Ultra-Fast Heating Process of Cu-Pd Bimetallic Nanoparticles Unraveled by Molecular Dynamics Simulation
Abstract
1. Introduction
2. Materials and Methods
3. Result and Discussion
3.1. Macroscopic Sintering Process
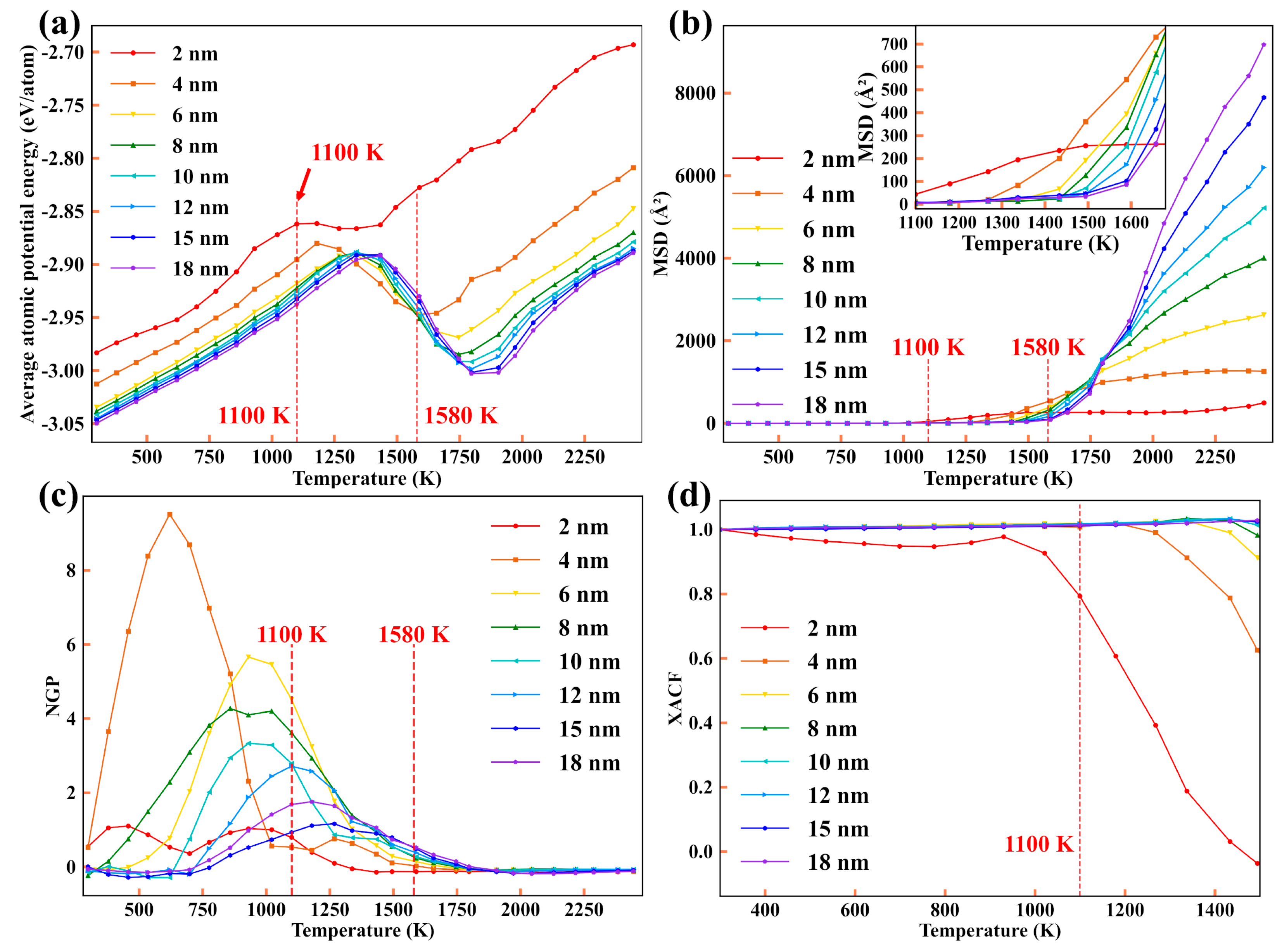
3.1.1. The Potential Energy Evolution and Structure Evolution
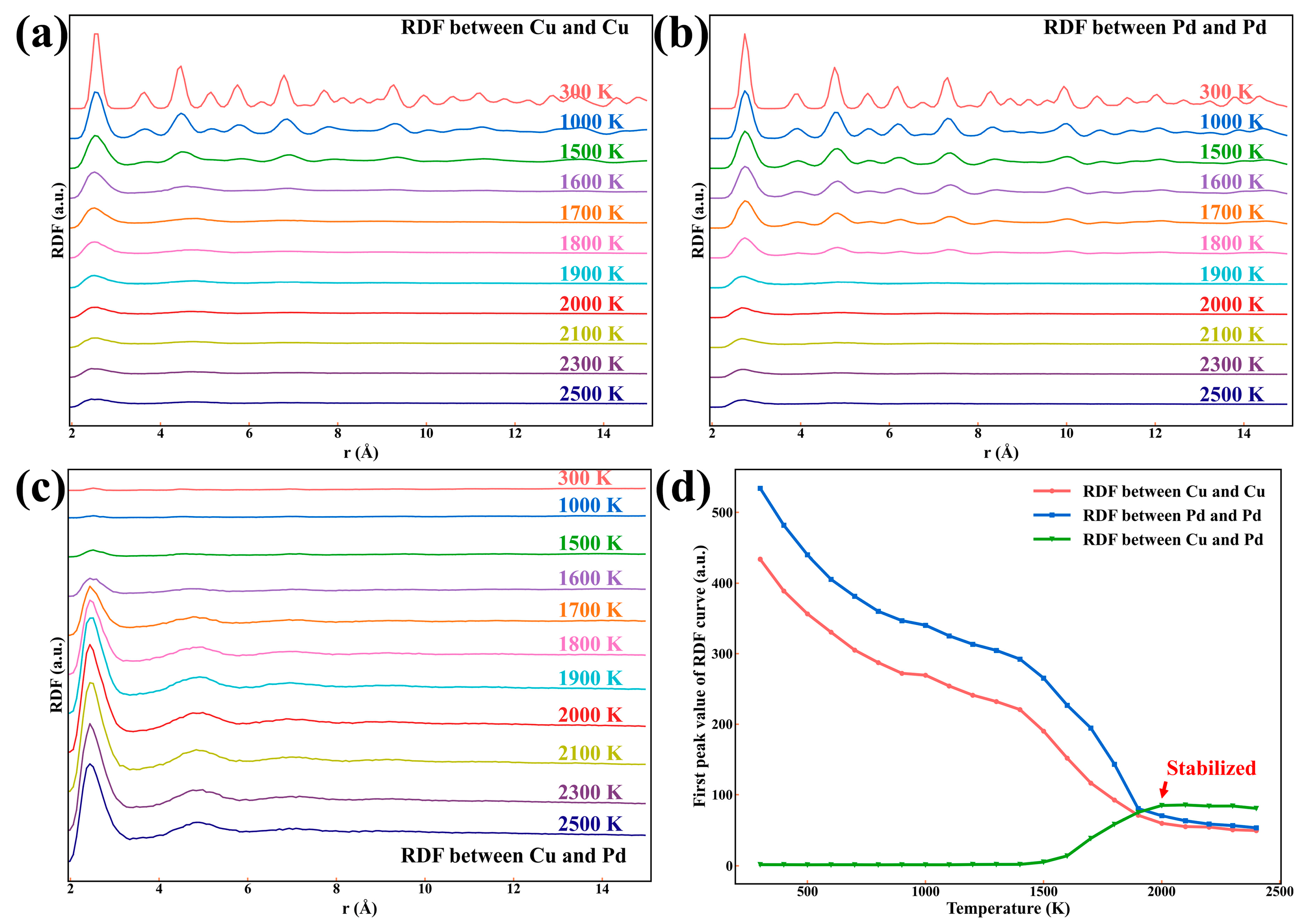
3.1.2. The Average Crystal Structure Evolution
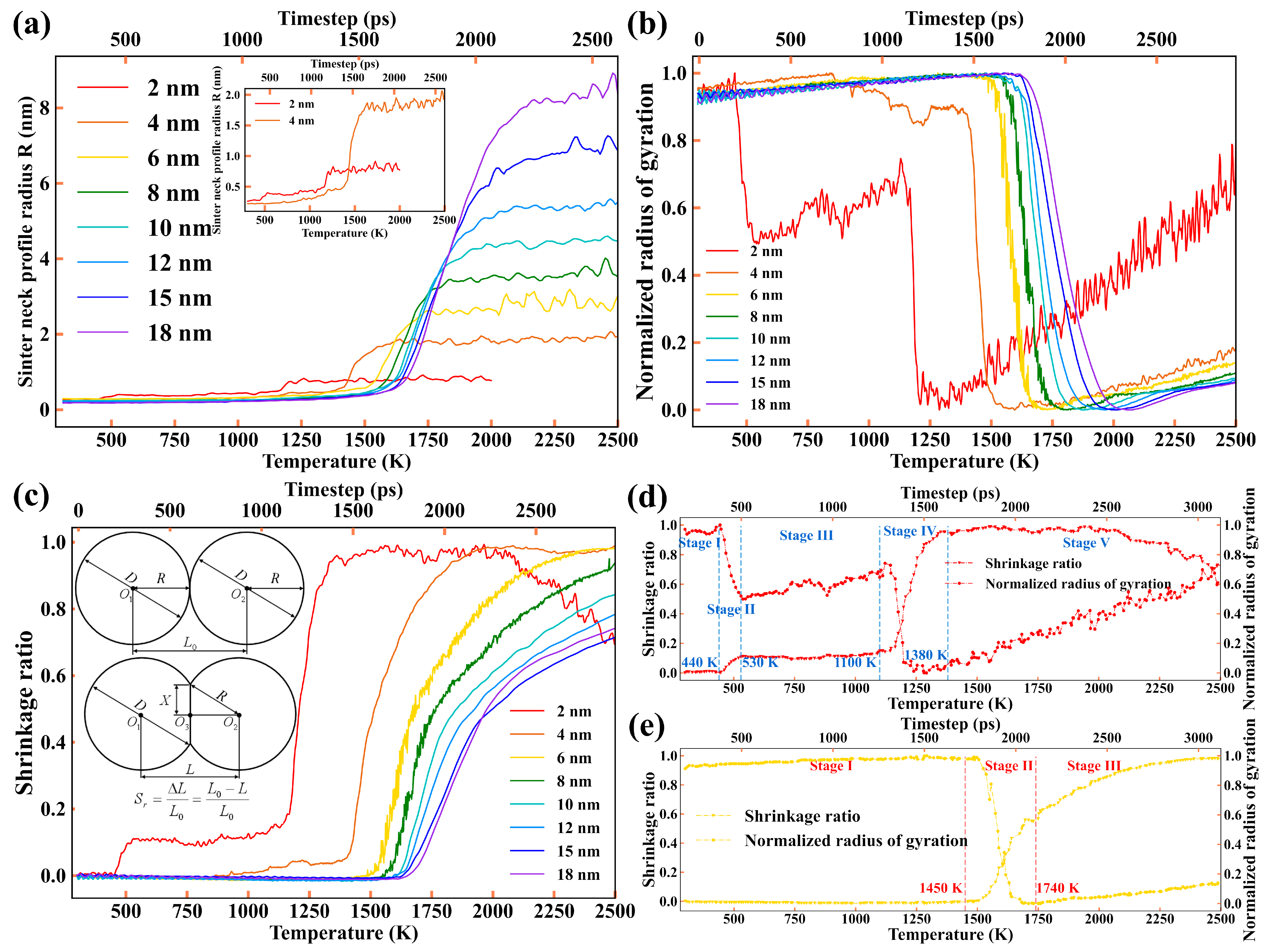
3.1.3. Sintering Neck Radius and Shrinkage Ratio
3.2. Sintering Mechanism
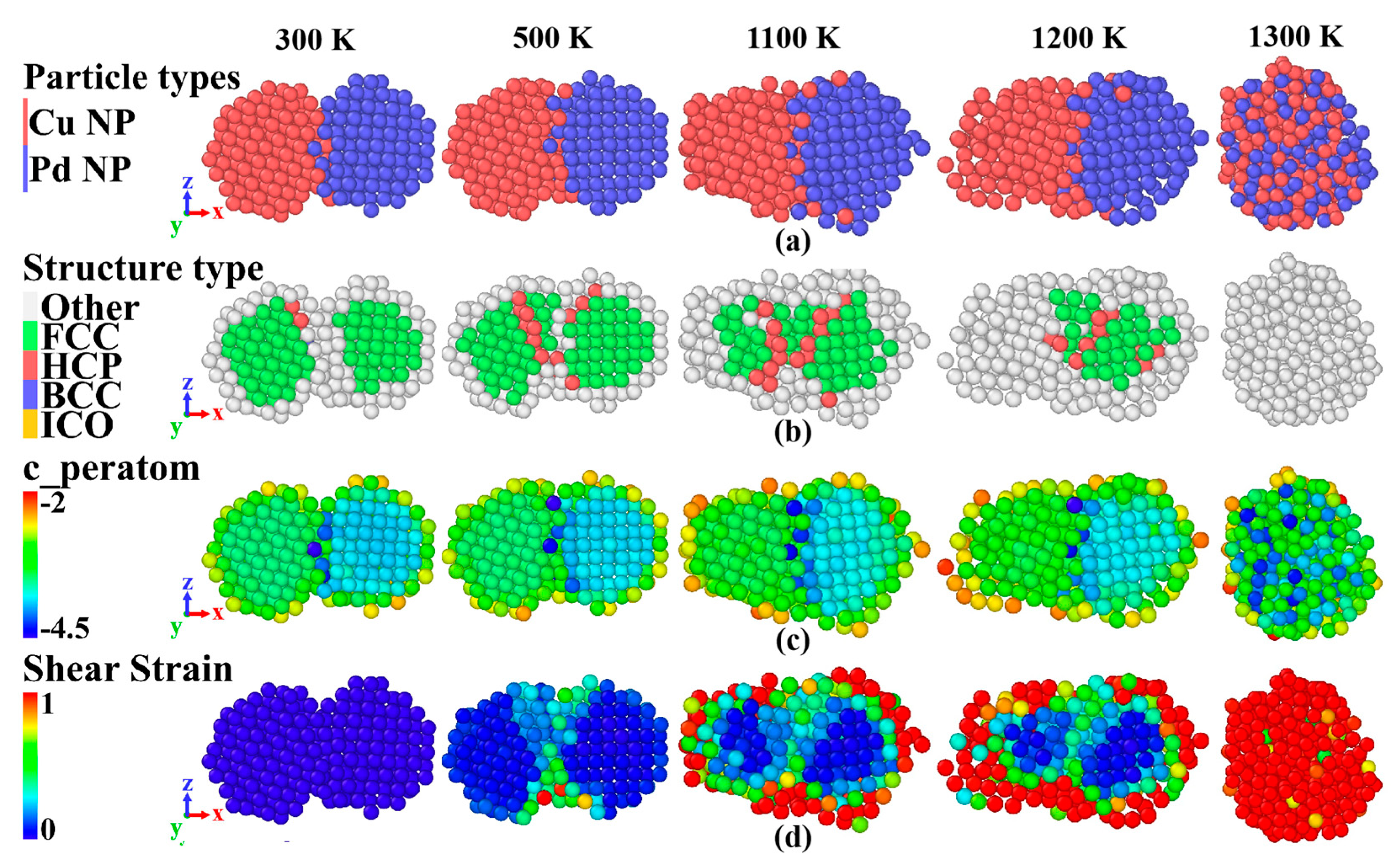
3.2.1. Atomic Characterization
3.2.2. The Shell Atomic Energy and Motion Pattern
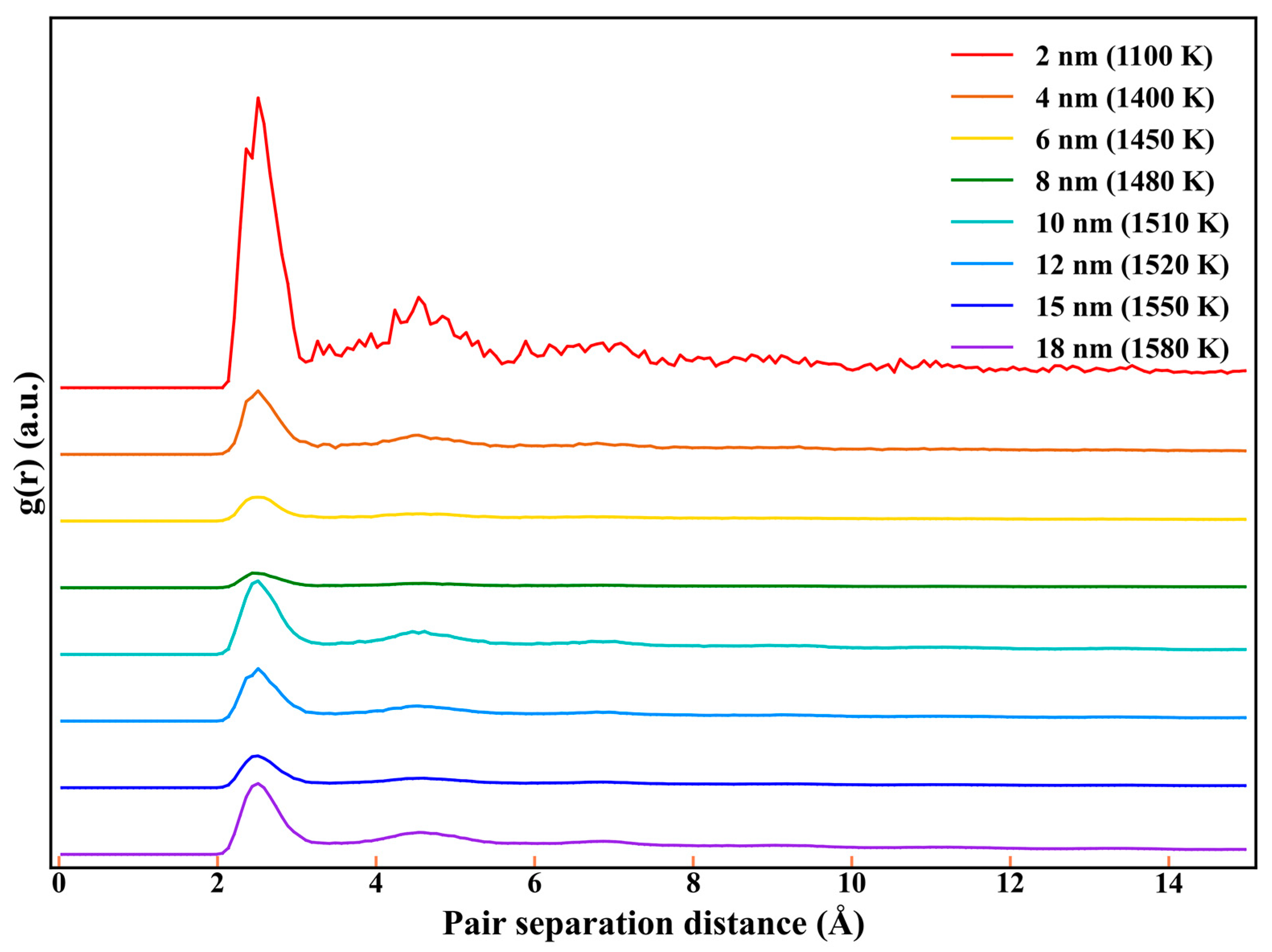
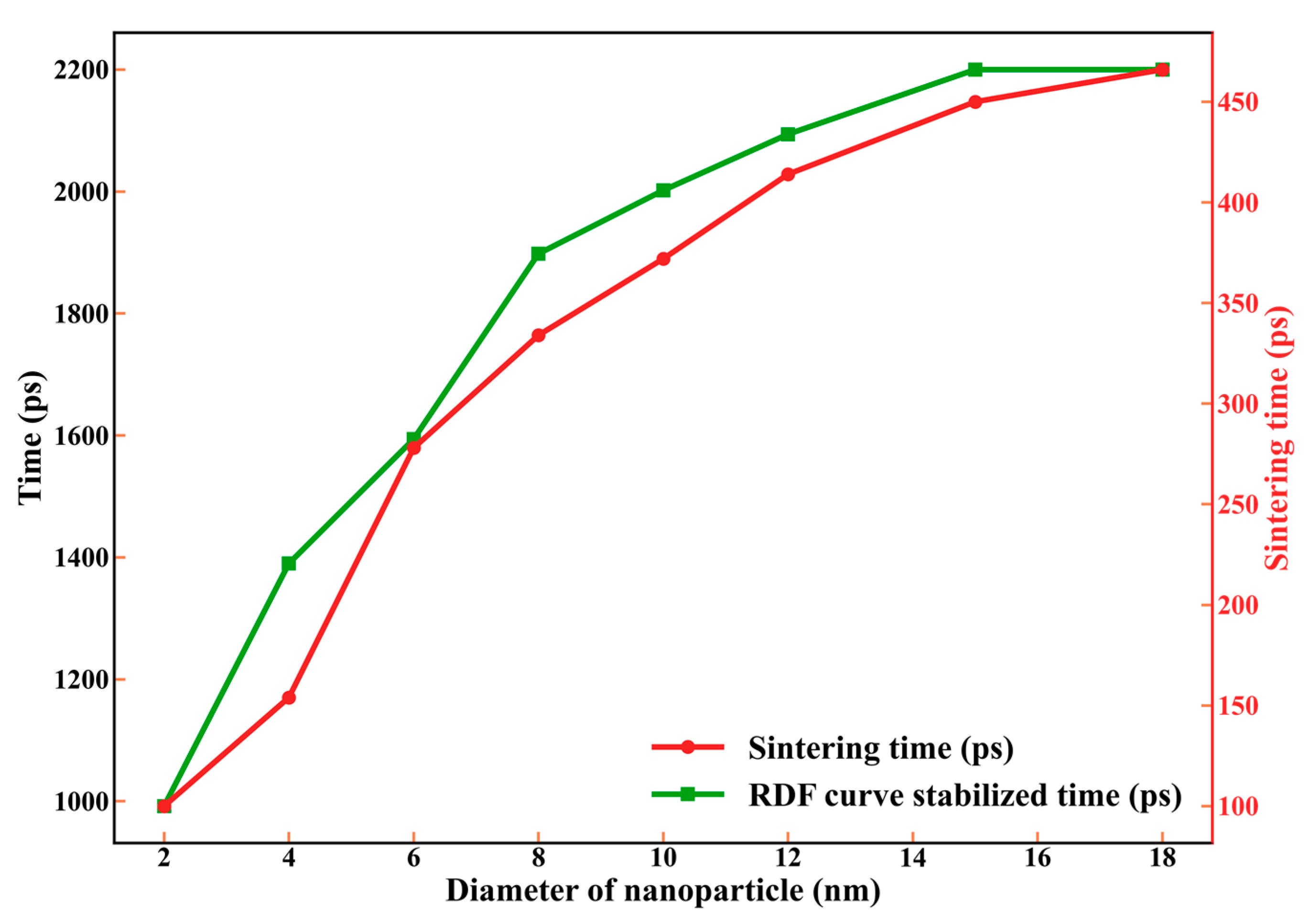
3.2.3. The Size Dependence of Sintering Time
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tobias, K.; Laurent, M.; Heinz, S.; Walter, R.; Nicholas, D.S.; Heiko, W. Nanoparticle printing with single-particle resolution. Nat. Nanotechnol. 2007, 2, 9. [Google Scholar]
- Jingyu, W.; Tao, X.; Jiahui, G.; Xing, Z.; Yong, Y. A nanogenerator based on metal nanoparticles and magnetic ionic gradients. NPG Asia Mater. 2023, 15, 1. [Google Scholar]
- Lichen, L.; Avelino, C. Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles. Chem. Rev. 2018, 118, 10. [Google Scholar]
- Debroy, T.; Mukherjee, T.; Wei, H.; Elmer, J.W.; Milewski, J.O. Metallurgy, mechanistic models and machine learning in metal printing. Nat. Rev. Mater. 2020, 6, 1. [Google Scholar] [CrossRef]
- Baptiste, G.; Ann, C.; Oana, C.M.; Patrick, S.; Renelle, D.; Christoph, F.; Surendra, K.M.; Tong, L.; Michael, M.; Julie, M.C. Atom probe tomography. Rev. Sci. Instrum. 2007, 78, 31101. [Google Scholar]
- Parul, C.; Lukman, A.; Anuj, C.; Govind, K.; Wen-Juan, C.; Shaohua, C. Nanoparticle-mediated bioremediation as a powerful weapon in the removal of environmental pollutants. J. Environ. Chem. Eng. 2023, 11, 109591. [Google Scholar]
- Jonas, G.C.; Kimberly, S.B.; Jeffrey, I.Z.; Brinker, C.J. Synthetic amorphous silica nanoparticles: Toxicity, biomedical and environmental implications. Nat. Rev. Mater. 2020, 5, 886–909. [Google Scholar]
