Application of Magnesium Hydroxide/Diphenoxy Phosphate in Silicone Rubber Flame Retardant Cable Material
Abstract
:1. Introduction
2. Materials
2.1. Analytical Methods and Test Standards
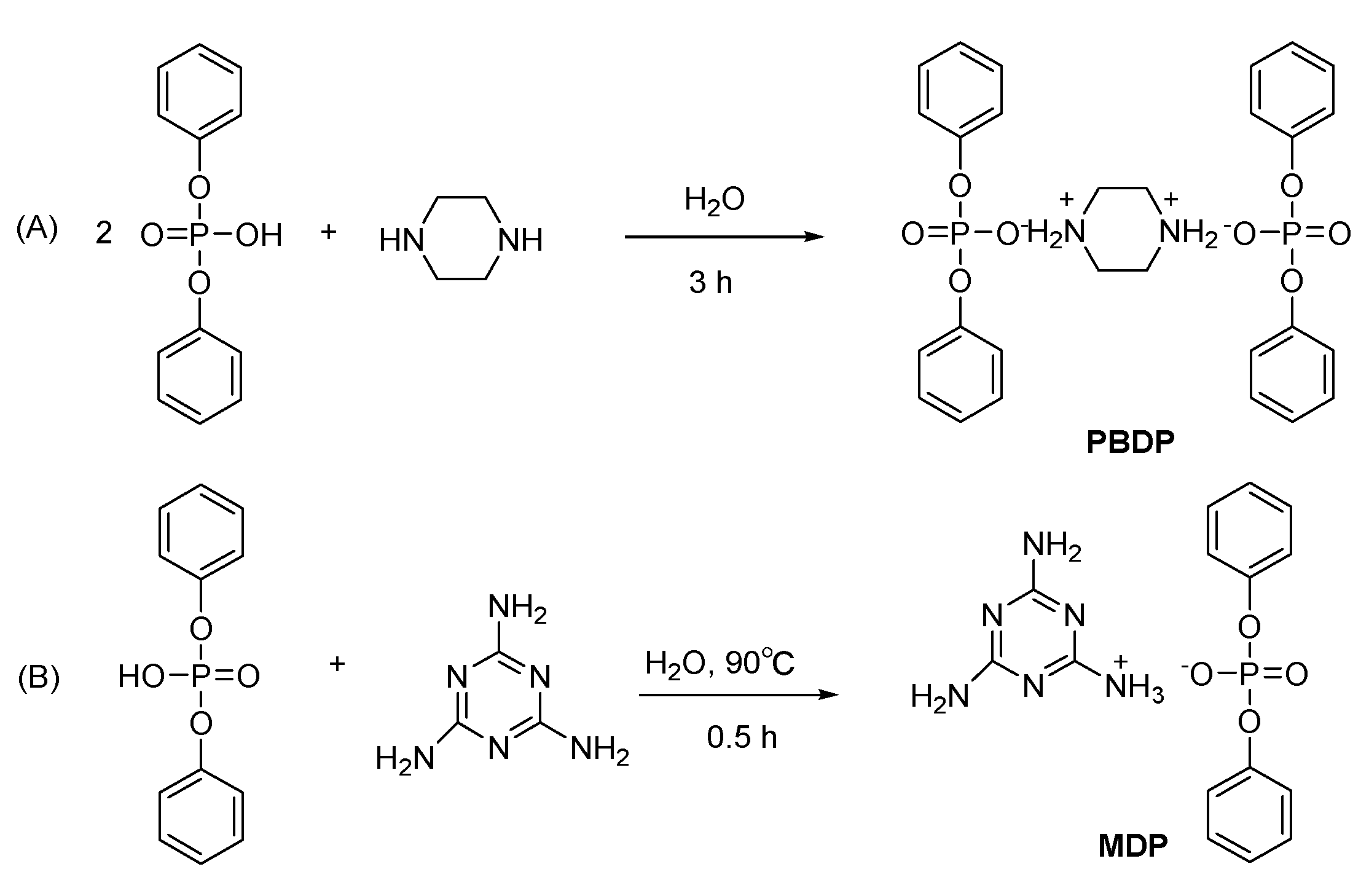
2.2. Synthesis of Piperazine bis (Diphenoxy Phosphate) Salt (PBDP) and Melamine Diphenoxy Phosphate (MDP)
2.3. Production Processes for Insulating Coatings
3. Results
3.1. Structural Characterization of Phosphorus–Based Flame Retardants PBDP and MDP
3.2. Effect of Different Amounts of Carbon Black on the Mechanical Properties of the Samples
3.3. Effects of Different Quantities of Carbon Black on the Surface Drying Time and Deep Curing Performance of the Coating
3.4. Effects of Different Flame Retardants on the Flame–Retardant Properties of Room–Temperature Vulcanized Rubber
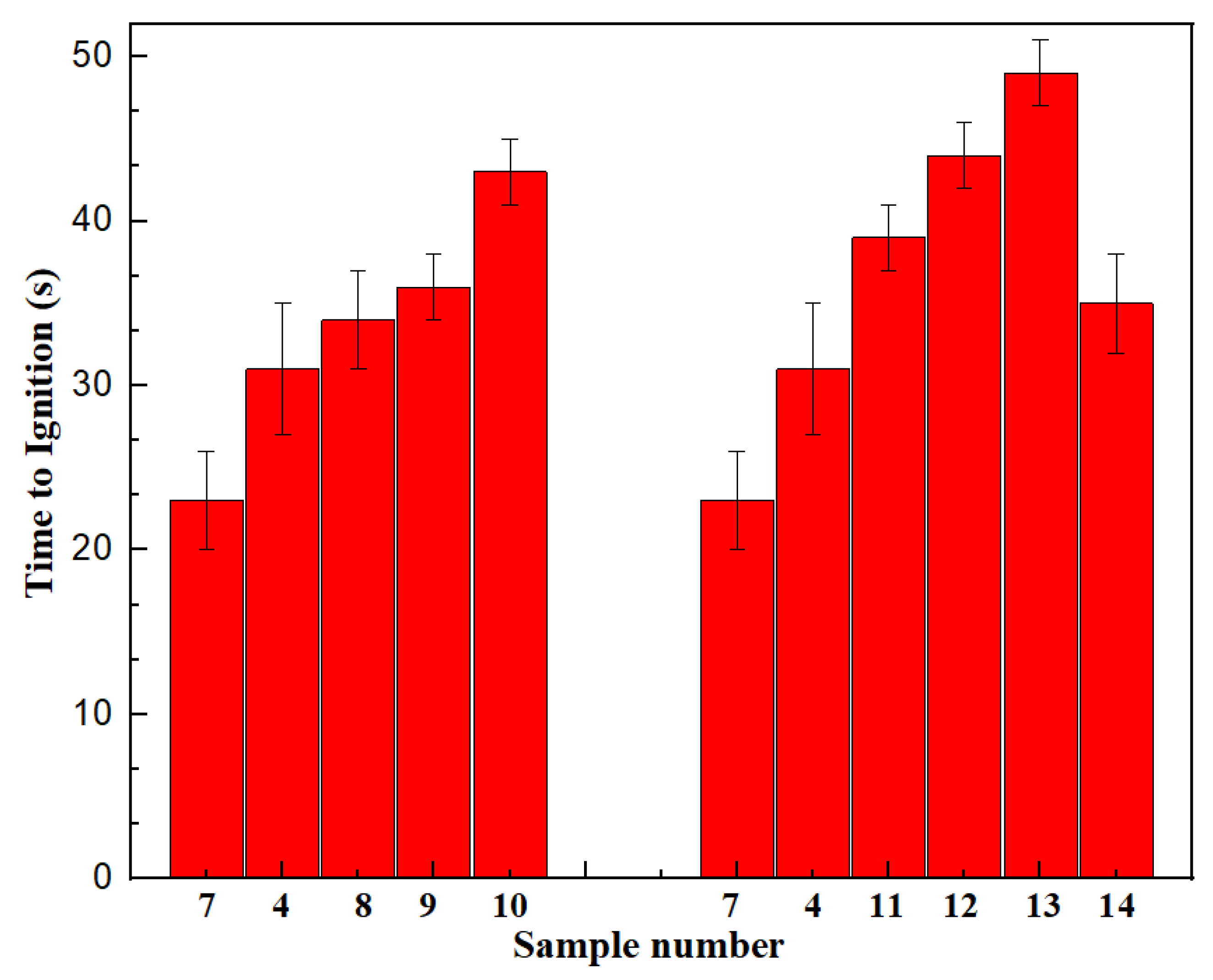
3.4.1. Ignition Time of Adding Different Flame Retardants
3.4.2. Heat Release Rate (HRR) with Different Flame Retardants
3.4.3. Total Heat Release (THR) with Different Flame Retardants
3.4.4. Vertical Combustion Test with Different Flame Retardants
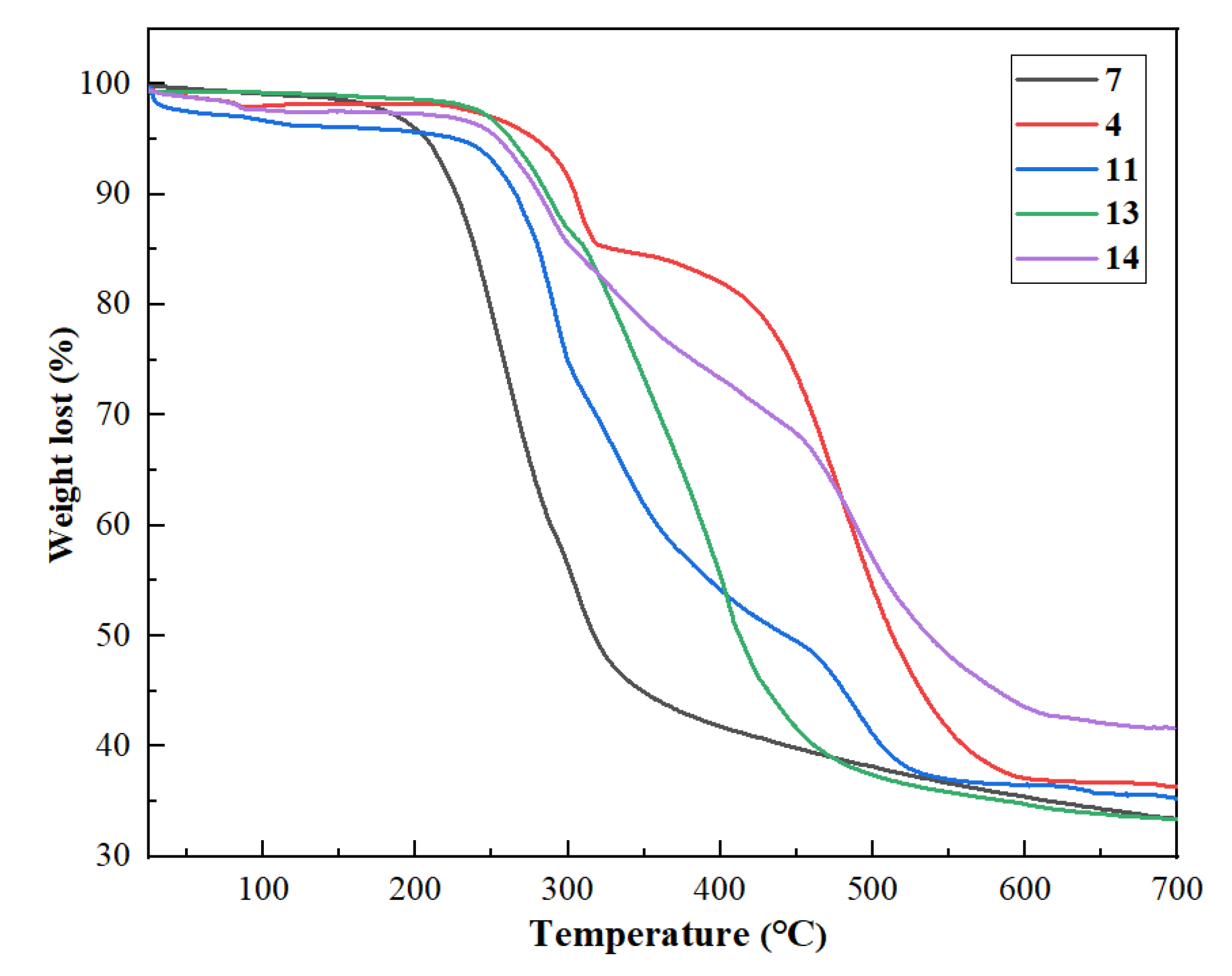
3.5. Thermogravimetric Analysis with Different Flame Retardants
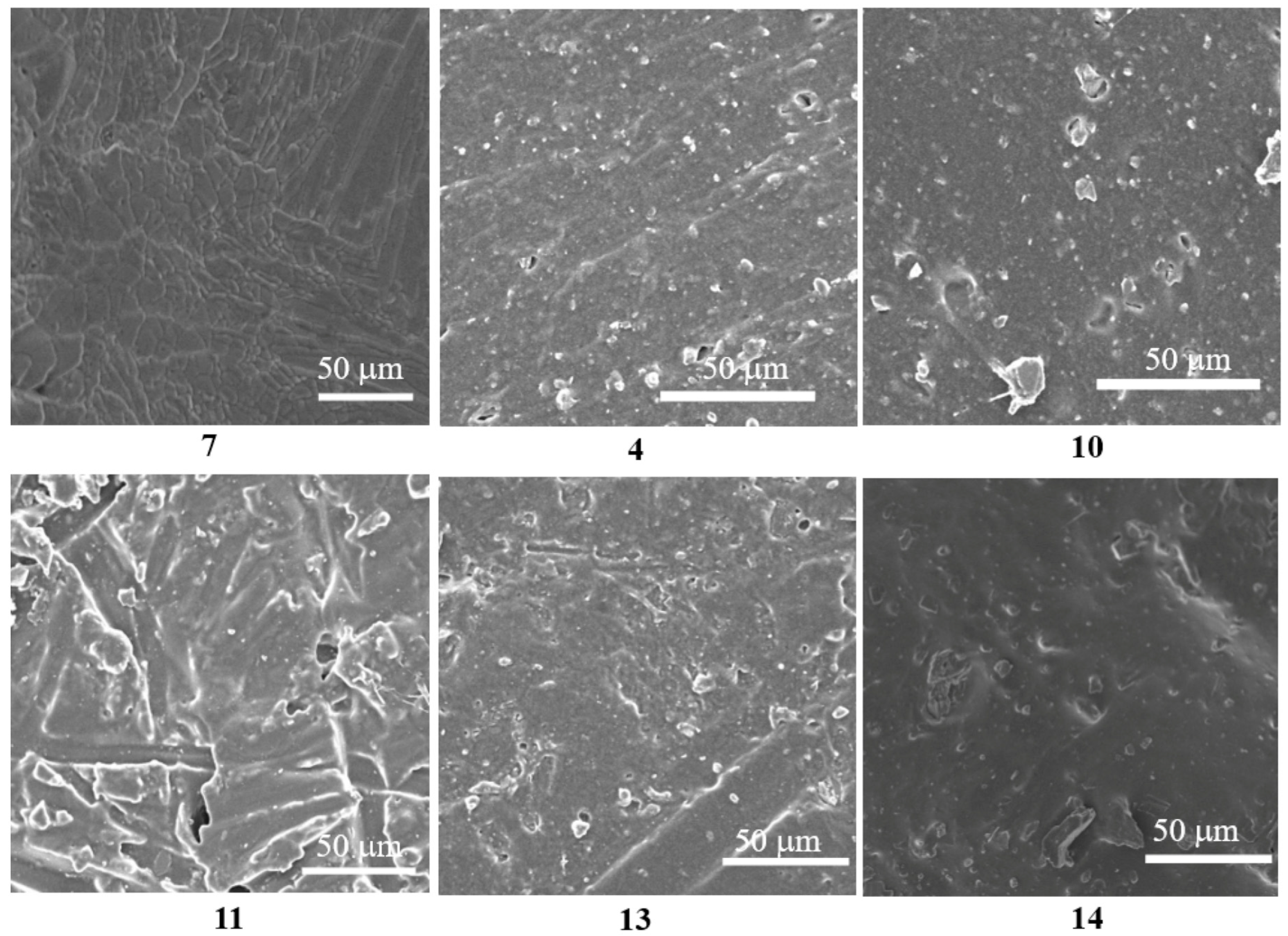
3.6. Microscopic Morphology of Coatings Containing Different Flame Retardants
3.7. Mechanical Properties and Electrical Properties of the Samples
