Biocidal Coatings against Gram-Positive Bacteria from Linear and Branched Polycations: The Decisive Role of the Diffusion Coefficients of Macromolecules
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Determination of the Diffusion Coefficients
2.2.2. Coatings Resistance to Wash-Off with Water
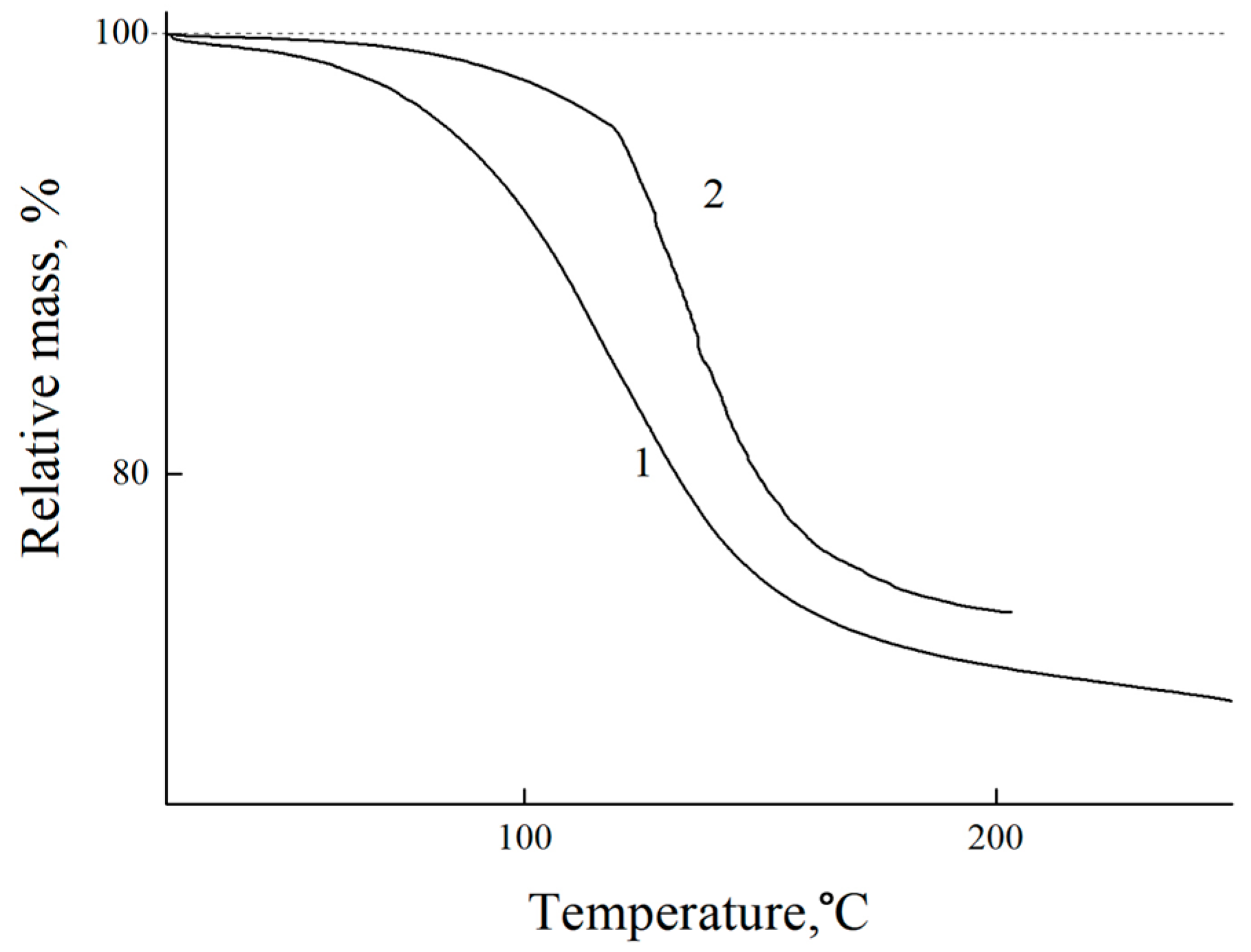
2.2.3. Water Content in Coatings Analysis
2.2.4. Polycation Coatings Adhesive Properties Analysis
2.2.5. Antibacterial Action of Polycations
2.2.6. Measurements of Morphology of Coatings
3. Results
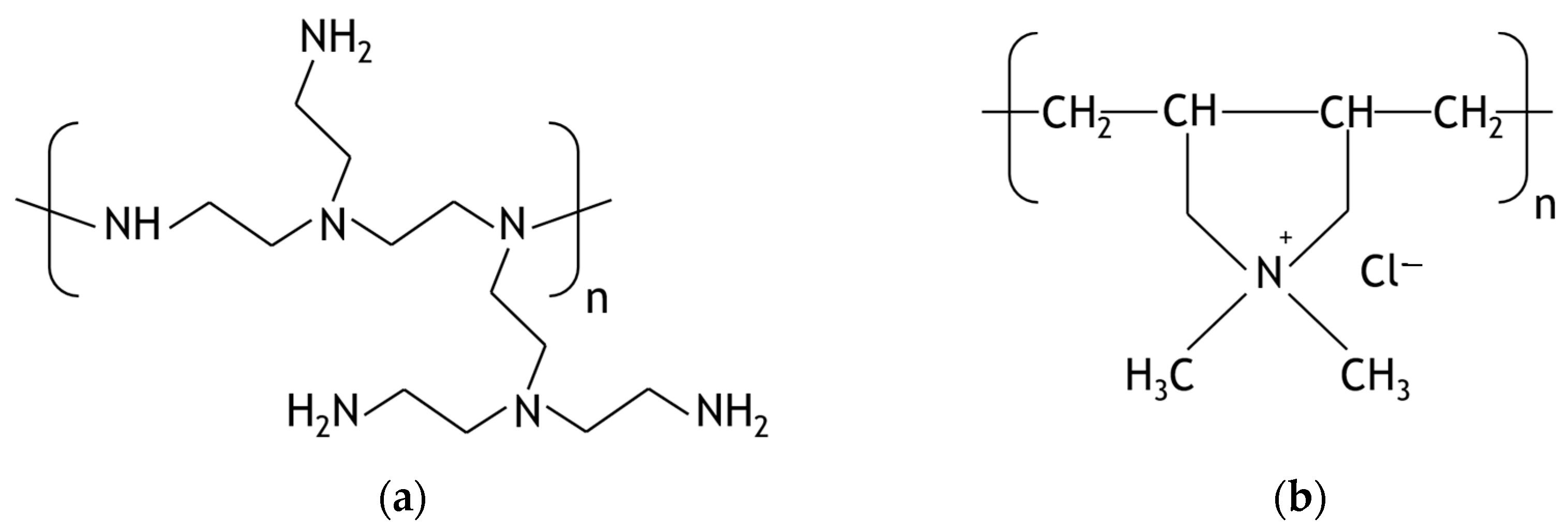
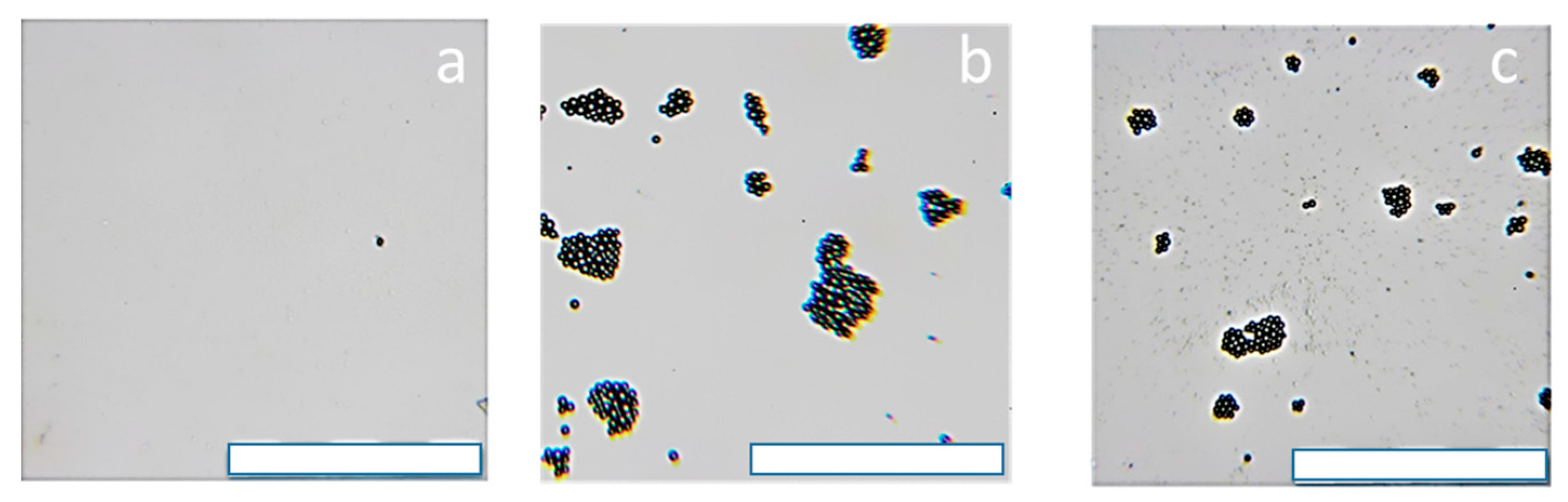
3.1. Samples Characterization
3.2. Screening of the Antibacterial Activity of PEI and PDADMAC
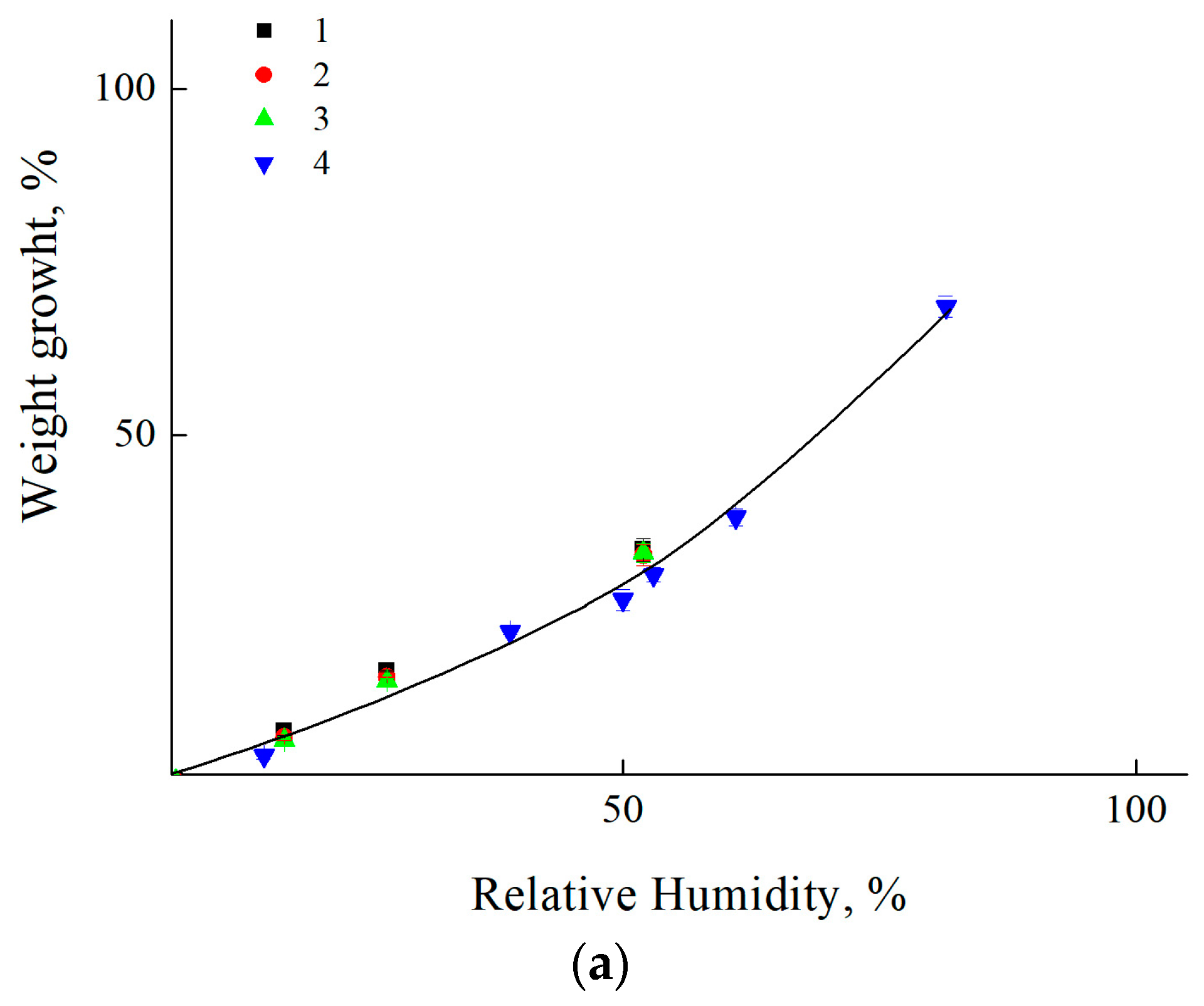
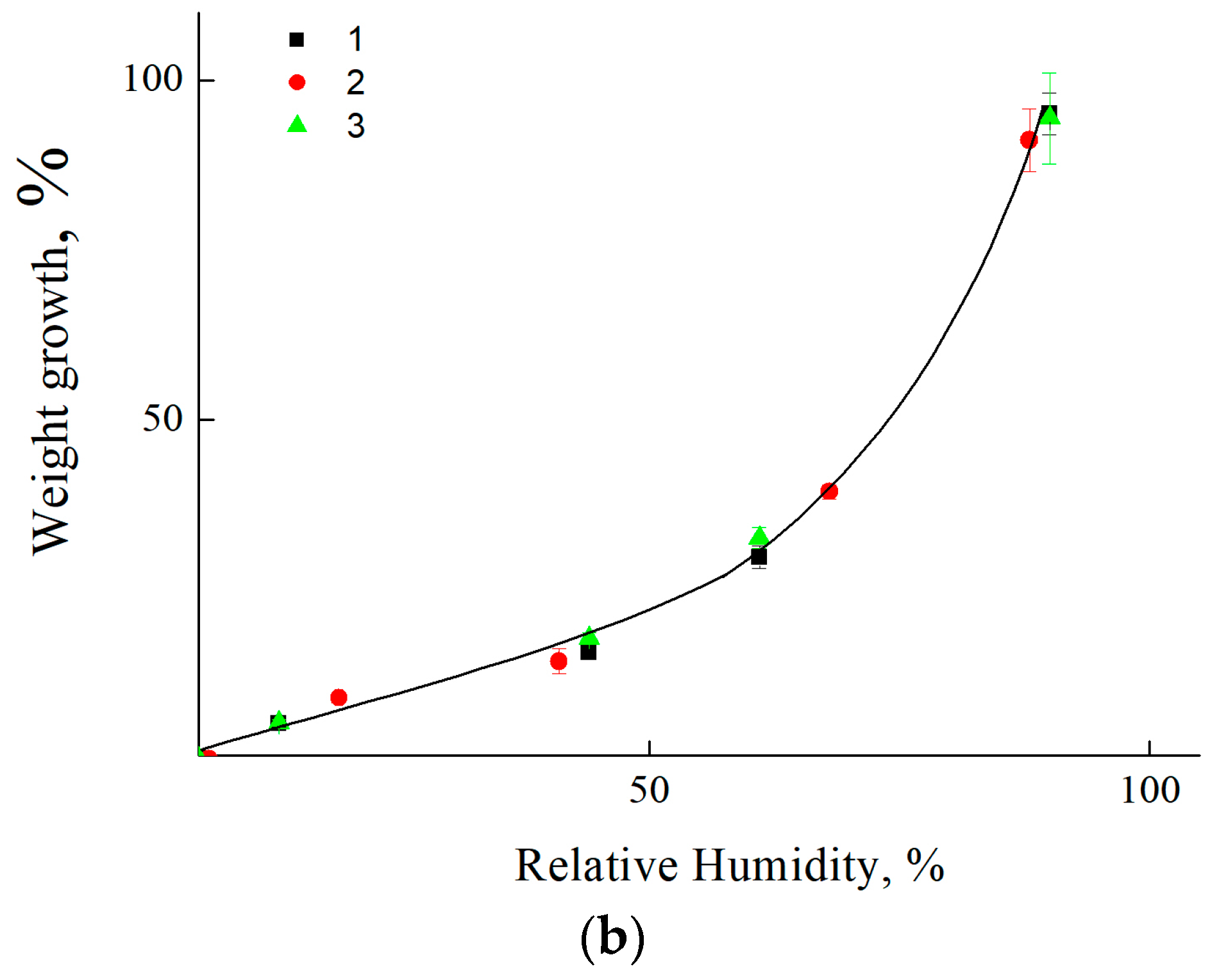
3.3. Estimation of Moisture Saturation of Coatings
3.4. Wash-Off Resistance of Polycation Coatings
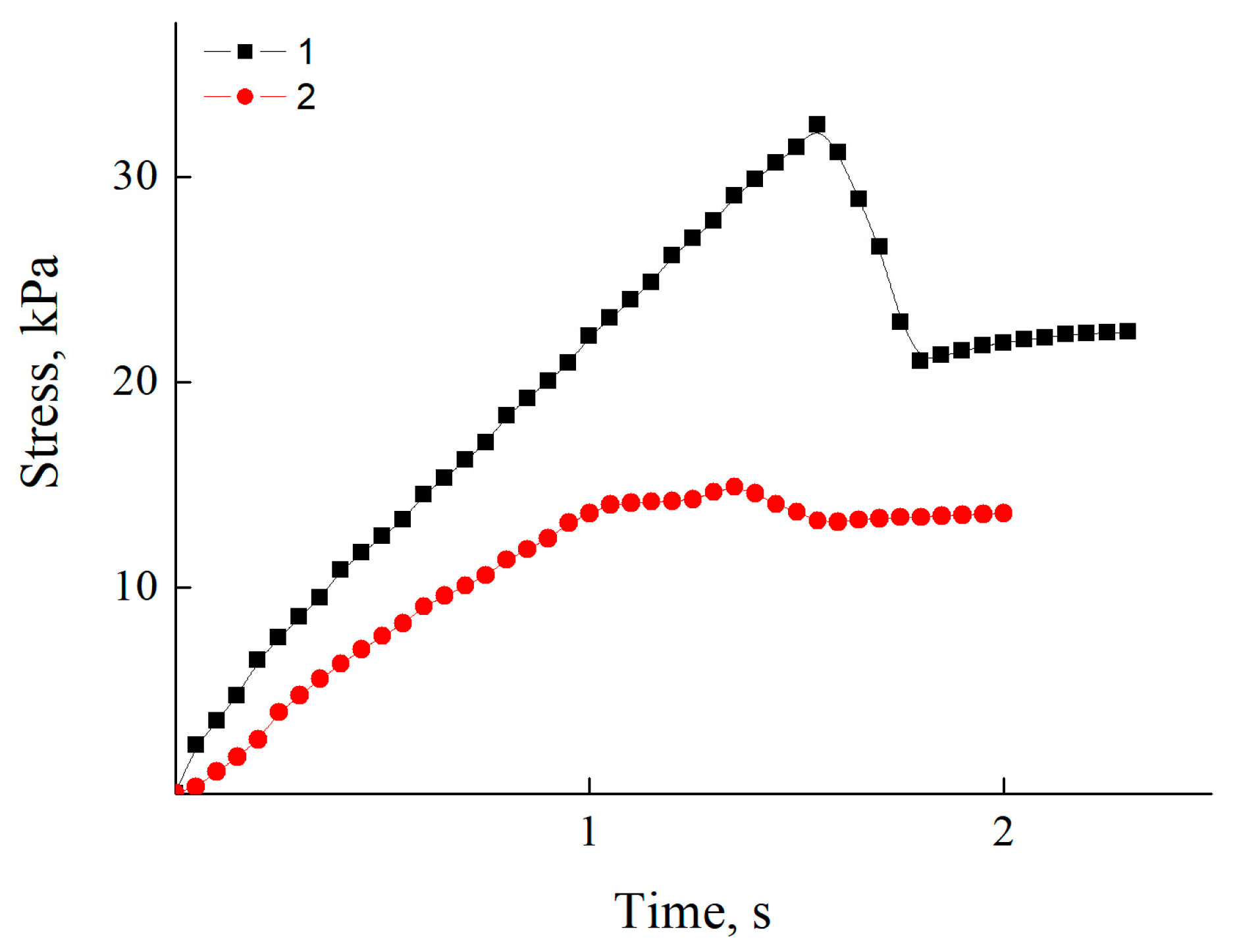
3.5. Adhesive Properties of Polycation Coatings
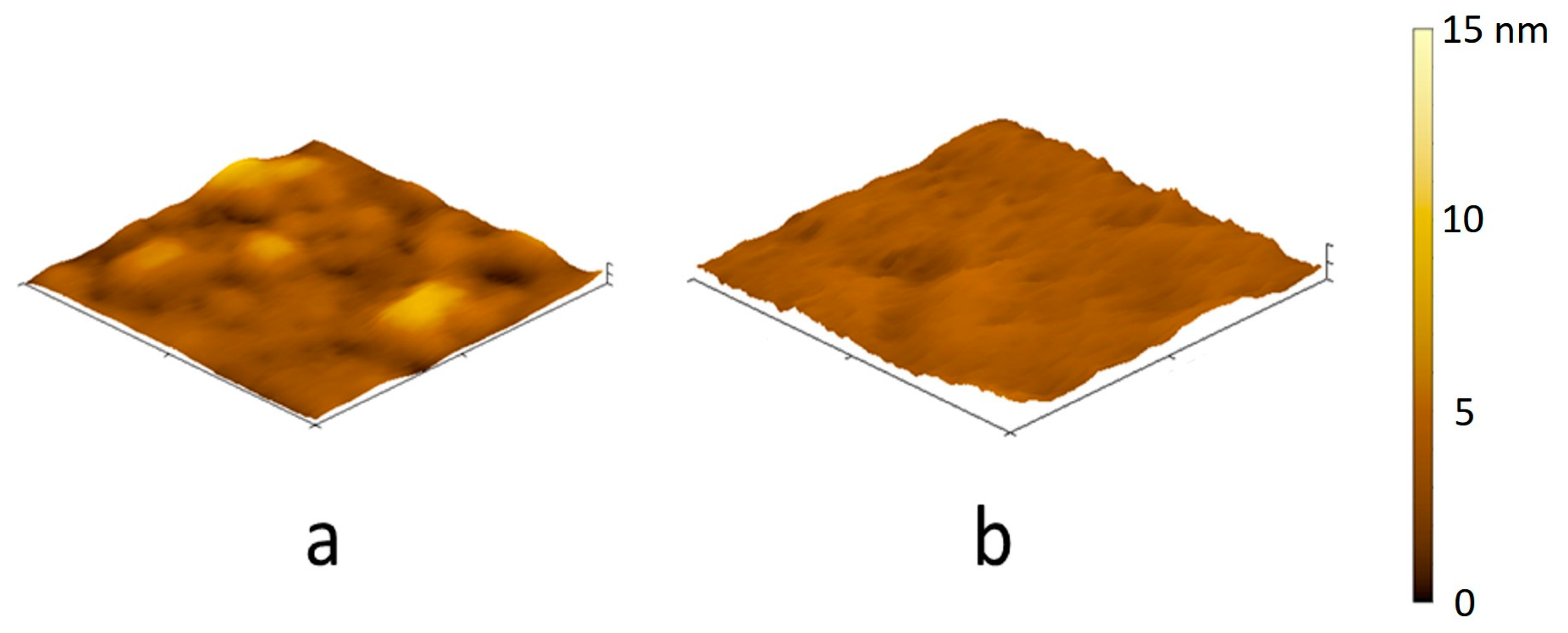
3.6. The Structure of the Polycationic Coatings
3.7. Interaction of the Polycationic Coatings with Model Cell Membranes
3.8. Biocidal Properties of the Polycationic Coatings against Food-Born Bacteria
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pérez-Escamilla, R. Food Security and the 2015–2030 Sustainable Development Goals: From Human to Planetary Health: Perspectives and Opinions. Curr. Dev. Nutr. 2017, 1, e000513. [Google Scholar] [CrossRef] [PubMed]
- López-Gálvez, F.; Gómez, P.A.; Artés, F.; Artés-Hernández, F.; Aguayo, E. Interactions between Microbial Food Safety and Environmental Sustainability in the Fresh Produce Supply Chain. Foods 2021, 10, 1655. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, K.; Verran, J. Formation, architecture and functionality of microbial biofilms in the food industry. Curr. Opin. Food Sci. 2015, 2, 84–91. [Google Scholar] [CrossRef]
