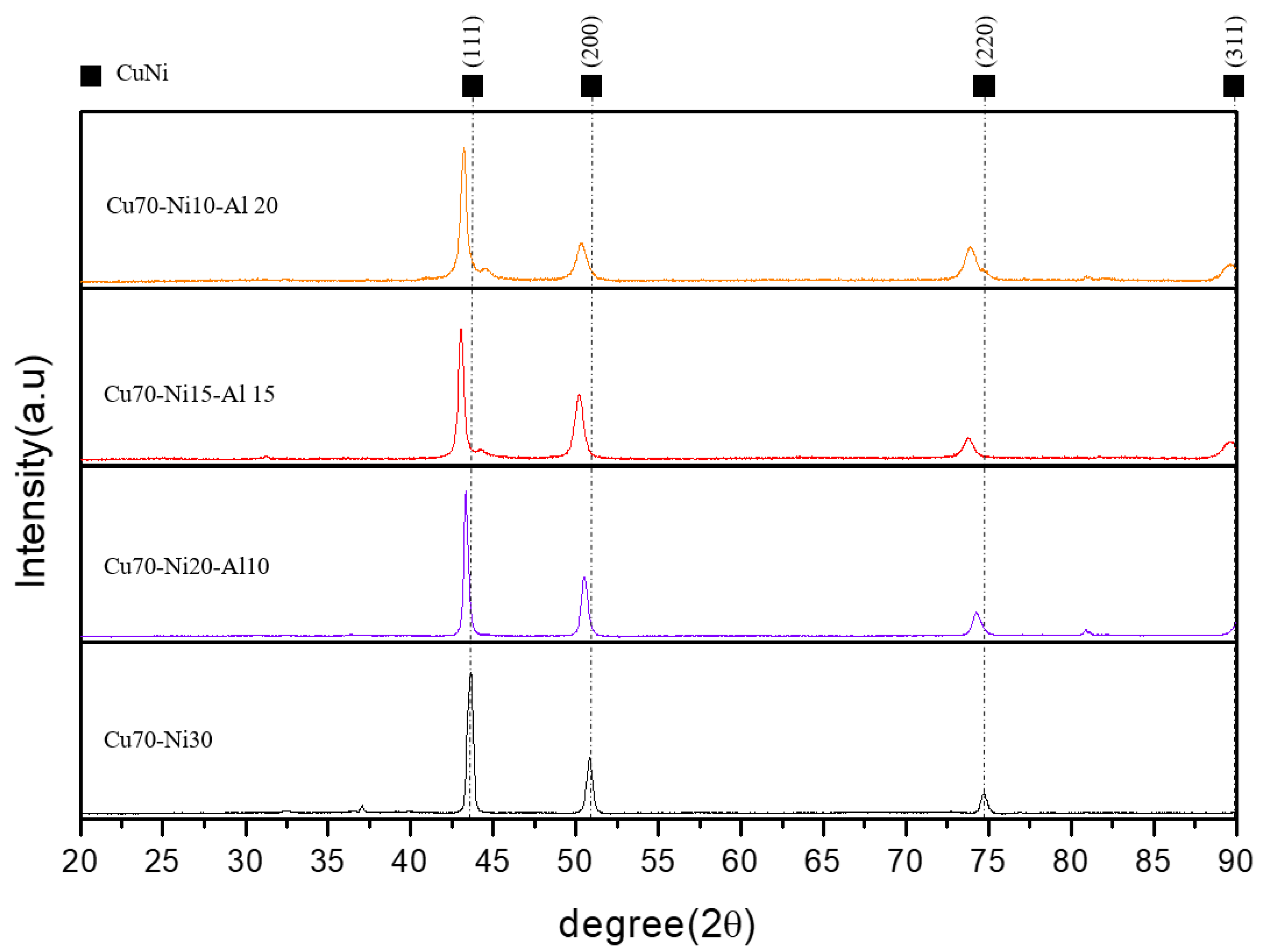
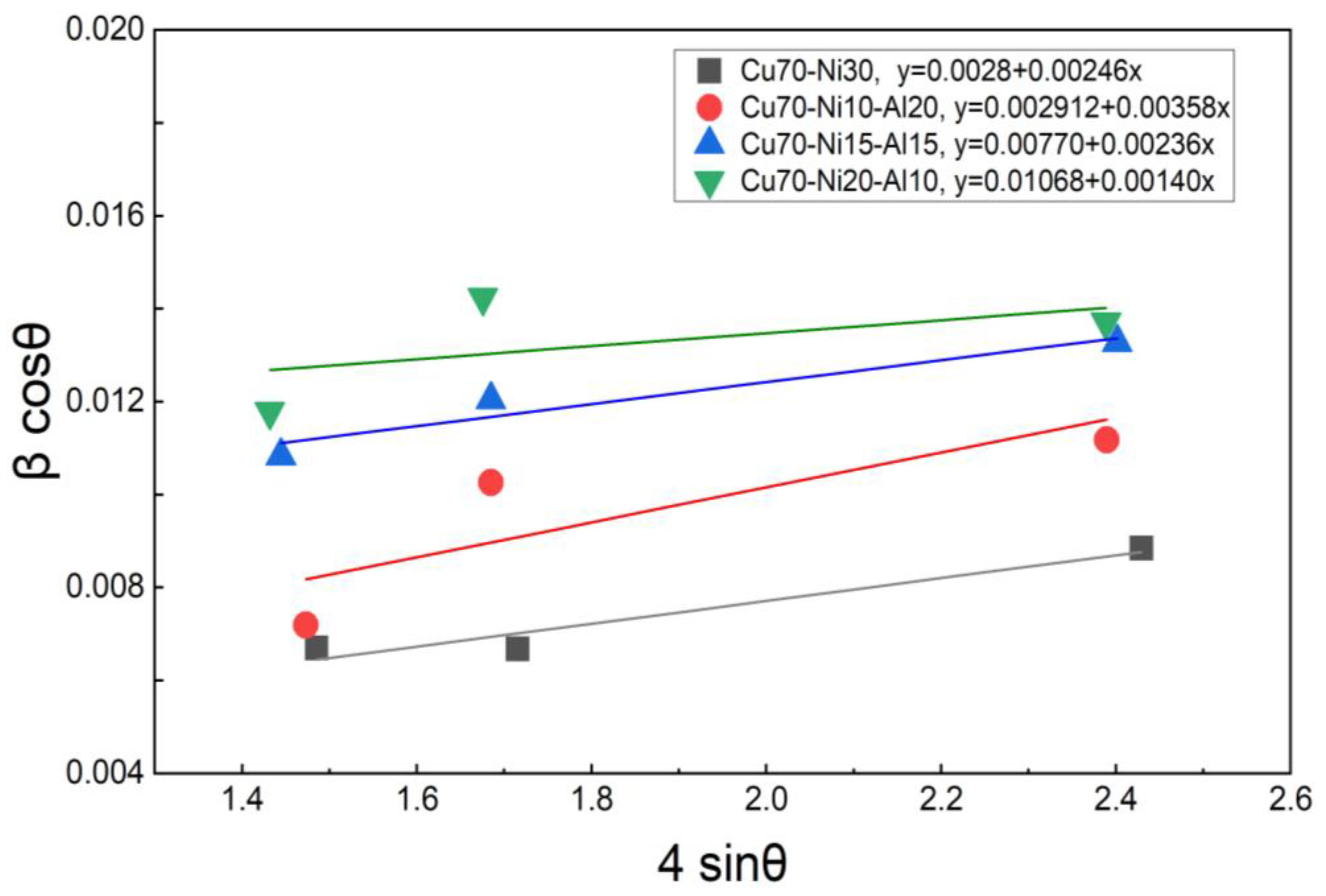
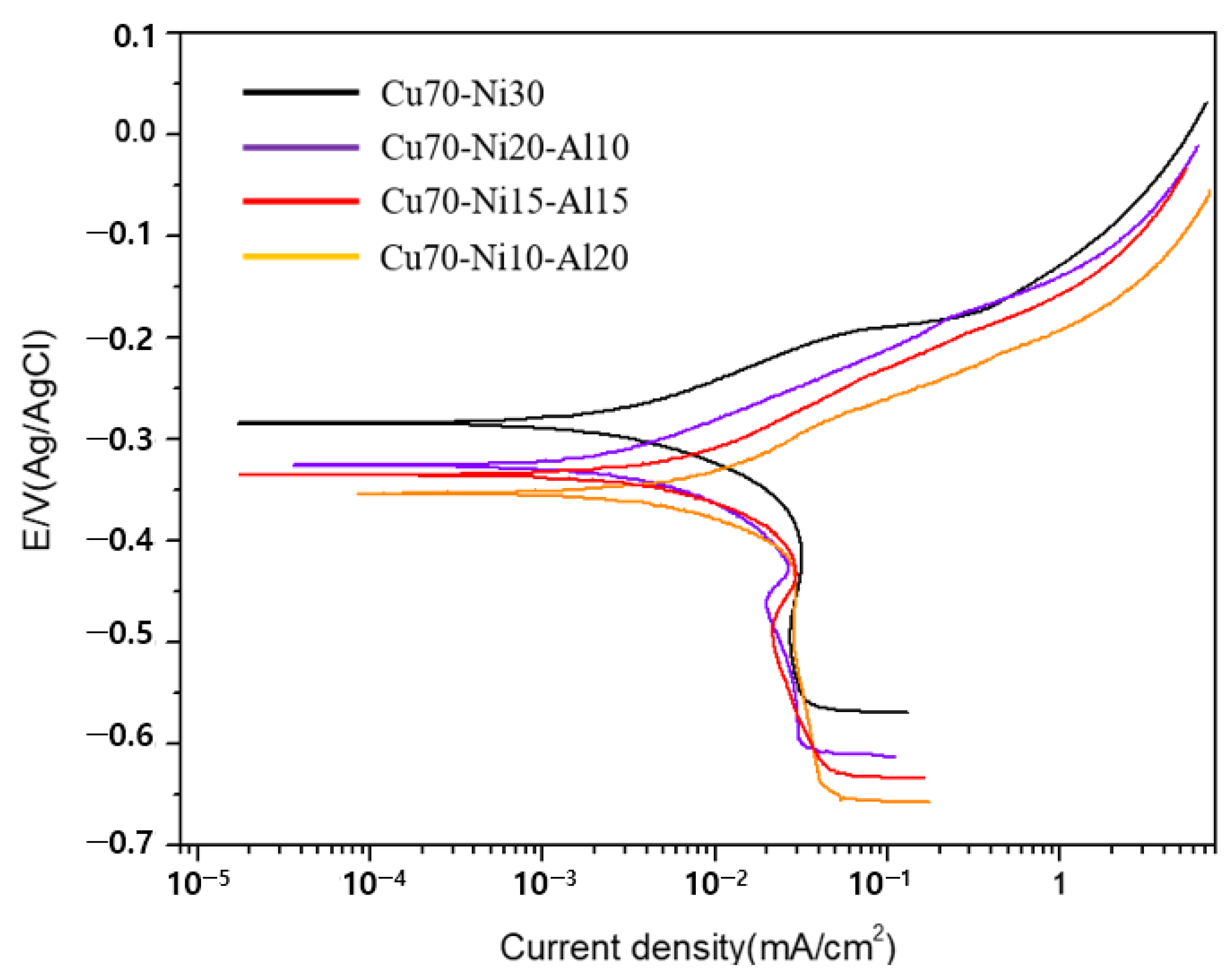
3.2. Crystal Structure and Cathodic and Anodic Reaction
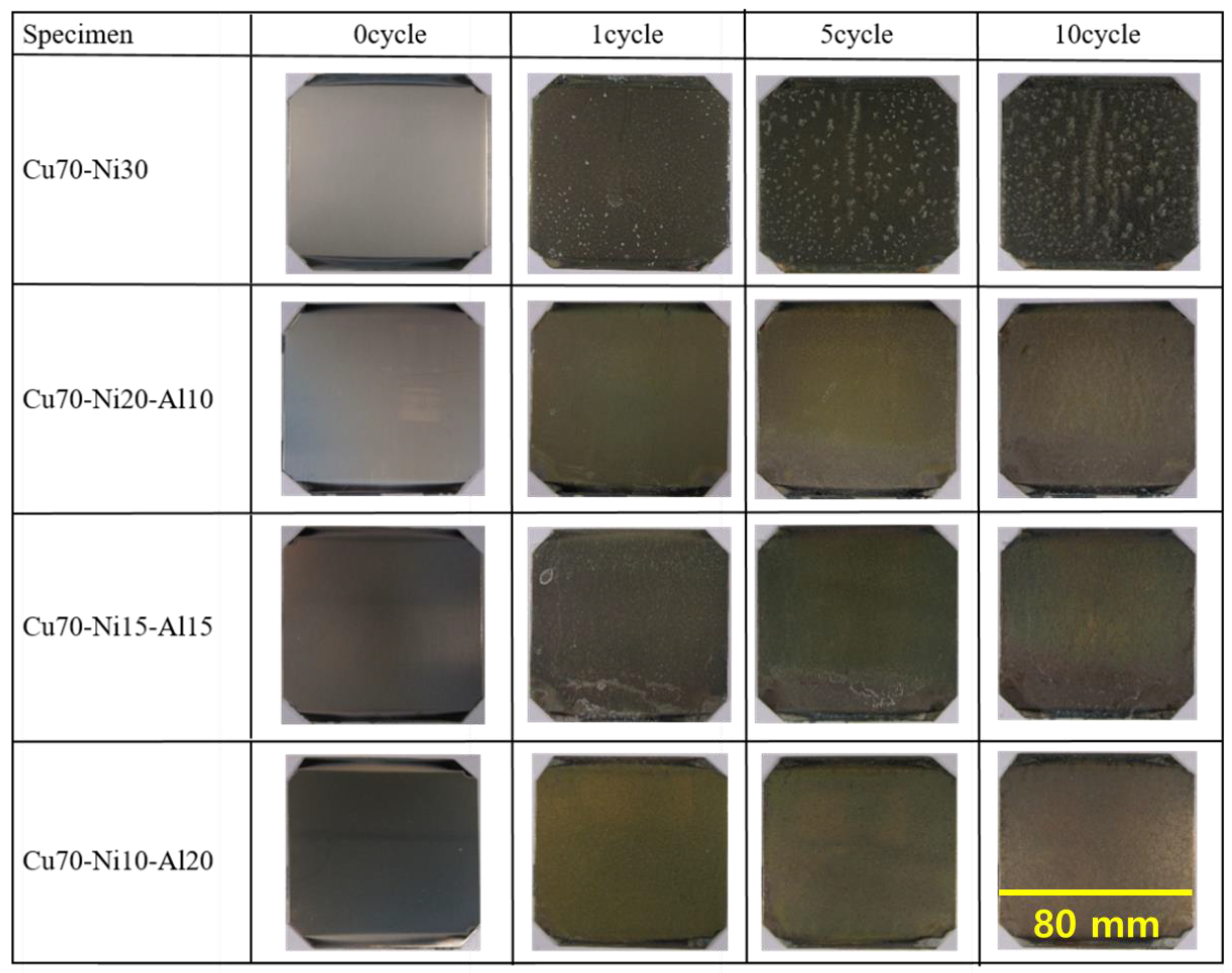
3.3. Evaluation of Corrosion Resistance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parvizi, M.S.; Aladjem, A.; Castle, J.E. Behaviour of 90–10 cupronickel in sea water. Int. Mater. Rev. 1988, 33, 169–200. [Google Scholar] [CrossRef]
- Jin, T.; Zhang, W.; Li, N.; Liu, X.; Han, L.; Dai, W. Surface Characterization and Corrosion Behavior of 90/10 Copper-Nickel Alloy in Marine Environment. Materials 2019, 12, 1869. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Choong, A.M.; Pehkonen, S. The influence of the marine aerobic Pseudomonas strain on the corrosion of 70/30 Cu–Ni alloy. Corros. Sci. 2007, 49, 4352–4385. [Google Scholar] [CrossRef]
- Yuan, S.; Pehkonen, S. Surface characterization and corrosion behavior of 70/30 Cu–Ni alloy in pristine and sulfide-containing simulated seawater. Corros. Sci. 2007, 49, 1276–1304. [Google Scholar] [CrossRef]
- Glover, T.J. Copper-nickel alloy for the construction of ship and boat hulls. Br. Corros. J. 1982, 17, 155–158. [Google Scholar] [CrossRef]
- Rao, B.A.; Kumar, K.C. Kumar, 5-(3-Aminophenyl) tetrazole—A new corrosion inhibitor for Cu–Ni (90/10) alloy in seawater and sulphide containing seawater. Arab. J. Chem. 2017, 10, S2245–S2259. [Google Scholar] [CrossRef]
