Synthesis and Characterization of Hydrophobic and Low Surface Tension Polyurethane
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Polyurethane and Polyurethane–Co–Perfluoropolyether
2.3. Coating and Curing
2.4. Instrumental
2.5. Surface Hydrophobicity and Critical Surface Tension
2.6. Statistics
3. Results and Discussion
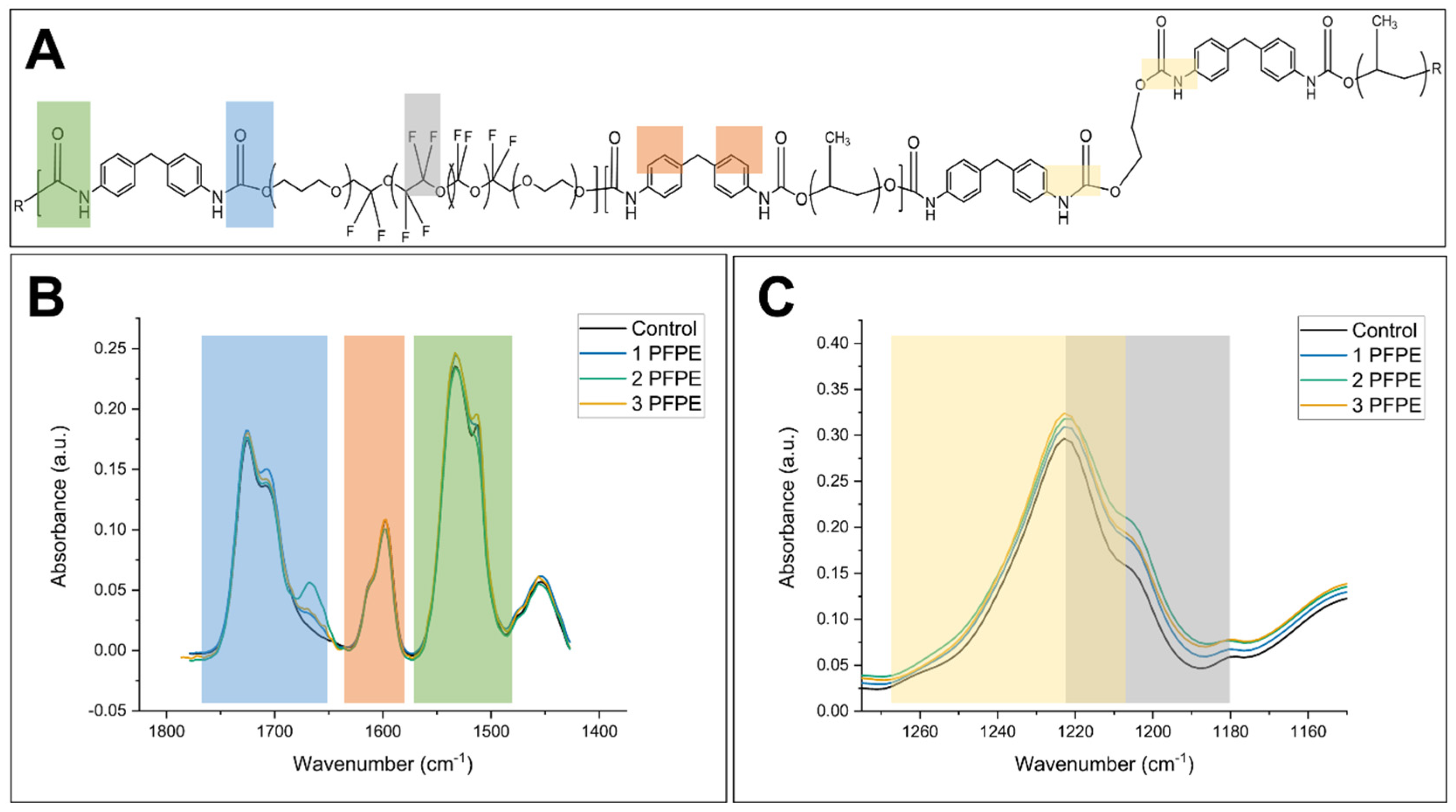
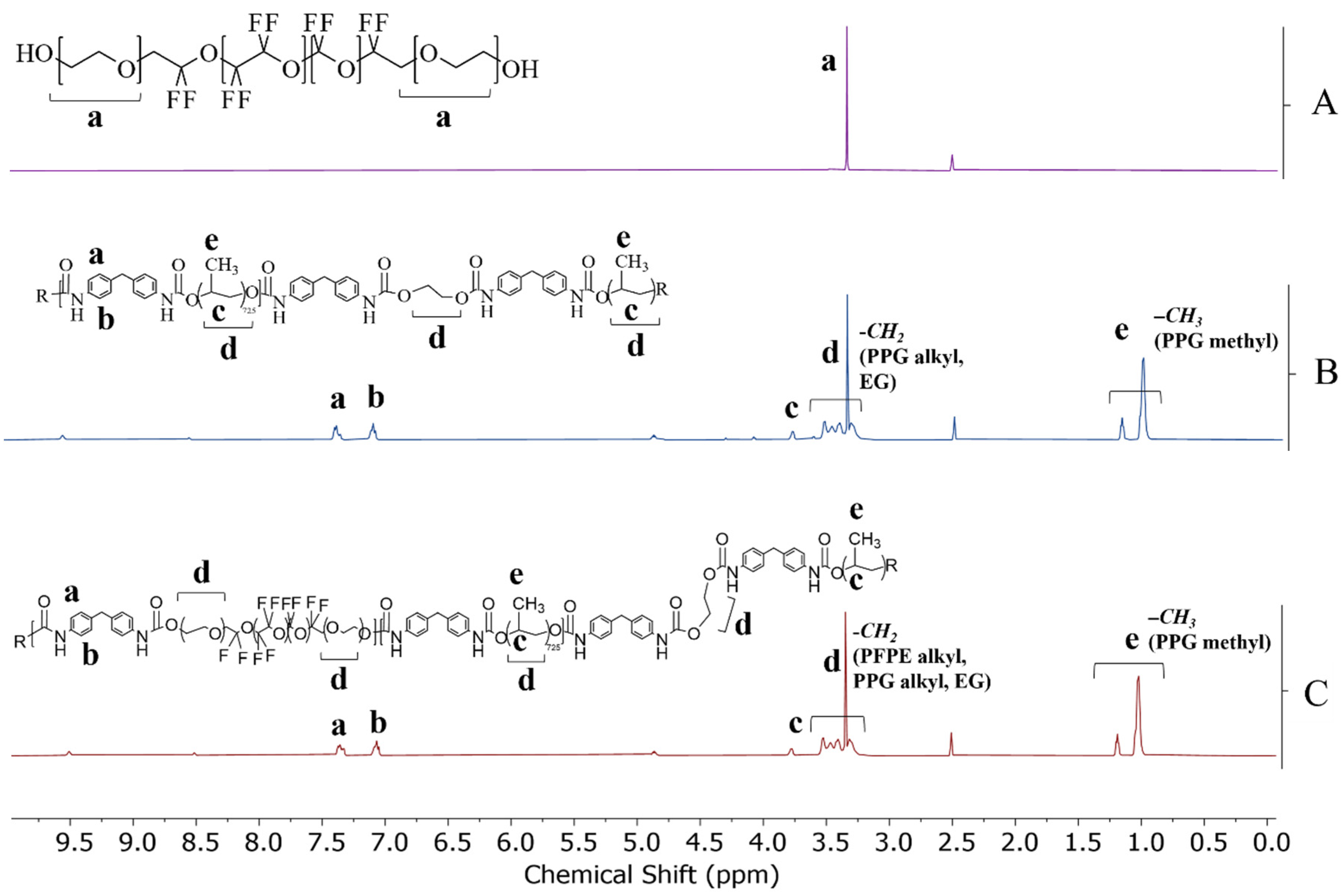
3.1. Synthesis
3.2. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Das, A.; Mahanwar, P. A brief discussion on advances in polyurethane applications. Adv. Ind. Eng. Polym. Res. 2020, 3, 93–101. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane types, synthesis and applications—A review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef]
- Oertel, G. Polyurethane Handbook: Chemistry, Raw Materials, Processing, Application, Properties; Hanser/Gardner [Distributor]: Munich, Germany; New York, NY, USA; Cincinnati, OH, USA, 1994. [Google Scholar]
- Hu, P.; Greiner, A.; Agarwal, S. Synthesis and properties evaluation of quaternized polyurethanes as antibacterial adhesives. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 752–757. [Google Scholar] [CrossRef]
- Hwang, H.D.; Kim, H.J. UV-curable low surface energy fluorinated polycarbonate-based polyurethane dispersion. J Colloid Interface Sci. 2011, 362, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.J.; Cheah, C.; Sarkar, D. Interfacial energetics approach for analysis of endothelial cell and segmental polyurethane interactions. Colloids Surf. B Biointerfaces 2016, 144, 46–56. [Google Scholar] [CrossRef]
- Young, T. An essay on the cohesion of fluids. Philos. Trans. R. Soc. 1805, 95, 65–87. [Google Scholar]
- Friesen, C.M.; Améduri, B. Outstanding telechelic perfluoropolyalkylethers and applications therefrom. Prog. Polym. Sci. 2018, 81, 238–280. [Google Scholar] [CrossRef]