- Rather, M.A.; Gupta, K.; Bardhan, P.; Borah, M.; Sarkar, A.; Eldiehy, K.S.H.; Bhuyan, S.; Mandal, M. Microbial biofilm: A matter of grave concern for human health and food industry. J. Basic Microbiol. 2021, 61, 380–395. [Google Scholar] [CrossRef] [PubMed]
- Abebe, G.M. The Role of Bacterial Biofilm in Antibiotic Resistance and Food Contamination. Int. J. Microbiol. 2020, 2020, 1705814. [Google Scholar] [CrossRef]
- Mevo, S.I.U.; Ashrafudoulla, M.; Mizan, M.F.R.; Park, S.H.; Ha, S.D. Promising strategies to control persistent enemies: Some new technologies to combat biofilm in the food industry—A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5938–5964. [Google Scholar] [CrossRef]
- Toushik, S.H.; Roy, A.; Alam, M.; Rahman, U.H.; Nath, N.K.; Nahar, S.; Matubber, B.; Uddin, M.J.; Roy, P.K. Pernicious Attitude of Microbial Biofilms in Agri-Farm Industries: Acquisitions and Challenges of Existing Antibiofilm Approaches. Microorganisms 2022, 10, 2348. [Google Scholar] [CrossRef]
- Yong, L.X.; Calautit, J.K. A Comprehensive Review on the Integration of Antimicrobial Technologies onto Various Surfaces of the Built Environment. Sustainability 2023, 15, 3394. [Google Scholar] [CrossRef]
- Bhattacharjee, B.; Ghosh, S.; Patra, D.; Haldar, J. Advancements in release-active antimicrobial biomaterials: A journey from release to relief. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1745. [Google Scholar] [CrossRef]
- Cloutier, M.; Tolouei, R.; Lesage, O.; Lévesque, L.; Turgeon, S.; Tatoulian, M.; Mantovani, D. On the long term antibacterial features of silver-doped diamondlike carbon coatings deposited via a hybrid plasma process. Biointerphases 2014, 9, 029013. [Google Scholar] [CrossRef]