- Mousavi, M.; Baghgoli, T. Application of interaction energy in quantitative structure-inhibition relationship study of some benzenethiol derivatives on copper corrosion. Corros. Sci. 2016, 105, 170–176. [Google Scholar] [CrossRef]
- Kong, D.; Dong, C.; Zheng, Z.; Mao, F.; Xu, A.; Ni, X.; Man, C.; Yao, J.; Xiao, K.; Li, X. Surface monitoring for pitting evolution into uniform corrosion on Cu-Ni-Zn ternary alloy in alkaline chloride solution: Ex-situ LCM and in-situ SECM. Appl. Surf. Sci. 2018, 440, 245–257. [Google Scholar] [CrossRef]
- Metikoš-Huković, M.; Babić, R.; Škugor, I.; Grubač, Z. Copper–nickel alloys modified with thin surface films: Corrosion behaviour in the presence of chloride ions. Corros. Sci. 2011, 53, 347–352. [Google Scholar] [CrossRef]
- North, R.; Pryor, M. The influence of corrosion product structure on the corrosion rate of Cu-Ni alloys. Corros. Sci. 1970, 10, 297–311. [Google Scholar] [CrossRef]
- Chandra, K.; Mahanti, A.; Singh, A.; Kain, V.; Gujar, H. Microbiologically influenced corrosion of 70/30 cupronickel tubes of a heat-exchanger. Eng. Fail. Anal. 2019, 105, 1328–1339. [Google Scholar] [CrossRef]
- Abouswa, K.; Elshawesh, F.; Elragei, O.; Elhood, A. Corrosion investigation of Cu–Ni tube desalination plant. Desalination 2007, 205, 140–146. [Google Scholar] [CrossRef]
- Asrar, N.; Malik, A.U.; Ahmad, S.; Al-Sheikh, A.H.H.; Al-Ghamdi, F.; Al-Thobiety, M.A. Early failure of cupro-nickel condenser tubes in thermal desalination plant. Desalination 1998, 116, 135–143. [Google Scholar] [CrossRef]