- Wei, D.; Bassem, K.; Hong, G.; Hong, L. Roles of nanoparticles in oil lubrication. Tribol. Int. 2016, 102, 88–98. [Google Scholar]
- Tarasov, S.; Kolubaev, A.; Belyaev, S.; Lerner, M.; Tepper, F. Study of friction reduction by nanocopper additives to motor oil. Wear 2002, 252, 63–69. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Tsui, W.C.; Liu, T.C. Experimental analysis of tribological properties of lubricating oils with nanoparticle additives. Wear 2007, 262, 819–825. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, C.; Hwang, Y.; Park, M.; Lee, J.; Choi, C.; Jung, M. Tribological behavior of copper nanoparticles as additives in oil. Curr. Appl. Phys. 2009, 9, e124–e127. [Google Scholar] [CrossRef]
- Chengzhi, H.; Minli, B.; Jizu, L.; Hao, L.; Xiaojie, L. Molecular dynamics investigation of the effect of copper nanoparticle on the solid contact between friction surfaces. Appl. Surf. Sci. 2014, 321, 302–309. [Google Scholar]
- Samuel, B.; Leanne, M.; Jingyi, C.; Robert, A.F.; Min, Z. The effects of polydopamine coated Cu nanoparticles on the tribological properties of polydopamine/PTFE coatings. Tribol. Int. 2016, 103, 87–94. [Google Scholar]
- Guangyuan, L.; Yifei, P.; Zhilei, D.; Ding-Bang, X. Tribology behavior of high-content graphene/nanograined Cu bulk composites from core/shell nanoparticles. Compos. Commun. 2021, 25, 100777. [Google Scholar]
- Tan, H.; Guo, Y.; Wang, D.; Cui, Y. The development of a Cu@Graphite solid lubricant with excellent anti-friction and wear resistant performances in dry condition. Wear 2022, 488, 204181. [Google Scholar] [CrossRef]
- Sreenivasa, R.P.; Yong, K.W.; Satyanarayana, N.; Sinha, S.K.; Srinivasan, M.P. Tribological properties of nanoparticle-laden ultrathin films formed by covalent molecular assembly. Langmuir 2007, 23, 8299–8303. [Google Scholar]
- Baker, C.C.; Hu, J.J.; Voevodin, A.A. Preparation of Al2O3/DLC/Au/MoS2 chameleon coatings for space and ambient environments. Surf. Coat. Technol. 2008, 201, 4224–4229. [Google Scholar] [CrossRef]
- Reza, S.; Mohsen, A.; Bandar, A.; Roya, K. New nanocomposites containing metal nanoparticles, carbon nanotube and polymer. J. Nanopart. Res. 2008, 10, 1309–1318. [Google Scholar]