- Rakhmatullayeva, D.; Ospanova, A.; Bekissanova, Z.; Jumagaziyeva, A.; Savdenbekova, B.; Seidulayeva, A.; Sailau, A. Development and characterization of antibacterial coatings on surgical sutures based on sodium carboxymethyl cellulose/chitosan/chlorhexidine. Int. J. Biol. Macromol. 2023, 236, 124024. [Google Scholar] [CrossRef]
- Grohmann, S.; Menne, M.; Hesse, D.; Bischoff, S.; Schiffner, R.; Diefenbeck, M.; Liefeith, K. Biomimetic multilayer coatings deliver gentamicin and reduce implant-related osteomyelitis in rats. Biomed. Tech. 2019, 64, 383–395. [Google Scholar] [CrossRef]
- Salta, M.; Dennington, S.P.; Wharton, J.A. Biofilm Inhibition by Novel Natural Product- and Biocide-Containing Coatings Using High-Throughput Screening. Int. J. Mol. Sci. 2018, 19, 1434. [Google Scholar] [CrossRef]
- Li, M.; Wang, M.; Hu, S.; Sun, J.; Zhu, M.; Ni, Y.; Wang, J. Advanced Coatings with Antioxidant and Antibacterial Activity for Kumquat Preservation. Foods 2022, 11, 2363. [Google Scholar] [CrossRef]
- Samuel, M.S.; Moghaddam, S.T.; Shang, M.; Niu, J. A Flexible Anti-Biofilm Hygiene Coating for Water Devices. ACS Appl. Bio Mater. 2022, 5, 3991–3998. [Google Scholar] [CrossRef]
- de Brito, F.A.E.; de Freitas, A.P.P.; Nascimento, M.S. Multidrug-Resistant Biofilms (MDR): Main Mechanisms of Tolerance and Resistance in the Food Supply Chain. Pathogens 2022, 11, 1416. [Google Scholar] [CrossRef]
- Ejaz, H.; Junaid, K.; Yasmeen, H.; Naseer, A.; Alam, H.; Younas, S.; Qamar, M.U.; Abdalla, A.E.; Abosalif, K.O.A.; Ahmad, N.; et al. Multiple Antimicrobial Resistance and Heavy Metal Tolerance of Biofilm-Producing Bacteria Isolated from Dairy and Non-Dairy Food Products. Foods 2022, 11, 2728. [Google Scholar] [CrossRef]
- Dimitrakellis, P.; Kaprou, G.D.; Papavieros, G.; Mastellos, D.C.; Constantoudis, V.; Tserepi, A.; Gogolides, E. Enhanced antibacterial activity of ZnO-PMMA nanocomposites by selective plasma etching in atmospheric pressure. Micro Nano Eng. 2021, 13, 100098. [Google Scholar] [CrossRef]
