Abstract
The amount of waste electrical and electronic equipment (WEEE) has been intensely increasing over the recent decades. In this view, the efficient recovery of metals from WEEE will allow a secure supply of raw materials and will contribute to a circular economy. Among many factors currently affecting the contribution of recycling, is the lack of suitable technologies for WEEE treatment in an environmentally friendly way. Current trends in eco-friendly technologies applied for gold, silver, copper, and tin recovery by electrowinning are reviewed in this paper. In addition, a case study on the perspectives of tin electrowinning has been evaluated. Tin can be present in rather high quantities in WEEE; moreover, its price is about three times higher than that for copper. The electrorecovery of tin has been carried out in cooperation with JSC “Elektronikos perdirbimo technologijos”. The eco-friendly process based on electrowinning from citric acid-containing leachates is elaborated. The citrate-based solutions have been chosen because citric acid is considered to be an environmentally friendly component. A high deposition rate and current efficiency have been achieved at a deposition potential −0.85 V at 60 °C. However, additional steps would be beneficial to diminish the interference of metals present in the scraps, such as Pb(II) and Cu(II), on tin electrorecovery.
1. Introduction
The amount generated by waste electrical and electronic equipment (WEEE) is increasing dramatically, especially in economically developed countries where markets are overwhelmed by huge quantities of new electronic goods. In order to control the environmental problems, a number of EU directives has been issued [1]. In this view, the efficient recovery of metals from WEEE will allow a secure supply of raw materials and will contribute to a circular economy. Advanced circular economy systems and sophisticated recycling technologies build the backbone for the development of a resource efficient and sustainable society [2]. Recycling is also regarded as a tool that improves sustainability due to the potentially lower environmental impacts of secondary material provision in comparison with production from primary raw materials. Sustainable recycling has many advantages that include energy efficiency, less influence on air, soil, and water, etc. The full spectra of environmental parameters are included in life cycle assessments [2]. Furthermore, the diverse range of materials found in WEEE makes it difficult to give a generalized material composition for the entire waste stream in order to design different recycling chains for the same group of waste.
The key factors that currently limit the input of recycling to meet demands for raw materials can be summarized as: (1) recycling of many materials from end-of-life products and waste streams is currently not economically feasible; (2) there is a lack of suitable environmentally friendly technologies for collection and separation of valuable metals; (3) some materials are embedded in products that are in use for long time periods (e.g., buildings or wind turbines); and (4) the demand for many metals is growing [3].
The presence of precious metals in WEEE such as Au, Pt, and Pd and trends in the market prices of metals, makes it economically attractive to recycle metals from WEEE, i.e., it is wiser for e-waste to be treated to recover these precious metals. In addition, evaluating the results obtained in [4], it is evident that the percentage of precious metals in WEEE has decreased a few times during last 20 years. This fact suggests that the elaboration of new technologies for metal recovery from WEEE with relatively small percentages of valuable metals is required.
There are a number of WEEE treatment routes comprising mechanical shredding following the various chemical leaching processes to obtain the metals via reduction [5]. Recovery of leached metal via the electrowinning technique is one of the alternative ways for metal recovery. In most cases, this method does not require additional chemicals (“chemical-free”) and can be considered an eco-friendly method for metal recovery.
The set-up used for electrowinning is simple in nature. Namely, a bath resistant to chemicals with anodes and cathodes submersed in a solution with current passing through the electrodes as a fundamental process unit. Besides ores, electrowinning is also used to recover valuable metals from electronic and galvanic industrial waste. The most common metals recovered using electrowinning are gold, silver, copper, cadmium, and zinc due to their relative value.
Actually, tin is also a valuable metal and its price is approx. three times higher than that for copper. The electrowinning of Sn is an attractive refining process because its electrochemical equivalent is relatively higher (6.2 × 10−4 g/C) than that for Cu (3.3 × 10−4 g/C) and Zn (3.5 × 10−4 g/C), and it is comparable with the electrochemical equivalent for Au (6.8 × 10−4 g/C). Therefore, in this paper a brief review on electrowinning of metals is provided. In addition, the case study of tin electrowinning from citrate-based baths has been carried out, and the perspectives of its industrial application are discussed here. The citrate-based solutions have been chosen since citric acid is considered an environmentally friendly component. It is food-safe, easily biodegradable, requires lower acid concentrations, and does not generate toxic fumes or hazardous waste [6]. The elaborated process of electrowinning of tin might be implemented by small and medium enterprises because of the relatively simple and affordable technologies for electrolysis and the usage of non-hazardous chemicals.
