Abstract
As a novel post-harvesting strategy, edible films and coatings for fruits and vegetables offer preservation measures to meet the growing needs of hunger and agricultural management. The functionality of edible films and coatings is distinctly the same. However, edible films and coatings differ in their processing and physicomechanical characteristics as they are designed to improve the shelf life, barrier, and nutritional properties of the food. With emerging concerns about sustainability, biomacromolecules have been widely considered in preparing edible films and coatings, which are Generally Recognized as Safe (GRAS) substances. Biopolymers, including polysaccharides, proteins, and lipids, are the main sources of preparing edible films and coatings. These biomacromolecules make stable colloidal dispersions that deliver processing convenience with various formulation, blending, casting, coating, and film-forming methods. Edible films and coating from biopolymers require improvements for their extended performance due to several structural and barrier limitations. Therefore, preparing blends and composites, incorporating target molecules to introduce different functionalities, and designing complex multilayers are among the many recent research approaches developed to overcome those limitations. These recent research approaches ensure enhanced food preservation and extended shelf life, essential requirements of food waste management, with or without minimal influence on the texture, flavor, and nutritional value of food and vegetables. This review focuses on the recent developments in edible films and coatings for fruits and vegetables. Furthermore, this review includes characteristics and functionalities, processing, structural and chemical significance, different sources and their performances, health effects, and recent trends related to edible films and coatings.
1. Introduction
Rapid population growth and the food supply chain crisis have exacerbated global hunger management. In addition, many geopolitical, socio-economical, and concurrent post-pandemic events have further aggravated the global food crisis. Global food waste generation in 2009 was 1.3 billion tons. It has been reported that 32% of food produced for human consumption is wasted across the worldwide supply chain [1,2]. As per the United Nations’ Food and Agriculture Organization (FAO), approximately one-third (or 1.3 billion tons) of the global food production designated for human consumption is wasted annually [3]. The main challenge in modern agronomy is resolving the hunger crisis while delivering adequate agricultural products and services. As a result, there is significant importance in developing novel food preservation and waste management strategies.
The magnitude of food waste can be equally expressed in terms of 3.3 gigatons (or 8% of the world’s total) of CO2 [4] or 250 km3 of blue water consumption that would spread across 1.4 billion hectares (28% of the world’s total) of agricultural landscape [4,5,6,7]. Global food waste is responsible for nearly USD 2 trillion, including environmental costs (USD 700 billion) and social costs (USD 900 billion) [8], which compounds to approximately 10% of the USD 23 trillion gross domestic product (GDP) of the United States of America [9]. Across the globe, developed and developing countries contribute between USD 680 billion and less than USD 310 billion in food waste on average [10]. As a result, under the sustainable development goals in 12.3, the United Nations has vowed to reduce per-capita food waste by 50% by 2030 through enhancements in food security and environmental sustainability as a hunger management strategy amid projected exponential population growth [11]. Therefore, sustainable, innovative, and high-performance packaging and coating solutions are required to accomplish the defined sustainable development goal.
Primary food commodities that generate food losses and wastes are classified into 10 subcategories according to FAO [12,13]: (a) cereal products (wheat, maize and rice), (b) roots and tubers (potatoes, sweet potatoes and cassava), (c) oilseeds and pulses (from various sources such as peanuts, soybeans and olives), (d) fruits, (e) vegetables, (f) meat, fish, and seafood, (g) dairy and dairy-related products, (h) eggs, and (i) products that cannot be specified. Among the abovementioned 10 subcategories, fruits and vegetables undergo the largest fraction of food losses and wastes in all regions, from high- to low-income countries [3,14]. On average, the total weight of vegetables (25%) and fruits (12%) contributes to total food waste [14]. Assessing wastage measures relevant to fruits and vegetables has many challenges. However, the mechanisms of waste generation that spread throughout the entire food chain can be indicated as follows [15]:
- primary production (in agricultural production and harvesting),
- secondary production (in postharvest handling and storage, processing, distribution, and retailing),
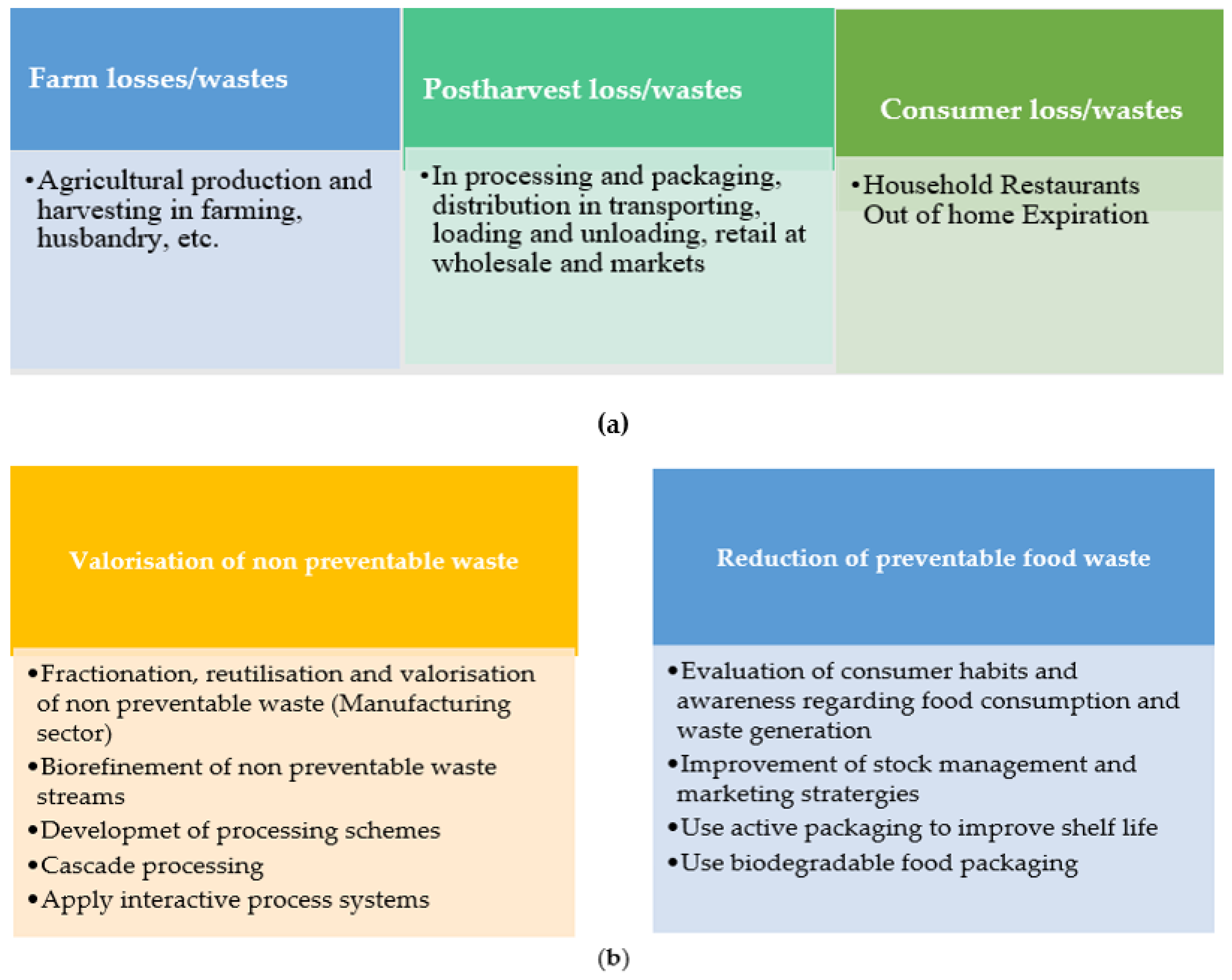
- consumption (in-household and out-of-home). Figure 1a depicts the major wastes and losses in food consumption.
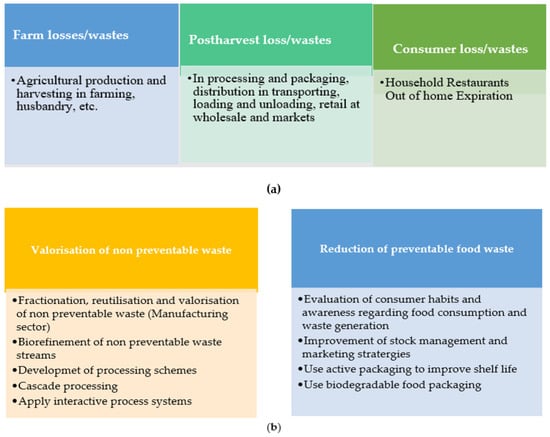 Figure 1. (a) Main wastes and losses in food consumption [16]. (b) Main approaches to promoting food waste reduction and valorization [16].
Figure 1. (a) Main wastes and losses in food consumption [16]. (b) Main approaches to promoting food waste reduction and valorization [16].
Food that fails to meet the quality measures is considered food waste, and food losses lead to a decrease in food quantity or quality. Food waste and losses arise for various reasons, including contamination, poor handling and storage, spoilage, microbial-fungal growth, and other factors [17,18]. Figure 1b shows two major approaches to improve food quality and mitigate food losses and waste, including reducing preventable food waste and the valorization of non-preventable waste.
Edible films and coatings have recently been investigated as a commercially viable post-harvesting preservation strategy for fruits and vegetables. Interestingly, food can be consumed without removing the film or coating [19,20,21]. Edible films and coatings act as a thin protective barrier that extends shelf life of food in the post-harvesting, processing, transportation, storage, and consumption stages; prevents dehydration, deterioration, and spoilage; and preserves the color, freshness, flavor, and nutrients of the food [22,23]. Furthermore, edible films and coatings do not devalue or alter the nutritional value of fruits and vegetables.
The applications of edible coatings and films are subjected to extreme safety guidelines. These involve good manufacturing practices and the use of food-safety materials monitored by the Food and Drug Administration (FDA) [24,25]. Another safety measure is the use of Generally Recognized as Safe (GRAS) materials already approved by the FDA [26]. However, not all GRAS substances are considered consumer safe, as there can be rare allergic reactions caused by them, including lactose intolerance from milk and Celiac disease from wheat gluten. Apart from the FDA, the International Organization for Standardization (ISO) and the European Union (EU) are established regulatory organizations that maintain guidelines for the safe application of edible films and coatings [27].
Edible films and coatings for fruits and vegetables exhibit the potential to minimize preventable losses and waste and thus offer sustainable solutions to the global food crisis and hunger management. Therefore, this review explores recent developments in edible films and coatings for fruits and vegetables. Furthermore, comprehensive coverage on characteristics and functionalities, processing, major types, and their structural and chemical significance, different sources and their performances, health effects of edible films and coatings, and recent trends in edible films and coatings specific to fruits and vegetables are also discussed.
2. Characteristics and Functionalities of Edible Films and Coatings
The definition of edible films and coatings is generally a thin barrier with a thickness of 0.3 mm and made from material that can be directly consumed [28]. Edible films and coatings are widely applied in contact with minimally processed fruits and vegetables. Even though the main functionality of edible films and coatings is similar, their processing techniques are different. For instance, edible films are pre-formed before contact with fruits and vegetables. In contrast, edible coatings are usually formed on the surfaces of fruits and vegetables, and layer formation occurs directly [29,30]. Both techniques form thin barriers from rigid matrices that can be further functionalized for extended applications.
When developing edible films and coatings, assessment of the following considerations is critical [31]:
- (a)
- interactions with the food texture and surface;
- (b)
- aging and prolonged performance of the shelf life of the food in contact;
- (c)
- changes in flavor, color, and texture of food due to interactions with edible films and coatings;
- (d)
- response and sensitivity under storage/environmental conditions;
- (e)
- processing conditions, including temperature, color and thickness.
2.1. Key Functionalities of Edible Films and Coatings
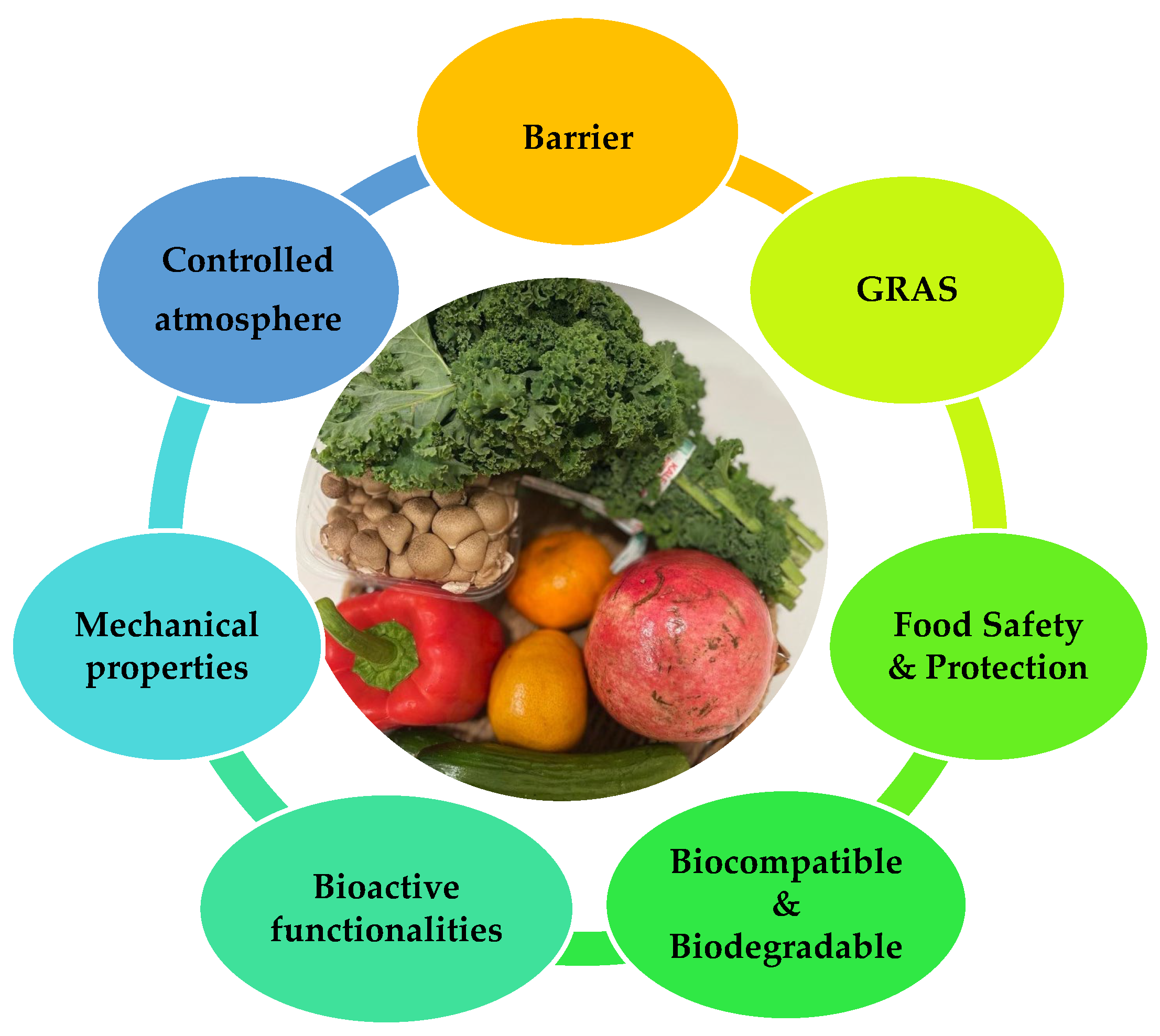
Edible films and coatings act as protective barriers and provide a controlled atmosphere around fruits and vegetables. Figure 2 summarizes the key functionalities of edible films and coatings in fruit and vegetable preservation.
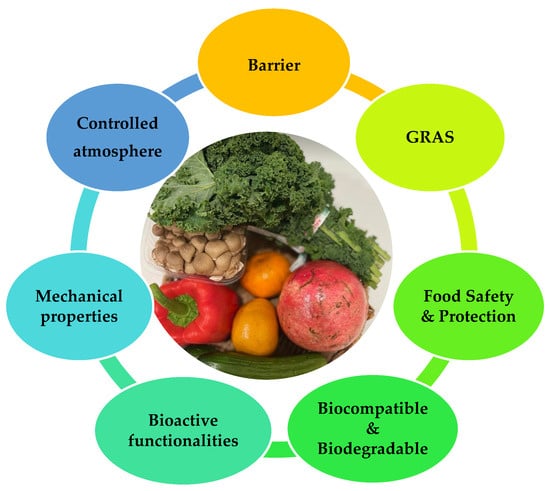
Figure 2.
Major functionalities of edible films and coatings in fruit and vegetable preservation.
The major functionalities of edible films and coatings are as follows [31,32,33]:
- Protection from transport, handling, mechanical damages, and UV radiation;
- Barrier properties:
- (a)
- Moisture barrier: minimizes water vapor transmission to prevent dehydration;
- (b)
- Gas barrier: controls oxygen and carbon dioxide levels as they pass through the protective layer;
- (c)
- Volatile organic compounds (VOC) barrier: protects against organic vapors such as aromas and solvents and other additives and pigments.
- Prolongation of shelf life;
- Bioactivity: shows antimicrobial and antifungal properties and acts as probiotics;
- Biodegradability;
- Structural integrity: melts above 40 °C without decomposition, water resistant, easily emulsifiable, non-sticky, or non-tacky, and delivery efficient drying;
- Maintenance of food quality: minimal influence on texture, flavor, or color;
- Formulated from economical, relatively abundant, consumer-safety GRAS materials.
2.2. Common Preparation Methods of Edible Films and Coatings
When processing edible films and coatings, the raw material and number of layers used play a significant role. In addition, the nature of the edible film may determine the overall film strength, solubility, surface activity, appearance, flavor, and texture in the mouth [24]. Edible films are primarily manufactured as sheets or thin wrap films and later used to cover food intact. Edible films and coatings are commonly prepared using melt extrusion and solvent casting techniques.
2.2.1. Melt Extrusion Method
The main steps in the melt extrusion method include formulation, melt blending, extrusion, cooling, and storage. Sheet extrusion, blown film extrusion, and reaction extrusion are commercialized melt extrusion techniques applied to prepare edible films [34,35]. Generally, the extruder temperature and operating conditions are determined by the thermal and rheological properties of polymers. Interestingly, biopolymers with thermo-plastic properties exhibit superior performance in melt extrusion. However, process aids and plasticizers are added to enhance polymer melt flow during the extrusion [36]. The film quality and clarity are ultimately determined by the degree of crystallinity of the polymeric matrix and the cooling rate. The polymeric matrix in the melt state undergoes re-crystallization when cooling and may change its polymorphic state [37]. Higher crystallinity may deliver extended barrier performance in highly amorphous polymers. However, the amorphous fraction of the polymers may significantly influence the optical properties and thereby control the clarity and transparency of films. The thickness of the films can be adjusted from the rotor speed and extruder parameters. However, formulation errors may lead to phase separation, non-uniform distributions, and irregularities in thickness.