- des Ligneris, E.; Dumée, L.F.; Al-Attabi, R.; Castanet, E.; Schütz, J.; Kong, L. Mixed Matrix Poly(Vinyl Alcohol)-Copper Nanofibrous Anti-Microbial Air-Microfilters. Membranes 2019, 9, 87. [Google Scholar] [CrossRef]
- Isopencu, G.; Mocanu, A. Recent Advances in Antibacterial Composite Coatings. Coatings 2022, 12, 1504. [Google Scholar] [CrossRef]
- Rashki, S.; Asgarpour, K.; Tarrahimofrad, H.; Hashemipour, M.; Ebrahimi, M.S.; Fathizadeh, H.; Khorshidi, A.; Khan, H.; Marzhoseyni, Z.; Salavati-Niasari, M.; et al. Chitosan-based nanoparticles against bacterial infections. Carbohydr. Polym. 2021, 251, 117108. [Google Scholar] [CrossRef]
- Kopiasz, R.J.; Tomaszewski, W.; Kuźmińska, A.; Chreptowicz, K.; Mierzejewska, J.; Ciach, T.; Jańczewski, D. Hydrophilic Quaternary Ammonium Ionenes-Is There an Influence of Backbone Flexibility and Topology on Antibacterial Properties? Macromol. Biosci. 2020, 20, e2000063. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; You, W.; Wang, H.L.; Zhang, Z.; Nie, X.; Wang, F.; You, Y.Z. Cyclic topology enhances the killing activity of polycations against planktonic and biofilm bacteria. J. Mater. Chem. B 2022, 10, 4823–4831. [Google Scholar] [CrossRef] [PubMed]
- Pigareva, V.A.; Senchikhin, I.N.; Bolshakova, A.V.; Sybachin, A.V. Modification of Polydiallyldimethylammonium Chloride with Sodium Polystyrenesulfonate Dramatically Changes the Resistance of Polymer-Based Coatings towards Wash-Off from Both Hydrophilic and Hydrophobic Surfaces. Polymers 2022, 14, 1247. [Google Scholar] [CrossRef] [PubMed]
- Misin, V.M.; Zezin, A.A.; Klimov, D.I.; Sybachin, A.V.; Yaroslavov, A.A. Biocidal Polymer Formulations and Coatings. Polym. Sci. Ser. B 2021, 63, 459–469. [Google Scholar] [CrossRef]
- Bhattacharjee, B.; Jolly, L.; Mukherjee, R.; Haldar, J. An easy-to-use antimicrobial hydrogel effectively kills bacteria, fungi, and influenza virus. Biomater. Sci. 2022, 10, 2014–2028. [Google Scholar] [CrossRef]