3.8. Field Trials and Applications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gomez, F.A.; De Maria, J.G.; Puertas, D.G.; Baïri, A.; Arrabe, R.G. Numerical study of the thermal behaviour of bare overhead conductors in electrical power lines. Recent Res. Commun. Electr. Comput. Eng. 2011, 149–153. [Google Scholar]
- Pompodakis, E.E.; Ahmed, A.; Alexiadis, M.C. A Three–Phase Weather–Dependent Power Flow Approach for 4–Wire Multi–Grounded Unbalanced Microgrids with Bare Overhead Conductors. IEEE Trans. Power Syst. 2020, 36, 2293–2303. [Google Scholar] [CrossRef]
- Zainuddin, N.M.; Rahman, M.S.A.; Kadir, M.Z.A.A.; Ali, N.H.N.; Ali, Z.; Osman, M.; Mansor, M.; Ariffin, A.M.; Nor, S.F.M.; Nasir, N.A.F.M. Review of Thermal Stress and Condition Monitoring Technologies for Overhead Transmission Lines: Issues and Challenges. IEEE Access 2020, 8, 120053–120081. [Google Scholar] [CrossRef]
- Shang, R.; Fan, H.; Wang, L.; Yin, F.; Farzaneh, M. Analysis of high–temperature wideband dielectric properties of ath–filled silicone rubber used for on–site insulation of bare overhead conductors. IEEE Trans. Dielectr. Electr. Insul. 2022, 29, 2171–2180. [Google Scholar] [CrossRef]