- Ezuber, H.M.; Al Shater, A. Influence of environmental parameters on the corrosion behavior of 90/10 cupronickel tubes in 3.5% NaCl. Desalination Water Treat. 2016, 57, 6670–6679. [Google Scholar] [CrossRef]
- Zhang, J.; Klasky, M.; Letellier, B.C. The aluminum chemistry and corrosion in alkaline solutions. J. Nucl. Mater. 2009, 384, 175–189. [Google Scholar] [CrossRef]
- Nakagawa, N.; Ohtsubo, H.; Mitani, A.; Shimizu, K.; Waku, Y. High temperature strength and thermal stability for melt growth composite. J. Eur. Ceram. Soc. 2005, 25, 1251–1257. [Google Scholar] [CrossRef]
- Hirano, K. Application of eutectic composites to gas turbine system and fundamental fracture properties up to 1700 °C. J. Eur. Ceram. Soc. 2005, 25, 1191–1199. [Google Scholar] [CrossRef]
- Waku, Y.; Sakata, S.; Mitani, A.; Shimizu, K.; Hasebe, M. Temperature dependence of flexural strength and microstructure of Al2O3/Y3Al5O12/ZrO2 ternary melt growth composites. J. Mater. Sci. 2002, 37, 2975–2982. [Google Scholar] [CrossRef]
- Barron, L.W.; Neidrich, J.; Kurinec, S.K. Optical, electrical, and structural properties of sputtered aluminum alloy thin films with copper, titanium and chromium additions. Thin Solid Films 2007, 515, 3363–3372. [Google Scholar] [CrossRef]
- Diler, E.; Rouvellou, B.; Rioual, S.; Lescop, B.; Vien, G.N.; Thierry, D. Characterization of corrosion products of Zn and Zn–Mg–Al coated steel in a marine atmosphere. Corros. Sci. 2014, 87, 111–117. [Google Scholar] [CrossRef]