2.2.2. Solvent Casting Method
Solvent casting is another widely used film processing method [38]. Unlike melt extrusion, solvent casting can be complicated depending on the solvent system and intended application [39]. Hence, stable colloidal systems need to be designed based on the solubility parameters of the polymers and the pre-selected solvent system. Moreover, to obtain stable and uniform films, essential process aids, such as emulsion stabilizers and oil- or wax-based surfactants, are added to facilitate the mixing. In addition, wetting and leveling agents are added to control the uniform wetting, surface tension, and surface defects, such as bubbles, pinholes, craters, and defoaming agents, to avoid the aeration of colloids [40,41]. Solvent removal and solidification are critical factors in the solvent-casting method. The solidification process is vital for the quality of the film or coating and is facilitated by a precise drying system because drying transforms fluid into a solid-state transition. Factors including coating formulation, number of layers, wet film/coating thickness, viscosity, solution solids, solution temperature, coating accuracy, and coating substrate must be considered when designing the coating or film using the solvent casting method.
Various drying systems are used in the solvent casting method. The drying system that is used depends on the requirements. Hot air convection, hot air impingement, steam, infrared, hot air flotation, and zoned drying are commonly employed drying systems. In addition, the rod, knife, and spin-coating methods are used in the batch film and coating processes [42]. In recent approaches, multilayer films and coating have been tested to enhance performance [43]. Moreover, lamination, calendaring, slot extrusion coating methods, dip coating, and spraying have also been employed in edible films and coating processes [44].
3. Types of Edible Films and Coatings and Their Structural and Chemical Significance
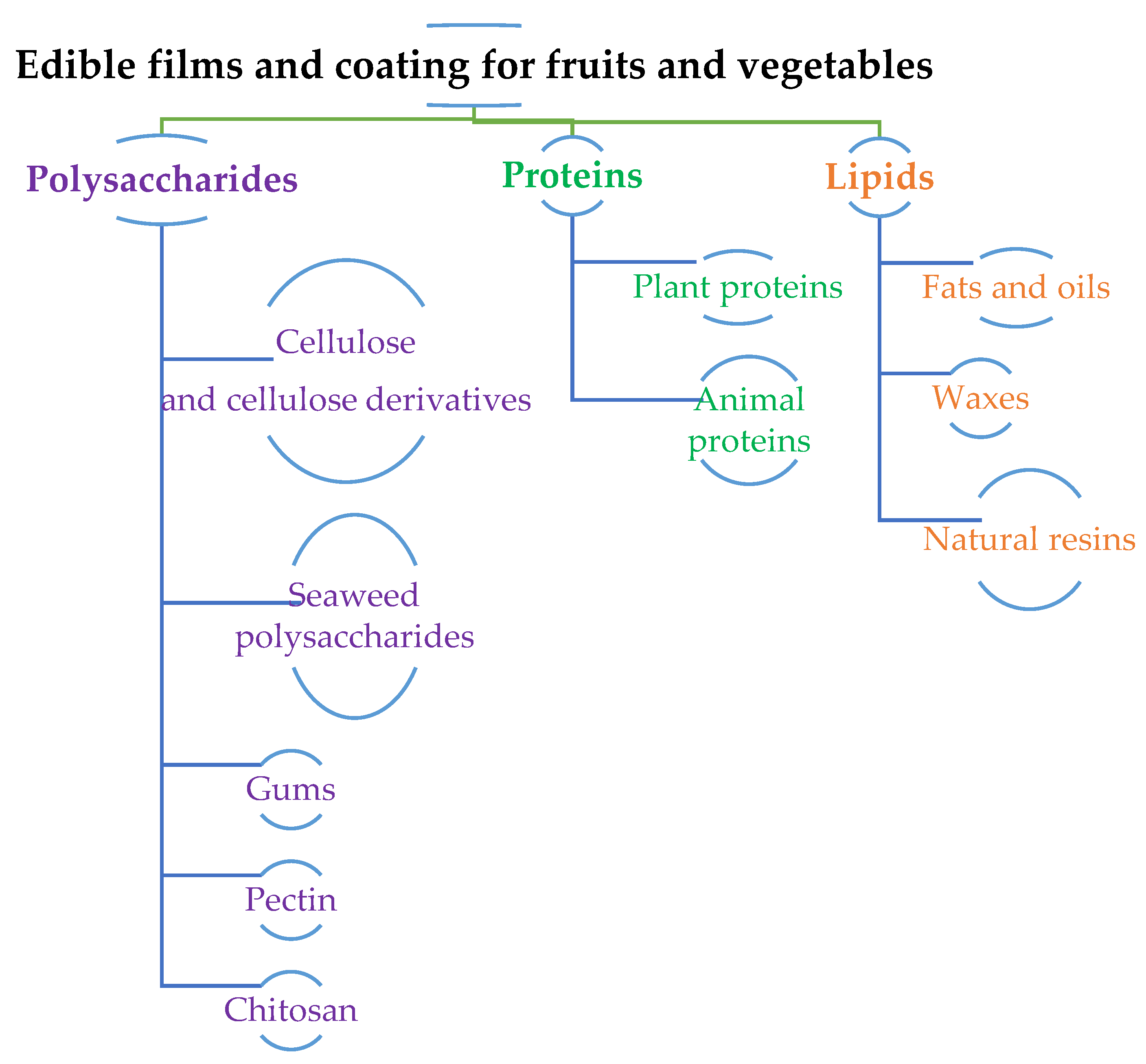
Most edible films and coatings are made from biomacromolecules as these originate from natural sources with minimal toxicity and comply with GRAS. Moreover, biomacromolecules provide extra processing convenience due to their biocompatibility and biodegradability. Matrices of biomacromolecules used for edible films and coatings can be segmented into three main categories that consider their physicochemical properties: hydrocolloids (polysaccharides and proteins); lipid colloids (fatty acids, acylglycerol, waxes); and composites. These are illustrated in Figure 3.
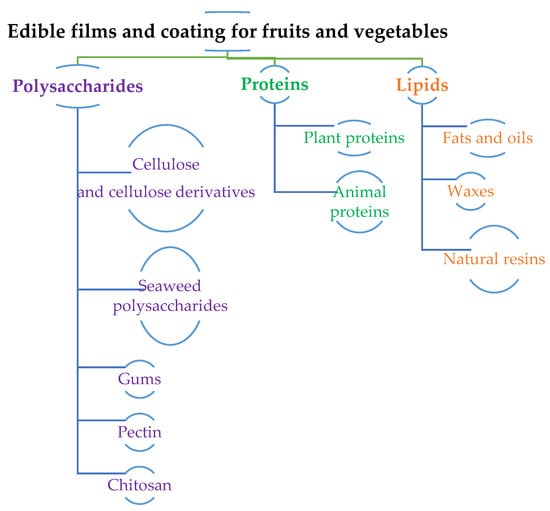
Figure 3.
Sources of Edible films and edible coatings.
3.1. Hydrocolloids
Hydrocolloids are made through the hydrophilic dispersion of colloids comprised of long-chain polymers. Hydrocolloids may be dispersed entirely or partially and tend to swell when in contact with water [45]. The dispersion of hydrocolloids results in changes to their physicochemical properties through the formation of stable gels or changes in their properties, including to their viscosity, thickness, emulsion, and stability [46]. Hydrophilic functional groups in biomacromolecules and hydrogen bonding play a crucial role in forming a stable hydrocolloid system [47]. Due to the presence of hydroxyl functional groups in their structure, polysaccharides are widely employed to prepare hydrocolloids. However, not all polysaccharides form hydrocolloids because of water insolubility. Indeed, the formation of hydrocolloids using water-soluble sugars is limited by their low molecular weight [48]. Proteins are also classified as hydrocolloids in some contexts due to the structural hydrophilicity and intrinsic polydispersity of protein colloids [45,49]. Protein sources, including gelatin, milk, eggs, and isolates of proteins derived from vegetables, form stable hydrocolloids.
Based on origin, hydrocolloids can be divided into (a) plant-based, (b) animal-based, and (c) modified hydrocolloids. Plant-based hydrocolloids are prominent in edible films and coatings due to their natural abundance and nontoxicity. Plant-based sources of hydrocolloids include pectin, starch, guar gum, locust bean gum, mannan, gum arabic, gum ghatti, tragacanth, agar, alginates, and carrageenan. Animal-based hydrocolloids include gelatin, collagen, whey, egg, and milk protein [50,51]. Among the many animal-based hydrocolloids, gelatin is extensively used due to its relative abundance and easy extraction. Chitosan is another prominent animal-based hydrocolloid [52].
Hydrocolloids have also been modified to enhance stability, quality, safety, and nutritional values. For instance, hydrocolloids prepared from polysaccharides, including cellulose, pectin, and starch, have been modified to achieve extended performance [51]. Cellulose derivatives, including nano-fibrillated cellulose (NFC), nanocrystalline cellulose (CNC), carboxymethyl cellulose, methylcellulose (MC), hydroxypropyl methylcellulose (HPMC), and hydroxyethyl cellulose, have been tested as hydrocolloids in the food industry [53]. Food hydrocolloids have been prepared using modified starch with different granule sizes, amylose-to-amylopectin ratios, and functional properties, such as gelling, thermal, and textural [49]. Pectin extracted from fruits and vegetables is modified through esterification into low methyl ether pectin. High methyl ether pectins are used industrially as gelling and thickening agents [54,55].
Hydrocolloids of polysaccharide edible films (EF) and edible coatings (EC) for F&V are formulated from various high to low molecular weights. They often exhibit thixotropic rheology. The gums of polysaccharides form micelles when dispersed in aqueous media through solvent–polymer hydrogen bonds [56]. As per the degree of hydrogen bonding and the intermolecular association between micelles and water, gums form a stable polymer–solvent system immobilized by water molecules [57].
Hydrocolloid systems of polysaccharides, hydrogels, and gums lead to highly viscous shear-thinning colloidal dispersions that are ideal for coating and film formation methods [58]. These hydrocolloids provide processing convenience and flexibility for continuous and batch production. However, formulation parameters such as concentration, drying conditions, and solidification methods (for water evaporation) are crucial to achieve quality EF&EC. During drying, EF&EC from polysaccharide molecules may rearrange into characteristic structural matrixes forming semicrystalline domains. The semi-crystallinity determines the vital characteristics of EF&EC stability, solubility, durability, and viscoelasticity and physicomechanical, optical, and barrier performance [59].
3.2. Lipid Colloids
Lipid colloids exhibit hydrophobic and emulsion properties. Lipid matrixes used for edible films and coatings are mostly fatty acids, glycerides, and waxes. Colloids from lipids are stabilized by physicochemically compatible surfactants [60]. The surface reactions and surface adsorption of lipid colloids are determined by the physical state of the hydrophobic matrix. Even though the lipid matrix is hydrophobic, it provides an excellent affinity for binding other molecules, such as proteins, to its surface [61].
In lipid colloids, the lipid–water interface provides a highly active reaction surface and moisture barrier performance. Common lipid colloids used in edible films and coatings include liposomes, micelles, nanoemulsions, microemulsions, and solid lipid nanoparticles [62]. Furthermore, lipid colloids can be broadly categorized into [63] (i) solid-in-liquid dispersions, (ii) liquid-in-liquid dispersions, and (iii) dispersions of self-assembled molecules. For edible films and coatings, lipid colloids are reliable due to their structural effectiveness in being absorbed in our digestive system and their nontoxicity, favorable emulsion properties, physicochemical stability of lipid carriers, and surface reactivity [62,64]. Surface reactivity and surface binding of lipids are unique properties that have been extensively considered in designing edible films and coatings [65].
3.3. Composites
Composite films are heterogenous in nature, and a combination of biopolymers is used to achieve a distinct functional property from each ingredient used [65]. When combined, hydrophobic and hydrophilic materials are blended to achieve the desired properties [66]. Biopolymers, carbohydrates, proteins, and lipids are widely used in combinations [67]. Generally, fats are used to reduce water transmission, proteins are used to provide mechanical stability, and carbohydrates are used to control the exchange of gasses [22].
According to the number of biopolymers combined, composite edible films and edible coatings are categorized as binary or ternary [68]. Combinations of composites and synthetic and natural polymers are also used [65]. Binary films and coatings comprise combinations such as protein–protein, carbohydrate–carbohydrate, carbohydrate–protein, and lipid-based binary films.
In order to achieve a packaging preservation that necessitates high performance, complex solutions such as multilayer, smart, and intelligent EF & EC have been designed. For such applications, natural polymer composites are used to achieve synchronized property enhancements and introduce functionalities. The composite applied for EC & EF is a combination of two distinct phases: continuous and discontinuous [68].
In conventional EF&EC composites, the continuous phase consists of an edible biocompatible polymer, and the discontinuous phase consists of a filler or modifier. Modern EF&EC can be formulated from multiple components. Furthermore, there can be metaphases to deliver the complex functionalities required for EC & EF. All the elements in EC&EF must be covered by FDA and GRAS guidelines. Moreover, composites for EC & EF can be designed for controlled release and active delivery of different molecules that are vital for F & V, such as antioxidants, vitamins, and antimicrobial compounds [68].
Bilayer composites of EC & EF used for F&V have been researched over the years to lessen the disadvantages of EC & EF made from single layers made from natural hydro and lipid colloids. Often, context composites of EF & EC refer to a binary component system with two or more layer with distinct performances and made from lipid–hydrocolloids or different combinations of protein–protein, carbohydrate–carbohydrate, and protein–carbohydrate [69].
Composite films can be prepared using two main methods: layer form or emulsion of film-forming materials. Based on the number of polymers used, the layered composites are classified as binary or ternary. The emulsion film-forming method is considered more effective than the layer form because the layered method requires more casting and drying processes and because delamination can reduce the effectiveness of this method over time [66].
Bilayer films can be developed using two main methods [70,71]: (1) two-steps method, or the continuous application over another matrix, and (2) single-step method, or the homogeneous dispersal of distinct emulsions or colloidal systems. However, bilayer films from the two-steps method are described as the best performing because the films from the dispersion method tend to shrink. The method of application affects the characteristics of the films and coatings, including barrier properties [65].
4. Polysaccharide-Based Edible Films and Coatings
Polysaccharides can be found in abundance in renewable sources, such as plants, sea weeds, algae, and microbial. However, the categorization of polysaccharides is complex. Based on their composition, polysaccharides are classified as homoglycans, with a single constituent in the main polymeric chain, or heteroglycans, with two or more constituents in the main polymeric chain, and as having an alternate or irregular sequence [72,73]. The physicochemical properties of polysaccharides vary by the degree of polymerization, molecular weight, ring size, anomeric configuration, linkage type, and absence or branching [72,73]. Hence, solvent polysaccharide interactions make them soluble, insoluble, or partially soluble. Insoluble polysaccharides are compact chains that tend to crystallize partially. Polysaccharides can act as sacrificing agents that can preserve the moist nature of foods [65].
Polysaccharides can contain various linkage patterns, such as C (1→3), C (1→4), and C (1→6). The linkage pattern affects the solubility. Among the linkages, the C (1→6) linkage provides easier solubility [74]. The degree of branching and linearity of polysaccharides influences their gel-forming ability and the stability of gels [75,76].
The solubility of polysaccharides is determined by many factors. The hydrocolloids of polysaccharides form stable hydrogen bonds with water because of their extremely hydrophilic nature [45]. Due to heterogeneity in natural polysaccharides’ plant cell walls, certain pre- and post-treatments and non-hazardous modifications may be required for processing EF & EC [77,78]. Polysaccharidal solutions deliver green processing from EF & EC; such green formulation and processing routes can be established based on hydrocolloid chemistry in aqueous media [79]. This chemistry leads to a reversible colloidal transformation from a solution to a gel, causing most polysaccharide hydrocolloids to form physical hydrogels [80].
Polysaccharides EF & EC are highly compatible with F & V because they are colorless and provide excellent barrier protection against oils and organic molecules [29,68,81]. However, polysaccharides EF&EC are less resistant to moisture and do not deliver a sufficient water barrier, which is essential for F & V. Polysaccharides EF & EC are efficient at modifying the controlled environment in the preservation of F & V by reducing the respiration rate from moderate permeability to O2 and CO2 [82]. In recent research, active components, such as antimicrobials, vitamins, antioxidants, bactericides, and preservatives, are incorporated into polysaccharide matrixes. Active EF & EC from polysaccharide solutions can be designed through functionalization, crosslinking, and composites for advanced preservation, control release, bioactivity, protection, and water barrier properties [83]. The application of polysaccharides may still be economically reliable as a low-cost preservation strategy for F & V applications with a short shelf life expectancy and extremely thick natural protection peels. However, EF & EC may not interfere with taste due to oxidation or rancidity and, when needed, dope other chemicals for extended performance.
4.1. Cellulose and Cellulosic Derivatives
Cellulose is the most abundant renewable material on earth. It can be extracted from the cell walls of plants, algae, tunicates, and some bacteria [84]. The linear homo-polymer/homoglycan structure of cellulose is low density with crystallinity varying between 40%–70%; moreover, it has disordered, loosely packed amorphous regions that are susceptible to surface reactions [85,86]. In densely packed crystalline domains, intramolecular hydrogen bonding is prominent [87]. The cellulose and glucose units of D-Glycose are linked through β-1,4-glycosidic bonds [88]. The ultimate structure–property relationship between cellulosic edible films and coatings is determined by the degree of crystallinity, polymerization, and polymeric chain length [89]. Importantly, the degree of crystallinity of cellulosic fibers represents the toughness, strength, and fiber-fibril characteristics of cellulosic fibers [90]. Furthermore, the degree of crystallinity and the ratio of amorphous to crystalline domains directly impact the physicomechanical, optical, and barrier properties of cellulosic fibers.
In the processing and formation of EF & EC, hydroxyl chemistry is responsible for the hydrophilicity, chirality, chemical functionalization, insolubility in most aqueous solvents, infusibility, and solvent resistance of cellulose hydrocolloids [91]. Cellulose is insoluble in water, alkalines, and modifications to it may alter the solubility of hydrocolloids [92]. The low film stability and poor oxygen and carbon dioxide barrier are key challenges with cellulosic hydrocolloids. Therefore, several research approaches have been executed to overcome the challenges of EF & EC from cellulosic and cellulose derivatives, such as modification, introducing functionalization, and composites. Cellulose extraction from high plants involves multistep chemical processing, drawing concerns for EF & EC under GRAS guidelines. Cellulose can be processed into hydrogels that are commercially used as micro cellulose or nanocellulose and that differ from the average fiber dimension of hydrogel fibers. Nanocellulose from microbial sources is the purest form of cellulose; hence, it is ideal for EF & EC. Crystalline nanocellulose, a product of the chemical and enzymatic digestion of amorphous regions, has enhanced mechanical and barrier properties resulting from a high degree of crystallinity.