- Jiang, J.; Jia, Z.; Wang, X.; Wang, S.; Yang, C. Analysis of conductor sag change after bare overhead conductor is covered with insulation material. In Proceedings of the 2019 2nd International Conference on Electrical Materials and Power Equipment (ICEMPE) 2019 IEEE, Guangzhou, China, 7–10 April 2019; pp. 439–442. [Google Scholar]
- Jiang, L.; Ning, K.; Qing, P. Adjustment Method of Insulation Level Difference between 10kV Overhead Line and Distribution Transformer. In Proceedings of the 2022 China International Conference on Electricity Distribution (CICED) 2022 IEEE, Changsha, China, 7–8 September 2022; pp. 134–141. [Google Scholar]
- Shit, S.C.; Shah, P. A review on silicone rubber. Natl. Acad. Sci. Lett. 2013, 36, 355–365. [Google Scholar] [CrossRef]
- Wei, Z.; He, Q.; Zhang, F.; Dai, F. Preparation and analysis of conductive and superhydrophobic silicone rubber. Sensors Actuators A Phys. 2023, 350, 114123. [Google Scholar] [CrossRef]
- Wang, G.; Li, A.; Zhao, W.; Xu, Z.; Ma, Y.; Zhang, F.; Zhang, Y.; Zhou, J.; He, Q. A Review on Fabrication Methods and Research Progress of Superhydrophobic Silicone Rubber Materials. Adv. Mater. Interfaces 2021, 8, 2001460. [Google Scholar] [CrossRef]
- Adupa, S.; Pulla, S.; Alavala, C.R. Effect of Filler Materials in Silicone Rubber for the Electrical Insulation Applications: A Review. Int. J. Appl. Eng. Res. 2021, 16, 721–729. [Google Scholar] [CrossRef]
- Pohmer, K. Molded silicone rubber automotive parts. Autotechnology 2001, 1, 32–35. [Google Scholar] [CrossRef]
