Study on Mechanical Properties of AlFeCrMoNi1.8Nb1.5 Eutectic High-Entropy Alloy Coating Prepared by Wide-Band Laser Cladding
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Laser Cladding Process Parameters Design
2.3. Microstructure Characterization
2.4. Microhardness and Wear Performance Experiments
3. Results
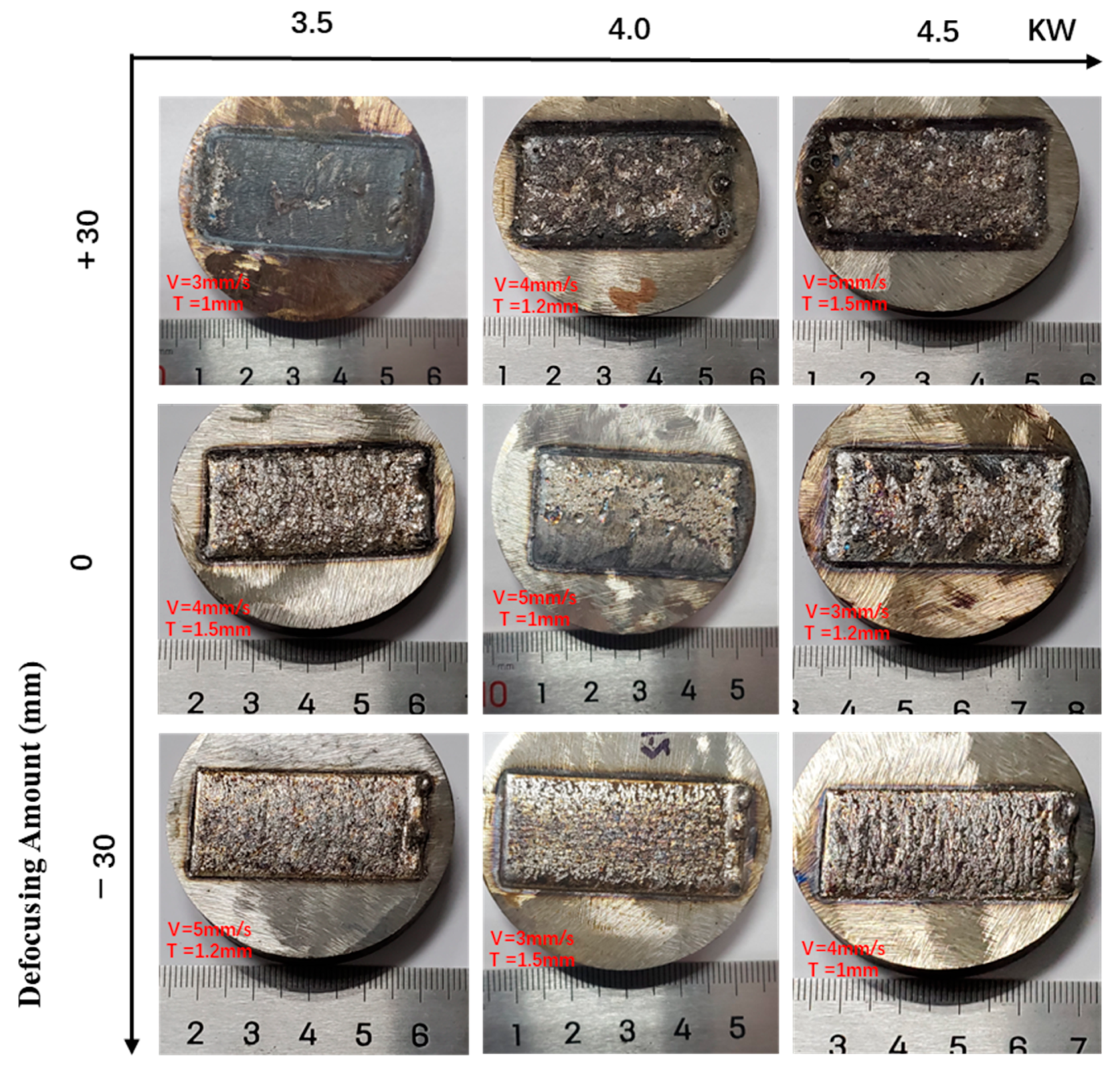
3.1. Macroscopic Morphology of Coatings
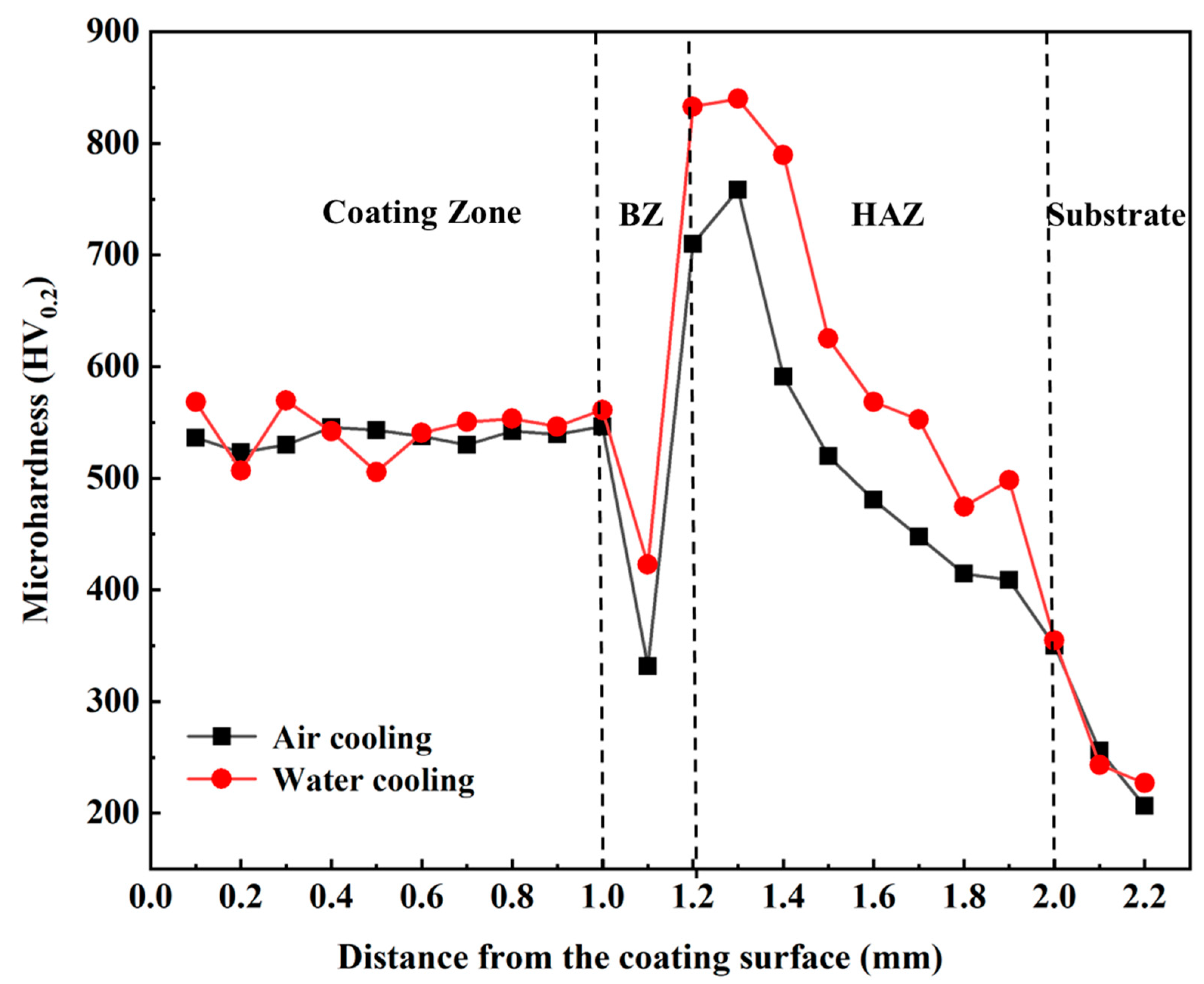
3.2. Effect of Cooling Rate on Coating
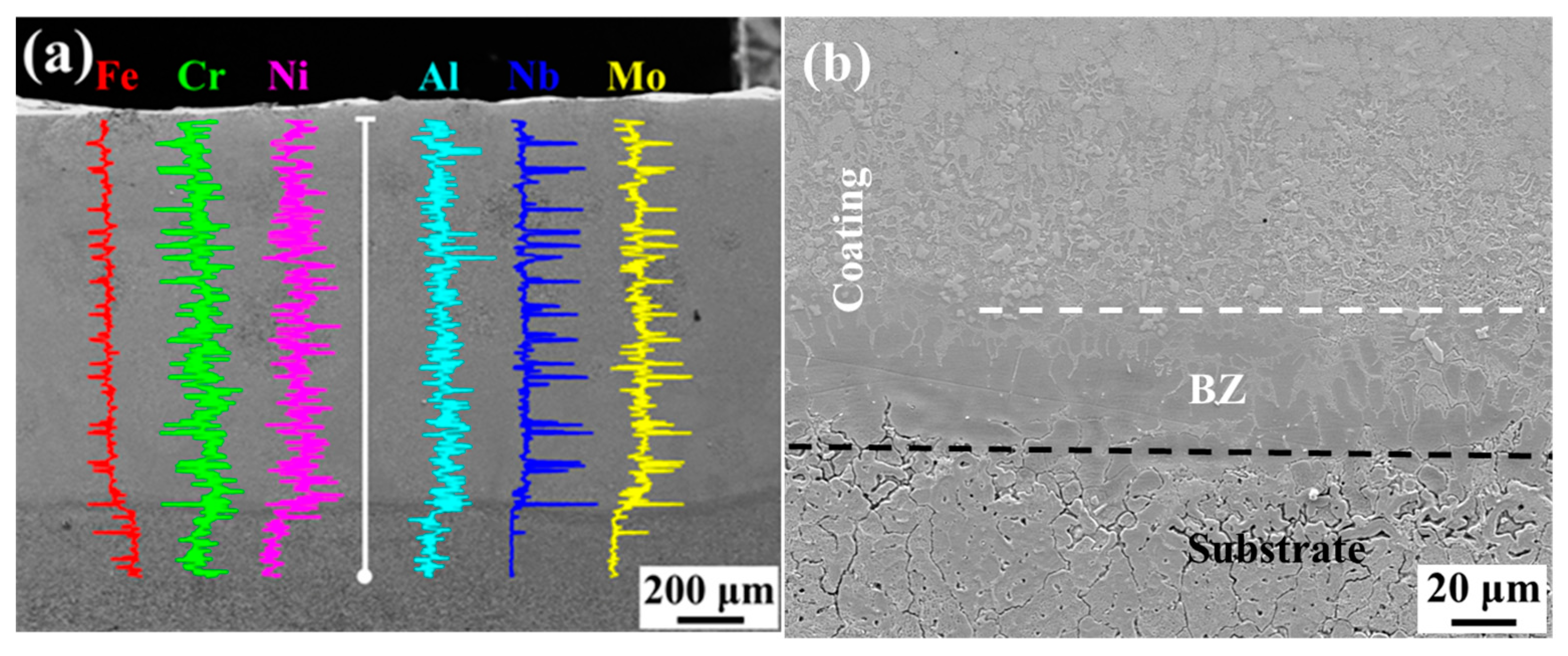
3.3. Phase and Microstructure Analysis
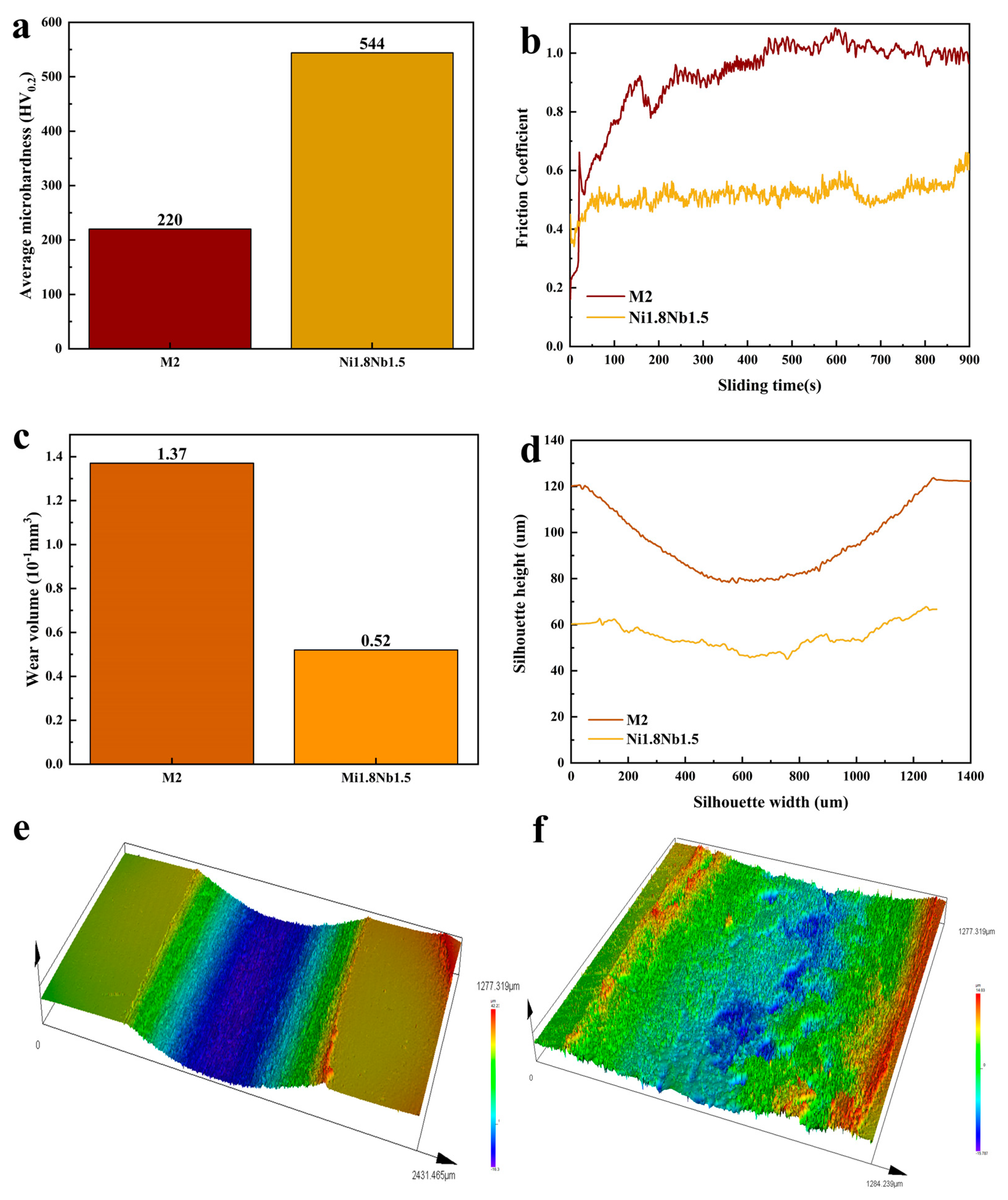
3.4. Mechanical Properties and Characterizations
4. Discussion
4.1. HEA Criterion
4.2. Phase Formation and Strengthening Mechanisms
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Wear volume | |
| Wear trace cross-section area | |
| The length of the wear track; | |
| G | The temperature gradient |
| R | The solidification rate |
References
- Maia, C.; Lima, R.C. Comparative study of surface modification techniques through average flank wear in high speed steel tools coated with thin TiN film. Surf. Coat. Technol. 2019, 366, 124–130. [Google Scholar] [CrossRef]
- Cao, H.; Dong, X.; Chen, S.; Dutka, M.; Pei, Y. Microstructure evolutions of graded high-vanadium tool steel composite coating in-situ fabricated via atmospheric plasma beam alloying. J. Alloy. Compd. 2017, 720, 169–181. [Google Scholar] [CrossRef]
- Wang, P.; He, W.; Mauer, G.; Mücke, R.; Vaßen, R. Monte Carlo simulation of column growth in plasma spray physical vapor deposition process. Surf. Coat. Technol. 2018, 335, 188–197. [Google Scholar] [CrossRef]