- Subramonian, B.; Kato, K.; Adachi, K.; Basu, B. Experimental evaluation of friction and wear properties of solid lubricant coatings on SUS440C steel in liquid nitrogen. Tribol. Int. 2005, 20, 263–272. [Google Scholar] [CrossRef]
- Hannel, S.; Fouvry, S.; Kapsa, P.; Vincent, L. The fretting sliding transition as a criterion for electrical contact performance. Wear 2001, 249, 761–770. [Google Scholar] [CrossRef]
- Chanaka, K.; Donovan, N.L.; Harry, M.M.; Huimin, L.; Beth L., A.; Jun, Q. Palladium Nanoparticle-Enabled Ultrathick Tribofilm with Unique Composition. ACS Appl. Mater. Interfaces 2018, 10, 31804–31812. [Google Scholar]
- Jianyong, O.; Yang, Y. Polymer: Metal nanoparticle devices with electrode-sensitive bipolar resistive switchings and their application as nonvolatile memory devices. Appl. Phys. Lett. 2010, 96, 063506. [Google Scholar]
- Wu, Z.P.; Shan, S.Y.; Xie, Z.H.; Kang, N.; Park, K.; Hopkins, E.; Yan, S.; Sharma, A.; Luo, J.; Wang, J. Revealing the Role of Phase Structures of Bimetallic Nanocatalysts in the Oxygen Reduction Reaction. ACS Catal. 2018, 8, 11302–11313. [Google Scholar] [CrossRef]
- Wang, Y.B.; Tang, W.K.; Wang, F.; Ding, C.P.; Xu, S.M.; Yu, R.H. Effects of surface coating with Cu-Pd on electrochemical properties of A2B7- type hydrogen storage alloy. Int. J. Hydrog. Energy 2018, 43, 3244–3252. [Google Scholar] [CrossRef]
- Simchi, A. Effects of lubrication procedure on the consolidation, sintering and microstructural features of powder compacts. Mater. Des. 2003, 24, 585–594. [Google Scholar] [CrossRef]
- Brahma, N.; Talbot, J. Effects of chemical mechanical planarization slurry additives on the agglomeration of alumina nanoparticles II: Aggregation rate analysis. J. Colloid Interface Sci. 2014, 419, 25–30. [Google Scholar] [CrossRef]
- Suvankar, G.; Suman, C. Tribological properties of nanoparticle-laden ultrathin films formed by covalent molecular assembly. Phys. Lett. 2011, 375, 2394–2399. [Google Scholar]
- Sajjadnejad, M.; Haghshenas, S.M.S.; Badr, P.; Setoudeh, N.; Hosseinpour, S. Wear and tribological characterization of nickel matrix electrodeposited composites: A review. Wear 2021, 486, 204098. [Google Scholar] [CrossRef]