- Pillai, S.K.R.; Reghu, S.; Vikhe, Y.; Zheng, H.; Koh, C.H.; Chan-Park, M.B. Novel Antimicrobial Coating on Silicone Contact Lens Using Glycidyl Methacrylate and Polyethyleneimine Based Polymers. Macromol. Rapid Commun. 2020, 41, e2000175. [Google Scholar] [CrossRef]
- Mkrtchyan, K.V.; Pigareva, V.A.; Zezina, E.A.; Kuznetsova, O.A.; Semenova, A.A.; Yushina, Y.K.; Tolordava, E.R.; Grudistova, M.A.; Sybachin, A.V.; Klimov, D.I.; et al. Preparation of Biocidal Nanocomposites in X-ray Irradiated Interpolyelectolyte Complexes of Polyacrylic Acid and Polyethylenimine with Ag-Ions. Polymers 2022, 14, 4417. [Google Scholar] [CrossRef]
- Hoque, J.; Akkapeddi, P.; Ghosh, C.; Uppu, D.S.; Haldar, J. A Biodegradable Polycationic Paint that Kills Bacteria in Vitro and in Vivo. ACS Appl. Mater. Interfaces 2016, 8, 29298–29309. [Google Scholar] [CrossRef]
- Jain, A.; Duvvuri, L.S.; Farah, S.; Beyth, N.; Domb, A.J.; Khan, W. Antimicrobial polymers. Adv. Healthc. Mater. 2014, 3, 1969–1985. [Google Scholar] [CrossRef]
- Neuber, S.; Sill, A.; Efthimiopoulos, I.; Nestler, P.; Fricke, K.; Helm, C.A. Influence of molecular weight of polycation polydimethyldiallylammonium and carbon nanotube content on electric conductivity of layer-by-layer films. Thin Solid Films 2022, 745, 139103. [Google Scholar] [CrossRef]
- Rougier, V.; Cellier, J.; Duchemin, B.; Gomina, M.; Bréard, J. Influence of the molecular weight and physical properties of a thermoplastic polymer on its dynamic wetting behavior. Chem. Eng. Sci. 2023, 269, 118442. [Google Scholar] [CrossRef]
- Assem, Y.; Chaffey-Millar, H.; Barner-Kowollik, C.; Wegner, G.; Agarwal, S. Controlled/Living Ring-Closing Cyclopolymerization of Diallyldimethylammonium Chloride via the Reversible Addition Fragmentation Chain Transfer Process. Macromolecules 2007, 40, 3907–3913. [Google Scholar] [CrossRef]