- Chaus, A.S.; Čaplovič, L.; Porubský, J. Microstructure and Properties of CBN Diffusion Coating on High-Speed Steel. Defect Diffus. Forum 2011, 312–315, 542–547. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, Y.; He, Y. Grain refinement and boundary misorientation transition by annealing in the laser rapid solidified 6FeNiCoCrAlTiSi multicomponent ferrous alloy coating. Surf. Coat. Technol. 2011, 205, 4068–4072. [Google Scholar] [CrossRef]
- Qi, K.; Yang, Y.; Hu, G.; Lu, X.; Li, J. Thermal expansion control of composite coatings on 42CrMo by laser cladding. Surf. Coat. Technol. 2020, 397, 125983. [Google Scholar] [CrossRef]
- Kuo, Y.L.; Chang, K.H. Atmospheric pressure plasma enhanced chemical vapor deposition of SiOx films for improved corrosion resistant properties of AZ31 magnesium alloys. Surf. Coat. Technol. 2015, 283, 194–200. [Google Scholar] [CrossRef]
- García, J.; Fernández, J.; Cuetos, J.; Costales, F. Fatigue effect of WC coatings thermal sprayed by HVOF and laser treated, on medium carbon steel. Eng. Fail. Anal. 2011, 18, 1750–1760. [Google Scholar] [CrossRef]
- Goncharov, O.; Sapegina, I.; Faizullin, R.; Baldaev, L. Tantalum chemical vapour deposition on steel and tungsten substrates in the TaBr5-Cd-He system. Surf. Coat. Technol. 2019, 377, 124893. [Google Scholar] [CrossRef]
- Fotovvati, B.; Namdari, N.; Dehghanghadikolaei, A. On Coating Techniques for Surface Protection: A Review. J. Manuf. Mater. Process. 2019, 3, 28. [Google Scholar] [CrossRef]