2. Brief Review on Experimental Set-Up for Electrowinning
2.1. Cathode Configurations
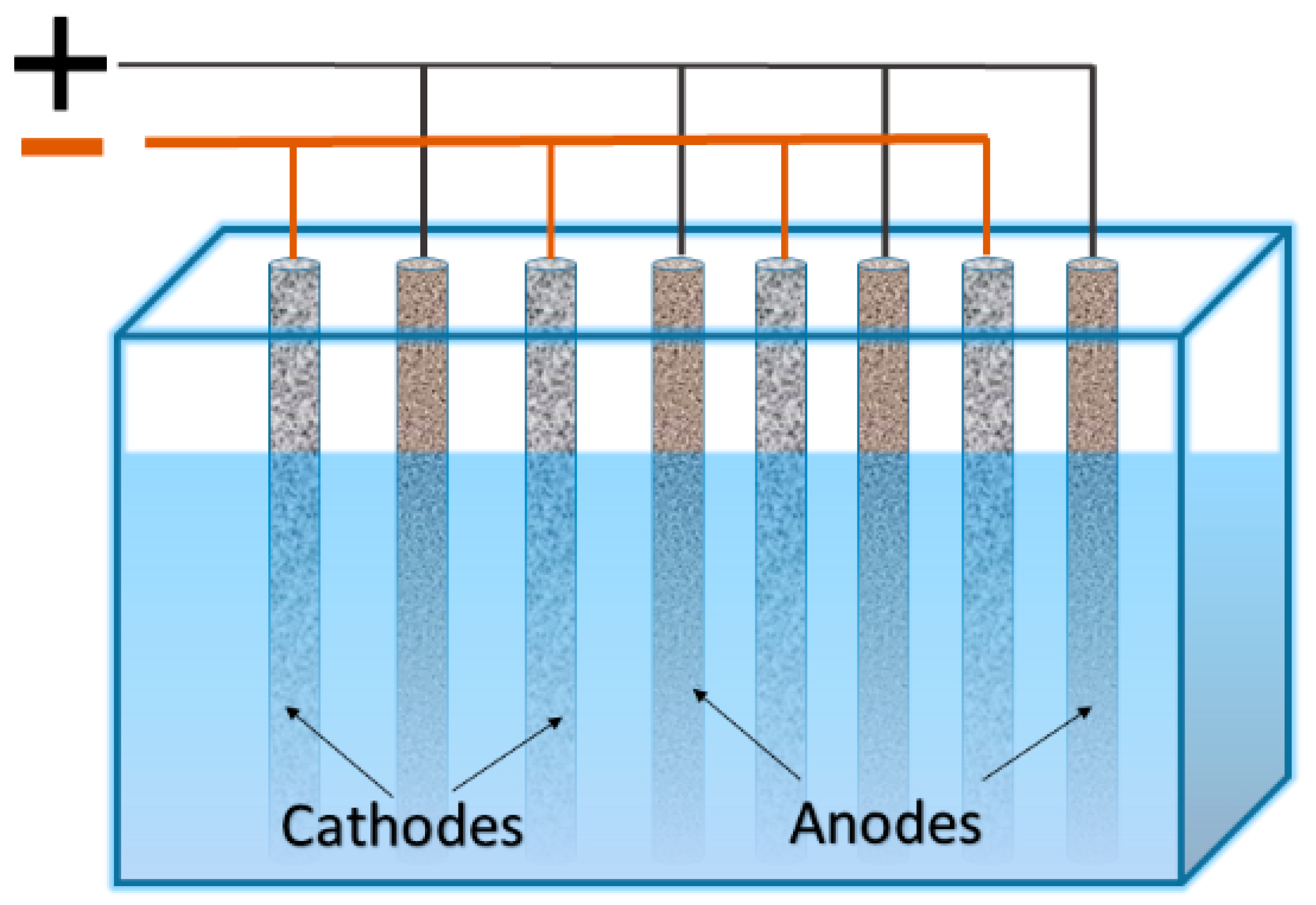
Electrowinning is a heterogeneous process. Therefore, the rate of mass gain on the electrodes depends on the total area of the electrodes. Hence, a number of attempts have been made to increase the specific area of the cathodes or to increase the “area of cathode per volume of solution” ratio. The simplest and conventional way to increase the mentioned ratio is the usage of a series of anodes and cathodes arranged in a “sandwich” configuration, where cathodes and anodes are typically arranged in alternating order on either side of the cathode compartments (see Figure 1). Increasing the metal concentration and solution flow rate has the largest effect on the metal deposition rate (particularly for copper) [7].
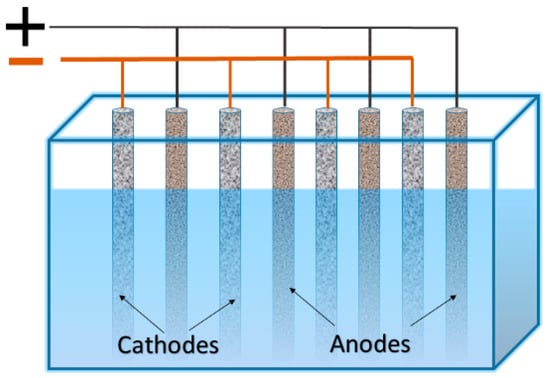
Figure 1.
Arrangement of cathodes and anodes in “sandwich” configuration.
Another option for cathode configuration is spiral-wound electrodes. Theoretically, for any scale-up, the spiral electrode maintains a high specific surface area and low internal resistance [8]. In a cell with the spiral cathodes, a sufficient amount of metal ions is supplied to the cathode surface at very low concentrations of the metal. This high mass transport of ions to the cathode surface enables the cell to operate outside of the mass transport limited regime of conventional electrowinning cells [9].