- Sybachin, A.; Pigareva, V. Ensembles of carboxymethyl cyclodextrins on cationic liposomes as highly efficient nanocontainers for the delivery of hydrophobic compounds. Biochim. Biophys. Acta Gen. Subj. 2023, 1867, 130363. [Google Scholar] [CrossRef]
- Panova, T.V.; Efimova, A.A.; Berkovich, A.K.; Efimov, A.V. Plasticity control of poly(vinyl alcohol)–graphene oxide nanocomposites. RSC Adv. 2020, 10, 24027–24036. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Pearson, R.D.; Steigbigel, R.T.; Davis, H.T.; Chapman, S.W. Method for reliable determination of minimal lethal antibiotic concentrations. Antimicrob. Agents Chemother. 1980, 18, 699–708. [Google Scholar] [CrossRef]
- Faÿ, F.; Champion, M.; Guennec, A.; Moppert, X.; Simon-Colin, C.; Elie, M. Biobased Anti-Adhesive Marine Coatings from Polyhydroxyalkanoates and Polysaccharides. Coatings 2023, 13, 766. [Google Scholar] [CrossRef]
- Hamouda, R.A.; Qarabai, F.A.K.; Shahabuddin, F.S.; Al-Shaikh, T.M.; Makharita, R.R. Antibacterial Activity of Ulva/Nanocellulose and Ulva/Ag/Cellulose Nanocomposites and Both Blended with Fluoride against Bacteria Causing Dental Decay. Polymers 2023, 15, 1047. [Google Scholar] [CrossRef]
- Qiu, H.; Si, Z.; Luo, Y.; Feng, P.; Wu, X.; Hou, W.; Zhu, Y.; Chan-Park, M.B.; Xu, L.; Huang, D. The Mechanisms and the Applications of Antibacterial Polymers in Surface Modification on Medical Devices. Front. Bioeng. Biotechnol. 2020, 8, 910. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Y. Bacteria-Removing and Bactericidal Efficiencies of Pdadmac Composite Coagulants in Enhanced Coagulation Treatment. Clean-Soil Air Water 2013, 41, 37–42. [Google Scholar] [CrossRef]
- Kyzioł, A.; Khan, W.; Sebastian, V.; Kyzioł, K. Tackling microbial infections and increasing resistance involving formulations based on antimicrobial polymers. Chem. Eng. J. 2020, 385, 123888. [Google Scholar] [CrossRef]