- Qiu, X.; Zhang, Y.; Liu, C. Effect of Ti content on structure and properties of Al2CrFeNiCoCuTix high-entropy alloy coatings. J. Alloy. Compd. 2013, 585, 282–286. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, Y.; He, Y.-Z. Synthesis and characterization of FeCoNiCrCu high-entropy alloy coating by laser cladding. Mater. Des. 2011, 32, 1910–1915. [Google Scholar] [CrossRef]
- Zhang, J. Process optimization and interface microstructure and performance analysis of laser cladding IN718 alloy. China Laser 2022, 49, 16. (In Chinese) [Google Scholar]
- Gong, Y.L.; Xu, X.D. Research on geometric modeling of single-layer process parameters of laser cladding. Manuf. Autom. 2022, 44, 59–62. (In Chinese) [Google Scholar]
- Dou, B.; Zhang, H.; Zhu, J.-H.; Xu, B.-Q.; Zhou, Z.-Y.; Wu, J.-L. Uniformly Dispersed Carbide Reinforcements in the Medium-Entropy High-Speed Steel Coatings by Wide-Band Laser Cladding. Acta Met. Sin. (Engl. Lett.) 2020, 33, 1145–1150. [Google Scholar] [CrossRef]
- Ma, Q.; Dong, Z.; Ren, N.; Hong, S.; Chen, J.; Hu, L.; Meng, W. Microstructure and Mechanical Properties of Multiple In-Situ-Phases-Reinforced Nickel Composite Coatings Deposited by Wide-Band Laser. Coatings 2021, 11, 36. [Google Scholar] [CrossRef]
- Zhang, P.; Pang, Y.; Yu, M. Effects of WC Particle Types on the Microstructures and Properties of WC-Reinforced Ni60 Composite Coatings Produced by Laser Cladding. Metals 2019, 9, 583. [Google Scholar] [CrossRef]
- Cai, Y.C.; Luo, Z.; Feng, M.N.; Liu, Z.M.; Huang, Z.Y.; Zeng, Y.D. The effect of TiC/ Al2O3 composite ceramic reinforcement on tribological behavior of laser cladding Ni60 alloys coatings. Surf. Coat. Technol. 2016, 291, 222–229. [Google Scholar] [CrossRef]