- Abbasi, F.; Mirzadeh, H.; Katbab, A.-A. Bulk and surface modification of silicone rubber for biomedical applications. Polym. Int. 2002, 51, 882–888. [Google Scholar] [CrossRef]
- Rey, T.; Le Cam, J.-B.; Chagnon, G.; Favier, D.; Rebouah, M.; Razan, F.; Robin, E.; Didier, P.; Heller, L.; Faure, S.; et al. An original architectured NiTi silicone rubber structure for biomedical applications. Mater. Sci. Eng. C 2014, 45, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Zare, M.; Ghomi, E.R.; Venkatraman, P.D.; Ramakrishna, S. Silicone–based biomaterials for biomedical applications: Antimicrobial strategies and 3D printing technologies. J. Appl. Polym. Sci. 2021, 138, 50969. [Google Scholar] [CrossRef]
- Kumar, V.; Lee, J.-Y.; Lee, D.-J. Synergistic effects of hybrid carbon nanomaterials in room–temperature–vulcanized silicone rubber. Polym. Int. 2017, 66, 450–458. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, W.; Xie, Z. Highly thermally conductive room–temperature–vulcanized silicone rubber and silicone grease. J. Appl. Polym. Sci. 2003, 89, 2397–2399. [Google Scholar] [CrossRef]
- Yang, X.; Li, Q.; Li, Z.; Xu, X.; Liu, H.; Shang, S.; Song, Z. Preparation and Characterization of Room–Temperature–Vulcanized Silicone Rubber Using Acrylpimaric Acid–Modified Aminopropyltriethoxysilane as a Cross–Linking Agent. ACS Sustain. Chem. Eng. 2019, 7, 4964–4974. [Google Scholar] [CrossRef]