- Popov, A.A.; Shubin, Y.V.; Plyusnin, P.E.; Sharafutdinov, M.R.; Korenev, S.V. Experimental redetermination of the Cu-Pd phase diagram. J. Alloys Compd. 2019, 777, 204–212. [Google Scholar] [CrossRef]
- Yingying, J.; Martial, D.; Shi, J.A.; Hongwei, Y.; Teck, L.T.; Utkur, M. Dynamics of the fcc-to-bcc phase transition in single-crystalline PdCu alloy nanoparticles. Nat. Commun. 2023, 14, 104. [Google Scholar]
- Kateryna, L.; Marc, H.; Matthias, E. Synthesis, Structure, Properties, and Applications of Bimetallic Nanoparticles of Noble Metals. Adv. Funct. Mater. 2020, 30, 1–14. [Google Scholar]
- Panagiotis, G.; Mukhles, S.; Joseph, K. Computational Modeling of Nanoparticle Coalescence. Adv. Theor. Simul. 2019, 2, 1900013. [Google Scholar]
- Junhua, Z.; Guangbin, Y.; Chunli, Z.; Yujuan, Z.; Shengmao, Z.; Pingyu, Z. Synthesis of water-soluble Cu nanoparticles and evaluation of their tribological properties and thermal conductivity as a water-based additive. Friction 2019, 7, 246–259. [Google Scholar]
- Huabing, Y.; Xuecheng, Z.; Zhiwei, G.; Yicong, X.; Xiang, R.; Chengqing, Y. Synergetic effects of surface textures with modified copper nanoparticles lubricant additives on the tribological properties of cylinder liner-piston ring. Tribol. Int. 2023, 178, 108085. [Google Scholar]
- Sánchez-López, J.C.; Abad, M.D.; Kolodziejczyk, L.; Guerrero, E.; Fernández, A. Surface-modified Pd and Au nanoparticles for anti-wear applications. Tribol. Int. 2011, 44, 720–726. [Google Scholar] [CrossRef]
- Abad, M.D.; Sánchez-López, J.C. Tribological properties of surface-modified Pd nanoparticles for electrical contacts. Wear 2013, 297, 943–951. [Google Scholar] [CrossRef]
- Kart, H.H.; Yildirim, H.; Kart, S.O.; Çağin, T. Physical properties of Cu nanoparticles: A molecular dynamics study. Mater. Chem. Phys. 2014, 147, 204–212. [Google Scholar] [CrossRef]
- Motlagh, M.B.; Kalteh, M. Molecular dynamics simulation of nanoflfluid convective heat transfer in a nanochannel: Effect of nanoparticles shape, aggregation and wall roughness. J. Mol. Liq. 2020, 318, 114028. [Google Scholar] [CrossRef]
- Maryam, A.; Movaffaq, K.; Pirooz, M. Determining phase transition using potential energy distribution and surface energy of Pd nanoparticles. Comp. Mater. Sci. 2019, 171, 109214. [Google Scholar]