- Xu, D.; Su, Y.; Zhao, L.; Meng, F.; Liu, C.; Guan, Y.; Zhang, J.; Luo, J. Antibacterial and antifouling properties of a polyurethane surface modified with perfluoroalkyl and silver nanoparticles. J. Biomed. Mater. Res. Part A 2017, 105, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Brady, R.F.; Aronson, C.L. Elastomeric fluorinated polyurethane coatings for nontoxic fouling control. Biofouling 2003, 19 (Suppl. S1), 59–62. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Y.; Sheng, J.; Wang, X.; Liu, L.; Yu, J.; Ding, B. Environmentally Friendly and Breathable Fluorinated Polyurethane Fibrous Membranes Exhibiting Robust Waterproof Performance. ACS Appl. Mater. Interfaces 2017, 9, 29302–29310. [Google Scholar] [CrossRef]
- Wouters, M.; van Zanten, J.; Vereijken, T.; Bakker, D.; Klijnstra, J. Fluorinated polyurethane coatings with adaptable surface properties. Surf. Coat. Int. Part B Coat. Trans. 2006, 89, 23–30. [Google Scholar] [CrossRef]
- Tonelli, C.; Ajroldi, G. New Fluoro-Modified Thermoplastic Polyurethanes. J. Appl. Polym. Sci. 2004, 87, 2279–2294. [Google Scholar] [CrossRef]
- Liu, P.; Ye, L.; Liu, Y.; Nie, F. Preparation and properties of the main-chain-fluorinated thermoplastic polyurethane elastomer. Polym. Bull. 2010, 66, 503–515. [Google Scholar] [CrossRef]
- Su, S.K.; Gu, J.H.; Lee, H.T.; Wu, C.L.; Hwang, J.J.; Suen, M.C. Synthesis and properties of novel biodegradable polyurethanes containing fluorinated aliphatic side chains. J. Polym. Res. 2017, 24, 142. [Google Scholar] [CrossRef]
- Yang, W.; Pan, P.; Wang, H.; Cheng, X.; Du, Z. Effect of hydroxyl-terminated poly(fluoroalkyl methacrylate) main-chain length on the microphase separation of waterborne fluorinated polyurethane. Polym. Int. 2018, 67, 1054–1061. [Google Scholar] [CrossRef]
- Li, J.W.; Lee, H.T.; Tsai, H.A.; Suen, M.C.; Chiu, C.W. Synthesis and Properties of Novel Polyurethanes Containing Long-Segment Fluorinated Chain Extenders. Polymers 2018, 10, 1292. [Google Scholar] [CrossRef]