Processing convenience and formulation of cellulose-based edible films and coatings can be improved by modifying cellulose following esterification routes in the presence of chloroacetic acid, methyl chloride, or propylene oxide to achieve carboxymethyl cellulose (CMC), methylcellulose (MC), hydroxypropyl cellulose (HPMC), or hydroxypropyl cellulose (HPC), respectively [29]. Edible films and coatings prepared from cellulose esters possess the following properties: odorless and tasteless, flexibility with moderate strength, optical features, repellent resistance to oils and fats, water solubility, moderate moisture, oxygen transmission, controlled release of bioactive, non-toxicity [93], compatibility with composites and laminates, and efficient membranes and separation [93,94]. Cellulose and cellulose derivatives provide strong adhesion between fibers and cellulosic interfaces, making them ideal for edible films and coatings for fruits and vegetables [94].
Cellulose and its derivatives have been widely investigated as edible films and coatings. For instance, hydroxypropyl methylcellulose (HPMC) was successfully tested as an edible coating for blueberries [95]. Sodium carboxymethyl cellulose (CMC) and hydroxyethyl cellulose (HEC) were crosslinked with citric acid for probiotic entrapment in food, including fruits and vegetables [96]. Functionalized encapsulation is a promising, inexpensive, and environmentally friendly approach to improve the preservative properties of edible food and coatings. The edible films and coatings of plant-based essential oils, such as lemongrass (Cymbopogon citratus), rosemary pepper (Lippia sidoides), and basil (Ocimum gratissimum) encapsulated cellulose acetate, cellulose acetate propionate, and cellulose acetate butyrate, were investigated for their fragrance and air freshening effects [97]. Cellulose esters in the essential oils enhance the physicomechanical properties and plasticizing effects of edible films and coatings. Oils embedded in cellulose ester minimize moisture loss and improve the water and gas barrier [98,99]. For example, TEMPO-oxidized cellulose esters were developed to enhance the biorefractory of edible films [100]. Cellulose esters were also substituted with acyl groups containing C2 to C18 to enhance the water and oxygen barrier properties and hydrophobicity [101]. The dispersed phase effect of steric acid was studied to improve the water barrier of CMC edible films. It has been concluded that the water vapor transmittance rate (WVTR) significantly dropped when loading more steric acid to CMC [102]. The mechanical and barrier characteristics of microcrystalline cellulose (MCC) modified with lipid coating and unmodified composites of hydroxypropyl methylcellulose (HMC) were investigated. Up to a 50% increase in mechanical properties and a 40% to 50% improvement in the water barrier when loading unmodified and modified MCC were reported [102]. Composite biofilms of wheat gluten and cellulose acetate phthalate have also been tested for better permeability toward water and oxygen [103]. The preparation of polysaccharide and lipid bilayers is another approach to improve the barrier performance of edible films from cellulose and cellulose derivatives. Here, polysaccharides facilitate the film forming, and lipid matrixes act as barriers against moisture transfers [104]. Edible films from MC and HPMC, with saturated fatty acids containing carbon chains ranging from C16 to C18, have been designed with a thin lamination of beeswax at the cellulose–lipid interface to improve moisture barrier properties [105]. Bilayers of edible films from Corn Zein and MC have been reported to reduce WVTR [106]. In related research, corn zein fatty acid was cast onto MC films, and the effect of corn zein fatty acid concentration on the mechanical properties and water vapor permeability of bilayer laminated edible films of cellulose ethers has been studied [107]. Hydrophobic ethyl cellulose (EC) and hydrophilic carboxymethyl chitosan hydrogel were used to design one-way bilayer films as humidity regulators to extend the browning of white button mushrooms [108].
Edible films of bacterial nanocellulose and konjac glucomannan composites were reported as having good blending dispersion and film formation properties due to the strong hydrogen bonding between the two compatible matrixes [109]. Similar research has developed edible composites using sago starch and CMC nanoparticles to improve mechanical properties for applications in fruits and vegetables [110]. Another study has introduced CMC as a reinforcing filler to strengthen the mechanical properties of gelatin edible films [111]. In addition, antibacterial and edible film and coating cellulose esters were developed by preparing composites with chitosan [112] and silver nanoparticles [113]. Moreover, ginger and olive plant oils with bacterial cellulose and CMC composites have been studied as an antimicrobial edible coating for oranges and tomatoes [114]. Cellulose-based composites demonstrate excellent results.
4.2. Carrageenan
Carrageenan exists in three forms: kappa carrageenan (κ-carrageenan), iota carrageenan (ι-carrageenan), and lambda carrageenan (λ-carrageenan) [115]. Edible films and coatings from carrageenan are known for their excellent mechanical and barrier properties [116]. For example, edible films and coatings prepared from iota carrageenan exhibit excellent barrier properties against oxygen, prevent the deterioration of fruits and vegetables, and preserve the flavors of fruits and vegetables [117]. However, lambda carrageenan does not form stable gels and has limited use in preparing edible films and coatings [118]. The double-helical conformation and linear structure of kappa carrageenan form efficient three-dimensional (3-D) gels under standard cationic colloidal conditions. Therefore, kappa carrageenan is widely used in edible films and coatings. Carrageenan-based films and coatings also help minimize moisture loss, turgor, and oxidation [119]. Carrageenan-based edible coatings show enhanced properties upon ultraviolet treatment on post-harvested longan fruits [120]. Carrageenan gum has been blended with different starch sources to achieve desired features. For instance, edible films developed from starch/carrageenan displayed improved mechanical and WVTR properties [121].
Edible films from rice starch and ι-carrageenan plasticized with stearic acids exhibited high physicomechanical and barrier properties when the concentration of carrageenan was increased and a small amount of stearic acid was used [122]. Following a similar trend, edible composite films were prepared from pearl millet starch and carrageenan gum using glycerol as a processing aid; the increased starch reduced the water vapor permeability and mechanical properties [123]. Blends and composites have been used to improve the performance of edible carrageenan films and coatings. For instance, transparent and stable films and coatings prepared from carrageenan and rice starch hybrids demonstrated enhanced UV protection, oxygen barrier, and hydrophobic characteristics [124]. The surface properties of iota carrageenan edible films blended with glycerol plasticizer and glycerol monostearate surfactant; additionally, the fat showed improved surface properties [125]. Edible films prepared from κ-carrageenan, ι-carrageenan, and alginate blends have demonstrated improved optical, barrier, and tensile performances [126]. I-carrageenan and sodium alginate blends exhibited good mechanical characteristics with emulsion stabilizers [127].
Agar is a heteroglycan with gelling and non-gelling fractions of agarose and agaropectin, and it is readily soluble in a hot aqueous medium [128,129]. Gels from agar are known for making thermoreversible gels up to 55–60 °C with lower viscosity profiles which are ideal for edible films and coatings [129]. Agar-based edible films and coatings are generally stable and transparent and have good mechanical properties [130]. However, edible films and coatings prepared from pure agar may draw concerns in industrial applications due to brittleness, low elasticity, less thermal stability, relatively medium gas barrier performance, and high water vapor permeability [131]. Interestingly, edible films prepared from starch–agar–maltodextrin blends displayed improved barrier properties due to extensive hydrogen bonding and hydrophobic aggregations [132].
Blended films prepared using binary combinations of agar, cassava starch, and arabinoxylan have reported a decline in mechanical properties at higher loadings of cassava and arabinoxylan. The water barrier significantly improved in agar–arabinoxylan films [133]. A similar study reported that edible agar films and coatings for fruits and vegetables required an optimum concentration of glycerol plasticizer to achieve good physical and mechanical properties [134,135]. Edible films and coatings of agar doped with essential oils exhibited improved mechanical and water barrier properties with antimicrobial and antioxidant activities [136]. Agar-based composites with nanoparticles and natural active ingredients have also been investigated to improve the physicomechanical, thermal, and antioxidant characteristics suited for edible films and coatings [137].
4.3. Pectin
Pectin is one of the main constituents of plant cell walls with an anionic polysaccharide structure of β-1,4-linked α-D-Galacturonic acid in which uronic and carboxyls can be fully or partially methyl esterified [138]. It is produced as a byproduct of the industrial processing of lignocellulosic biomass and is used to develop active food packaging materials [67]. Edible films and coatings prepared from pectin exhibit excellent mechanical properties and low water barrier characteristics [139]. For instance, pectin extracted from pineapple peels was tested as a natural plasticizer for biopolymer-based edible films and coatings [140]. Edible films and coatings prepared from pectins display improved O2, CO2, and ethylene barrier properties in the presence of hydrophobic additives [141]. The natural plasticizing effect of pectin has been further investigated using alginate/pectin blends for edible films and coatings [142]. It was reported that the WVTR of the films and coatings improved with increases in plasticizer and probiotic storage at different temperatures [142].
Pectin-based edible films and coating were used as the carrier for oregano essential oils to improve their antimicrobial properties against food-related microorganisms [143]. Novel red color pectin extracted as a byproduct of Hibiscus sabdariffa L. exhibited excellent film-forming properties and was studied for strawberry preservation [144]. In a similar study, edible films and coatings prepared from pectin–whey protein blends cross-linked with transglutaminase were applied to preserve fresh-cut fruits and vegetables [145]. This research demonstrated that fresh-cut apples, potatoes, and carrots coated with pectin–whey protein blends were preserved for ten days [143]. Conversely, edible films and coatings developed from pectin–protein blends crosslinked with transglutaminase caused a reduction in the hardness and chewiness of the fruits and vegetables they covered. Candelilla wax and pectin blends were also developed to improve hydrophobicity and barrier performance [146].
4.4. Chitin and Chitosan
Chitin is the second most available natural biopolymer on earth and is extracted from the exoskeleton of crustaceans, cell walls of fungi, and other species [147]. The repeating unit of chitin consists of poly (β-(1-4)-2-acetamide D-Glucose) [148]. Chitosan is derived from the deacetylation of chitin under alkali conditions, consisting of (β-(1-4)-2-acetamido-D-Glucose and (β-(1-4)-2-acetamide-D-Glucose units [149]. Chitin and chitosan are animal-based polysaccharides. The structural properties of chitosan depend on the degree of deacetylation and the average molecular weight. Edible films and coatings of chitosan are transparent, tough, and flexible, with good oxygen barrier properties ideal for fruit and vegetable packaging. Chitosan also exhibits excellent antimicrobial properties against fungi, algae, and bacteria due to its polycationic nature [150,151]. Compared to other biopolymers, chitosan has antibacterial potential. Chitosan has been described as bacteriostatic rather than bactericidal [152].
Edible films and coatings developed from chitosan and plasticized with 30% glycerol demonstrated enhanced the biological and mechanical protection of strawberries from fungal attacks without altering the aroma, flavor, texture, or appearance of the strawberries [153]. Moreover, edible films and coatings prepared using modified diethylaminoethylchitosan were studied for maintaining freshness in perishable fruits [154]. Strawberries and bananas coated with modified diethylaminoethylchitosan exhibited antimicrobial protection against fungi that affect various fruits and extended the shelf life of selected fruits compared to pure chitosan [154].
In similar research, edible films and coatings developed from biguanide modified chitosan and an alginate blend exhibited improved thermal and mechanical properties and antibacterial activity against gram-positive and gram-negative bacteria and reduced WVTR with increasing biguanide modified chitosan [155]. Edible films of multilayered emulsion composites of chitosan and beeswax crosslinked with tripolyphosphate exhibited reduced WVTR and adequate mechanical properties [156]. Sweet cherries coated with different ratios of chitosan effectively prevented moisture losses at 20 °C and improved shelf life by reducing microbial growth [157].
4.5. Gums
Gums are polysaccharides with a significant molecular weight and are soluble in aqueous systems making hydrocolloids through solvent–polymer hydrogen bonding [158]. In aqueous systems, polymer–gum molecules form micelles, leading to hydrocolloid formation with high viscosity [159]. These hydrocolloids can easily be cast into films and coatings, making them suitable for fruit and vegetable packaging. Gum polymers can be homoglycans or heteroglycans with linear or branched structures. Edible films and coatings prepared from gums exhibit excellent mechanical, transparency, tear resistance, and plasticity. Moreover, edible films and coatings of plant gums, including gum arabic, guar gum, xantham gum, and basil seed gums, possess good barrier properties against oxygen, carbon dioxide, and moisture [160].
Gum arabic is extracted from gummy extrudes of Acacia species [161]. Gum arabic has a heteroglycan structure with a backbone composed of (1,3)-linked β-D-galactopyranosyl residues, with side chains comprising of 2–5 (1,3)-linked β-D-galactopyranosyl units attached to the primary chain by (1,6) linkages. The primary and side chains of gum arabic also contain other carbohydrate units, including l-arabinose, l-rhamnose, and glucuronic acid [162]. The composition and related physicochemical properties of gum arabic may vary from source to source. In most cases, gum arabic has been used as a component in blends. Edible films prepared from the blends of gum arabic and chitosan infused with cinnamon essential oil exhibited improved WVTR performance and low mechanical properties [163]. Edible coatings of gum arabic/starch for fruits formulated using glycerol and sorbitol as plasticizers exhibited effectiveness against moisture loss by 30%, preserving firmness, facilitating respiration, and delaying the ripening process [164]. Emulsion-based edible films and coatings of gum acacia exhibited antioxidant and antimicrobial activity and also contributed to improved inter-molecular interactions [165]. Grapefruit-encapsulated edible films and coatings from emulsion-based seed proteins and gum acacia exhibited an enhanced water vapor barrier, surface hydrophobicity, mechanical properties, and thermal stability [166].
Galactomannans are linear chains made from (1→4)-β-D-mannopyranosyl units with single-side chains in a 3:1 ratio of (1→6)-α-D-galactopyranosyl [167]. Galactomannans form highly viscous water-binding colloidal systems that interact efficiently with the polymers [168]. Guar gum and tara gum are widely studied galactomannans for preparing edible films and coatings. However, tara gums have poor mechanical and barrier performance and require improvements with plasticizers, including glycerol [169].
Xanthan gum is an extracellular polysaccharide used to prepare highly viscous colloids at low concentrations [170]. Generally, xanthan gum is used as an additive in edible films and coatings. Guar gum is hydrophilic, linear polymer with β (1→4) linkages of D-mannose and single-linked α (1→6)-D-galactose [171]. Guar gum is widely employed in preparing blends with other biopolymers, such as starch [160,172,173,174]. Active packaging blends of guar gum and sago starch infused with carvacrol and citral exhibited improved mechanical properties and inhibition of Bacillus cereus and E. coli [175]. Monosaccharides have also been investigated as plasticizers for developing edible films from guar gum and pea starch blends [176]. Edible films of guar gum and pea starch incorporated with natural antimicrobial agents have demonstrated changes in mechanical and antimicrobial properties following a concentration dependent trend [177]. For instance, four different natural antioxidants—namely, epigallocatechin gallate, blueberry ash fruit extract, macadamia peel extract, and banana peel extract—were studied with edible films and coatings of guar gum and starch blends [178].
Basil seed gum is an acidic anionic gum that has a glucomannan (43%) structure with a glucose-to-mannose ratio of 10:2, (1→4) linked xylan (24.29%), and a minor fraction of glucan (2.31%) [179]. Basil seed gum has been studied for preparing active edible films and coatings because of its mechanical, antioxidant, and antimicrobial properties. It has been reported that the edible films of basil seed gum infused with oregano essential oils exhibited enhanced physicomechanical properties [180].
Moreover, adding glycerol plasticizer into edible films and coatings prepared from basil seed gum hydrocolloids improved the physical, mechanical, microstructural, and thermal characteristics of the edible films [181]. In addition, edible films from nanoemulsions of Zataria multiflora essential oil with basil seed gum displayed high mechanical properties and strong antimicrobial activity against gram-positive and gram-negative bacteria [182]. Nanoemulsion also delayed the release of volatile compounds [182].
Gellan gum is a bacterial exopolysaccharide produced from the aerobic fermentation of carbohydrate substrates in bacteria [183]. Gellan gum has a linear, anionic polymer structure with a degree of polymerization (DP) of about 50,000. The chemical structure of gellan gum comprises of repeating units of β-d-glucose, l-rhamnose, and d-glucuronic acid and two acyl groups, acetate, and glycerate, attached to the glucose residue adjacent to glucuronic acid [184]. De-esterification of gellan gum produces stronger films and also alters gel texture [159]. Polymer blends of gellan gum have been widely investigated for developing edible films and coatings [185]. For example, edible films prepared from the blends of gellan gum and aloe vera gel showed improved mechanical properties [186]. Edible films and coatings of gellan gum and aloe vera blends have been designed for active food packaging [187]. Moreover, edible films of gellan gum integrated into proteins exhibited excellent mechanical properties and barrier performance [188].
4.6. Starch
Starch is a semicrystalline homopolymer with up to 20%–40% crystallinity and consists of two major constituents: amylose and amylopectin [189]. Amylose is a liner polysaccharide structure made up of α-1,4 bonds, while amylopectin is a branched molecule in which the branch points consist of α-1,6 glyosidic bonds [190]. Co-crystallization favors crystallization in amylose into single helixes [191]. The hydrocolloid properties of starch in edible films and coatings depend on the crystallinity, amylopectin ratio, moisture content, molecular mass, degree of branching, and polymeric chain length of the source of origin [189,192]. Edible films and coatings prepared from starch exhibit mechanical, oil, and oxygen barrier properties [193]. Moreover, modified starch enhances the physicomechanical characteristics of films and coatings and processing processes [194]. However, due to its high hydrophilicity, antioxidant, antimicrobial, and other food additive agents are incorporated into the starch matrix.
Starch composites and blends have been investigated extensively for the active packaging of fruits and vegetables. For example, edible coatings were developed using a combination of cassava starch, glycerol, carnauba wax, and stearic acid as plasticizers for fresh-cut fruits and vegetables [195]. Bioactive edible starch films and coatings were formulated using phenolic compounds [196]. Starch–chitosan blends were studied for antimicrobial properties against lactobacillus spp. [153]. This study demonstrated reduced aerobic mesophilic and psychrophilic cell counts while maintaining pH and weight loss in refrigerated storage and extending product life beyond 6 days [153]. The effect of the amylose-to-amylopectin ratio of different starch species, wheat, corn, and potato on the physicomechanical properties of edible films and coatings has been studied [197]. Edible films prepared from starch with higher amylose content exhibited better mechanical resistance and barrier properties with higher moisture sensitivity due to their hydrophilic nature [197]. Among many starch sources, wheat starch has the least surface wettability because of its low surface hydrophilicity at elevated temperatures [197].