- Zhang, Z.; Farahmand, P.; Kovacevic, R. Laser cladding of 420 stainless steel with molybdenum on mild steel A36 by a high power direct diode laser. Mater. Des. 2016, 109, 686–699. [Google Scholar] [CrossRef]
- Yao, J.H.; Zhang, J.; Wu, G.L.; Wang, L.; Zhang, Q.L.; Liu, R. Microstructure and wear resistance of laser cladded composite coatings prepared from pre-alloyed WC- NiCrMo powder with different laser spots, Opt. Laser. Technol. 2017, 101, 520–530. [Google Scholar] [CrossRef]
- Yeh, J.-W.; Chen, S.K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Huang, P.-K.; Yeh, J.-W.; Shun, T.-T.; Chen, S.-K. Multi-Principal-Element Alloys with Improved Oxidation and Wear Resistance for Thermal Spray Coating. Adv. Eng. Mater. 2004, 6, 74–78. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Chuang, M.-H.; Tsai, M.-H.; Wang, W.-R.; Lin, S.-J.; Yeh, J.-W. Microstructure and wear behavior of AlxCo1.5CrFeNi1.5Tiy high-entropy alloys. Acta Mater. 2011, 59, 6308–6317. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Sheu, T.-S.; Yeh, J.-W.; Chen, S.-K. Effect of iron content on wear behavior of AlCoCrFexMo0.5Ni high-entropy alloys. Wear 2010, 268, 653–659. [Google Scholar] [CrossRef]
- Wu, J.-M.; Lin, S.-J.; Yeh, J.-W.; Chen, S.-K.; Huang, Y.-S.; Chen, H.-C. Adhesive wear behavior of AlxCoCrCuFeNi high-entropy alloys as a function of aluminum content. Wear 2006, 261, 513–519. [Google Scholar] [CrossRef]