- Chen, K.; Kuo, J. Synthesis and properties of novel fluorinated aliphatic polyurethanes with fluoro chain extenders. Macromol. Chem. Phys. 2000, 201, 2676–2686. [Google Scholar] [CrossRef]
- Takakura, T.; Kato, M.; Yamabe, M. Fluorinated polyurethanes, 1 Synthesis and characterization of fluoroine-containing segmented poly(urethane-urea)s. Macromol. Chem. Phys. 1990, 191, 625–632. [Google Scholar] [CrossRef]
- Choi, Y.S.; Kim, N.K.; Kang, H.; Jang, H.-K.; Noh, M.; Kim, J.; Shon, D.-J.; Kim, B.-S.; Lee, J.-C. Antibacterial and biocompatible ABA-triblock copolymers containing perfluoropolyether and plant-based cardanol for versatile coating applications. RSC Adv. 2017, 7, 38091–38099. [Google Scholar] [CrossRef]
- Gu, X.; Gao, T.; Meng, X.; Zhu, Y.; Wang, G. Enhanced hydrophobicity of polyurethane with the self-assembly of perfluoropolyether-based triblock copolymers. Prog. Org. Coat. 2022, 162, 106561. [Google Scholar] [CrossRef]
- Jiang, J.; Fu, Y.; Zhang, Q.; Zhan, X.; Chen, F. Novel amphiphilic poly(dimethylsiloxane) based polyurethane networks tethered with carboxybetaine and their combined antibacterial and anti-adhesive property. Appl. Surf. Sci. 2017, 412, 1–9. [Google Scholar] [CrossRef]
- Korhonen, J.T.; Huhtamaki, T.; Ikkala, O.; Ras, R.H. Reliable measurement of the receding contact angle. Langmuir 2013, 29, 3858–3863. [Google Scholar] [CrossRef] [PubMed]
- Combe, E. A protocol for determining the surface free energy of dental materials. Dent. Mater. 2004, 20, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Kabza, K.; Gestwicki, J.E.; McGrath, J.L. Contact Angle Goniometry as a Tool for Surface Tension Measurements of Solids, Using Zisman Plot Method. A Physical Chemistry Experiment. J. Chem. Educ. 2000, 77, 63–65. [Google Scholar] [CrossRef]