- Schürz, S.; Luckeneder, G.; Fleischanderl, M.; Mack, P.; Gsaller, H.; Kneissl, A.; Mori, G. Chemistry of corrosion products on Zn–Al–Mg alloy coated steel. Corros. Sci. 2010, 52, 3271–3279. [Google Scholar] [CrossRef]
- Feng, X.; Wang, H.; Liu, X.; Wang, C.; Cui, H.; Song, Q.; Huang, K.; Li, N.; Jiang, X. Effect of Al content on wear and corrosion resistance of Ni-based alloy coatings by laser cladding. Surf. Coat. Technol. 2021, 412, 126976. [Google Scholar] [CrossRef]
- Somjit, V.; Yildiz, B. Doping α-Al2O3 to reduce its hydrogen permeability: Thermodynamic assessment of hydrogen defects and solubility from first principles. Acta Mater. 2019, 169, 172–183. [Google Scholar] [CrossRef]
- Roberts, R.M.; Elleman, T.S.; Iii, H.P.; Verghese, K. Hydrogen Permeability of Sintered Aluminum Oxide. J. Am. Ceram. Soc. 1979, 62, 495–499. [Google Scholar] [CrossRef]
- Wang, G.; Zhu, L.; Liu, H.; Li, W. Galvanic corrosion of Ni–Cu–Al composite coating and its anti-fouling property for metal pipeline in simulated geothermal water. Surf. Coat. Technol. 2012, 206, 3728–3732. [Google Scholar] [CrossRef]
- Abdallah, B.; Kakhia, M.; Alssadat, W.; Zetoun, W. Study of Power Effect on Structural, Mechanical Properties and Corrosion Behavior of CrN thin Films Deposited by Magnetron Sputtering. Prot. Met. Phys. Chem. Surfaces 2021, 57, 80–87. [Google Scholar] [CrossRef]
- Huang, B.; Zhang, C.; Zhang, G.; Liao, H. Wear and corrosion resistant performance of thermal-sprayed Fe-based amorphous coatings: A review. Surf. Coat. Technol. 2019, 377, 124896. [Google Scholar] [CrossRef]