- Felipe, J.V.; Benjamín, P.; Miguel, K.; Carlos, J.R.; Eduardo, M.B.; José, R. Nanoindentation of polycrystalline Pd hollow nanoparticles: Grain size role. Comp. Mater. Sci. 2020, 179, 109642. [Google Scholar]
- Kang, H.Y.; Wang, H.P. Growth of CuPd nanoalloys encapsulated in carbon-shell. J. Nanopart. Res. 2013, 15, 1672. [Google Scholar] [CrossRef]
- Caroline, D.; Jerome, D. Effect of the Composition on the Free Energy of Crystal Nucleation for CuPd Nanoalloys. J. Phys. Chem. A 2016, 120, 27657–27664. [Google Scholar]
- Fatih, A.C.; Ebru, T.K. Molecular dynamic investigation of the effect of atomic polyhedrons on crystallization mechanism for Cu-based Cu-Pd and Cu-Pt alloys. J. Mol. Liq. 2020, 314, 113636. [Google Scholar]
- Aidan, P.T.; Aktulga, H.M.; Richard, B.; Dan, S.B.; Brown, W.M.; Paul, S.C.; Pieter, J.V.; Axel, K.; Stan, G.M.; Trung, D.N.; et al. LAMMPS—A flexible simulation tool for particle-based materials modeling at the atomic, meso, and continuum scales. Comput. Phys. Commun. 2022, 271, 108171. [Google Scholar]
- Ga-Un, J.; Chang, S.P.; Hyeon-Seok, D.; Seul-Mi, P.; Byeong-Joo, L. Second nearest-neighbor modified embedded-atom method interatomic potentials for the Pd-M (M = Al, Co, Cu, Fe, Mo, Ni, Ti) binary systems. CALPHAD 2018, 62, 172–186. [Google Scholar]
- Panagiotis, G.; Cathal, C.; Vidyadhar, S.; Mukhles, S. Coalescence-induced crystallisation wave in Pd nanoparticles. Sci. Rep. 2014, 4, 5779. [Google Scholar]
- Denis, J.E.; Brad, L.H. The Nose-Hoover thermostat. J. Phys. Chem. A 1985, 83, 4069–4074. [Google Scholar]
- Berendsen, H.J.; Postma, J.V.; Van Gunsteren, W.F.; DiNola, A.R.H.J.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684. [Google Scholar] [CrossRef]
- William, C.S.; Hans, C.A.; Peter, H.B.; Kent, R.W. A computer simulation method for the calculation of equilibrium constants for the formation of physical clusters of molecules: Application to small water clusters. J. Chem. Phys. 1982, 76, 637–649. [Google Scholar]
- Andrew, R.L. Molecular Modelling: Principles and Applications, 2nd ed.; Pearson Education-Hall: London, UK, 2001. [Google Scholar]