- Khatoon, S.; Khan, A.A.; Singh, S. A review of the flashover performance of high voltage insulators constructed with modern insulating materials. Trans. Electr. Electron. Mater. 2017, 18, 246–249. [Google Scholar]
- Pylarinos, D.; Siderakis, K.; Thalassinakis, E. Comparative investigation of silicone rubber composite and room temperature vulcanized coated glass insulators installed in coastal overhead transmission lines. IEEE Electr. Insul. Mag. 2015, 31, 23–29. [Google Scholar] [CrossRef]
- Amin, M.; Khattak, A.; Ali, M. Accelerated aging investigation of silicone rubber/silica composites for coating of high–voltage insulators. Electr. Eng. 2018, 100, 217–230. [Google Scholar] [CrossRef]
- Hualing, W.; Yueju, Z.; Jinbiao, S.; Chunfeng, Z.; Xiwei, X.; Jianhui, W.; Yongli, Z.; Qiang, C.; Yuan, G. Reasch on Insulation Modification of Overhead Bare Wires online by Room Temperature Vulcanized Silicone Rubber. In Proceedings of the 2021 IEEE Sustainable Power and Energy Conference (iSPEC) 2021 IEEE, Nanjing, China, 25–27 November 2021; pp. 2254–2256. [Google Scholar]
- Yang, J.; Huang, Z.; Ma, Z.; Zheng, M.; Su, J. Research on the Effect of Reinforcing Filler on The Mechanical and Electrical Properties of Insulating Coated Silicone Rubber. J. Physics Conf. Ser. 2021, 2083, 022013. [Google Scholar] [CrossRef]