- Kozlovskiy, A.; Shlimas, I.; Dukenbayev, K.; Zdorovets, M. Structure and corrosion properties of thin TiO2 films obtained by magnetron sputtering. Vacuum 2019, 164, 224–232. [Google Scholar] [CrossRef]
- Ariza, E.; Rocha, L.A.; Vaz, F.; Cunha, L.; Ferreira, S.C.; Carvalho, P.; Rebouta, L.; Alves, E.; Goudeau, P.; Rivière, J.P. Corrosion resistance of ZrNxOy thin films obtained by rf reactive magnetron sputtering. Thin Solid Films 2004, 469, 274–281. [Google Scholar] [CrossRef]
- Taweesup, K.; Visuttipitukul, P.; Yongvanich, N.; Lothongkum, G. Corrosion behavior of Ti-Cr-N coatings on tool steel substrates prepared using DC magnetron sputtering at low growth temperatures. Surf. Coat. Technol. 2018, 358, 732–740. [Google Scholar] [CrossRef]
- Gollapudi, S. Grain size distribution effects on the corrosion behaviour of materials. Corros. Sci. 2012, 62, 90–94. [Google Scholar] [CrossRef]
- Tan, Z.J.; Da Ma, T.; Zhang, L.M.; Zhang, W.M.; Jia, R.G.; Cao, D.D.; Ji, H. Relationship between Corrosion Resistance and Microstructure of Copper-Nickel Alloy Pipes in Marine Engineering. Mater. Sci. Forum 2018, 944, 389–397. [Google Scholar] [CrossRef]
- Chung, M.-K.; Choi, Y.-S.; Kim, J.-G.; Kim, Y.-M.; Lee, J.-C. Effect of the number of ECAP pass time on the electrochemical properties of 1050 Al alloys. Mater. Sci. Eng. A 2004, 366, 282–291. [Google Scholar] [CrossRef]
- Kus, E.; Lee, Z.; Nutt, S.; Mansfeld, F. A Comparison of the Corrosion Behavior of Nanocrystalline and Conventional Al 5083 Samples. Corrosion 2006, 62, 152–161. [Google Scholar] [CrossRef]