- Helio, T.; Paulo, S.B.; José, P.R. Structural characterization of deformed crystals by analysis of common atomic neighborhood. Comp. Mater. Sci. 2007, 177, 518–523. [Google Scholar]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO-the Open Visualization Tool. Model. Simul. Mater. Sci. 2010, 18, 015012. [Google Scholar] [CrossRef]
- Huilong, Z.; Averback, R.S. Sintering processes of two nanoparticles: A study by molecular dynamics simulations. Philos. Mag. Lett. 1996, 73, 27–33. [Google Scholar]
- Jun, J.; Pengwan, C.; Jiali, Q.; Weifu, S.; Sergei, A.C.; Alexander, A.M.; Galina, B.M.; Tatiana, A.K. The effect of heating rate on the sintering of aluminum nanospheres. Phys. Chem. Chem. Phys. 2021, 23, 11684–11697. [Google Scholar]
- Qiang, J.; Guisheng, Z.; Hongqiang, Z.; Wengan, W.; Hui, R.; Zhanwen, A.; Zhongyang, D.; Shaohua, Y.; Daozhi, S.; Lei, L. Sintering mechanism of Ag-Pd nanoalloy film for power electronic packaging. Appl. Surf. Sci. 2021, 554, 149579. [Google Scholar]
- Hamed, A.; Hamzeh, Y.; Amir, N.S.; Farid, T. Effects of gas adsorption on the graphite-supported Ag nanoclusters: A molecular dynamics study. J. Phys. Chem. C 2013, 117, 26287–26294. [Google Scholar]
- Song, P.; Wen, D. Molecular dynamics simulation of the sintering of metallic nanoparticles. J. Nanoparticle Res. 2010, 12, 823–829. [Google Scholar] [CrossRef]
- Jun, J.; Pengwan, C.; Jiali, Q.; Weifu, S.; Sergei, A.C.; Alexander, A.M.; Galina, B.M.; Tatiana, A.K. Dynamic mechanical contact behaviors and sintering mechanism of Al nanoparticles subjected to high-speed impact. Mater. Chem. Phys. 2021, 273, 125111. [Google Scholar]
- Bai, X.M.; Li, M. Ring-diffusion mediated homogeneous melting in the superheating regime. Phys. Rev. B 2008, 77, 134109. [Google Scholar] [CrossRef]
- Surajit, S.; Soumya, C. Classical relaxation in a double well. Phys. A 1994, 209, 410–421. [Google Scholar]
- Walter, K.; Claudio, D.; Steven, J.P.; Peter, H.P.; Sharon, C.G. Dynamical heterogeneities in a supercooled Lennard-Jones liquid. Phys. Rev. Lett. 1997, 79, 2827–2830. [Google Scholar]
- Rahman, A. Correlations in the motion of atoms in liquid argon. Phys. Rev. 1964, 136, A405–A411. [Google Scholar] [CrossRef]