- Santos, O.; Nylander, T.; Rosmaninho, R.; Rizzo, G.; Yiantsios, S.; Andritsos, N.; Karabelas, A.; Müller-Steinhagen, H.; Melo, L.; Boulangé-Petermann, L.; et al. Modified stainless steel surfaces targeted to reduce fouling—Surface characterization. J. Food Eng. 2004, 64, 63–79. [Google Scholar] [CrossRef]
- Yuan, Y.; Lee, T.R. Contact Angle and Wetting Properties. In Surface Science Techniques; Springer: Berlin/Heidelberg, Germany, 2013; pp. 3–34. [Google Scholar]
- Owens, D.K.; Wendt, R.C. Estimation of the Surface Free Energy of Polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Fox, H.W.; Zisman, W.A. The Spreading of Liquids on Low Energy Surfaces. I Polytetrafluoroethylene. J. Colloid Sci. 1950, 5, 514–531. [Google Scholar] [CrossRef]
- Trigwell, S.; Selvaduary, G. Effect of Surface Treatment on the Surface Characteristics of AISI 316L Stainless Steel; NASA: Washington, DC, USA, 2004. [Google Scholar]
- Potschke, P.; Pionteck, J.; Stutz, H. Surface tension, interfacial tension, and morphology in blends of thermoplastic polyurethanes and polyolefins. Part I. Surface tension of melts of TPU model substances and polyolefins. Polymer 2002, 43, 6965–6972. [Google Scholar] [CrossRef]
- Erceg, T.; Tanasić, J.; Banjanin, B.; Baloš, S.; Cvetinov, M.; Cakić, S.; Ristić, I. Surface, structural, and thermal properties of polydimethylsiloxane-based polyurethanes and their blends with thermoplastic polyurethane elastomer. Polym. Bull. 2022, 79, 10909–10929. [Google Scholar] [CrossRef]


| MDI (mmol) | PPG (mmol) | EG (mmol) | PFPE (mmol) | |
|---|---|---|---|---|
| Polyurethane | 10.27 | 6.90 | 4.03 | - |
| PU–co–1PFPE | 10.27 | 6.90 | 4.03 | 0.03 |
| PU–co–2PFPE | 10.31 | 6.90 | 4.03 | 0.06 |
| PU–co–3PFPE | 10.35 | 6.90 | 4.03 | 0.09 |
| Mn (Daltons) | Mw (Daltons) | Polydispersity | |
|---|---|---|---|
| Polyurethane | 26,000 | 41,890 | 1.61 |
| PU–co–1PFPE | 28,900 | 45,912 | 1.59 |
| PU–co–2PFPE | 29,200 | 49,341 | 1.69 |
| PU–co–3PFPE | 26,400 | 43,628 | 1.65 |
| Carbon (at. %) | Oxygen (at. %) | Nitrogen (at. %) | Fluorine (at. %) | |