The most interesting material for cathodes applied to electrowinning processes might be metal foams. The characteristics of Cu foams (see Figure 2) for electrowinning have been comprehensively studied in [10].

Figure 2.
SEM image of 3D copper foam.
Using various electrochemical methods, it was determined that the rate-limiting step in copper deposition is the diffusion. The main processes occurring on the electrode are the charging-up of the double electric layer, charge transfer in the electrochemical reaction, and diffusion. The specific electrochemically active area of Cu foam has been estimated from electrochemical impedance spectroscopy (EIS) data, and based on the values of the double electric layer. It was shown that this area could be 7–14 times higher than that for a plane electrode. In addition, based on the EIS data, it was determined that the charge transfer resistance of the Cu foam electrode is 1.5–1.7 times lower than that of a Cu plane electrode, which results in an increase in the charge transfer rate by approximately 2 times. Based on the analysis of the diffusion impedance and chronopotentiometry data, it was found that Cu2+ mass transfer and the copper deposition rate was up to 3 times faster on the foam surface in comparison with a flat one having the same geometric area under the same deposition potential range.
Other types of cathodes can be used for the treatment of wastewater containing copper ions and various surfactants. In the case of anionic surfactants, the negatively charged hydrophilic parts can electrostatically attract positively charged metal ions, leading to the interference of electrolytic metal recovery [11]. Additionally, if nonionic surfactants coexist with the anionic surfactant in the wastewater, they will attract each other with their hydrophobic tails. The mixed anionic and nonionic surfactants may inhibit the mass transfer of metal ions and reduce the metal deposition rate per electrode area unit. Therefore, the use of steel wool as cathodes with enhanced active surface areas is attractive for electrowinning applications [12]. On the steel wool cathodes (Figure 3) metals could be rapidly recovered from dilute solutions with an acceptable current efficiency in comparison to a parallel-plate reactor, e.g., the time of copper recovery was shortened up to 40 min [13].

Figure 3.
An example of stainless steel wool cathodes.
Another type of electrodes for electrowinning are powder cathodes. The use of powder materials makes it possible to combine the advantages of fibrous electrodes with their enhanced surface with the advantages of compact cathodes including low cost and reprocessing simplicity. Powder cathodes may be made not only of iron or titanium, but also of more electro-negative metals such as aluminum or magnesium. In turn, the use of these powders in the Au-containing solutions makes it possible to extract precious metal by electrowinning and cementation simultaneously [14].
2.2. Solutions for Electrowinning
Electrowinning is often combined with leaching of WEEE [15,16,17]. Different solutions for leaching and for electrowinning have been tested and briefly discussed in [18]. An evolving interest has grown in organic acids as leaching agents due to their biodegradability and stability under leaching conditions. The examples of organic acids used in previous studies to substitute the strong toxic leaching agents and reduce the environmental pollution include: ascorbic acid, citric acid, succinic acid, oxalic acid, formic acid, phosphoric acid, acetic acid, DL-malic acid, and L-tartaric acid [19,20].
The electrowinning of gold and silver in many cases is an important way to obtain pure metals using cyanide-based solutions of Au, and in some cases of Ag. This process had been used safely for many years, but currently the application of the cyanide-based methods is restricted in many countries. In this paper, we review some alternative solutions for gold and silver electrowinning.
Attempts have been made to implement thiosulfate-based leaching technology for industrial Au production, as it may reduce the use of cyanide. The electrowinning of leachate was carried out at rather high constant current density of ~12 A/m2. However, the drawback of this approach is the low recovery efficiency of Au, because some amount of Au is cemented on the anodes when the electrolytic bath is off [21].
In addition, the obtained results revealed that, when an excess of thiosulfate is present in the electrolyte, cathodic side reactions, such as hydrogen evolution or thiosulfate decomposition, occur. This leads to the electrodeposition of gold layers having smaller grains and rougher surfaces in comparison with sulfite-based electrolytes. Consequently, sulfite can represent a better-controlled electroreduction reaction, resulting in the formation of finer grains, and purer and more even layers of gold [22]. A selective recovery by electrowinning of Au and Cu was described in [23]. The process was carried out from the solutions obtained from the leaching of printed circuit boards of mobile phones in an ammonia solution containing thiosulphate ions.
Another alternative method for Au recovery is electrowinning of leachates obtained during leaching of gold-containing raw materials in acidic solutions of thiourea. However, if leachate contains gold at low concentrations, the electrowinning process is uneconomical. Instead, the process in which Au is specifically adsorbed in the form of gold–thiourea complexes onto activated carbon and eluted by dilute acidic thiourea solutions containing alcohol can be adopted. Such eluates contain 5–50 mg dm−3 of Au or more, which can then be recovered by electrowinning [24].