- Sikora, E.; Wei, X.J.; Shaw, B.A. Corrosion Behavior of Nanocrystalline Bulk Al-Mg-Based Alloys. Corrosion 2004, 60, 387–398. [Google Scholar] [CrossRef]
- Tsai, T.; Chuang, T. Role of grain size on the stress corrosion cracking of 7475 aluminum alloys. Mater. Sci. Eng. A 1997, 225, 135–144. [Google Scholar] [CrossRef]
- Suhadi, A.; Febriyanti, E.; Sari, L.N. Analysis of denickelification phenomenon on heat exchanger tube. Maj. Ilm. Pengkaj. Ind. 2018, 12, 57–66. [Google Scholar] [CrossRef]
- Schüssler, A.; Exner, H. The corrosion of nickel-aluminium bronzes in seawater—II. The corrosion mechanism in the presence of sulphide pollution. Corros. Sci. 1993, 34, 1803–1815. [Google Scholar] [CrossRef]


| Temperature | Salt Solutions | Humidity | Run Time | |
|---|---|---|---|---|
| Salt mist conditions | 35 °C ± 1 °C | pH6.5 to 7.2 salt concentration 50 g/L ±5 g/L | – | 2 h |
| Dry conditions | 60 °C ± 1 °C | <30% | 4 h | |
| Wet conditions | 50 °C ± 1 °C | >95% | 2 h |
| Specimen Name | Cu | Ni | Al |
|---|---|---|---|
| Cu70-Ni30 | Rem. | 29–31 | |
| Cu70-Ni20-Al10 | Rem. | 20–25 | 5–10 |
| Cu70-Ni15-Al15 | Rem. | 14–16 | 14–16 |
| Cu70-Ni10-Al20 | Rem. | 9–11 | 19–21 |
| Specimen Name | Cu | Ni | Al | Cl | O |
|---|---|---|---|---|---|
| Cu70-Ni30 | 45−46 | 14−15 | 1−2 | 39−40 | |
| Cu70-Ni20-Al10 | 48−49 | 15−16 | 4−5 | 1−2 | 29−30 |
| Cu70-Ni15-Al15 | 43−44 | 12−13 | 10−11 | 2−3 | 31−32 |