- Xue, F.; Deng, P.; Mo, L. Rethinking Lindemann criterion: A molecular dynamics simulation of surface mediated melting. Acta Mater. 2020, 193, 280–290. [Google Scholar]
- Watanabe, M.; Adachi, M.; Fukuyama, H. Correlation between excess volume and thermodynamic functions of liquid Pd-X (X = Fe, Cu and Ni) binary systems. J. Chem. Thermodyn. 2019, 130, 9–16. [Google Scholar] [CrossRef]
- Liang, T.S.; Zhou, D.J.; Wu, Z.H.; Shi, P.P. Size-dependent melting modes and behaviors of Ag nanoparticles: A molecular dynamics study. Nanotechnology 2017, 28, 485704. [Google Scholar] [CrossRef]
- Wu, R.; Zhao, X.; Liu, Y. Atomic insights of Cu nanoparticles melting and sintering behavior in Cu-Cu direct bonding. Mater. Des. 2021, 197, 109240. [Google Scholar] [CrossRef]
- Musazadeh, M.H.; Dehghani, K. The Effect of Crystallographic Orientation on Sintering Behavior of Ni Nanoparticles: A Molecular Dynamic Study. J. Comput. Theor. Nanosci. 2013, 10, 1497–1502. [Google Scholar] [CrossRef]
- Lange, A.P.; Samanta, A.; Majidi, H.; Mahajan, S.; Ging, J.; Olson, T.Y.; Benthem, V.K.; Elhadj, S. Dislocation mediated alignment during metal nanoparticle coalescence. Acta Mater. 2016, 120, 364–378. [Google Scholar] [CrossRef]
- Tavakol, M.; Mahnama, M.; Naghdabadi, R. Mechanisms Governing Microstructural Evolution during Consolidation of Nanoparticles. Mater. Manuf. Process. 2015, 30, 1397–1402. [Google Scholar] [CrossRef]
- Jun, J.; Pengwan, C.; Weifu, S. Monitoring micro-structural evolution during aluminum sintering and understanding the sintering mechanism of aluminum nanoparticles: A molecular dynamics study. J. Mater. Sci. Technol. 2020, 57, 92–100. [Google Scholar]
- Abedini, A.; Montazeri, A.; Malti, A.; Kardani, A. Mechanical properties are affected by coalescence mechanisms during sintering of metal powders: Case study of Al-Cu nanoparticles by molecular dynamics simulation. Powder Technol. 2022, 405, 117567. [Google Scholar] [CrossRef]
- Eleskandarany, M.S. Mechanical Alloying: Nanotechnology, Materials Science and Powder Metallurgy, 2nd ed.; Elsevier: Waltham, MA, USA, 2015. [Google Scholar]
- Yi, Z.; Jing, Z. Sintering phenomena and mechanical strength of nickel based materials in direct metal laser sintering process: A molecular dynamics study. J. Mater. Res. 2016, 31, 2233–2243. [Google Scholar]