Gold and silver recovery by electrowinning from various electronic connector waste was studied in [25]. Thus, from the solution containing ~100 mg/L of Au and Ag, the metals were successfully recovered by electrowinning with high yields (89.95% for Au and 87.98% for Ag) after 1.5 h of electrolysis. Such an effective process could be achieved due to a copper cathode, which favors reduction processes for both noble metals. The only issue when using a copper electrode is its proneness to react with thiourea and decompose it. This will lead to a 20% decrease in thiourea concentration at the end of the process, whereas it was possible to achieve 95% recovery on a graphite cathode within 1.5 h at room temperature.
The recovery of Ag by electrowinning is also considered in the case of treatment of spent batteries. Thus, an environmentally friendly process for recycling of such batteries was developed in [26]. A high electrowinning rate and recovery efficiency (98.5%) of ultra-pure Ag (≥99.9%) was achieved under optimized operating potentials. The elaborated process can be considered as a sustainable technology for precious metal recovery from scraps containing silver oxide batteries.
One of the effective way to recover Cd from metallic mixtures is the hydrometallurgical process described in [27]. Sulfuric acid is often used as the most common agent to dissolve base metals, and in this case, it is possible to regulate different levels of impurities. Namely, it is possible to selectively leach Cd and Zn from a mixture of Cd, Zn, Cu, and Pb. After the leaching, Cd and Zn dissolve and remain in the filtrate, while Cu and Pb are precipitated and can be separated by filtration. Then, the metallic Cd will be obtained by cementation with zinc dust. In order to remove any co-precipitated Zn, electrowinning can be performed.
Currently, high purity tin is generally produced by an electrolytic method using an acidic tin solution such as from leachates based on sulfuric acid [28], alkaline solutions [29], or a mixture of fluorosilicic acid and sulfuric acid [30]. Notably, Sn can be separated from sulfuric acid leachate containing Cu by its precipitation in the form of SnO2 at a pH of 3, and the pure Cu can be recovered by electrowinning [31]. Citric acid-based solutions are very promising for metal leaching and electrowinning due to its biodegradability. Citrate ions form stable complexes with many metals and it is widely used for metal and alloy electrodeposition, including electrowinning of metals after treatment of WEEE (e.g., Cu [32], Ni [33], Sn [34], Pb and Zn [35], Sn-Zn [36], Cu-Sn [37], FeCoNiCr [38]). The agents facilitating metals leaching are EDTA [39] and H2O2 [40], which can also be used for electrowinning.
3. Results
3.1. Methodology of Experiments
Two leachates of base metals (“L1” and “L2”) were prepared on the premises of JSC Elektronikos Perdirbimo Technologijos (EPT) throughout WEEE treatment. Namely, leachates “L1” and “L2” were obtained after treatment of telecommunication boards and random access memory (RAM) cards, respectively. It should be noted that the scrap had been shredded up into 1–5 mm size pieces. Then, the leaching was carried out in the rotating reactor RRM 7 (producer EMAK, Turkey). The load of 7 kg of scrap was treated for 2 h at 60 °C in the leaching solution with a total volume 12 L, which contained 200 g/L citric acid and 10 g/L ammonium persulfate at pH 3.2–6.5. The pH of the solutions had been adjusted using sodium hydroxide.
In addition, for comparison, a reference solution (“Ref”) containing citric acid (200 g/L) and Sn (II) (20 g/L) at pH 3.5 was prepared and evaluated (see Table 1).

Table 1.
The composition of investigated solutions. Leachates “L1” and “L2” are obtained after treatment of WEEE.
The composition of the leachates was determined using inductively coupled plasma optical emission spectroscopy, ICP-OES, using a Perkin Elmer Optima 7000 DV spectrometer (Waltham, MA, USA). The composition of leachates is provided in Table 1. The main difference between “L1” and “L2” arise from the Cu(II) concentration, which is comparable with Sn(II) for the last case.
The electrochemical studies at a lab scale were carried out in a standard 3-electrode cell using a programmable potentiostat/galvanostat AUTOLAB PGSTAT 302N (Metrohm, Utrecht, the Netherlands).
All potentials were recorded and presented vs. a saturated Ag/AgCl/KCl electrode. The stainless steel (type 304) plates served both as the anode and cathode. In the last case, the working area was 4 cm2. After electrolysis, the deposits were evaluated by SEM equipped with EDS for structural and compositional analyses.