- Chai, W.; Su, X.; Xia, Y.; Gao, M.; Li, Y.; Liao, C.; Zheng, Z. An eco–friendly, bio–inspired and synergistic flame retardant system with outstanding water tolerance and ultra–low addition for silicone rubber. Constr. Build. Mater. 2023, 365, 130005. [Google Scholar] [CrossRef]
- Huang, X.; Tian, Z.; Zhang, D.; Jing, Q.; Li, J. The synergetic effect of antimony (Sb2O3) and melamine cyanurate (MCA) on the flame–retardant behavior of silicon rubber. Polym. Bull. 2021, 78, 185–202. [Google Scholar] [CrossRef]
- Zhang, Q.; Tian, M.; Wu, Y.; Lin, G.; Zhang, L. Effect of particle size on the properties of Mg(OH)2–filled rubber composites. J. Appl. Polym. Sci. 2004, 94, 2341–2346. [Google Scholar] [CrossRef]
- Asaad, J.; El–Nashar, D.E.; Mansour, S. Effect of modified clay and Mg(OH)2 on the properties of ethylene propylene diene monomer/silicon rubber nanocomposites. Proc. Inst. Mech. Eng. Part N J. Nanoeng. Nanosyst. 2015, 229, 121–130. [Google Scholar] [CrossRef]
- Bright, D.A.; Dashevsky, S.; Moy, P.Y.; Williams, B. Resorcinol bis (diphenyl phosphate), a non–halogen flame–retardant additive. J. Vinyl Addit. Technol. 1997, 3, 170–174. [Google Scholar] [CrossRef]
- Suparanon, T.; Phetwarotai, W. Fire–extinguishing characteristics and flame retardant mechanism of polylactide foams: Influence of tricresyl phosphate combined with natural flame retardant. Int. J. Biol. Macromol. 2020, 158, 1090–1101. [Google Scholar] [CrossRef] [PubMed]