Sunflower oil added corn starch edible films and coatings demonstrated improvements in mechanical and water barrier properties due to the low crystallinity of starch and microstructural changes with the loading of sunflower oils as a plasticizer [198]. The combined effect of plasticizers and surfactants on starch-based edible films and coatings was investigated using glycerol, Tween 20, and Spam 80 as plasticizers and soy lecithin as a surfactant [199]. This study confirmed the synergistic contribution of the plasticizer and the surfactants in achieving high mechanical and water barrier properties, and further concluded that a high loading of plasticizers led to a decline in mechanical properties and higher WVTR and that surfactants contributed to improving mechanical properties in the absence of glycerol [199]. Transfer properties of glycerol-loaded edible starch films were reported, confirming higher diffusivity/transfer properties for 55% glycerol-loaded edible films over 33% glycerol-loaded films [200]. Moreover, glycerol impacts the water diffusivity, oxygen permeability, and water vapor permeability performances in edible films and coatings. The same trend was observed with the edible film of native wheat prepared from varying concentrations of glycerol (0, 20, 30, 40, and 50, wt%) [201]. The lowest WVTR was reported at 30% glycerol loading, but the degree of crystallinity was reduced. With increased glycerol loading in the starch matrix, stress at break and Young’s modulus decreased, and elongation increased [201]. Edible films of highly carboxymethylated starch (HCMS) plasticized using sorbitol, xylitol, mannitol, and glycerol showed reduced WVTR and decreased solubility with increasing plasticizer concentration [202,203,204]. Edible films and coatings have been developed from non-conventional arrowroot starch using the casting method prepared for plum packaging [205]. Results displayed an increase in WVP, from 2.20 to 3.68 g mm/m2 day kPa, an increase in moisture content from 3.22% to 7.95%, and a decrease in solubility in water from 22.45% to 13.89%, delivering extended post-harvesting preservation up to 35 days [205]. Furthermore, these edible films and coatings exhibited good film-forming capacity, with homogeneous, transparent, and manageable appearances. The 2% coating indicated good adhesion, the successful minimization of moisture loss, and control of the respiration rate [205]. In another study, the rheological properties of corn starch methylcellulose and glycerol edible films and coatings exhibited high total viscoelastic recovery at a high shear rate, similar to entangled polymer dispersions, due to the interactions of topological entanglements and dispersion stability [206].
5. Protein-Based Edible Films and Coatings
Edible films and coatings from protein sources may have an animal and plant origin [207]. Protein-based edible films and coatings are formulated with three main components: protein, plasticizer, and solvent. Most of the proteins in edible food packaging are insoluble fibrous or globular water-soluble proteins [29]. Proteins make a cohesive film matrix with string interactions stabilized from uniformly distributed attractive forces of hydrogen, Van der Waals forces, and covalent and disulfide bonding [208]. Protein-based edible films and coatings are biodegradable and compostable. The degradation process of protein-based edible films generates nitrogen in the soil, enhances soil nitrogen content, and functions as a fertilizer, delivering more benefits than non-protein-based edible films and coatings [209]. Protein-based edible films act as bioactive peptides in digestion and deliver health benefits through antihypertensive and radical scavenging [210,211]. Characteristics of edible films and coatings from proteins are interlinked with composition, components, and processing techniques [34,212]. The performances of protein-based edible films and coatings are associated with their structural factors, including their crystallinity, amino acid composition, additives, plasticizer content, hydrophobicity/hydrophilicity, nature of the surface charge, molecular size distribution, and three-dimensional shape [213,214].
5.1. Animal Proteins—Casein
Casein is the major dairy protein group in bovine or goat milk. There are four main subunits of casein: alpha s1-casein, alpha s2-casein, beta-casein, and kappa-casein, which make up 38%, 10%, 36%, and 13% of casein composition, respectively [215]. The primary, secondary, and tertiary structures of casein are less ordered and more flexible than those of typical globular proteins [216,217]. The structure of casein contains polar and hydrophobic domains with a degree of flexibility, giving limited proteolysis and enhanced functionality [218]. Each of the four protein fractions possesses unique properties that influence the film-forming ability of casein [219]. Edible films and coatings of casein in fruits and vegetables have been widely investigated due to the processing convenience of emulsion preparation in amphipathic dispersion systems [220]. The complex intermolecular binding of milk protein in edible films and coatings is well known for providing a good barrier against gas permeation and for its nutritional value [221]. Cohesive edible films and coatings can be designed from total milk proteins, wherein the continuous 3D film-coating network is determined by protein–protein interaction [220,222].
Edible films and coatings from casein and beeswax blends were developed from emulsions to reduce white blush and increase water vapor resistance in processed carrots by integrating stearic acid or acetylated monoglyceride [223]. Edible films prepared from casein and natamycin controlled mold growth and microbial activity efficiently [224]. Generally, the WVTR of caseinate edible films is affected by several factors, such as pH, calcium crosslinking, and lipid content [225]. A study assessing the permeability of casein and wax heat-sealed edible film reported that the WVTR decreased as the wax content increased without a difference in oxygen permeability with loading waxes [226]. Extruded casein and wax blends for active edible films incorporated with potassium sorbate as the carrier exhibited good bacteriostatic properties, inhibiting E. coli growth for up to 20 days [227].
5.2. Animal Proteins—Whey Protein
Whey protein is a byproduct of serum coagulation from casein extraction in cheese production [228]. Whey protein in edible films and coatings can be found as protein isolate or whey protein concentrate, which forms an interactive 3D polymeric network upon drying [229]. Compared to polysaccharides and other proteins, whey protein can be used as an emulsifying agent, thickening agent, gelling agent, foaming agent, and water-binding agent. Films and coatings of whey protein inherit good mechanical, odorless, flexible, and transparent characteristics with moderate water vapor permeability and an excellent oxygen gas barrier [230]. The film formation and gel characteristics of whey protein are determined by its secondary, tertiary, or quaternary globular structures and the presence of various combinations of cross-sulfur bonds, making them heat-labile, dephosphorylated, and less calcium sensitive [231]. Whey protein coatings are proposed for microbial growth reduction, shelf life extension, and moisture loss and spoilage minimization for fruits and vegetables, without changing texture [232]. Furthermore, edible films and coatings from whey proteins can be used as an efficient carrier for antimicrobials, antioxidants, or other nutraceuticals [233].
The influence of protein-to-glycerol ratio in mechanical, optical, and moisture sorption properties of films and coatings prepared from whey protein isolates were analyzed, further justifying that increasing protein concentration leads to transparent films, higher moisture adsorption and enhanced tensile properties [234]. Edible films and coatings of whey proteins modified with almond and walnut oils have been reported for decreased WVTR due to enhanced hydrophobicity. Further, the incorporation of almond oils provided a plasticizing effect to the film [235]. Blends of whey protein and pullan plasticized using glycerol have been studied to determine optimum blending ratios for potential edible films and coatings applications [236]. The physical properties of edible films from whey proteins loaded with rapeseed oils have been studied for their emulsion properties. Significant improvements in tensile properties were reported because the mechanical resistance decreased with lipids, while the opacity increased [237]. The blend of whey protein and pectin crosslinked with transglutaminase minimized microbial growth control and moisture loss, with minimal impact on the texture properties, chewiness, and hardness [145].
5.3. Animal Proteins—Collagen
Proteins of animal origin are also considered for edible films and coatings due to their excellent mechanical and oxygen and carbon dioxide barrier properties in low-humidity conditions [238]. Collagen is extracted from the connective tissues of animals and is a major structural protein of the extracellular matrix [239]. The triple helical structure of collagen is known for determining its physicochemical and colloidal properties [240]. A colloidal dispersion of collagen is desirable for making EF & EC via casting or extrusion and may require salt coagulation or covalent crosslinking for extended packaging requirements [241]. A recent study used an acetic acid extraction technique for collagen. EF & EC properties proved to be acceptable after evaluating the film’s mechanical and tensile strength, Young’s modulus, elongation, and WVR [242]. Chitosan–collagen edible films emulsified with cinnamon perilla essential oil exhibited improved film properties and shelf life, resulting from Pickering nanoemulsification [243]. Coating food with chitosan films lowers the oxygen partial pressure in the package [244].
In another approach, hydrolyzed collagen and cocoa butter edible films plasticized from sucrose proved to have satisfactory optical properties, improvements in mechanical properties, and significant changes in WVTR [245]. Blends of collagen and alginates were used to prepare uniform films with no phase separation and exhibited good tensile, elongation at break, and WVTR properties [246]. Edible films and coatings developed from collagen and polysaccharide blends exhibited antimicrobial efficacy for staphylococcus aureus and Escherichia coli when 12 mg/mL cell-free supernatants were loaded into the films [247]. In similar research, hydrolyzed collagen and sodium alginate edible films and coatings EF & EC were developed as a low-cost alternative to byproducts with increased thickness [248]. WVTR was reduced up to 50%, with slight inhomogeneity across the film [248]. Transglutaminase-crosslinked edible films and coatings from collagen were developed to improve the stability of the structure and packing of collagen fibers, improving mechanical and barrier properties, thermal stability, and morphology compared with the uncrosslinked [249]. Chitosan–lemon–essential oil nanoparticles incorporated in collagen composites exhibited lower oxygen permeability, higher tensile strength (TS), and higher elongation at break [250]. Furthermore, collagen–chitosan–lemon essential oil composites preferentially inhibited lipid oxidation, microbial growth, and food deterioration [250].
5.4. Animal Proteins—Gelatin
Gelatin is a secondary product derived from the hydrolysis of primary collagen proteins under mild heat or acidic or alkaline conditions into a partially denatured unstructured domain triple helical structural form [251]. Type A gelatins are derived from the acid processing of collagens, and type B gelatins are a product of alkaline or lime processing [252]. Blends of gelatin and starch have been widely tested for edible films and coatings [253]. For example, edible films and coatings prepared from gelatin–starch blends plasticized using sorbitol and glycerol displayed improved transparency, mechanical properties, and satisfactory WVTR among crimson red grapes [254]. Furthermore, the edible films and coatings of crimson red grapes act as an effective post-harvest treatment, maintaining fruit quality and preventing moisture loss without interfering with product taste [254]. Manioc starch and gelatin blends with different plasticizer combinations exhibited enhanced transparency, tensile performance, and WVTR [255]. However, as the sorbitol concentration increased, the stability of the edible film decreased due to a broader glass transition and phase separation [256].
Antioxidant and antimicrobial edible films and coatings were also prepared from gelatin and chitosan blends by integrating plant ethanolic extracts. It was reported that increasing chitosan increased the elasticity of the EF. According to the tolerance equivalent antioxidant test, these infused blends exhibited good antioxidant performance and excellent microbial growth inhibition against Escherichia coli and Staphylococcus aureus [257]. A similar study that assessed the mechanical performance of gelatin and chitosan blends indicated that chitosan loading significantly increased tensile strength and elastic modulus but reduced elongation at break, rendering films brittle (p < 0.05) [258]. Furthermore, loading chitosan drastically improved the water barrier and water stability of the gelatin films (p < 0.05) and the light barrier properties at 600 nm against UV light [258]. In another approach, olive oil was incorporated into the gelatin matrix, and it delivered enhanced barrier properties and tensile performance, with high stability against UV light in emulsified films, rendering them potentially useful for fruits and vegetable packaging [259]. Edible films from gelatin and casein blends crosslinked using transglutaminase enzymatic crosslinker exhibited elevated elongation (p < 0.05) compared to gelatin or casein alone. Crosslinks have improved water barrier as casein and gelatin (75:25) reported the lowest WVTR (5.06 ± 0.31 g mm/m2 d kPa) [260].
5.5. Plant Proteins—Soy and Wheat Proteins
Soy and wheat are the two main plant-based proteins used for edible films and coatings in food [261]. Soy protein isolates are hydrocolloids. Films and coatings can be processed following film-forming and casting methods. Films prepared from soy proteins are an excellent barrier against oxygen and moisture [262]. Moreover, the properties, including appearance, tensile strength, and low WVTR of soy hydrocolloid films, can be enhanced with alkaline treatment [263]. Wheat glutens are also used for edible films and coatings. However, the application of wheat glutens in edible films and coatings is limited due to increasing gluten-free food consumption. In general, plant proteins in edible films and coating are less common than animal proteins due to food security concerns, limited availability, and processing difficulties.
6. Lipid-Based Edible Films and Coatings
Edible films and coatings prepared from lipids are critical for minimizing moisture loss. Moreover, they act as a hydrophobic barrier [104]. For fruits and vegetables, moisture regulation preserves and extends shelf life through a method similar to desiccation. In addition, lipids act as carriers for active molecules and keep the texture and flavors of the food. Additionally, they are utilized as varnishes. Table 1 summarizes the applications of edible films and coatings for fruits and vegetables. Table 2 summarizes the different types of lipids used in edible films and coatings.

Table 1.
Applications of edible films and coatings for fruits and vegetables.

Table 2.
Lipids in edible films and edible coatings.
Lipids are incorporated with hydrocolloids such as polysaccharides, proteins, cellulose, starches, and their derivatives in composites. Lipid blends and hydrocolloids can be prepared mainly through solvent blending and mixing. Other methods used for making composites are the casting and film-forming methods, with multilayer and bilayer films. In lipid–hydrocolloid composites, the hydrocolloid fraction contributes to moisture absorbance, while lipids are responsible for moisture regulation. Additives are doped into lipids to introduce various functionalities to edible films and coatings. Functionalization of lipids helps improve the film-forming ability of colloidal emulsions and suspension phases and promotes adherence of films and coatings on the food surfaces. In addition, lipids act as emulsifiers, texturing agents, antioxidants, antimicrobial agents, enzymes, process aid, and gelling promoters. Plant based oils such as soybean, sunflower, high oleic linseed, rapeseed, and palm have been studied as edible films and coatings due to their moisture barrier properties, wide availability, smaller flavor impact, and low cost [268].
6.1. Essential Oils
Essential oils are volatile components obtained from plants and exhibit distinct anti-microbial properties [269]. Furthermore, essential oils in edible films and coatings prevent chemical reactions, lipid oxidation, endogenous enzyme activity and microbial growth [136]. Natural essential oils also considered GRAS help improve the mechanical, antimicrobial, antioxidant, and sensory properties of the inedible films and coatings in fruits and vegetables. To enhance the shelf life of fresh fruits and fruit cuts, essential oils with monoterpenes and sesquiterpenes are incorporated into films and coatings [270]. It was reported that essential plant oils are the main additive used as antimicrobial and antioxidant agents in edible films and coatings. The active components found in essential oils include various spices and herbs, specifically thymol, cinnamaldehyde, carvacrol, 1,8-cineole, and eugenol, which give a greater extent of antimicrobial and antioxidant performance [271]. Essential oils cause stable interactions between hydroxyl groups in biomacromolecules through ethers, aldehydes, and ketones pendant groups in their structure. Essential oils give hydrophobicity to edible films and coatings with improved water and moisture barrier properties [272,273]. Essential oils may improve tensile strength and elongation at the break due to the plasticizing effect and their capability to make crosslinks [274,275,276].
6.2. Waxes and Resins
Waxes, referred to as esterified long-chain alcohols and fatty acids, belong to a diverse class of organic compounds that may be solid or semi-solid at ambient temperatures [277]. Based on the source of origin, waxes can be divided into three classes [267]:
- (a)
- Animal waxes: bees wax, spermaceti wax, shellac wax, lanolin wax, Chinese insect wax;
- (b)
- Vegetable waxes: carnauba wax, candelilla wax, bayberry wax, rosin wood wax, sugarcane wax, palm wax, esparto wax, cotton seed wax, oricury wax, rice bran wax, Japan wax, and waxol;
- (c)
- Mineral and synthetic waxes: montan wax, paraffin wax, ozocerite, synthetic wax, and microcrystalline wax.
According to the FDA, paraffin wax on raw fruits and vegetables is permitted [278]. Paraffin, carnauba, beeswax, candelilla, and polyethylene waxes are widely used in food-related applications alone or in combination [279]. Edible wax coatings are commonly applied in the post-harvest preservation of fruits and vegetables. Waxes provide good moisture and gas properties and preserve the surface appearance of fruits and vegetables. Edible waxes exhibit high durability, stretching, and super-hydrophobicity [280]. However, it has been reported that waxes such as paraffin, carnauba, candelilla, and bees wax act as humidity barriers for edible films and coatings [281]. Wax-based micro-emulsions are widely used in fruit and vegetable coatings [282]. For instance, micro-emulsion coatings prepared from candelilla, beeswax, carnauba wax, polyethylene, and petroleum exhibit distinct moisture barrier properties [283]. However, polyethylene and carnauba waxes were the glossiest and most brittle coatings [283].
Compared to ammonia-based emulsion wax coatings, edible coatings from morpholine wax exhibited a low barrier to oxygen and water vapor. Candelilla wax edible coating combined with biocontrol bacteria improved the quality and the shelf life of strawberries and acted against Rhizopus stolonifer bacteria [284]. Carnauba wax incorporated in edible coatings enhanced the shelf life of mangos by decelerating the ripening process during storage [285]. Candelilla wax incorporated with bioactive compounds preserved the quality of tomatoes for a prolonged period [286]. Blends of waxes and fermented extract of tarbush improved the quality of apples by minimizing weight loss and ensuring firmness without altering the appearance and taste of apples [287].
Resins are another component of edible films and coatings. Terpene resin is obtained from the polymerization of terpene hydrocarbons derived from wood and is approved as a direct food additive [269]. Natural resins are added to the formulations of edible films and coatings to provide hydrophobicity, cohesiveness, and flexibility [266].
7. Health Effects
Edible films and coatings are primary packaging. According to Regulation No. 1935/2004, food contact materials should not transfer hazardous compounds to health into food [288]. Edible films and coatings were developed as an alternative to chemicals and synthetic materials, which are harmful to human health [289]. Edible coatings can help to preserve the antioxidants, phenolics, and pigments of the food for a long time [289]. Probiotics can be added through the microencapsulation technique [290]. Furthermore, edible coatings which are supplemented with bioactive compounds, such as probiotics, can provide benefits to human health, and, if the food coating has a certain level of probiotics, the food can be considered a functional food [289,291]. Waxes and paraffins are considered safe coatings for fruits and vegetables [291]. Cellulose monomers and starch-based biopolymer monomers are considered to be health safe and cause no health problems [292] However, nanocellulose, which is used in edible coatings, is non- toxic at a concentration of 0–50 μg/mL in human endothelial cells, and above the stated physiological changes [293]. Certain studies have demonstrated that collagen exhibited discoloration, skin necrosis, granuloma formation, blindness, and foreign body reactions as clinical manifestations [293]. However, further detailed studies are required to assess the full effect of edible films and coatings on human health to ensure food safety.