3.2. Perspectives of Tin Electrowinning from Citrate-Based Solutions
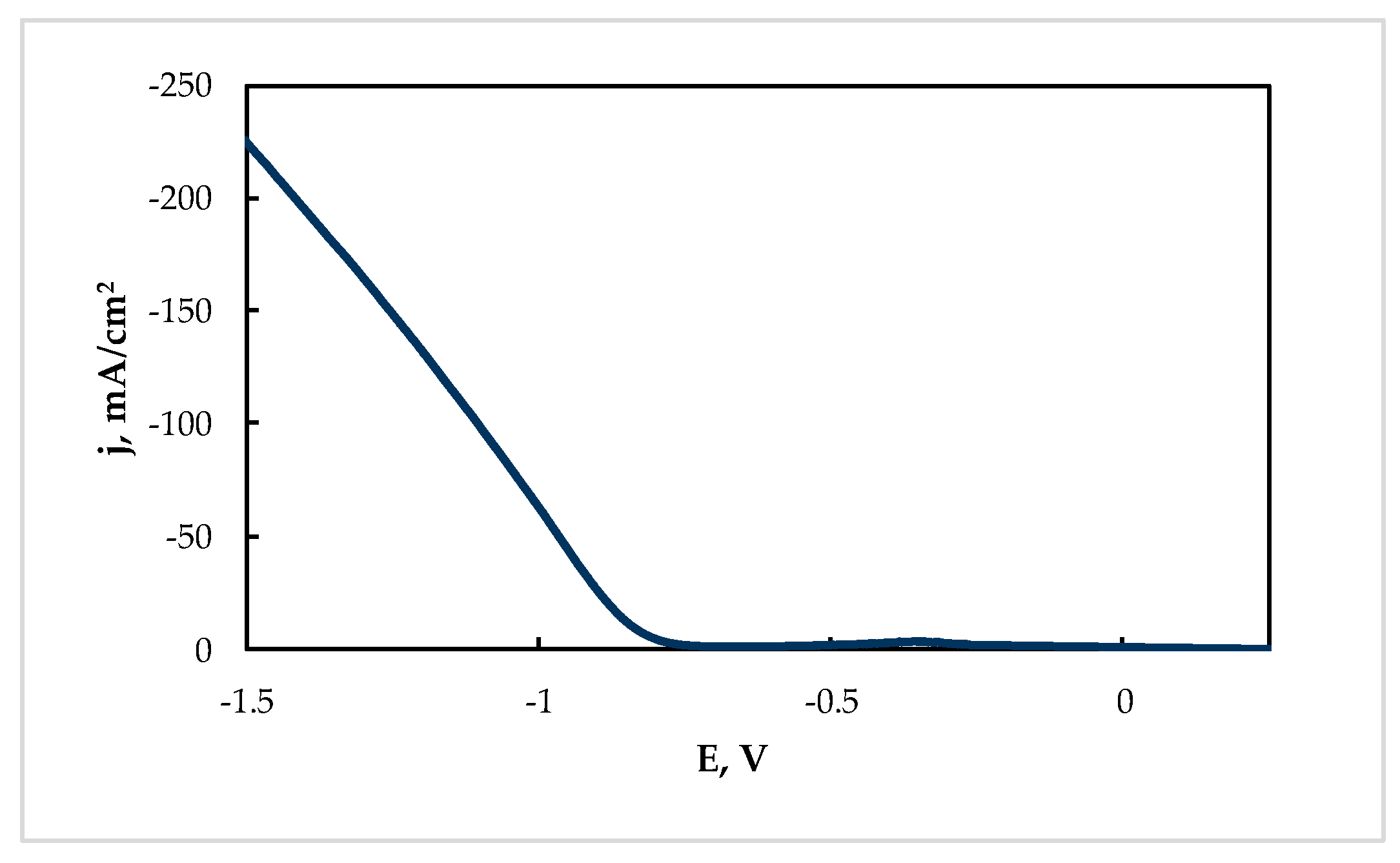
The cathodic current densities for Sn electroreduction from the “Ref” solution were rather high at the potentials more negative than −0.7 V at 60 °C (see Figure 4).
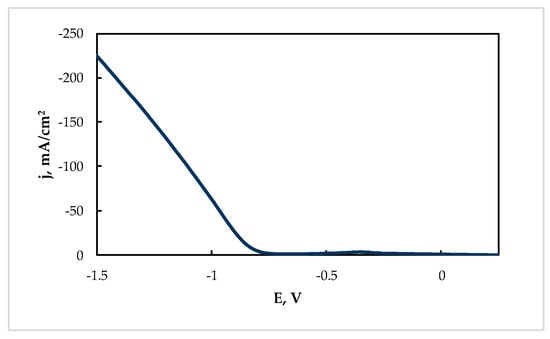
Figure 4.
Cathodic polarization curve of Sn electroreduction at 60 °C from the “Ref” solution (g/L): Sn(II)—20; citric acid—200.