- Tsai, M.-H.; Wang, C.-W.; Tsai, C.-W.; Shen, W.-J.; Yeh, J.-W.; Gan, J.-Y.; Wu, W.-W. Thermal Stability and Performance of NbSiTaTiZr High-Entropy Alloy Barrier for Copper Metallization. J. Electrochem. Soc. 2011, 158, H1161–H1165. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Juan, C.-C.; Wang, W.-R.; Sheu, T.-S.; Yeh, J.-W.; Chen, S.-K. On the superior hot hardness and softening resistance of AlCoCrxFeMo0.5Ni high-entropy alloys. Mater. Sci. Eng. A 2011, 528, 3581–3588. [Google Scholar] [CrossRef]
- Liu, Y.; Jin, B.; Lu, J. Mechanical Properties, Microstructure and Thermal Stability of a Nanocrystalline CoCrFeMnNi High-Entropy Alloy After Severe Plastic Deformation. Acta. Mater. 2015, 96, 258–268. [Google Scholar]
- Gorr, B.; Azim, M.; Christ, H.J.; Mueller, T.; Schliephake, D.; Heilmaier, M. Phase equilibria, microstructure, and high temper-ature oxidation resistance of novel refractory high-entropy alloys. J. Alloy. Compd. 2015, 624, 270–278. [Google Scholar] [CrossRef]
- Gorr, B.; Mueller, F.; Christ, H.-J.; Mueller, T.; Chen, H.; Kauffmann, A.; Heilmaier, M. High temperature oxidation behavior of an equimolar refractory metal-based alloy 20Nb 20Mo 20Cr 20Ti 20Al with and without Si addition. J. Alloy. Compd. 2016, 688, 468–477. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, S.; Ritchie, R.O.; Meyers, M.A. Mechanical properties of high-entropy alloys with emphasis on face-centered cubic alloys. Prog. Mater. Sci. 2018, 102, 296–345. [Google Scholar] [CrossRef]
- Couzinié, J.-P.; Dirras, G. Body-centered cubic high-entropy alloys: From processing to underlying deformation mechanisms. Mater. Charact. 2018, 147, 533–544. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiao, J.; Ma, S.; Gao, M.; Yang, H.; Chen, M.; Zhang, Y. A hexagonal close-packed high-entropy alloy: The effect of entropy. Mater. Des. 2016, 96, 10–15. [Google Scholar] [CrossRef]