8. Conclusions and Current Trends
Edible films and coatings are a pivotal part of food preservation amid unprecedented global hunger and supply chain crises. Moreover, edible films and coatings prepared from renewable biomacromolecules have been widely explored due to their relative abundance, cost-effectiveness, biocompatibility, nontoxicity, and biodegradability. The colloidal chemistry of biomacromolecules, including polysaccharides, proteins, and lipids, determines their processing and film-forming capacity, casting, lamination, and other properties. Hydrocolloids of biopolymers exhibit excellent air and gas barrier properties, making them excellent candidates for developing edible films and coatings.
Sustainable production approaches, materials from renewable sources, green synthesis, eco, and biotoxicity are crucial aspects in the modern packaging of edible films and coatings. Therefore, biomacromolecules, including polysaccharides, proteins, and lipids, are researched extensively for future food packaging advancements. There are some challenges presented by edible films that limit industrial applications, such as their structural characteristics, aroma, and flavors. In addition, some edible films exhibit instability of bioactive compounds, poor weak film-forming properties, and surface adhesion limited commercial applications. The incorporation of essential oil to improve the antimicrobial properties of edible coatings results in poor water solubility, intense odor, and high volatility. At the industry level, edible packaging materials from biobased sources face challenges due to their low performance in moisture barrier, temperature resistance, and relative humidity control. Hence, using natural molecules in designing efficient films and coatings for fruits and vegetables may have chemical and structural limitations. Regulation and safety-related issues are other challenges with the utilization of edible films and coatings (no recommended standards).
Biopolymer blends, composites, and complex multilayers have recently emerged as novel techniques for developing edible films and coatings for fruits and vegetables. Furthermore, crosslinking, functionalization, and surface modifications using green routes have gained significant attention for enhancing the physical, chemical, and biological properties of biopolymer-based edible films and coatings. For instance, active edible films and coatings have been developed by doping active molecules, such as antimicrobial, antifungal, enzymes, vitamins, essential oils, and plasticizers. Moreover, active packaging, intelligent packing, and nanotechnology-based approaches are trending in edible food packing applications. Edible coatings and films with nanoparticles improve the strength and performance and increase the prolonged shelf life of perishable food items. Consumers, food processors, and environmental regulating agencies should promote the use of edible films and coatings engineered from biobased alternatives to reduce food losses and minimize the environmental impacts of plastic packaging as a sustainable circular measure.
Author Contributions
Writing—original draft A.L., A.G., A.M. and R.S.D. Review and editing, A.L., A.G., A.M., T.M., O.M., Y.J., R.R.K. and P.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAO. Food Wastage Footprint: Impacts on Natural Resources; FAO: Rome, Italy, 2013. [Google Scholar]
- Liu, C.; Hotta, Y.; Santo, A.; Hengesbaugh, M.; Watabe, A.; Totoki, Y.; Allen, D.; Bengtsson, M. Food waste in Japan: Trends, current practices and key challenges. J. Clean. Prod. 2016, 133, 557–564. [Google Scholar] [CrossRef]
- Gustavsson, J.; Cederberg, C.; Sonesson, U.; Van Otterdijk, R.; Meybeck, A. Global Food Losses and Food Waste; FAO: Rome, Italy, 2011. [Google Scholar]
- Amicarelli, V.; Lagioia, G.; Bux, C. Global warming potential of food waste through the life cycle assessment: An analytical review. Environ. Impact Assess. Rev. 2021, 91, 106677. [Google Scholar] [CrossRef]
- Poore, J.; Nemecek, T. Reducing food’s environmental impacts through producers and consumers. Science 2018, 360, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, H.; Roser, M. Environmental impacts of food production. 2020. Available online: https://ourworldindata.org/environmental-impacts-of-food (accessed on 28 May 2023).
- Racz, A.; Vasiljev Marchesi, V.; Crnković, I. Economical, environmental and ethical impact of food wastage in hospitality and other global industries. Jahr Eur. Časopis Bioetiku 2018, 9, 25–42. [Google Scholar]
- FAO. Food Wastage Footprint Full-Cost Accounting; FAO: Rome, Italy, 2014. [Google Scholar]
- Gross Domestic Product, Fourth Quarter and Year 2021 (Second Estimate). 2022. Available online: https://www.bea.gov/news/2023/gross-domestic-product-fourth-quarter-and-year-2022-second-estimate (accessed on 28 May 2023).
- United Nations Environment Programme. Think, Eat, Save. Reduce Your Footprint; UNEP: Nairobi, Kenya, 2021. [Google Scholar]
- United Nations General Assembly. Transforming Our World: The 2030 Agenda for Sustainable Development; A/RES/70/1; United Nations: New York, NY, USA, 2015.
- Xue, L.; Liu, G. 1—Introduction to global food losses and food waste. In Saving Food; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 1–31. [Google Scholar]
- Xue, L.; Liu, G.; Parfitt, J.; Liu, X.; Van Herpen, E.; Stenmarck, Å.; O’Connor, C.; Östergren, K.; Cheng, S. Missing Food, Missing Data? A Critical Review of Global Food Losses and Food Waste Data. Environ. Sci. Technol. 2017, 51, 6618–6633. [Google Scholar] [CrossRef]
- Chen, C.; Chaudhary, A.; Mathys, A. Nutritional and environmental losses embedded in global food waste. Resour. Conserv. Recycl. 2020, 160, 104912. [Google Scholar] [CrossRef]
- Blakeney, M. Food Loss and Food Waste: Causes and Solutions; Edward Elgar Publishing: Cheltenham, UK, 2019. [Google Scholar]
- Gustafsson, J.; Cederberg, C.; Sonesson, U.; Emanuelsson, A. The Methodology of the FAO Study: Global Food Losses and Food Waste-Extent, Causes and Prevention-FAO, 2011; FAO: Rome, Italy, 2013. [Google Scholar]
- Sachdeva, S.; Sachdev, T.R.; Sachdeva, R. Increasing fruit and vegetable consumption: Challenges and opportunities. Indian J. Community Med. Off. Publ. Indian Assoc. Prev. Soc. Med. 2013, 38, 192. [Google Scholar] [CrossRef]
- Southon, S.; Faulks, R. Health benefits of increased fruit and vegetable consumption. Fruit Veg. Process. Improv. Qual. 2002, 1, 2–20. [Google Scholar]
- FAO. International Year of Fruits and Vegetables 2021; Global Action Plan: London, UK, 2021. [Google Scholar]
- Morone, P.; Koutinas, A.; Gathergood, N.; Arshadi, M.; Matharu, A. Food waste: Challenges and opportunities for enhancing the emerging bio-economy. J. Clean. Prod. 2019, 221, 10–16. [Google Scholar] [CrossRef]
- Lintas, C. Nutritional aspects of fruits and vegetables consumption. Options Mediterraennes 1992, 19, 79–87. [Google Scholar]
- Embuscado, M.E.; Huber, K.C. Edible Films and Coatings for Food Applications; Springer: Berlin/Heidelberg, Germany, 2009; Volume 9. [Google Scholar]
- Baldwin, E.A.; Hagenmaier, R.; Bai, J.; Krochta, J.M. Edible Coatings and Films to Improve Food Quality; Taylor & Francis: Abingdon, UK, 1994. [Google Scholar]
- Rossman, J.M. Commercial Manufacture of Edible Films. In Edible Films and Coatings for Food Applications; Huber, K.C., Embuscado, M.E., Eds.; Springer New York: New York, NY, USA, 2009; pp. 367–390. [Google Scholar]
- United States Food and Drug Administration. CFR—Code of Federal Regulations Title 21; FDA: Montgomery, MD, USA, 2022.
- United States Food and Drug Administration. Generally Recognized as Safe (GRAS); FDA: Montgomery, MD, USA, 2019.
- Díaz-Montes, E.; Castro-Muñoz, R. Edible Films and Coatings as Food-Quality Preservers: An Overview. Foods 2021, 10, 249. [Google Scholar] [CrossRef]
- Davis, G.; Song, J. Biodegradable packaging based on raw materials from crops and their impact on waste management. Ind. Crops Prod. 2006, 23, 147–161. [Google Scholar] [CrossRef]
- Bourtoom, T. Edible films and coatings: Characteristics and properties. Int. Food Res. J. 2008, 15, 237–248. [Google Scholar]
- Guimaraes, A.; Abrunhosa, L.; Pastrana, L.M.; Cerqueira, M.A. Edible films and coatings as carriers of living microorganisms: A new strategy towards biopreservation and healthier foods. Compr. Rev. Food Sci. Food Saf. 2018, 17, 594–614. [Google Scholar] [CrossRef] [PubMed]
- Olivas, G.I.I.; Barbosa-Cánovas, G. Edible Films and Coatings for Fruits and Vegetables. In Edible Films and Coatings for Food Applications; Huber, K.C., Embuscado, M.E., Eds.; Springer: New York, NY, USA, 2009; pp. 211–244. [Google Scholar]
- Rossi-Márquez, G.; Dávalos-Saucedo, C.A.; Di Pierro, P. Edible Films and Coatings Applied in the Food Industry. Coatings 2023, 13, 670. [Google Scholar] [CrossRef]
- Han, J.H. Edible films and coatings: A review. In Innovations in Food Packaging; Academic Press: Cambridge, MA, USA, 2014; pp. 213–255. [Google Scholar]
- Hernandez-Izquierdo, V.; Krochta, J. Thermoplastic processing of proteins for film formation—A review. J. Food Sci. 2008, 73, R30–R39. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Castellanos, W.; Martínez-Bustos, F.; Rodrigue, D.; Trujillo-Barragán, M. Extrusion blow molding of a starch–gelatin polymer matrix reinforced with cellulose. Eur. Polym. J. 2015, 73, 335–343. [Google Scholar] [CrossRef]
- Dahiya, M.; Saha, S.; Shahiwala, A.F. A review on mouth dissolving films. Curr. Drug Deliv. 2009, 6, 469–476. [Google Scholar] [CrossRef]
- Pavlath, A.E.; Orts, W. Edible films and coatings: Why, what, and how? In Edible Films and Coatings for Food Applications; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–23. [Google Scholar]
- Mellinas, C.; Valdés, A.; Ramos, M.; Burgos, N.; Garrigos, M.d.C.; Jiménez, A. Active edible films: Current state and future trends. J. Appl. Polym. Sci. 2016, 133, 42631. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Food applications of emulsion-based edible films and coatings. Trends Food Sci. Technol. 2015, 45, 273–283. [Google Scholar] [CrossRef]
- Bhattacharya, T. Techniques of Preparing Edible Protein Films. Asian J. Sci. Technol. 2013, 4, 39–41. [Google Scholar]
- Safaya, M.; Rotliwala, Y. Nanoemulsions: A review on low energy formulation methods, characterization, applications and optimization technique. Mater. Today Proc. 2020, 27, 454–459. [Google Scholar] [CrossRef]
- Gutoff, E.B.; Cohen, E.D. Water-and solvent-based coating technology. In Multilayer Flexible Packaging; Elsevier: Amsterdam, The Netherlands, 2016; pp. 205–234. [Google Scholar]
- Wang, Q.; Chen, W.; Zhu, W.; McClements, D.J.; Liu, X.; Liu, F. A review of multilayer and composite films and coatings for active biodegradable packaging. NPJ Sci. Food 2022, 6, 18. [Google Scholar] [CrossRef]
- Gupta, V.; Biswas, D.; Roy, S. A Comprehensive Review of Biodegradable Polymer-Based Films and Coatings and Their Food Packaging Applications. Materials 2022, 15, 5899. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E. Hydrocolloids at interfaces and the influence on the properties of dispersed systems. Food Hydrocoll. 2003, 17, 25–39. [Google Scholar] [CrossRef]
- Li, J.-M.; Nie, S.-P. The functional and nutritional aspects of hydrocolloids in foods. Food Hydrocoll. 2016, 53, 46–61. [Google Scholar] [CrossRef]
- Phillips, G.O.; Williams, P.A. Handbook of Hydrocolloids; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Nishinari, K.; Zhang, H.; Ikeda, S. Hydrocolloid gels of polysaccharides and proteins. Curr. Opin. Colloid Interface Sci. 2000, 5, 195–201. [Google Scholar] [CrossRef]
- Goff, H.D.; Guo, Q. Chapter 1 The Role of Hydrocolloids in the Development of Food Structure. In Handbook of Food Structure Development; The Royal Society of Chemistry: London, UK, 2020; pp. 1–28. [Google Scholar]
- Godoi, F.C.; Ningtyas, D.W.; Geoffroy, Z.; Prakash, S. Protein-based hydrocolloids: Effect on the particle size distribution, tribo-rheological behaviour and mouthfeel characteristics of low-fat chocolate flavoured milk. Food Hydrocoll. 2021, 115, 106628. [Google Scholar] [CrossRef]
- Yemenicioğlu, A.; Farris, S.; Turkyilmaz, M.; Gulec, S. A review of current and future food applications of natural hydrocolloids. Int. J. Food Sci. Technol. 2020, 55, 1389–1406. [Google Scholar] [CrossRef]
- Kristl, J.; Šmid-Korbar, J.; Štruc, E.; Schara, M.; Rupprecht, H. Hydrocolloids and gels of chitosan as drug carriers. Int. J. Pharm. 1993, 99, 13–19. [Google Scholar] [CrossRef]
- Wüstenberg, T. Cellulose and Cellulose Derivatives in the Food Industry: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Picot-Allain, M.C.N.; Ramasawmy, B.; Emmambux, M.N. Extraction, characterisation, and application of pectin from tropical and sub-tropical fruits: A review. Food Rev. Int. 2022, 38, 282–312. [Google Scholar] [CrossRef]
- Chen, J.; Liu, W.; Liu, C.-M.; Li, T.; Liang, R.-H.; Luo, S.-J. Pectin modifications: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1684–1698. [Google Scholar] [CrossRef] [PubMed]
- Nieto, M.B. Structure and function of polysaccharide gum-based edible films and coatings. In Edible Films and Coatings for Food Applications; Springer: Berlin/Heidelberg, Germany, 2009; pp. 57–112. [Google Scholar]
- Alsabagh, A.; Abdou, M.; Khalil, A.; Ahmed, H.; Aboulrous, A. Investigation of some locally water-soluble natural polymers as circulation loss control agents during oil fields drilling. Egypt. J. Pet. 2014, 23, 27–34. [Google Scholar] [CrossRef]
- Marcotte, M.; Hoshahili, A.R.T.; Ramaswamy, H. Rheological properties of selected hydrocolloids as a function of concentration and temperature. Food Res. Int. 2001, 34, 695–703. [Google Scholar] [CrossRef]
- Kester, J.J.; Fennema, O. Edible films and coatings: A review. Food Technol. 1986, 40, 47–59. [Google Scholar]
- Bunjes, H. Structural properties of solid lipid based colloidal drug delivery systems. Curr. Opin. Colloid Interface Sci. 2011, 16, 405–411. [Google Scholar] [CrossRef]
- Gordillo-Galeano, A.; Ponce, A.; Mora-Huertas, C.E. Surface structural characteristics of some colloidal lipid systems used in pharmaceutics. J. Drug Deliv. Sci. Technol. 2021, 62, 102345. [Google Scholar] [CrossRef]
- Heurtault, B.; Saulnier, P.; Pech, B.; Proust, J.-E.; Benoit, J.-P. Physico-chemical stability of colloidal lipid particles. Biomaterials 2003, 24, 4283–4300. [Google Scholar] [CrossRef]
- Patel, A.R.; Velikov, K.P. Colloidal delivery systems in foods: A general comparison with oral drug delivery. LWT—Food Sci. Technol. 2011, 44, 1958–1964. [Google Scholar] [CrossRef]
- Wilde, P.J.; Chu, B.S. Interfacial & colloidal aspects of lipid digestion. Adv. Colloid Interface Sci. 2011, 165, 14–22. [Google Scholar]
- Bourtoom, T.; Chinnan, M. Improvement of water barrier property of rice starch-chitosan composite film incorporated with lipids. Food Sci. Technol. Int. 2009, 15, 149–158. [Google Scholar] [CrossRef]
- Ajesh, K.V.; Hasan, M.; Shukadev, M.; Pravitha, M. Deepak Kumar Verma, Prem Prakash Srivastav, Trends in Edible Packaging Films and its Prospective Future in Food: A Review. Appl. Food Res. 2022, 2, 100118. [Google Scholar]
- Baranwal, J.; Barse, B.; Fais, A.; Delogu, G.L.; Kumar, A. Biopolymer: A Sustainable Material for Food and Medical Applications. Polymers 2022, 14, 983. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, T.J. Surface and nutraceutical properties of edible films made from starchy sources with and without added blackberry pulp. Carbohydr. Polym. 2017, 165, 169–179. [Google Scholar] [CrossRef]
- Dhumal, C.V.; Sarkar, P. Composite edible films and coatings from food-grade biopolymers. J. Food Sci. Technol. 2018, 55, 4369–4383. [Google Scholar] [CrossRef]
- Fu, X.; Chang, X.; Ding, Z.; Xu, H.; Kong, H.; Chen, F.; Wang, R.; Shan, Y.; Ding, S. Fabrication and Characterization of Eco-Friendly Polyelectrolyte Bilayer Films Based on Chitosan and Different Types of Edible Citrus Pectin. Foods 2022, 11, 3536. [Google Scholar] [CrossRef]