- Yang, Y.; Zhang, H.; Douglas, J.F. Origin and nature of spontaneous shape fluctuations in “small” nanoparticles. ACS Nano 2014, 8, 7465–7477. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Deng, P.; Mo, L. Melting of bcc crystal Ta without the Lindemann criterion. J. Phys-Condens. Matter 2019, 31, 095402. [Google Scholar]
- Gao, M.C.; Ouyang, L.Z.; Dogan, O.N. First principles screening of B2 stabilizers in CuPd-based hydrogen separation membranes: (1) Substitution for Pd. J. Alloys Compd. 2013, 574, 368–376. [Google Scholar] [CrossRef]
- Ercolessi, F.; Andreoni, W.; Tosatti, E. Melting of small gold particles: Mechanism and size effects. Phys. Rev. Lett. 1991, 66, 911–914. [Google Scholar] [CrossRef]
- Pernilla, T.; Paul, E. Hydrogen-Driven Surface Segregation in Pd Alloys from Atomic Scale Simulations. J. Phys. Chem. C 2021, 125, 17248–17260. [Google Scholar]
- Gao, X.; Tang, J.L.; Zuo, Y.; Tang, Y.M.; Xiong, J.P. The electroplated palladium–copper alloy film on 316L stainless steel and its corrosion resistance in mixture of acetic and formic acids. Corros. Sci. 2009, 51, 1822–1827. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Guo, X.; Jia, H.; Li, G.; Fan, X.; Ding, S. Ultra-Fast Heating Process of Cu-Pd Bimetallic Nanoparticles Unraveled by Molecular Dynamics Simulation. Coatings 2023, 13, 1078. https://doi.org/10.3390/coatings13061078
Zhou Z, Guo X, Jia H, Li G, Fan X, Ding S. Ultra-Fast Heating Process of Cu-Pd Bimetallic Nanoparticles Unraveled by Molecular Dynamics Simulation. Coatings. 2023; 13(6):1078. https://doi.org/10.3390/coatings13061078
Chicago/Turabian StyleZhou, Zhukun, Xing Guo, Helin Jia, Guangxian Li, Xue Fan, and Songlin Ding. 2023. "Ultra-Fast Heating Process of Cu-Pd Bimetallic Nanoparticles Unraveled by Molecular Dynamics Simulation" Coatings 13, no. 6: 1078. https://doi.org/10.3390/coatings13061078
APA StyleZhou, Z., Guo, X., Jia, H., Li, G., Fan, X., & Ding, S. (2023). Ultra-Fast Heating Process of Cu-Pd Bimetallic Nanoparticles Unraveled by Molecular Dynamics Simulation. Coatings, 13(6), 1078. https://doi.org/10.3390/coatings13061078