- Jiang, L.; Lu, Y.; Wu, W.; Cao, Z.; Li, T. Microstructure and Mechanical Properties of a CoFeNi2V0.5Nb0.75 Eutectic High Entropy Alloy in As-cast and Heat-treated Conditions. J. Mater. Sci. Technol. 2016, 32, 245–250. [Google Scholar] [CrossRef]
- Han, L.; Xu, X.; Li, Z.; Liu, B.; Liu, C.T.; Liu, Y. A novel equiaxed eutectic high-entropy alloy with excellent mechanical properties at elevated temperatures. Mater. Res. Lett. 2020, 8, 373–382. [Google Scholar] [CrossRef]
- Kuang, S.H.; Zhou, F.; Liu, W.C.; Liu, Q.B. Al2O3/MC particles reinforced MoFeCrTiWNbx high-entropy-alloy coatings pre-pared by laser cladding. Surf. Eng. 2021, 38, 158–165. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Cai, J.; Ji, S.; Geng, J.; Sun, X.; Li, D. High-strength NbMoTaX refractory high-entropy alloy with low stacking fault energy eutectic phase via laser additive manufacturing. Mater. Des. 2021, 201, 109462. [Google Scholar] [CrossRef]
- Yang, J.X.; Bai, B.; Ke, H.; Cui, Z.; Liu, Z.; Zhou, Z.; Xu, H.C.; Xiao, J.H.; Liu, Q.; Li, H.X. Effect of metallurgical behavior on mi-crostructure and properties of FeCrMoMn coatings prepared by high-speed laser cladding. Opt. Laser Technol. 2021, 144, 107431. [Google Scholar] [CrossRef]
- Archard, J.F. Contact and Rubbing of Flat Surfaces. J. Appl. Phys. 1953, 24, 981–988. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.J.; Lin, J.P.; Chen, G.L.; Liaw, P.K. Solid-Solution Phase Formation Rules for Multi-component Alloys. Adv. Eng. Mater. 2008, 10, 534–538. [Google Scholar] [CrossRef]