- Zhao, K.; Wang, W.; Teng, A.; Zhang, K.; Ma, Y.; Duan, S.; Li, S.; Guo, Y. Using cellulose nanofibers to reinforce polysaccharide films: Blending vs layer-by-layer casting. Carbohydr. Polym. 2020, 227, 115264. [Google Scholar] [CrossRef]
- Gamage, A.; Thiviya, P.; Mani, S.; Ponnusamy, P.G.; Manamperi, A.; Evon, P.; Merah, O.; Madhujith, T. Environmental Properties and Applications of Biodegradable Starch-Based Nanocomposites. Polymers 2022, 14, 4578. [Google Scholar] [CrossRef]
- Wang, B.; Yan, L.; Guo, S.; Wen, L.; Yu, M.; Feng, L.; Jia, X. Structural Elucidation, Modification, and Structure-Activity Relationship of Polysaccharides in Chinese Herbs: A Review. Front. Nutr. 2022, 9, 08175. [Google Scholar] [CrossRef]
- Guo, M.Q.; Hu, X.; Wang, C.; Ai, L. Polysaccharides: Structure and solubility. Solubility Polysacch. 2017, 2, 8–21. [Google Scholar]
- Ross-Murphy, S.; Shatwell, K. Polysaccharide strong and weak gels. Biorheology 1993, 30, 217–227. [Google Scholar] [CrossRef]
- Burchard, W. Structure formation by polysaccharides in concentrated solution. Biomacromolecules 2001, 2, 342–353. [Google Scholar] [CrossRef]
- Wettstein, S.G.; Alonso, D.M.; Gürbüz, E.I.; Dumesic, J.A. A roadmap for conversion of lignocellulosic biomass to chemicals and fuels. Curr. Opin. Chem. Eng. 2012, 1, 218–224. [Google Scholar] [CrossRef]
- Knauf, M.; Moniruzzaman, M. Lignocellulosic biomass processing: A perspective. Int. Sugar J. 2004, 106, 147–150. [Google Scholar]
- Tang, X.; Zuo, M.; Li, Z.; Liu, H.; Xiong, C.; Zeng, X.; Sun, Y.; Hu, L.; Liu, S.; Lei, T. Green processing of lignocellulosic biomass and its derivatives in deep eutectic solvents. Chem. Sus. Chem. 2017, 10, 2696–2706. [Google Scholar] [CrossRef]
- Xue, H.; Sun, M.; Zhao, X.; Wang, Y.; Yan, J.; Zhang, W. Preparation and Characterization of Polysaccharide-Based Hydrogels for Cutaneous Wound Healing. Polymers 2022, 14, 1716. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, B.; Li, C.; Xu, Y.; Luo, Y.; Liang, D.; Huang, C. Comprehensive Review of Polysaccharide-Based Materials in Edible Packaging: A Sustainable Approach. Foods 2021, 10, 1845. [Google Scholar] [CrossRef] [PubMed]
- Dhaka, R.; Upadhyay, A. Edible films and coatings: A brief overview. Pharma Innov. J. 2018, 7, 331–333. [Google Scholar]
- Lacroix, M.; Le Tien, C. 20—Edible films and coatings from nonstarch polysaccharides. In Innovations in Food Packaging; Han, J.H., Ed.; Academic Press: London, UK, 2005; pp. 338–361. [Google Scholar]
- Vazquez, A.; Foresti, M.L.; Moran, J.I.; Cyras, V.P. Extraction and production of cellulose nanofibers. In Handbook of Polymer Nanocomposites. Processing, Performance and Application; Springer: Berlin/Heidelberg, Germany, 2015; pp. 81–118. [Google Scholar]
- Dufresne, A. Nanocellulose: From Nature to High Performance Tailored Materials; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2017. [Google Scholar]
- Kargarzadeh, H.; Ahmad, I.; Thomas, S.; Dufresne, A. Handbook of Nanocellulose and Cellulose Nanocomposites; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Phanthong, P.; Reubroycharoen, P.; Hao, X.; Xu, G.; Abudula, A.; Guan, G. Nanocellulose: Extraction and application. Carbon Resour. Convers. 2018, 1, 32–43. [Google Scholar] [CrossRef]
- Burchard, W.; Schulz, L. Functionality of the β (1, 4) glycosidic linkage in polysaccharides. In Proceedings of the Macromolecular Symposia; Hüthig & Wepf Verlag: Basel, Switzerland, 1995; Volume 99, pp. 57–69. [Google Scholar]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Zhou, X.; Lyu, S.; Pan, D.; Dong, M.; Wu, S.; Ding, T.; Wei, X.; Seok, I.; Wei, S. Magnetic nanocellulose-magnetite aerogel for easy oil adsorption. J. Colloid Interface Sci. 2020, 560, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Hudson, S.M.; Cuculo, J.A. The solubility of unmodified cellulose: A critique of the literature. J. Macromol. Sci. Rev. Macromol. Chem. 1980, 18, 1–82. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Advances and Challenges in Biopolymer-Based Films. Polymers 2022, 14, 3920. [Google Scholar] [CrossRef]
- Tajeddin, B. Cellulose-based polymers for packaging applications. In Lignocellulosic Polymer Composites; Thakur, V.K., Ed.; Scrivener Publishing LLC: Beverly, MA, USA, 2014; pp. 477–498. [Google Scholar]
- Osorio, F.A.; Molina, P.; Matiacevich, S.; Enrione, J.; Skurtys, O. Characteristics of hydroxy propyl methyl cellulose (HPMC) based edible film developed for blueberry coatings. Procedia Food Sci. 2011, 1, 287–293. [Google Scholar] [CrossRef]
- Singh, P.; Magalhães, S.; Alves, L.; Antunes, F.; Miguel, M.; Lindman, B.; Medronho, B. Cellulose-based edible films for probiotic entrapment. Food Hydrocoll. 2019, 88, 68–74. [Google Scholar] [CrossRef]
- Bastos, M.d.S.R.; Laurentino, L.d.S.; Canuto, K.M.; Mendes, L.G.; Martins, C.M.; Silva, S.M.F.; Furtado, R.F.; Kim, S.; Biswas, A.; Cheng, H.N. Physical and mechanical testing of essential oil-embedded cellulose ester films. Polym. Test. 2016, 49, 156–161. [Google Scholar] [CrossRef]
- Kong, I.; Degraeve, P.; Pui, L.P. Polysaccharide-Based Edible Films Incorporated with Essential Oil Nanoemulsions: Physico-Chemical, Mechanical Properties and Its Application in Food Preservation—A Review. Foods 2022, 11, 555. [Google Scholar] [CrossRef]
- Ghiasi, F.; Golmakani, M.-T.; Eskandari, M.H.; Hosseini, S.M.H. A new approach in the hydrophobic modification of polysaccharide-based edible films using structured oil nanoparticles. Ind. Crops Prod. 2020, 154, 112679. [Google Scholar] [CrossRef]
- Zhuang, C.; Tao, F.; Cui, Y. Eco-friendly biorefractory films of gelatin and TEMPO-oxidized cellulose ester for food packaging application. J. Sci. Food Agric. 2017, 97, 3384–3395. [Google Scholar] [CrossRef]
- Bras, J.; Vaca-Garcia, C.; Borredon, M.-E.; Glasser, W. Oxygen and water vapor permeability of fully substituted long chain cellulose esters (LCCE). Cellulose 2007, 14, 367–374. [Google Scholar] [CrossRef]
- Bilbao-Sáinz, C.; Avena-Bustillos, R.J.; Wood, D.F.; Williams, T.G.; McHugh, T.H. Composite Edible Films Based on Hydroxypropyl Methylcellulose Reinforced with Microcrystalline Cellulose Nanoparticles. J. Agric. Food Chem. 2010, 58, 3753–3760. [Google Scholar] [CrossRef]
- Fakhouri, F.; Tanada-Palmu, P.; Grosso, C. Characterization of composite biofilms of wheat gluten and cellulose acetate phthalate. Braz. J. Chem. Eng. 2004, 21, 261–264. [Google Scholar] [CrossRef]
- Debeaufort, F.; Quezada-Gallo, J.-A.; Delporte, B.; Voilley, A. Lipid hydrophobicity and physical state effects on the properties of bilayer edible films. J. Membr. Sci. 2000, 180, 47–55. [Google Scholar] [CrossRef]
- Kester, J.J.; Fennema, O. An Edible Film of Lipids and Cellulose Ethers: Barrier Properties to Moisture Vapor Transmission and Structural Evaluation. J. Food Sci. 1989, 54, 1383–1389. [Google Scholar] [CrossRef]
- Koh, H.-Y.; MS, C. Characteristics of corn zein and methyl cellulose bilayer edible films according to preparation protocol. Food Sci. Biotechnol. 2002, 11, 310–315. [Google Scholar]
- Park, J.W.; Testin, R.F.; Park, H.J.; Vergano, P.J.; Weller, C.L. Fatty Acid Concentration Effect on Tensile Strength, Elongation, and Water Vapor Permeability of Laminated Edible Films. J. Food Sci. 1994, 59, 916–919. [Google Scholar] [CrossRef]
- Shao, P.; Wu, W.; Chen, H.; Sun, P.; Gao, H. Bilayer edible films with tunable humidity regulating property for inhibiting browning of Agaricus bisporus. Food Res. Int. 2020, 138, 109795. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, D.; Lopez-Sanchez, P.; Yang, X. Characterizations of bacterial cellulose nanofibers reinforced edible films based on konjac glucomannan. Int. J. Biol. Macromol. 2020, 145, 634–645. [Google Scholar] [CrossRef] [PubMed]
- Tabari, M. Characterization of a new biodegradable edible film based on Sago Starch loaded with Carboxymethyl Cellulose nanoparticles. Nanomed. Res. J. 2018, 3, 25–30. [Google Scholar]
- Tabari, M. Investigation of Carboxymethyl Cellulose (CMC) on Mechanical Properties of Cold Water Fish Gelatin Biodegradable Edible Films. Foods 2017, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Indumathi, M.; Sarojini, K.S.; Rajarajeswari, G. Antimicrobial and biodegradable chitosan/cellulose acetate phthalate/ZnO nano composite films with optimal oxygen permeability and hydrophobicity for extending the shelf life of black grape fruits. Int. J. Biol. Macromol. 2019, 132, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Bhopal, R. Investigation of water vapour permeation and antibacterial properties of nano silver loaded cellulose acetate film. Int. Food Res. J. 2010, 17, 623–639. [Google Scholar]
- Atta, O.M.; Manan, S.; Ul-Islam, M.; Ahmed, A.A.Q.; Ullah, M.W.; Yang, G. Development and characterization of plant oil-incorporated carboxymethyl cellulose/bacterial cellulose/glycerol-based antimicrobial edible films for food packaging applications. Adv. Compos. Hybrid Mater. 2022, 5, 973–990. [Google Scholar] [CrossRef]
- Necas, J.; Bartosikova, L. Carrageenan: A review. Vet. Med. 2013, 58, 187–205. [Google Scholar] [CrossRef]
- Park, H.-J.; Rhim, J.-W.; Jung, S.-T.; Kang, S.-G.; Hwang, K.-T.; Park, Y.-K. Mechanical properties of carrageenan-based biopolymer films. Korean J. Packag. Sci. Technol. 1995, 1, 38–50. [Google Scholar]
- Balqis, A.I.; Khaizura, M.N.; Russly, A.; Hanani, Z.N. Effects of plasticizers on the physicochemical properties of kappa-carrageenan films extracted from Eucheuma cottonii. Int. J. Biol. Macromol. 2017, 103, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Thomas, W. Carrageenan. In Thickening and Gelling Agents for Food; Springer: Berlin/Heidelberg, Germany, 1997; pp. 45–59. [Google Scholar]
- Baldwin, E.A.; Nisperos-Carriedo, M.O.; Baker, R.A. Use of edible coatings to preserve quality of lightly (and slightly) processed products. Crit. Rev. Food Sci. Nutr. 1995, 35, 509–524. [Google Scholar] [CrossRef]
- Lin, M.G.; Lasekan, O.; Saari, N.; Khairunniza-Bejo, S. The Effect of the Application of Edible Coatings on or before Ultraviolet Treatment on Postharvested Longan Fruits. J. Food Qual. 2017, 2017, 5454263. [Google Scholar] [CrossRef]
- Abdou, E.; Sorour, M. Preparation and characterization of starch/carrageenan edible films. Int. Food Res. J. 2014, 21, 189. [Google Scholar]
- Thakur, R.; Saberi, B.; Pristijono, P.; Golding, J.; Stathopoulos, C.; Scarlett, C.; Bowyer, M.; Vuong, Q. Characterization of rice starch-ι-carrageenan biodegradable edible film. Effect of stearic acid on the film properties. Int. J. Biol. Macromol. 2016, 93, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, K.S.; Sharma, L.; Kaur, M.; Kaur, R. Physical, structural and thermal properties of composite edible films prepared from pearl millet starch and carrageenan gum: Process optimization using response surface methodology. Int. J. Biol. Macromol. 2020, 143, 704–713. [Google Scholar] [CrossRef]
- Larotonda, F.D.S.; Torres, M.D.; Gonçalves, M.P.; Sereno, A.M.; Hilliou, L. Hybrid carrageenan-based formulations for edible film preparation: Benchmarking with kappa carrageenan. J. Appl. Polym. Sci. 2016, 133, 42263. [Google Scholar] [CrossRef]
- Campos, C.A.; Gerschenson, L.N.; Flores, S.K. Development of Edible Films and Coatings with Antimicrobial Activity. Food Bioprocess Technol. 2011, 4, 849–875. [Google Scholar] [CrossRef]
- Paula, G.A.; Benevides, N.M.B.; Cunha, A.P.; de Oliveira, A.V.; Pinto, A.M.B.; Morais, J.P.S.; Azeredo, H.M.C. Development and characterization of edible films from mixtures of κ-carrageenan, ι-carrageenan, and alginate. Food Hydrocoll. 2015, 47, 140–145. [Google Scholar] [CrossRef]
- Hambleton, A.; Voilley, A.; Debeaufort, F. Transport parameters for aroma compounds through i-carrageenan and sodium alginate-based edible films. Food Hydrocoll. 2011, 25, 1128–1133. [Google Scholar] [CrossRef]
- Thiviya, P.; Gamage, A.; Liyanapathiranage, A.; Makehelwala, M.; Dassanayake, R.S.; Manamperi, A.; Merah, O.; Mani, S.; Koduru, J.R.; Terrence Madhujith, T. Algal polysaccharides: Structure, preparation and applications in food packaging. Food Chem. 2022, 405, 134903. [Google Scholar] [CrossRef]
- Jayakody, M.M.; Vanniarachchy, M.P.G.; Wijesekara, I. Seaweed derived alginate, agar, and carrageenan based edible coatings and films for the food industry: A review. J. Food Meas. Charact. 2022, 16, 1195–1227. [Google Scholar] [CrossRef]
- Cebrián-Lloret, V.; Göksen, G.; Martínez-Abad, A.; López-Rubio, A.; Martínez-Sanz, M. Agar-based packaging films produced by melt mixing: Study of their retrogradation upon storage. Algal Res. 2022, 66, 102802. [Google Scholar] [CrossRef]
- Mostafavi, F.S.; Zaeim, D. Agar-based edible films for food packaging applications—A review. Int. J. Biol. Macromol. 2020, 159, 1165–1176. [Google Scholar] [CrossRef]
- Wongphan, P.; Harnkarnsujarit, N. Characterization of starch, agar and maltodextrin blends for controlled dissolution of edible films. Int. J. Biol. Macromol. 2020, 156, 80–93. [Google Scholar] [CrossRef]
- Phan The, D.; Debeaufort, F.; Voilley, A.; Luu, D. Biopolymer interactions affect the functional properties of edible films based on agar, cassava starch and arabinoxylan blends. J. Food Eng. 2009, 90, 548–558. [Google Scholar] [CrossRef]
- Arham, R.; Mulyati, M.; Metusalach, M.; Salengke, S. Physical and mechanical properties of agar based edible film with glycerol plasticizer. Int. Food Res. J. 2016, 23, 1669–1675. [Google Scholar]
- Sousa, A.M.; Sereno, A.M.; Hilliou, L.; Gonçalves, M.P. Biodegradable Agar Extracted from Gracilaria vermiculophylla: Film Properties and Application to Edible Coating. In Proceedings of the Materials Science Forum; Trans Tech Publications Ltd: Stafa-Zurich, Switzerland, 2010; Volume 636, pp. 739–744. [Google Scholar]
- Shahidi, F.; Hossain, A. Preservation of aquatic food using edible films and coatings containing essential oils: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 66–105. [Google Scholar] [CrossRef] [PubMed]
- Perera, K.Y.; Sharma, S.; Pradhan, D.; Jaiswal, A.K.; Jaiswal, S. Seaweed Polysaccharide in Food Contact Materials (Active Packaging, Intelligent Packaging, Edible Films, and Coatings). Foods 2021, 10, 2088. [Google Scholar] [CrossRef]
- Thakur, B.R.; Singh, R.K.; Handa, A.K.; Rao, M. Chemistry and uses of pectin—A review. Crit. Rev. Food Sci. Nutr. 1997, 37, 47–73. [Google Scholar] [CrossRef] [PubMed]
- Maftoonazad, N.; Ramaswamy, H.S.; Marcotte, M. Evaluation of factors affecting barrier, mechanical and optical properties of pectin-based films using response surface methodology. J. Food Process Eng. 2007, 30, 539–563. [Google Scholar] [CrossRef]
- Rodsamran, P.; Sothornvit, R. Preparation and characterization of pectin fraction from pineapple peel as a natural plasticizer and material for biopolymer film. Food Bioprod. Process. 2019, 118, 198–206. [Google Scholar] [CrossRef]
- Lazaridou, A.; Biliaderis, C.G. Edible Films and Coatings with Pectin. In Pectin: Technological and Physiological Properties; Kontogiorgos, V., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 99–123. [Google Scholar]
- Shahrampour, D.; Khomeiri, M.; Razavi, S.M.A.; Kashiri, M. Development and characterization of alginate/pectin edible films containing Lactobacillus plantarum KMC 45. LWT 2020, 118, 108758. [Google Scholar] [CrossRef]
- Alvarez, M.V.; Ortega-Ramirez, L.A.; Gutierrez-Pacheco, M.M.; Bernal-Mercado, A.T.; Rodriguez-Garcia, I.; Gonzalez-Aguilar, G.A.; Ponce, A.; Moreira, M.d.R.; Roura, S.I.; Ayala-Zavala, J.F. Oregano essential oil-pectin edible films as anti-quorum sensing and food antimicrobial agents. Front. Microbiol. 2014, 5, 699. [Google Scholar] [CrossRef]
- Estrada-Girón, Y.; Cabrera-Díaz, E.; Esparza-Merino, R.M.; Martín-del-Campo, A.; Valencia-Botín, A.J. Innovative edible films and coatings based on red color pectin obtained from the byproducts of Hibiscus sabdariffa L. for strawberry preservation. J. Food Meas. Charact. 2020, 14, 3371–3380. [Google Scholar] [CrossRef]
- Rossi Marquez, G.; Di Pierro, P.; Mariniello, L.; Esposito, M.; Giosafatto, C.V.L.; Porta, R. Fresh-cut fruit and vegetable coatings by transglutaminase-crosslinked whey protein/pectin edible films. LWT 2017, 75, 124–130. [Google Scholar] [CrossRef]