- Gaan, S.; Sun, G.; Hutches, K.; Engelhard, M.H. Effect of nitrogen additives on flame retardant action of tributyl phosphate: Phosphorus–nitrogen synergism. Polym. Degrad. Stab. 2008, 93, 99–108. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, Y.; Zhang, D.; Li, J.; Huang, G. Preparation and thermal degradation behavior of room temperature vulcanized silicone rubber–g–polyhedral oligomeric silsesquioxanes. Polymer 2013, 54, 6140–6149. [Google Scholar] [CrossRef]
- Ogawa, K.; Vogt, T.; Ullmann, M.; Johnson, S.; Friedlander, S.K. Elastic properties of nanoparticle chain aggregates of TiO2, Al2O3, and Fe2O3 generated by laser ablation. J. Appl. Phys. 2000, 87, 63–73. [Google Scholar] [CrossRef]
- Leblanc, J.L. Rubber–filler interactions and rheological properties in filled compounds. Prog. Polym. Sci. 2002, 27, 627–687. [Google Scholar] [CrossRef]
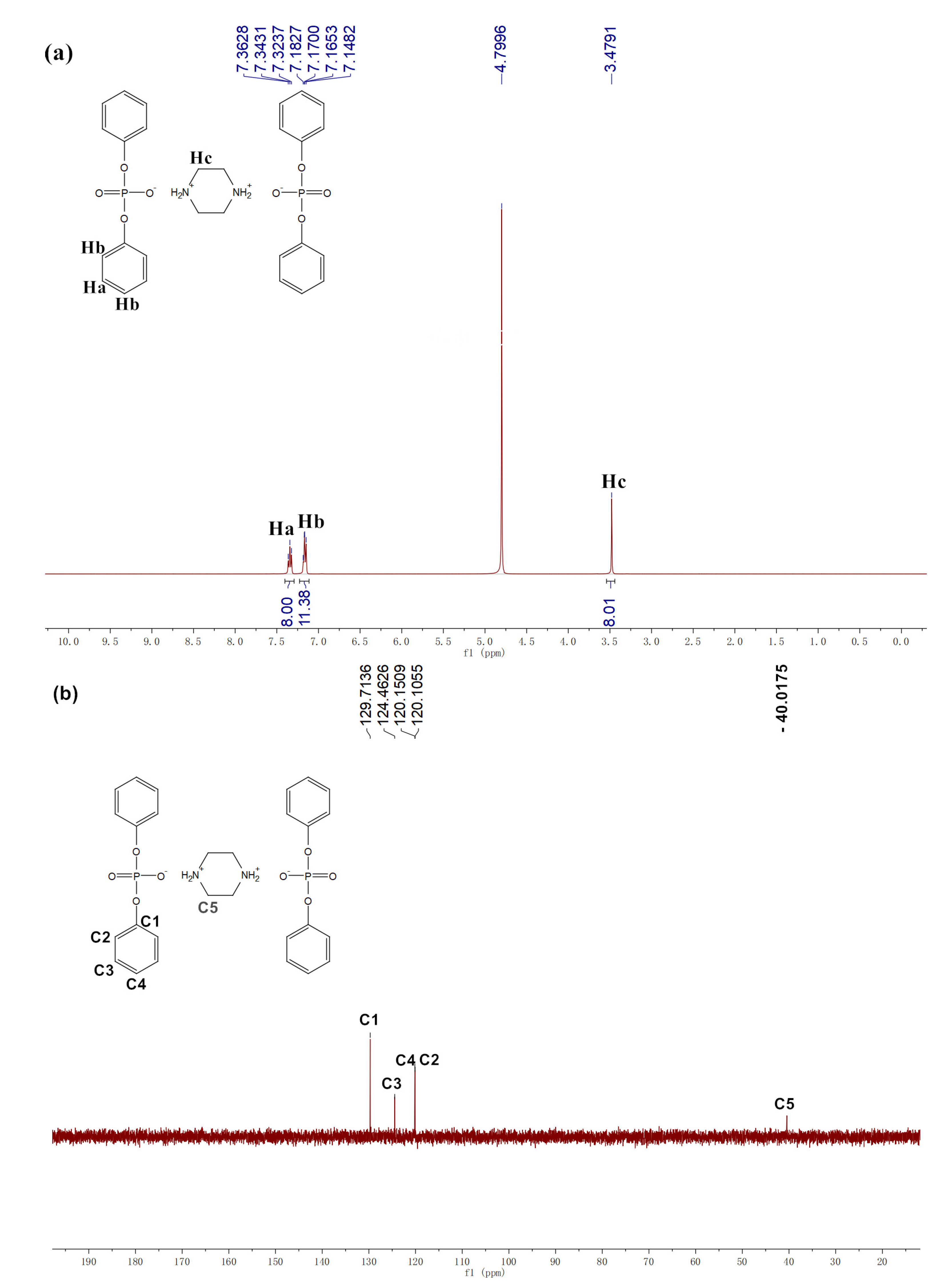



 for PBDP was added to the flame retardant,
for PBDP was added to the flame retardant,  for MDP was added to the flame retardant.
for MDP was added to the flame retardant.
 for PBDP was added to the flame retardant,
for PBDP was added to the flame retardant,  for MDP was added to the flame retardant.
for MDP was added to the flame retardant.