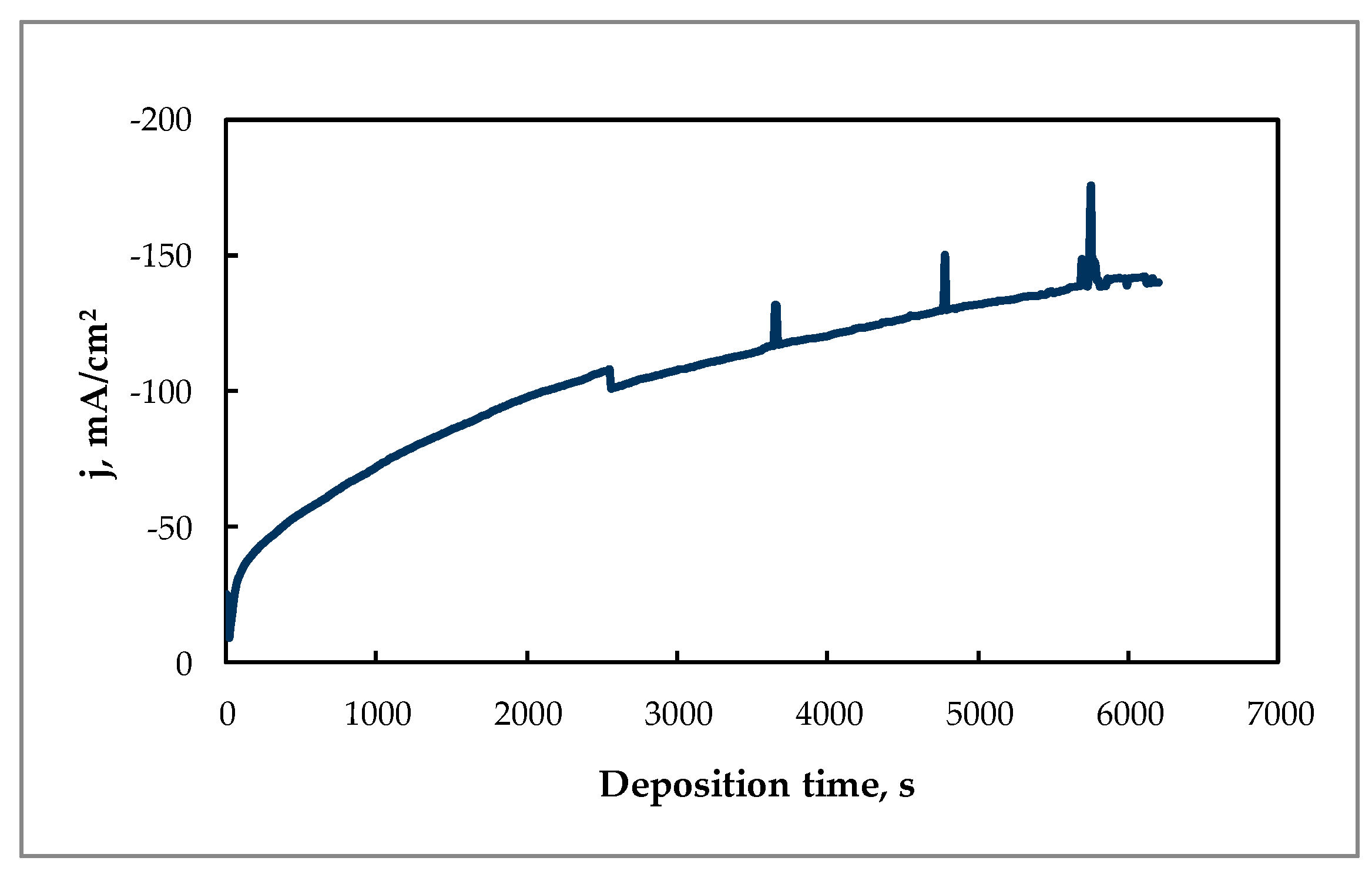
Furthermore, the electrowinning of tin under potentiostatic conditions at −0.85 V can be considered as optimal, because a high deposition rate (0.0035 g cm−2 min−1) and current efficiency (86%) were achieved. Under these conditions, the current increased sufficiently with deposition time (Figure 5).
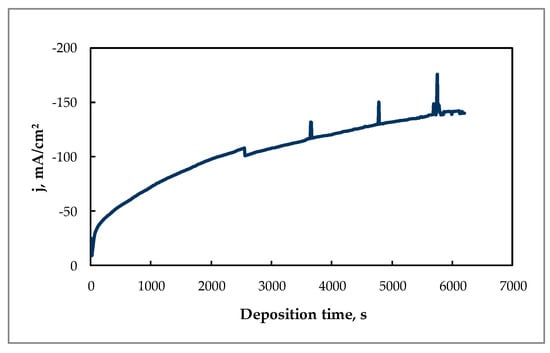
Figure 5.
Chronoamperogram of Sn electrodeposition at E = −0.85 V and 60 °C from the citrate solution. Content of the solution as indicated in Figure 4, substrate—Cu electrode.