- Alvarez-Pérez, O.B.; Montañez, J.; Aguilar, C.N.; Rojas, R. Pectin–Candelilla wax: An alternative mixture for edible films. J. Microbiol. Biotechnol. Food Sci. 2021, 2021, 167–171. [Google Scholar] [CrossRef]
- Piekarska, K.; Sikora, M.; Owczarek, M.; Jó ’zwik-Pruska, J.; Wiśniewska-Wrona, M. Chitin and Chitosan as Polymers of the Future—Obtaining, Modification, Life Cycle Assessment and Main Directions of Application. Polymers 2023, 15, 793. [Google Scholar] [CrossRef]
- Roberts, G.A.; Roberts, G.A. Chitin Chemistry; Springer: Berlin/Heidelberg, Germany, 1992. [Google Scholar]
- Hudson, S.; Smith, C. Polysaccharides: Chitin and chitosan: Chemistry and technology of their use as structural materials. In Biopolymers from Renewable Resources; Springer: Berlin/Heidelberg, Germany, 1998; pp. 96–118. [Google Scholar]
- Sivakanthan, S.; Rajendran, S.; Gamage, A.; Madhujith, T.; Mani, S. Antioxidant and antimicrobial applications of biopolymers: A review. Food Res. Int. 2020, 136, 109327. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Téllez, C.N.; Luque-Alcaraz, A.G.; Núñez-Mexía, S.A.; Cortez-Rocha, M.O.; Lizardi-Mendoza, J.; Rosas-Burgos, E.C.; Rosas-Durazo, A.d.J.; Parra-Vergara, N.V.; Plascencia-Jatomea, M. Relationship between the Antifungal Activity of Chitosan–Capsaicin Nanoparticles and the Oxidative Stress Response on Aspergillus parasiticus. Polymers 2022, 14, 2774. [Google Scholar] [CrossRef] [PubMed]
- Ardean, C.; Davidescu, C.M.; Neme¸s, N.S.; Negrea, A.; Ciopec, M.; Duteanu, N.; Negrea, P.; Duda-Seiman, D.; Musta, V. Factors Influencing the Antibacterial Activity of Chitosan and Chitosan Modified by Functionalization. Int. J. Mol. Sci. 2021, 22, 7449. [Google Scholar] [CrossRef] [PubMed]
- Pavinatto, A.; de Almeida Mattos, A.V.; Malpass, A.C.G.; Okura, M.H.; Balogh, D.T.; Sanfelice, R.C. Coating with chitosan-based edible films for mechanical/biological protection of strawberries. Int. J. Biol. Macromol. 2020, 151, 1004–1011. [Google Scholar] [CrossRef]
- da Mata Cunha, O.; Lima, A.M.F.; Assis, O.B.G.; Tiera, M.J.; de Oliveira Tiera, V.A. Amphiphilic diethylaminoethyl chitosan of high molecular weight as an edible film. Int. J. Biol. Macromol. 2020, 164, 3411–3420. [Google Scholar] [CrossRef]
- Salama, H.E.; Abdel Aziz, M.S.; Sabaa, M.W. Novel biodegradable and antibacterial edible films based on alginate and chitosan biguanidine hydrochloride. Int. J. Biol. Macromol. 2018, 116, 443–450. [Google Scholar] [CrossRef]
- Velickova, E.; Winkelhausen, E.; Kuzmanova, S.; Moldão-Martins, M.; Alves, V.D. Characterization of multilayered and composite edible films from chitosan and beeswax. Food Sci. Technol. Int. 2015, 21, 83–93. [Google Scholar] [CrossRef]
- Tokatlı, K.; Demirdöven, A. Effects of chitosan edible film coatings on the physicochemical and microbiological qualities of sweet cherry (Prunus avium L.). Sci. Hortic. 2020, 259, 108656. [Google Scholar] [CrossRef]
- Doublier, J.-L.; Garnier, C.; Cuvelier, G. Gums and hydrocolloids: Functional aspects. In Carbohydrates in Food; CRC Press: Boca Raton, FL, USA, 2017; pp. 307–354. [Google Scholar]
- Lim, W.; Shin, S.Y.; Cha, J.M.; Bae, H. Optimization of Polysaccharide Hydrocolloid for the Development of Bioink with High Printability/Biocompatibility for Coextrusion 3D Bioprinting. Polymers 2021, 13, 1773. [Google Scholar] [CrossRef] [PubMed]
- Khezerlou, A.; Zolfaghari, H.; Banihashemi, S.A.; Forghani, S.; Ehsani, A. Plant gums as the functional compounds for edible films and coatings in the food industry: A review. Polym. Adv. Technol. 2021, 32, 2306–2326. [Google Scholar] [CrossRef]
- Islam, A.; Phillips, G.; Sljivo, A.; Snowden, M.; Williams, P. A review of recent developments on the regulatory, structural and functional aspects of gum arabic. Food Hydrocoll. 1997, 11, 493–505. [Google Scholar] [CrossRef]
- Musa, H.H.; Ahmed, A.A.; Musa, T.H. Chemistry, biological, and pharmacological properties of gum Arabic. Bioactive Molecules in Food; Springer International Publishing AG: Cham, Switzerland, 2018; pp. 1–18. [Google Scholar]
- Xu, T.; Gao, C.; Feng, X.; Yang, Y.; Shen, X.; Tang, X. Structure, physical and antioxidant properties of chitosan-gum arabic edible films incorporated with cinnamon essential oil. Int. J. Biol. Macromol. 2019, 134, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Razak, A.S.; Lazim, A.M. Starch-based edible film with gum arabic for fruits coating. AIP Conf. Proc. 2015, 1678, 050020. [Google Scholar]
- Xue, F.; Gu, Y.; Wang, Y.; Li, C.; Adhikari, B. Encapsulation of essential oil in emulsion based edible films prepared by soy protein isolate-gum acacia conjugates. Food Hydrocoll. 2019, 96, 178–189. [Google Scholar] [CrossRef]
- Li, C.; Pei, J.; Xiong, X.; Xue, F. Encapsulation of Grapefruit Essential Oil in Emulsion-Based Edible Film Prepared by Plum (Pruni Domesticae Semen) Seed Protein Isolate and Gum Acacia Conjugates. Coatings 2020, 10, 784. [Google Scholar] [CrossRef]
- Mathur, N. Industrial Galactomannan Polysaccharides; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2012. [Google Scholar]
- Prajapati, V.D.; Jani, G.K.; Moradiya, N.G.; Randeria, N.P.; Nagar, B.J.; Naikwadi, N.N.; Variya, B.C. Galactomannan: A versatile biodegradable seed polysaccharide. Int. J. Biol. Macromol. 2013, 60, 83–92. [Google Scholar] [CrossRef]
- Antoniou, J.; Liu, F.; Majeed, H.; Qazi, H.J.; Zhong, F. Physicochemical and thermomechanical characterization of tara gum edible films: Effect of polyols as plasticizers. Carbohydr. Polym. 2014, 111, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Mudgil, D.; Barak, S.; Khatkar, B.S. Guar gum: Processing, properties and food applications—A review. J. Food Sci. Technol. 2014, 51, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Maji, B.; Moorthy, N.H.N.; Maiti, S. Xanthan gum derivatives: Review of synthesis, properties and diverse applications. RSC Adv. 2020, 10, 27103–27136. [Google Scholar] [CrossRef]
- Hashemi Gahruie, H.; Safdarianghomsheh, R.; Zamanifar, P.; Salehi, S.; Niakousari, M.; Hosseini, S.M.H. Characterization of novel edible films and coatings for food preservation based on gum cordia. J. Food Qual. 2020, 2020, 8883916. [Google Scholar] [CrossRef]
- Radev, R.; Pashova, S. Application of edible films and coatings for fresh fruit and vegetables. Qual. Access Success 2020, 21, 108–112. [Google Scholar]
- Dubey, N.K.; Dubey, R. Edible films and coatings: An update on recent advances. In Biopolymer-Based Formulations; Elsevier: Amsterdam, The Netherlands, 2020; pp. 675–695. [Google Scholar]
- Dhumal, C.V.; Ahmed, J.; Bandara, N.; Sarkar, P. Improvement of antimicrobial activity of sago starch/guar gum bi-phasic edible films by incorporating carvacrol and citral. Food Packag. Shelf Life 2019, 21, 100380. [Google Scholar] [CrossRef]
- Saberi, B.; Chockchaisawasdee, S.; Golding, J.B.; Scarlett, C.J.; Stathopoulos, C.E. Physical and mechanical properties of a new edible film made of pea starch and guar gum as affected by glycols, sugars and polyols. Int. J. Biol. Macromol. 2017, 104, 345–359. [Google Scholar] [CrossRef]
- Saberi, B.; Chockchaisawasdee, S.; Golding, J.B.; Scarlett, C.J.; Stathopoulos, C.E. Characterization of pea starch-guar gum biocomposite edible films enriched by natural antimicrobial agents for active food packaging. Food Bioprod. Process. 2017, 105, 51–63. [Google Scholar] [CrossRef]
- Saberi, B.; Vuong, Q.V.; Chockchaisawasdee, S.; Golding, J.B.; Scarlett, C.J.; Stathopoulos, C.E. Physical, Barrier, and Antioxidant Properties of Pea Starch-Guar Gum Biocomposite Edible Films by Incorporation of Natural Plant Extracts. Food Bioprocess Technol. 2017, 10, 2240–2250. [Google Scholar] [CrossRef]
- Naji-Tabasi, S.; Razavi, S.M.A. Functional properties and applications of basil seed gum: An overview. Food Hydrocoll. 2017, 73, 313–325. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Mousavi Khaneghah, A. Characterization of novel basil-seed gum active edible films and coatings containing oregano essential oil. Prog. Org. Coat. 2017, 110, 35–41. [Google Scholar] [CrossRef]
- Salehi, F. Characterization of New Biodegradable Edible Films and Coatings Based on Seeds Gum: A Review. J. Packag. Technol. Res. 2019, 3, 193–201. [Google Scholar] [CrossRef]
- Hashemi Gahruie, H.; Mostaghimi, M.; Ghiasi, F.; Tavakoli, S.; Naseri, M.; Hosseini, S.M.H. The effects of fatty acids chain length on the techno-functional properties of basil seed gum-based edible films. Int. J. Biol. Macromol. 2020, 160, 245–251. [Google Scholar] [CrossRef]
- Prajapati, V.D.; Jani, G.K.; Zala, B.S.; Khutliwala, T.A. An insight into the emerging exopolysaccharide gellan gum as a novel polymer. Carbohydr. Polym. 2013, 93, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Giavasis, I.; Harvey, L.M.; McNeil, B. Gellan gum. Crit. Rev. Biotechnol. 2000, 20, 177–211. [Google Scholar] [CrossRef] [PubMed]
- Falguera, V.; Quintero, J.P.; Jiménez, A.; Muñoz, J.A.; Ibarz, A. Edible films and coatings: Structures, active functions and trends in their use. Trends Food Sci. Technol. 2011, 22, 292–303. [Google Scholar] [CrossRef]
- Alvarado-González, J.; Chanona-Pérez, J.; Welti-Chanes, J.; Calderón-Domínguez, G.; Arzate-Vázquez, I.; Pacheco-Alcalá, S.; Garibay-Febles, V.; Gutiérrez-López, G. Optical, microstructural, functional and nanomechanical properties of Aloe vera gel/gellan gum edible films. Rev. Mex. Ing. Quím. 2012, 11, 193–210. [Google Scholar]
- Maan, A.A.; Reiad Ahmed, Z.F.; Iqbal Khan, M.K.; Riaz, A.; Nazir, A. Aloe vera gel, an excellent base material for edible films and coatings. Trends Food Sci. Technol. 2021, 116, 329–341. [Google Scholar] [CrossRef]
- Téllez-Rangel, E.C.; Rodríguez-Huezo, E.; Totosaus, A. Effect of gellan, xanthan or locust bean gum and/or emulsified maize oil on proteins edible films properties. Emir. J. Food Agric. 2018, 30, 404–412. [Google Scholar]
- Tester, R.F.; Karkalas, J.; Qi, X. Starch—Composition, fine structure and architecture. J. Cereal Sci. 2004, 39, 151–165. [Google Scholar] [CrossRef]
- van Soest, J.J.G.; Vliegenthart, J.F.G. Crystallinity in starch plastics: Consequences for material properties. Trends Biotechnol. 1997, 15, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Rindlav-Westling, Å.; Stading, M.; Gatenholm, P. Crystallinity and morphology in films of starch, amylose and amylopectin blends. Biomacromolecules 2002, 3, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Rempel, C.; Liu, Q. Thermoplastic Starch Processing and Characteristics—A Review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1353–1370. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z. 19—Edible films and coatings from starches. In Innovations in Food Packaging; Han, J.H., Ed.; Academic Press: London, UK, 2005; pp. 318–337. [Google Scholar]
- Shah, U.; Naqash, F.; Gani, A.; Masoodi, F.A. Art and Science behind Modified Starch Edible Films and Coatings: A Review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 568–580. [Google Scholar] [CrossRef]
- Chiumarelli, M.; Hubinger, M.D. Evaluation of edible films and coatings formulated with cassava starch, glycerol, carnauba wax and stearic acid. Food Hydrocoll. 2014, 38, 20–27. [Google Scholar] [CrossRef]
- Pedreiro, S.; Figueirinha, A.; Silva, A.S.; Ramos, F. Bioactive Edible Films and Coatings Based in Gums and Starch: Phenolic Enrichment and Foods Application. Coatings 2021, 11, 1393. [Google Scholar] [CrossRef]
- Basiak, E.; Lenart, A.; Debeaufort, F. Effect of starch type on the physico-chemical properties of edible films. Int. J. Biol. Macromol. 2017, 98, 348–356. [Google Scholar] [CrossRef]
- García, M.A.; Martino, M.N.; Zaritzky, N.E. Lipid Addition to Improve Barrier Properties of Edible Starch-based Films and Coatings. J. Food Sci. 2000, 65, 941–944. [Google Scholar] [CrossRef]
- Rodríguez, M.; Osés, J.; Ziani, K.; Maté, J.I. Combined effect of plasticizers and surfactants on the physical properties of starch based edible films. Food Res. Int. 2006, 39, 840–846. [Google Scholar] [CrossRef]
- Basiak, E.; Lenart, A.; Debeaufort, F. How Glycerol and Water Contents Affect the Structural and Functional Properties of Starch-Based Edible Films. Polymers 2018, 10, 412. [Google Scholar] [CrossRef]
- Farahnaky, A.; Saberi, B.; Majzoobi, M. Effect of Glycerol on Physical and Mechanical Properties of Wheat Starch Edible Films. J. Texture Stud. 2013, 44, 176–186. [Google Scholar] [CrossRef]
- Kim, K.W.; Ko, C.J.; Park, H.J. Mechanical Properties, Water Vapor Permeabilities and Solubilities of Highly Carboxymethylated Starch-Based Edible Films. J. Food Sci. 2002, 67, 218–222. [Google Scholar] [CrossRef]
- Charles, A.L.; Motsa, N.; Abdillah, A.A. A Comprehensive Characterization of Biodegradable Edible Films Based on Potato Peel Starch Plasticized with Glycerol. Polymers 2022, 14, 3462. [Google Scholar] [CrossRef] [PubMed]
- Wilpiszewska, K.; Adrian Krzysztof Antosik, A.K.; Schmidt, B.; Janik, J.; Rokicka, J. Hydrophilic Films Based on Carboxymethylated Derivatives of Starch and Cellulose. Polymers 2020, 12, 2447. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, G.F.; Leme, B.d.O.; Santos, G.R.S.d.; Silva, J.V.d.; Nascimento, P.B.; Soares, C.T.; Fakhouri, F.M.; de Oliveira, R.A. Edible Films and Coatings Formulated with Arrowroot Starch as a Non-Conventional Starch Source for Plums Packaging. Polysaccharides 2021, 2, 373–386. [Google Scholar] [CrossRef]
- Peressini, D.; Bravin, B.; Lapasin, R.; Rizzotti, C.; Sensidoni, A. Starch–methylcellulose based edible films: Rheological properties of film-forming dispersions. J. Food Eng. 2003, 59, 25–32. [Google Scholar] [CrossRef]
- Cuq, B.; Gontard, N.; Guilbert, S. Proteins as Agricultural Polymers for Packaging Production. Cereal Chem. 1998, 75, 1–9. [Google Scholar] [CrossRef]
- Mchugh, T.H.; Aujard, J.F.; Krochta, J. Plasticized whey protein edible films: Water vapor permeability properties. J. Food Sci. 1994, 59, 416–419. [Google Scholar] [CrossRef]
- Martucci, J.F.; Ruseckaite, R.A. Biodegradation of three-layer laminate films based on gelatin under indoor soil conditions. Polym. Degrad. Stab. 2009, 94, 1307–1313. [Google Scholar] [CrossRef]
- Jauregi, P. Bioactive peptides from food proteins: New opportunities and challenges. Food Sci. Technol. Bull. Funct. Foods 2009, 5, 11–25. [Google Scholar] [CrossRef]
- Alkan, D.; Yemenicioğlu, A. Potential application of natural phenolic antimicrobials and edible film technology against bacterial plant pathogens. Food Hydrocoll. 2016, 55, 1–10. [Google Scholar] [CrossRef]
- Banerjee, R.; Chen, H.; Wu, J. Milk protein-based edible film mechanical strength changes due to ultrasound process. J. Food Sci. 1996, 61, 824–828. [Google Scholar] [CrossRef]
- Panyam, D.; Kilara, A. Enhancing the functionality of food proteins by enzymatic modification. Trends Food Sci. Technol. 1996, 7, 120–125. [Google Scholar] [CrossRef]
- Yada, R.Y. Proteins in Food Processing; Woodhead Publishing: Sawston, UK, 2017. [Google Scholar]
- Audic, J.-L.; Chaufer, B.; Daufin, G. Non-food applications of milk components and dairy co-products: A review. Le Lait 2003, 83, 417–438. [Google Scholar] [CrossRef]
- Hinz, K.; O’Connor, P.M.; Huppertz, T.; Ross, R.P.; Kelly, A.L. Comparison of the principal proteins in bovine, caprine, buffalo, equine and camel milk. J. Dairy Res. 2012, 79, 185–191. [Google Scholar] [CrossRef]
- Petrova, S.Y.; Khlgatian, S.; Emel’yanova, O.Y.; Pishulina, L.; Berzhets, V. Current Data about Milk Caseins. Russ. J. Bioorg. Chem. 2022, 48, 273–280. [Google Scholar] [CrossRef]
- Swaisgood, H.E. Review and Update of Casein Chemistry. J. Dairy Sci. 1993, 76, 3054–3061. [Google Scholar] [CrossRef]
- Lacroix, M.; Cooksey, K. 18—Edible films and coatings from animal origin proteins. In Innovations in Food Packaging; Han, J.H., Ed.; Academic Press: London, UK, 2005; pp. 301–317. [Google Scholar]
- Shendurse, A.; Gopikrishna, G.; Patel, A.; Pandya, A. Milk protein based edible films and coatings–preparation, properties and food applications. J. Nutr. Health Food Eng. 2018, 8, 219–226. [Google Scholar] [CrossRef]