- Zhang, Y. High-Entropy Materials: A Brief Introduction; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Yang, X.; Zhang, Y. Prediction of high-entropy stabilized solid-solution in multi-component alloys. Mater. Chem. Phys. 2012, 132, 233–238. [Google Scholar] [CrossRef]
- Guo, S.; Ng, C.; Lu, J.; Liu, C.T. Effect of valence electron concentration on stability of fcc or bcc phase in high entropy alloys. J. Appl. Phys. 2011, 109, 103505. [Google Scholar] [CrossRef]
- Miedema, A. The electronegativity parameter for transition metals: Heat of formation and charge transfer in alloys. J. Less Common Met. 1973, 32, 117–136. [Google Scholar] [CrossRef]




| W6Mo5Cr4V2 | AlFeCrMoNi1.8Nb1.5 | |||
|---|---|---|---|---|
| Element | wt.% | Element | wt.% | at.% |
| W | 5.50~6.75 | Al | 0.12 | 0.38 |
| Mo | 4.50~5.50 | Fe | 11.73 | 18.11 |
| Cr | 3.80~4.40 | Cr | 10.93 | 18.11 |
| V | 1.75~2.20 | Mo | 20.16 | 18.11 |
| C | 1.05~1.20 | Nb | 29.29 | 27.16 |
| Al | 0.80~1.20 | Ni-coated Al composite powder | 27.75 | 18.11 |
| Si | ≤0.60 | |||
| Mn | ≤0.40 | |||
| S | ≤0.03 | |||
| P | ≤0.03 | |||
| Number | Defocus Amount (mm) | Laser Power (KW) | Scanning Speed (mm/s) | Preset Powder Thickness (mm) |
|---|---|---|---|---|
| 1 | +30 | 3.5 | 3 | 1 |
| 2 | 0 | 4 | 4 | 1.2 |
| 3 | −30 | 4.5 | 5 | 1.5 |
| Number | Defocus Amount (mm) | Laser Power (kW) | Scanning Speed (mm/s) | Preset Powder Thickness (mm) | Macroscopic Morphology | Formability |
|---|---|---|---|---|---|---|
| No. 1 | +30 | 3.5 | 3 | 1 | Uneven | Holes-rich and thinner coating |