|---|---|---|---|---|
| Polyurethane | 73.52 | 23.94 | 1.73 | 0.82 |
| PU–co–1PFPE | 59.08 | 20.11 | 2.46 | 18.35 |
| PU–co–2PFPE | 58.19 | 20.67 | 2.84 | 20.67 |
| PU–co–PFPE | 58.75 | 17.36 | 2.32 | 21.57 |
| C–C (at. %) | C–O (at. %) | C=O (at. %) | C–F2 (at. %) | |
|---|---|---|---|---|
| Polyurethane | 73.33 | 23.87 | 2.80 | - |
| PU–co–1PFPE | 57.48 | 27.29 | 4.20 | 11.02 |
| PU–co–2PFPE | 54.57 | 24.19 | 6.16 | 14.41 |
| PU–co–3PFPE | 49.53 | 17.81 | 6.65 | 13.96 |
| Advancing Contact Angle (θa) | Receding Contact Angle (θr) | Hysteresis | |
|---|---|---|---|
| Stainless Steel | 73.4 ± 17.8° A | 18.5 ± 9.8° A | 54.9° |
| Polyurethane | 93.6 ± 3.6° B | 34.4 ± 4.3° B | 59.2° |
| PU–co–1PFPE | 131.5 ± 8.0° C | 27.2 ± 7.1° AB | 104.3° |
| PU–co–2PFPE | 130.9 ± 5.8° C | 28.0 ± 5.2° AB | 102.9° |
| PU–co–3PFPE | 128.8 ± 5.2° C | 29.2 ± 5.9° B | 99.6° |
| cr (mN m−1) | Goodness of Fit (r2) | |
|---|---|---|
| Stainless Steel | 17.49 | 0.99 |
| Polyurethane | 17.19 | 0.99 |
| PU–co–1PFPE | 12.11 | 0.99 |
| PU–co–2PFPE | 12.25 | 0.99 |
| PU–co–3PFPE | 12.54 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudlong, A.M.; Goddard, J.M. Synthesis and Characterization of Hydrophobic and Low Surface Tension Polyurethane. Coatings 2023, 13, 1133. https://doi.org/10.3390/coatings13071133
Rudlong AM, Goddard JM. Synthesis and Characterization of Hydrophobic and Low Surface Tension Polyurethane. Coatings. 2023; 13(7):1133. https://doi.org/10.3390/coatings13071133
Chicago/Turabian StyleRudlong, Autumn M., and Julie M. Goddard. 2023. "Synthesis and Characterization of Hydrophobic and Low Surface Tension Polyurethane" Coatings 13, no. 7: 1133. https://doi.org/10.3390/coatings13071133
APA StyleRudlong, A. M., & Goddard, J. M. (2023). Synthesis and Characterization of Hydrophobic and Low Surface Tension Polyurethane. Coatings, 13(7), 1133. https://doi.org/10.3390/coatings13071133