- Melo, L.D.; Mamizuka, E.M.; Carmona-Ribeiro, A.M. Antimicrobial particles from cationic lipid and polyelectrolytes. Langmuir 2010, 26, 12300–12306. [Google Scholar] [CrossRef] [PubMed]
- Giano, M.C.; Ibrahim, Z.; Medina, S.H.; Sarhane, K.A.; Christensen, J.M.; Yamada, Y.; Brandacher, G.; Schneider, J.P. Injectable bioadhesive hydrogels with innate antibacterial properties. Nat. Commun. 2014, 5, 4095. [Google Scholar] [CrossRef] [PubMed]
- Helander, I.M.; Alakomi, H.L.; Latva-Kala, K.; Koski, P. Polyethyleneimine is an effective permeabilizer of gram-negative bacteria. Microbiology 1997, 143, 3193–3199. [Google Scholar] [CrossRef]
- Pigareva, V.; Stepanova, D.; Bolshakova, A.; Marina, V.; Osterman, I.; Sybachin, A. Hyperbranched Kaustamin as an antibacterial for surface treatment. Mendeleev Commun. 2022, 32, 561–563. [Google Scholar] [CrossRef]
- Sorzabal-Bellido, I.; Diaz-Fernandez, Y.A.; Susarrey-Arce, A.; Skelton, A.A.; McBride, F.; Beckett, A.J.; Prior, I.A.; Raval, R. Exploiting Covalent, H-Bonding, and π-π Interactions to Design Antibacterial PDMS Interfaces That Load and Release Salicylic Acid. ACS Appl. Bio Mater. 2019, 2, 4801–4811. [Google Scholar] [CrossRef]
- Dos Santos, R.L.O.; Sarra, G.; Lincopan, N.; Petri, D.F.S.; Aliaga, J.; Marques, M.M.; Dias, R.B.; Coto, N.P.; Sugaya, N.N.; Paula, C.R. Preparation, Antimicrobial Properties, and Cytotoxicity of Acrylic Resins Containing Poly(diallyldimethylammonium chloride). Int. J. Prosthodont. 2021, 34, 635–641. [Google Scholar] [CrossRef]
- Tran, P.L.; Huynh, E.; Hamood, A.N.; de Souza, A.; Schultz, G.; Liesenfeld, B.; Mehta, D.; Webster, D.; Reid, T.W. The ability of quaternary ammonium groups attached to a urethane bandage to inhibit bacterial attachment and biofilm formation in a mouse wound model. Int. Wound J. 2017, 14, 79–84. [Google Scholar] [CrossRef]
- Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=177&showFR=1 (accessed on 18 April 2023).