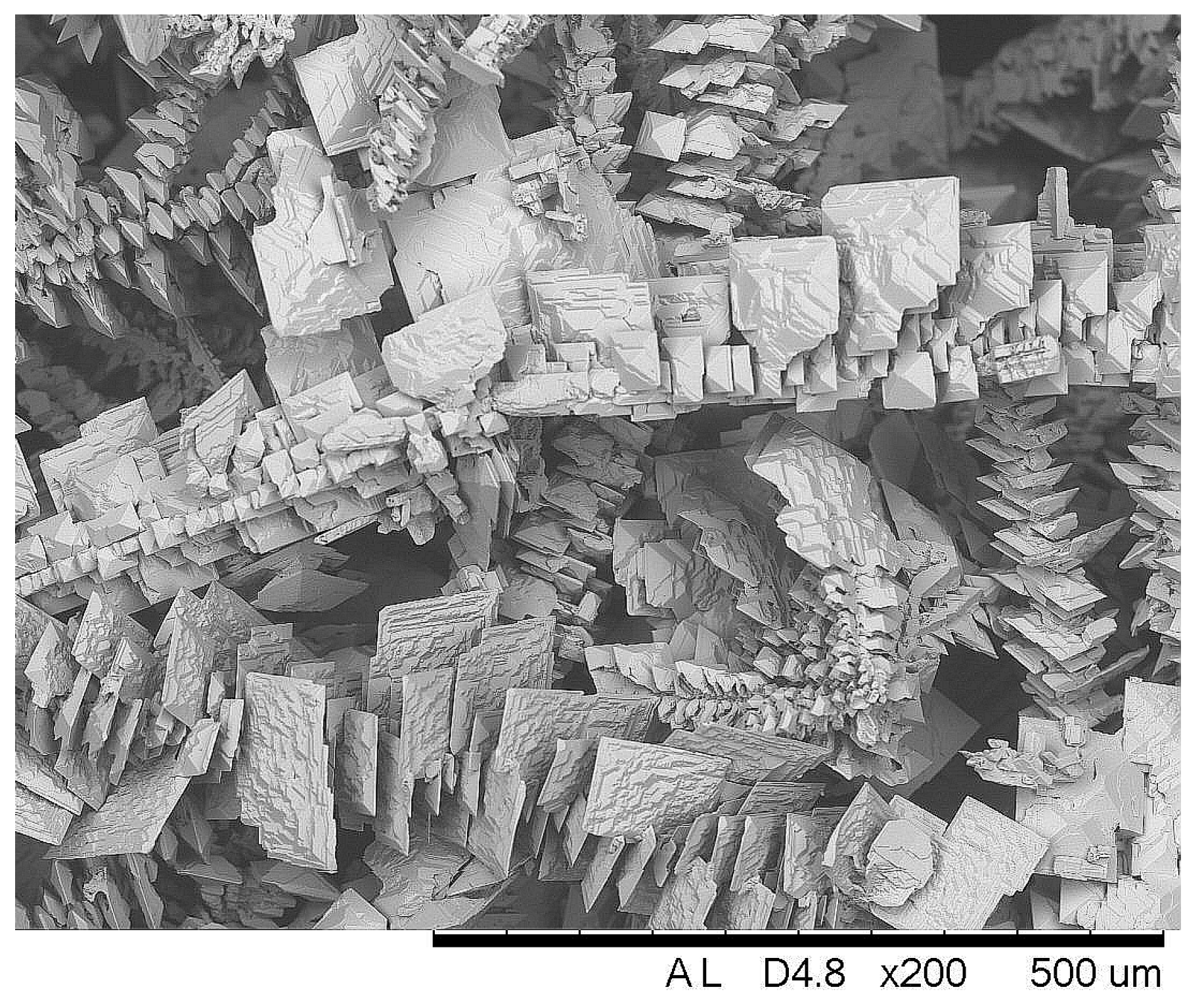
The rise of apparent current density during electrodeposition was triggered by the formation of a coarse crystalline deposit on the cathode, as shown in Figure 6. The growing of such crystallites increases the active area of the cathode, therefore the current increased under potentiostatic deposition conditions.
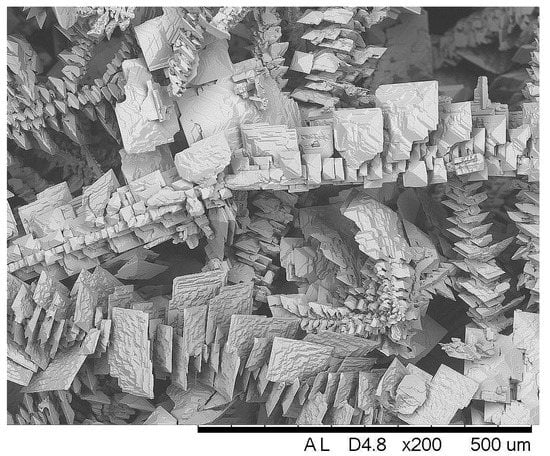
Figure 6.
SEM image of Sn electrodeposit obtained at E = −0.85 V and 60 °C from the solution “Ref”.
The solution “L1” obtained after treatment of WEEE contained a similar concentration of Sn(II) as the “Ref” solution, namely ~20 g/L (see Table 1). However, irrespectively of the pH, the obtained cathodic current densities were essentially lower, almost 10 times, than in the “Ref” solution, as is shown in Figure 7.
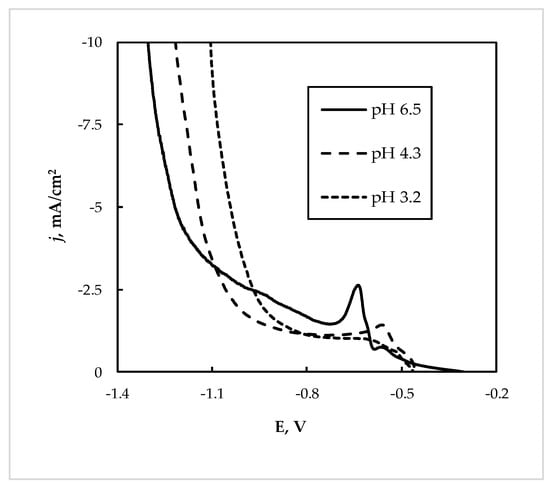
Figure 7.