| No. 2 | +30 | 4 | 4 | 1.2 | Uneven | Holes |
| No. 3 | +30 | 4.5 | 5 | 1.5 | Uneven | Cracks and holes |
| No. 4 | 0 | 3.5 | 4 | 1.5 | Uneven | Good combination and no defects |
| No. 5 | 0 | 4 | 5 | 1 | Uneven | Thinner coating |
| No. 6 | 0 | 4.5 | 3 | 1.2 | Uneven | Cracks |
| No. 7 | −30 | 3.5 | 5 | 1.2 | Slightly even | Good combination and no defects |
| No. 8 | −30 | 4 | 3 | 1.5 | Even and smooth | Good combination and no defects |
| No. 9 | −30 | 4.5 | 4 | 1 | Slightly even | Holes-rich and thinner coating |
| Compounds | Enthalpy of Formation (kJ/mol) |
|---|---|
| NiAl | −48.42 |
| Ni3Al | −33.54 |
| Fe2Nb | −20.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Zheng, S.; Zhou, F. Study on Mechanical Properties of AlFeCrMoNi1.8Nb1.5 Eutectic High-Entropy Alloy Coating Prepared by Wide-Band Laser Cladding. Coatings 2023, 13, 1077. https://doi.org/10.3390/coatings13061077
Li F, Zheng S, Zhou F. Study on Mechanical Properties of AlFeCrMoNi1.8Nb1.5 Eutectic High-Entropy Alloy Coating Prepared by Wide-Band Laser Cladding. Coatings. 2023; 13(6):1077. https://doi.org/10.3390/coatings13061077
Chicago/Turabian StyleLi, Feng, Shisong Zheng, and Fang Zhou. 2023. "Study on Mechanical Properties of AlFeCrMoNi1.8Nb1.5 Eutectic High-Entropy Alloy Coating Prepared by Wide-Band Laser Cladding" Coatings 13, no. 6: 1077. https://doi.org/10.3390/coatings13061077
APA StyleLi, F., Zheng, S., & Zhou, F. (2023). Study on Mechanical Properties of AlFeCrMoNi1.8Nb1.5 Eutectic High-Entropy Alloy Coating Prepared by Wide-Band Laser Cladding. Coatings, 13(6), 1077. https://doi.org/10.3390/coatings13061077