- Cahit, A.; Yildirim, M. Molecular weight dependent antistaphylococcal activities of oligomers/polymers synthesized from 3-aminopyridi. J. Serb. Chem. Soc. 2010, 75, 1203–1208. [Google Scholar]
- Ikeda, T.; Hirayama, H.; Yamaguchi, H.; Tazuke, S.; Watanabe, M. Polycationic biocides with pendant active groups: Molecular weight dependence of antibacterial activity. Antimicrob. Agents Chemother. 1986, 30, 132–136. [Google Scholar] [CrossRef]
- Hae Cho, C.A.; Liang, C.; Perera, J.; Liu, J.; Varnava, K.G.; Sarojini, V.; Cooney, R.P.; McGillivray, D.J.; Brimble, M.A.; Swift, S.; et al. Molecular Weight and Charge Density Effects of Guanidinylated Biodegradable Polycarbonates on Antimicrobial Activity and Selectivity. Biomacromolecules 2018, 19, 1389–1401. [Google Scholar] [CrossRef]

| Polycation | Diffusion Coefficient, cm2/s * | Pw |
|---|---|---|
| PDADMAC-100 | 4.2 × 10−7 | 620 |
| PDADMAC-300 | 3.0 × 10−8 | 1860 |
| PDADMAC-500 | 8.0 × 10−8 | 3100 |
| PEI-1.3 | 2.5 × 10−5 | 30 |
| PEI-2 | 6.0 × 10−7 | 50 |
| PEI-25 | 2.5 × 10−7 | 580 |
| PEI-40 | 1.3 × 10−7 | 930 |
| PEI-70 | 5.8 × 10−8 | 1630 |
| PEI-750 | N/A 1 | 17,440 |
| Polycation | MIC (μg mL−1) |
|---|---|
| PDADMAC-100 | 0.025 |
| PDADMAC-200 | 0.025 |
| PDADMAC-500 | 0.025 |
| PEI-1.3 | 0.2 |
| PEI-2 | 0.2 |
| PEI-25 | 0.05 |
| PEI-70 | 0.0125 |
| PEI-750 | 0.025 |
| Polycation | Stress, MPa |
|---|---|
| PDADMAC-100 | 26,600 |
| PDADMAC-300 | 30,900 |
| PDADMAC-500 | 31,500 |
| PEI-1.3 | 11,600 |
| PEI-70 | 18,000 |
| PEI-750 | 19,000 |
| Sample | CFU/mL |
|---|---|
| Control | 5.0 × 107 |
| PEI-750 | 0 |
| PDADMAC-500 | 0 |
| Polymers | L. monocytogenes | |
|---|---|---|
| MIC, mg/mL | MBC, mg/mL | |
| PEI-750 | 5 | 5 |
| PDADMAC-500 | 2.5 | 5 |
| Sample | Control | PEI-750 | PDADMAC-500 |
|---|---|---|---|
| CFU/mL | 4.0 × 107 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pigareva, V.A.; Marina, V.I.; Bolshakova, A.V.; Berkovich, A.K.; Kuznetsova, O.A.; Semenova, A.A.; Yushina, Y.K.; Bataeva, D.S.; Grudistova, M.A.; Sybachin, A.V. Biocidal Coatings against Gram-Positive Bacteria from Linear and Branched Polycations: The Decisive Role of the Diffusion Coefficients of Macromolecules. Coatings 2023, 13, 1076. https://doi.org/10.3390/coatings13061076
Pigareva VA, Marina VI, Bolshakova AV, Berkovich AK, Kuznetsova OA, Semenova AA, Yushina YK, Bataeva DS, Grudistova MA, Sybachin AV. Biocidal Coatings against Gram-Positive Bacteria from Linear and Branched Polycations: The Decisive Role of the Diffusion Coefficients of Macromolecules. Coatings. 2023; 13(6):1076. https://doi.org/10.3390/coatings13061076
Chicago/Turabian StylePigareva, Vladislava A., Valeria I. Marina, Anastasia V. Bolshakova, Anna K. Berkovich, Oksana A. Kuznetsova, Anastasia A. Semenova, Yulia K. Yushina, Dagmara S. Bataeva, Maria A. Grudistova, and Andrey V. Sybachin. 2023. "Biocidal Coatings against Gram-Positive Bacteria from Linear and Branched Polycations: The Decisive Role of the Diffusion Coefficients of Macromolecules" Coatings 13, no. 6: 1076. https://doi.org/10.3390/coatings13061076
APA StylePigareva, V. A., Marina, V. I., Bolshakova, A. V., Berkovich, A. K., Kuznetsova, O. A., Semenova, A. A., Yushina, Y. K., Bataeva, D. S., Grudistova, M. A., & Sybachin, A. V. (2023). Biocidal Coatings against Gram-Positive Bacteria from Linear and Branched Polycations: The Decisive Role of the Diffusion Coefficients of Macromolecules. Coatings, 13(6), 1076. https://doi.org/10.3390/coatings13061076