| Cu70-Ni10-Al20 | 45−46 | 8−9 | 14−15 | 1−2 | 29−30 |
| Specimen Name | Ecorr (mV) | Icorr (μA) | βa | βb |
|---|---|---|---|---|
| Cu70-Ni30 | −283.154 | 10.868 | 78.7 | 250.5 |
| Cu70-Ni20-Al10 | −320.530 | 4.451 | 75.6 | 103.3 |
| Cu70-Ni15-Al15 | −330.048 | 5.195 | 75.0 | 98.9 |
| Cu70-Ni10-Al20 | −340.994 | 4.213 | 70.2 | 84.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, S.-D.; Kim, Y.; Lee, J.-S.; Noh, J.-P.; Kim, B.-S.; Kwon, J.-S.; Choi, S.-W.; Yang, J.-H. Aluminum Co-Deposition via DC Magnetron Sputtering for Enhanced Pitting Resistance of Copper–Nickel Alloys. Coatings 2024, 14, 132. https://doi.org/10.3390/coatings14010132
Yun S-D, Kim Y, Lee J-S, Noh J-P, Kim B-S, Kwon J-S, Choi S-W, Yang J-H. Aluminum Co-Deposition via DC Magnetron Sputtering for Enhanced Pitting Resistance of Copper–Nickel Alloys. Coatings. 2024; 14(1):132. https://doi.org/10.3390/coatings14010132
Chicago/Turabian StyleYun, Sang-Du, Yeonwon Kim, Jun-Seok Lee, Jung-Pil Noh, Beom-Soo Kim, Jae-Sung Kwon, Sung-Woong Choi, and Jeong-Hyeon Yang. 2024. "Aluminum Co-Deposition via DC Magnetron Sputtering for Enhanced Pitting Resistance of Copper–Nickel Alloys" Coatings 14, no. 1: 132. https://doi.org/10.3390/coatings14010132
APA StyleYun, S.-D., Kim, Y., Lee, J.-S., Noh, J.-P., Kim, B.-S., Kwon, J.-S., Choi, S.-W., & Yang, J.-H. (2024). Aluminum Co-Deposition via DC Magnetron Sputtering for Enhanced Pitting Resistance of Copper–Nickel Alloys. Coatings, 14(1), 132. https://doi.org/10.3390/coatings14010132