- Chen, H. Functional Properties and Applications of Edible Films Made of Milk Proteins. J. Dairy Sci. 1995, 78, 2563–2583. [Google Scholar] [CrossRef]
- Phillips, L.G.; Whitehead, D.M.; Kinsella, J. Introduction to Functional Properties of Proteins. In Structure–Function Properties of Food Proteins; Phillips, L.G., Whitehead, D.M., Kinsella, J., Eds.; Academic Press: Cambridge, MA, USA, 1994; pp. 107–109. [Google Scholar]
- Avena-Bustillos, R.J.; Cisneros-Zevallos, L.A.; Krochta, J.M.; Saltveit, M.E. Application of casein-lipid edible film emulsions to reduce white blush on minimally processed carrots. Postharvest Biol. Technol. 1994, 4, 319–329. [Google Scholar] [CrossRef]
- Yangılar, F.; Oğuzhan Yıldız, P. Casein/natamycin edible films efficiency for controlling mould growth and on microbiological, chemical and sensory properties during the ripening of Kashar cheese. J. Sci. Food Agric. 2016, 96, 2328–2336. [Google Scholar] [CrossRef] [PubMed]
- Avena-Bustillos, R.J.; Krochta, J.M. Water Vapor Permeability of Caseinate-Based Edible Films as Affected by pH, Calcium Crosslinking and Lipid Content. J. Food Sci. 1993, 58, 904–907. [Google Scholar] [CrossRef]
- Chick, J.; Hernandez, R.J. Physical, Thermal, and Barrier Characterization of Casein-Wax-Based Edible Films. J. Food Sci. 2002, 67, 1073–1079. [Google Scholar] [CrossRef]
- Chevalier, E.; Chaabani, A.; Assezat, G.; Prochazka, F.; Oulahal, N. Casein/wax blend extrusion for production of edible films as carriers of potassium sorbate—A comparative study of waxes and potassium sorbate effect. Food Packag. Shelf Life 2018, 16, 41–50. [Google Scholar] [CrossRef]
- Yadav, J.S.S.; Yan, S.; Pilli, S.; Kumar, L.; Tyagi, R.D.; Surampalli, R.Y. Cheese whey: A potential resource to transform into bioprotein, functional/nutritional proteins and bioactive peptides. Biotechnol. Adv. 2015, 33, 756–774. [Google Scholar] [CrossRef]
- Schmid, M.; Müller, K. Whey protein-based packaging films and coatings. In Whey Proteins; Elsevier: Amsterdam, The Netherlands, 2019; pp. 407–437. [Google Scholar]
- de Castro, R.J.S.; Domingues, M.A.F.; Ohara, A.; Okuro, P.K.; dos Santos, J.G.; Brexó, R.P.; Sato, H.H. Whey protein as a key component in food systems: Physicochemical properties, production technologies and applications. Food Struct. 2017, 14, 17–29. [Google Scholar] [CrossRef]
- Kandasamy, S.; Yoo, J.; Yun, J.; Kang, H.-B.; Seol, K.-H.; Kim, H.-W.; Ham, J.-S. Application of Whey Protein-Based Edible Films and Coatings in Food Industries: An Updated Overview. Coatings 2021, 11, 1056. [Google Scholar] [CrossRef]
- Di Pierro, P.; Mariniello, L.; Giosafatto, V.L.; Esposito, M.; Sabbah, M.; Porta, R. Chapter 13—Dairy Whey Protein-Based Edible Films and Coatings for Food Preservation. In Food Packaging and Preservation; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 439–456. [Google Scholar]
- Ramos, Ó.L.; Fernandes, J.C.; Silva, S.I.; Pintado, M.E.; Malcata, F.X. Edible Films and Coatings from Whey Proteins: A Review on Formulation, and on Mechanical and Bioactive Properties. Crit. Rev. Food Sci. Nutr. 2012, 52, 533–552. [Google Scholar] [CrossRef]
- Galus, S.; Lenart, A. Optical, mechanical, and moisture sorption properties of whey protein edible films. J. Food Process Eng. 2019, 42, e13245. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Whey protein edible films modified with almond and walnut oils. Food Hydrocoll. 2016, 52, 78–86. [Google Scholar] [CrossRef]
- Gounga, M.E.; Xu, S.-Y.; Wang, Z. Whey protein isolate-based edible films as affected by protein concentration, glycerol ratio and pullulan addition in film formation. J. Food Eng. 2007, 83, 521–530. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Moisture Sensitivity, Optical, Mechanical and Structural Properties of Whey Protein-Based Edible Films Incorporated with Rapeseed Oil. Food Technol. Biotechnol. 2016, 54, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Dangaran, K.; Tomasula, P.M.; Qi, P. Structure and Function of Protein-Based Edible Films and Coatings. In Edible Films and Coatings for Food Applications; Huber, K.C., Embuscado, M.E., Eds.; Springer New York: New York, NY, USA, 2009; pp. 25–56. [Google Scholar]
- Linsenmayer, T.F. Collagen. In Cell Biology of Extracellular Matrix: Second Edition; Hay, E.D., Ed.; Springer US: Boston, MA, USA, 1991; pp. 7–44. [Google Scholar]
- Brodsky, B.; Ramshaw, J.A. The collagen triple-helix structure. Matrix Biol. 1997, 15, 545–554. [Google Scholar] [CrossRef]
- Deiber, J.A.; Peirotti, M.B.; Ottone, M.L. Rheological characterization of edible films made from collagen colloidal particle suspensions. Food Hydrocoll. 2011, 25, 1382–1392. [Google Scholar] [CrossRef]
- O’Sullivan, A.; Shaw, N.B.; Murphy, S.C.; van de Vis, J.W.; van Pelt-Heerschap, H.; Kerry, J.P. Extraction of Collagen from Fish Skins and Its Use in the Manufacture of Biopolymer Films. J. Aquat. Food Prod. Technol. 2006, 15, 21–32. [Google Scholar] [CrossRef]
- Zhao, R.; Guan, W.; Zhou, X.; Lao, M.; Cai, L. The physiochemical and preservation properties of anthocyanidin/chitosan nanocomposite-based edible films containing cinnamon-perilla essential oil pickering nanoemulsions. LWT 2022, 153, 112506. [Google Scholar] [CrossRef]
- Song, D.-H.; Hoa, V.B.; Kim, H.W.; Khang, S.M.; Cho, S.-H.; Ham, J.-S.; Seol, K.-H. Edible Films on Meat and Meat Products. Coatings 2021, 11, 1344. [Google Scholar] [CrossRef]
- Fadini, A.L.; Rocha, F.S.; Alvim, I.D.; Sadahira, M.S.; Queiroz, M.B.; Alves, R.M.V.; Silva, L.B. Mechanical properties and water vapour permeability of hydrolysed collagen–cocoa butter edible films plasticised with sucrose. Food Hydrocoll. 2013, 30, 625–631. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, S.; Wang, H. Scale-Up Preparation and Characterization of Collagen/Sodium Alginate Blend Films. J. Food Qual. 2017, 2017, 4954259. [Google Scholar] [CrossRef]
- Ma, D.; Jiang, Y.; Ahmed, S.; Qin, W.; Liu, Y. Antilisterial and physical properties of polysaccharide-collagen films embedded with cell-free supernatant of Lactococcus lactis. Int. J. Biol. Macromol. 2020, 145, 1031–1038. [Google Scholar] [CrossRef]
- Marangoni Júnior, L.; Rodrigues, P.R.; da Silva, R.G.; Vieira, R.P.; Alves, R.M.V. Sustainable Packaging Films Composed of Sodium Alginate and Hydrolyzed Collagen: Preparation and Characterization. Food Bioprocess Technol. 2021, 14, 2336–2346. [Google Scholar] [CrossRef]
- Wu, X.; Luo, Y.; Liu, Q.; Jiang, S.; Mu, G. Improved structure-stability and packaging characters of crosslinked collagen fiber-based film with casein, keratin and SPI. J. Sci. Food Agric. 2019, 99, 4942–4951. [Google Scholar] [CrossRef]
- Jiang, Y.; Lan, W.; Sameen, D.E.; Ahmed, S.; Qin, W.; Zhang, Q.; Chen, H.; Dai, J.; He, L.; Liu, Y. Preparation and characterization of grass carp collagen-chitosan-lemon essential oil composite films for application as food packaging. Int. J. Biol. Macromol. 2020, 160, 340–351. [Google Scholar] [CrossRef]
- Poppe, J. Gelatin. In Thickening and Gelling Agents for Food; Springer: Berlin/Heidelberg, Germany, 1992; pp. 98–123. [Google Scholar]
- Keenan, T.R. Gelatin. Kirk-Othmer Encyclopedia of Chemical Technology; J. Wiley & Sons: Hoboken, NJ, USA, 1998; Volume 26, pp. 517–541. [Google Scholar]
- Arvanitoyannis, I.; Psomiadou, E.; Nakayama, A.; Aiba, S.; Yamamoto, N. Edible films made from gelatin, soluble starch and polyols, Part 3. Food Chem. 1997, 60, 593–604. [Google Scholar] [CrossRef]
- Fakhouri, F.M.; Martelli, S.M.; Caon, T.; Velasco, J.I.; Mei, L.H.I. Edible films and coatings based on starch/gelatin: Film properties and effect of coatings on quality of refrigerated Red Crimson grapes. Postharvest Biol. Technol. 2015, 109, 57–64. [Google Scholar] [CrossRef]
- Fakhouri, F.M.; Maria Martelli, S.; Canhadas Bertan, L.; Yamashita, F.; Innocentini Mei, L.H.; Collares Queiroz, F.P. Edible films made from blends of manioc starch and gelatin—Influence of different types of plasticizer and different levels of macromolecules on their properties. LWT 2012, 49, 149–154. [Google Scholar] [CrossRef]
- Sobral, P.J.A.; Menegalli, F.C.; Hubinger, M.D.; Roques, M.A. Mechanical, water vapor barrier and thermal properties of gelatin based edible films. Food Hydrocoll. 2001, 15, 423–432. [Google Scholar] [CrossRef]
- Bonilla, J.; Sobral, P.J.A. Investigation of the physicochemical, antimicrobial and antioxidant properties of gelatin-chitosan edible film mixed with plant ethanolic extracts. Food Biosci. 2016, 16, 17–25. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Ghavi, F.F. Preparation and functional properties of fish gelatin–chitosan blend edible films. Food Chem. 2013, 136, 1490–1495. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Tang, C.-H.; Yin, S.-W.; Yang, X.-Q.; Wang, Q.; Liu, F.; Wei, Z.-H. Characterization of gelatin-based edible films incorporated with olive oil. Food Res. Int. 2012, 49, 572–579. [Google Scholar] [CrossRef]
- Chambi, H.; Grosso, C. Edible films produced with gelatin and casein cross-linked with transglutaminase. Food Res. Int. 2006, 39, 458–466. [Google Scholar] [CrossRef]
- Buffo, R.A.; Han, J.H. 17—Edible films and coatings from plant origin proteins. In Innovations in Food Packaging; Han, J.H., Ed.; Academic Press: London, UK, 2005; pp. 277–300. [Google Scholar]
- Chiralt, A.; González-Martínez, C.; Vargas, M.; Atarés, L. 18—Edible films and coatings from proteins. In Proteins in Food Processing, 2nd ed.; Yada, R.Y., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 477–500. [Google Scholar]
- Brandenburg, A.H.; Weller, C.L.; Testin, R.F. Edible Films and Coatings from Soy Protein. J. Food Sci. 1993, 58, 1086–1089. [Google Scholar] [CrossRef]
- Hill, K.; Höfer, R. Natural fats and oils. Sustainable Solutions for Modern Economies; The Royal Society of Chemistry: London, UK, 2009; pp. 167–237. [Google Scholar]
- Rhim, J.W.; Shellhammer, T.H. 21—Lipid-based edible films and coatings. In Innovations in Food Packaging; Han, J.H., Ed.; Academic Press: London, UK, 2005; pp. 362–383. [Google Scholar]
- Hernandez, E. Edible coatings from lipids and resins. In Edible Coatings and Films to Improve Food Quality; Krochta, J., Nisperos-Carriedo, M., Eds.; CRC Press: Boca Raton, FL, USA, 1994; pp. 279–303. [Google Scholar]
- Debeaufort, F.; Voilley, A. Lipid-Based Edible Films and Coatings. In Edible Films and Coatings for Food Applications; Huber, K.C., Embuscado, M.E., Eds.; Springer: New York, NY, USA, 2009; pp. 135–168. [Google Scholar]
- Gordon, M. Fats and Fatty Foods. In Food Industries Manual; Ranken, M.D., Kill, R.C., Eds.; Springer: Boston, MA, USA, 1993; pp. 288–327. [Google Scholar]
- Duarte, M.; Duarte, R.; Rodrigues, R.; Rodrigues, M. Essential oils and their characteristics. Essential Oils in Food Processing: Chemistry, Safety and Applications; IFT Press: Chicago, IL, USA, 2018; pp. 1–19. [Google Scholar]
- Antunes, M.; Gago, C.; Cavaco, A.; Miguel, M. Edible coatings enriched with essential oils and their compounds for fresh and fresh-cut fruit. Recent Pat. Food Nutr. Agric. 2012, 4, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Bassolé, I.H.N.; Juliani, H.R. Essential oils in combination and their antimicrobial properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef] [PubMed]
- Nisar, T.; Wang, Z.-C.; Yang, X.; Tian, Y.; Iqbal, M.; Guo, Y. Characterization of citrus pectin films integrated with clove bud essential oil: Physical, thermal, barrier, antioxidant and antibacterial properties. Int. J. Biol. Macromol. 2018, 106, 670–680. [Google Scholar] [CrossRef]
- Kavoosi, G.; Rahmatollahi, A.; Dadfar, S.M.M.; Purfard, A.M. Effects of essential oil on the water binding capacity, physico-mechanical properties, antioxidant and antibacterial activity of gelatin films. LWT-Food Sci. Technol. 2014, 57, 556–561. [Google Scholar] [CrossRef]
- Aitboulahsen, M.; El Galiou, O.; Laglaoui, A.; Bakkali, M.; Hassani Zerrouk, M. Effect of plasticizer type and essential oils on mechanical, physicochemical, and antimicrobial characteristics of gelatin, starch, and pectin-based films. J. Food Process. Preserv. 2020, 44, e14480. [Google Scholar] [CrossRef]
- Benbettaïeb, N.; Karbowiak, T.; Debeaufort, F. Bioactive edible films for food applications: Influence of the bioactive compounds on film structure and properties. Crit. Rev. Food Sci. Nutr. 2019, 59, 1137–1153. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Genipin-Crosslinked Gelatin/Chitosan-Based Functional Films Incorporated with Rosemary Essential Oil and Quercetin. Materials 2022, 15, 3769. [Google Scholar] [CrossRef]
- Shellhammer, T.; Rumsey, T.; Krochta, J. Viscoelastic properties of edible lipids. J. Food Eng. 1997, 33, 305–320. [Google Scholar] [CrossRef]
- Shit, S.C.; Shah, P.M. Edible polymers: Challenges and opportunities. J. Polym. 2014, 2014, 427529. [Google Scholar] [CrossRef]
- Baldwin, E.A. Surface treatments and edible coatings in food preservation. In Handbook of Food Preservation; CRC Press: Boca Raton, FL, USA, 2020; pp. 507–528. [Google Scholar]
- Wang, D.; Huang, J.; Guo, Z.; Liu, W. Durable mixed edible wax coating with stretching superhydrophobicity. J. Mater. Chem. A 2021, 9, 1495–1499. [Google Scholar] [CrossRef]
- Dhall, R.K. Advances in Edible Coatings for Fresh Fruits and Vegetables: A Review. Crit. Rev. Food Sci. Nutr. 2013, 53, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Hagenmaier, R.D. Wax microemulsion formulations used as fruit coatings. Proc. Fla. State Hortic. Soc. 1998, 111, 251–254. [Google Scholar]
- Hagenmaier, R.D.; Baker, R.A. Edible Coatings from Morpholine-Free Wax Microemulsions. J. Agric. Food Chem. 1997, 45, 349–352. [Google Scholar] [CrossRef]
- Oregel-Zamudio, E.; Angoa-Pérez, M.V.; Oyoque-Salcedo, G.; Aguilar-González, C.N.; Mena-Violante, H.G. Effect of candelilla wax edible coatings combined with biocontrol bacteria on strawberry quality during the shelf-life. Sci. Hortic. 2017, 214, 273–279. [Google Scholar] [CrossRef]
- Baldwin, E.; Burns, J.; Kazokas, W.; Brecht, J.; Hagenmaier, R.; Bender, R.; Pesis, E. Effect of two edible coatings with different permeability characteristics on mango (Mangifera indica L.) ripening during storage. Postharvest Biol. Technol. 1999, 17, 215–226. [Google Scholar] [CrossRef]
- Ruiz-Martínez, J.; Aguirre-Joya, J.A.; Rojas, R.; Vicente, A.; Aguilar-González, M.A.; Rodríguez-Herrera, R.; Alvarez-Perez, O.B.; Torres-León, C.; Aguilar, C.N. Candelilla Wax Edible Coating with Flourensia cernua Bioactives to Prolong the Quality of Tomato Fruits. Foods 2020, 9, 1303. [Google Scholar] [CrossRef]
- De León-Zapata, M.A.; Sáenz-Galindo, A.; Rojas-Molina, R.; Rodríguez-Herrera, R.; Jasso-Cantú, D.; Aguilar, C.N. Edible candelilla wax coating with fermented extract of tarbush improves the shelf life and quality of apples. Food Packag. Shelf Life 2015, 3, 70–75. [Google Scholar] [CrossRef]
- Cruz, R.M.; Krauter, V.; Krauter, S.; Agriopoulou, S.; Weinrich, R.; Herbes, C.; Scholten, P.B.; Uysal-Unalan, I.; Sogut, E.; Kopacic, S.; et al. Bioplastics for Food Packaging: Environmental Impact, Trends and Regulatory Aspects. Foods 2022, 11, 3087. [Google Scholar] [CrossRef]
- Pham, T.T.; Nguyen, L.L.P.; Dam, M.S.; Baranyai, L. Application of Edible Coating in Extension of Fruit Shelf Life: Review. AgriEngineering 2023, 5, 520–536. [Google Scholar] [CrossRef]
- Gupta, I.; Cherwoo, L.; Bhatia, R.; Setia, H. Biopolymers: Implications and application in the food industry. Biocatal. Agric. Biotechnol. 2022, 46, 102534. [Google Scholar] [CrossRef]
- Ding, D. A review about edible food coatings and films. Master’s Thesis, Cornell University, Ithaca, NY, USA, 2021. [Google Scholar]
- Pellicer, E.; Nikolic, D.; Sort, J.; Baró, M.; Zivic, F.; Grujovic, N.; Grujic, R.; Pelemis, S. Advances in Applications of Industrial Biomaterials; Springer International Publishing: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Ragitha, V.M.; Edison, L.K. Safety Issues, Environmental Impacts, and Health Effects of Biopolymers. In Handbook of Biopolymers; Springer Nature: Singapore, 2022; pp. 1–27. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).