| Entry | PDMS (g) | Carbon Black (phr) | CaCO3 (phr) | Mg(OH)2 (phr) | PBDP (phr) | MDP (phr) |
|---|---|---|---|---|---|---|
| 1 | 100 | 0 | 100 | 30 | 0 | 0 |
| 2 | 100 | 10 | 100 | 30 | 0 | 0 |
| 3 | 100 | 20 | 100 | 30 | 0 | 0 |
| 4 | 100 | 30 | 100 | 30 | 0 | 0 |
| 5 | 100 | 40 | 100 | 30 | 0 | 0 |
| 6 | 100 | 50 | 100 | 30 | 0 | 0 |
| 7 | 100 | 30 | 100 | 0 | 0 | 0 |
| 8 | 100 | 30 | 100 | 20 | 10 | 0 |
| 9 | 100 | 30 | 100 | 10 | 20 | 0 |
| 10 | 100 | 30 | 100 | 0 | 30 | 0 |
| 11 | 100 | 30 | 100 | 20 | 0 | 10 |
| 12 | 100 | 30 | 100 | 10 | 0 | 20 |
| 13 | 100 | 30 | 100 | 0 | 0 | 30 |
| 14 | 100 | 30 | 100 | 10 | 10 | 10 |
| Entry | First after–Flame Burn Time for 5 Specimens (t1,i)/s | Second after–Flame Burn Time for 5 Specimens (t2,i)/s | Total after–Flame Burn Time Per Specimen (t1,i + t2,i)/s | Total after–Flame Burn Time for 5 Specimens (tf)/s | Drip Ignition of Skimmed Cotton | Flame Retardant Grade |
|---|---|---|---|---|---|---|
| 1 | Burn | Burn | Burn | Burn | Yes | NR |
| 7 | Burn | Burn | Burn | Burn | Yes | NR |
| 4 | 3/4/2/3/2 | 6/6/5/7/5 | 9/10/8/10/7 | 44 | No | FV–0 |
| 10 | 22/16/19/23/20 | 26/25/25/27/34 | 48/41/44/50/54 | 237 | Yes | FV–2 |
| 11 | 5/4/5/3/7 | 7/9/11/6/12 | 12/13/16/9/19 | 69 | No | FV–1 |
| 13 | 6/6/5/7/7 | 10/9/11/13/14 | 16/15/16/20/21 | 88 | No | FV–1 |
| 14 | 14/11/13/13/12 | 13/10/15/17/14 | 27/28/28/30/26 | 6/6/5/7/4 | No | FV–1 |
| Entry | 7 0 phr Mg(OH)2 | 4 30 phr Mg(OH)2 | 10 30 phr PBDP | 11 20 phr Mg(OH)2 + 10 phr MDP | 13 30 phr MDP | 14 10 phr Mg(OH)2 + 10 phr PBDP + 10 phr MDP |
|---|---|---|---|---|---|---|
| Tensile strength (MPa) | 3.68 ± 0.32 | 3.07 ± 0.31 | 3.12 ± 0.27 | 3.28 ± 0.23 | 3.41 ± 0.22 | 3.14 ± 0.27 |
| Elongation at break (%) | 327 ± 29 | 244 ± 18 | 280 ± 14 | 291 ± 25 | 297 ± 31 | 253 ± 21 |
| Surface drying time (min) | 28.2 | 23.0 | 20.3 | 24.7 | 20.5 | 22.1 |
| Depth of cure (mm/24 h) | 3.3 | 3.7 | 3.4 | 3.5 | 3.5 | 3.6 |
| Volumetric resistivity (Ω·cm) | 9.16 × 1014 | 5.21 × 1014 | 7.19 × 1014 | 6.52 × 1014 | 7.36 × 1014 | 5.06 × 1014 |
| Breakdown voltage (KV/mm) | 24.3 | 21.4 | 22.5 | 22.1 | 22.7 | 21.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Yang, F.; Lu, Y.; Luo, Z.; Li, F.; Wu, Y.; Zhang, J.; Xiao, Z.; Li, W.; Qin, C. Application of Magnesium Hydroxide/Diphenoxy Phosphate in Silicone Rubber Flame Retardant Cable Material. Coatings 2023, 13, 934. https://doi.org/10.3390/coatings13050934
Wang W, Yang F, Lu Y, Luo Z, Li F, Wu Y, Zhang J, Xiao Z, Li W, Qin C. Application of Magnesium Hydroxide/Diphenoxy Phosphate in Silicone Rubber Flame Retardant Cable Material. Coatings. 2023; 13(5):934. https://doi.org/10.3390/coatings13050934
Chicago/Turabian StyleWang, Wei, Fan Yang, Yunlai Lu, Zhi Luo, Fangya Li, You Wu, Jianbing Zhang, Zufeng Xiao, Wei Li, and Caiqin Qin. 2023. "Application of Magnesium Hydroxide/Diphenoxy Phosphate in Silicone Rubber Flame Retardant Cable Material" Coatings 13, no. 5: 934. https://doi.org/10.3390/coatings13050934
APA StyleWang, W., Yang, F., Lu, Y., Luo, Z., Li, F., Wu, Y., Zhang, J., Xiao, Z., Li, W., & Qin, C. (2023). Application of Magnesium Hydroxide/Diphenoxy Phosphate in Silicone Rubber Flame Retardant Cable Material. Coatings, 13(5), 934. https://doi.org/10.3390/coatings13050934