Cathodic polarization curve on Sn electrode at 60 °C and scan rate 5 mV/s from the leachate “L1”.
The metal deposition rates from leachate “Ll” throughout the studied range of pH was also ~10 times lower. The elemental analysis of the deposits obtained at −0.85 V showed that the main component in the deposits was lead (see Table 2).

Table 2.
The composition of deposits obtained at −0.85 V and 60 °C from leachate “L1”at various pHs.
This means that Pb inhibited the electroreduction of Sn(II) in the citrate solutions, although at pH 3.2 the content of Sn was essentially increased up to 30.8%, compared to higher pHs. Further studies are needed in order to find the optimal conditions for electrowinning from the given leachate, i.e., when the electrodeposits ratio of Sn to Cu and Pb content would be closer to their concentration ratio in the solution.
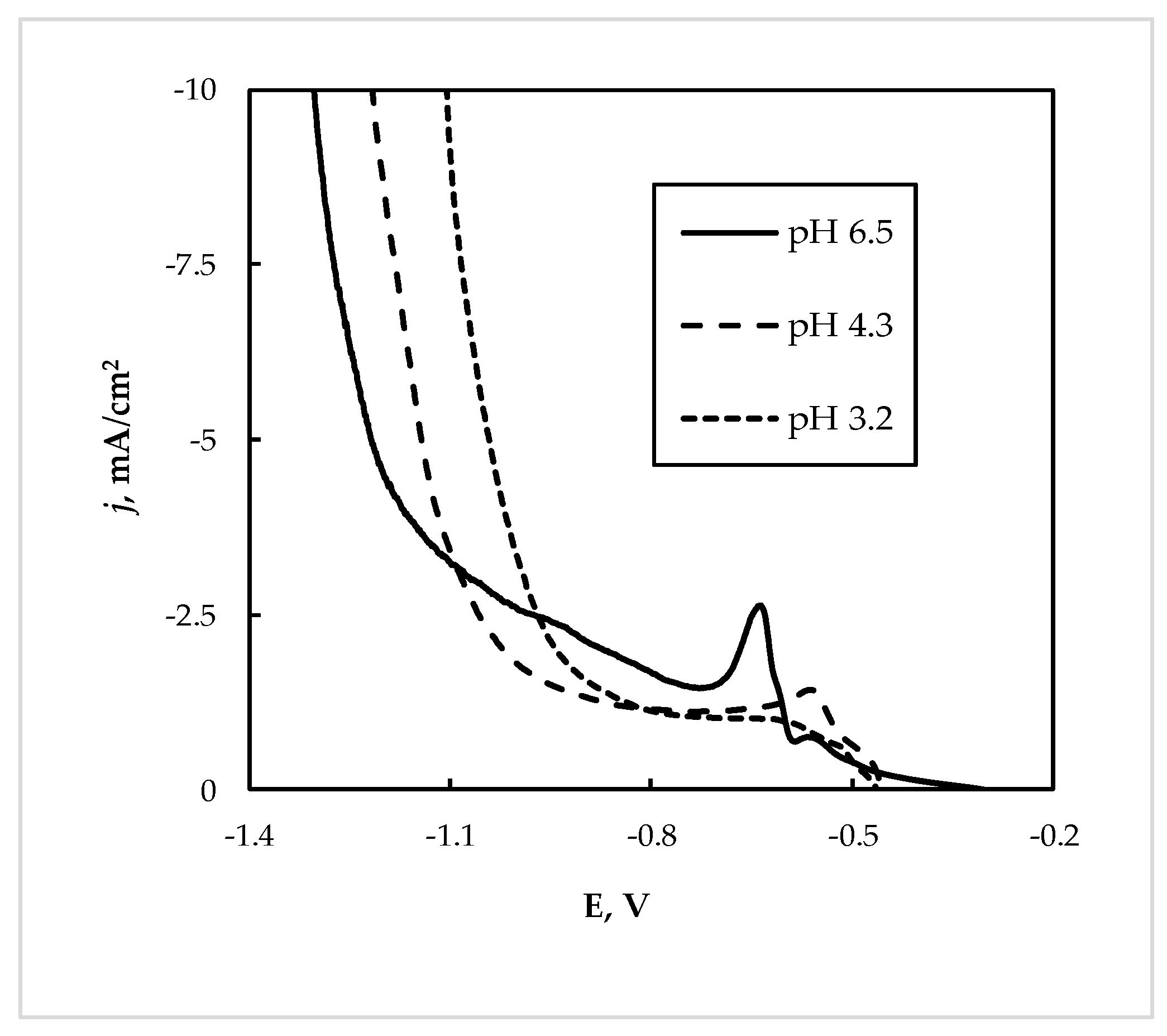
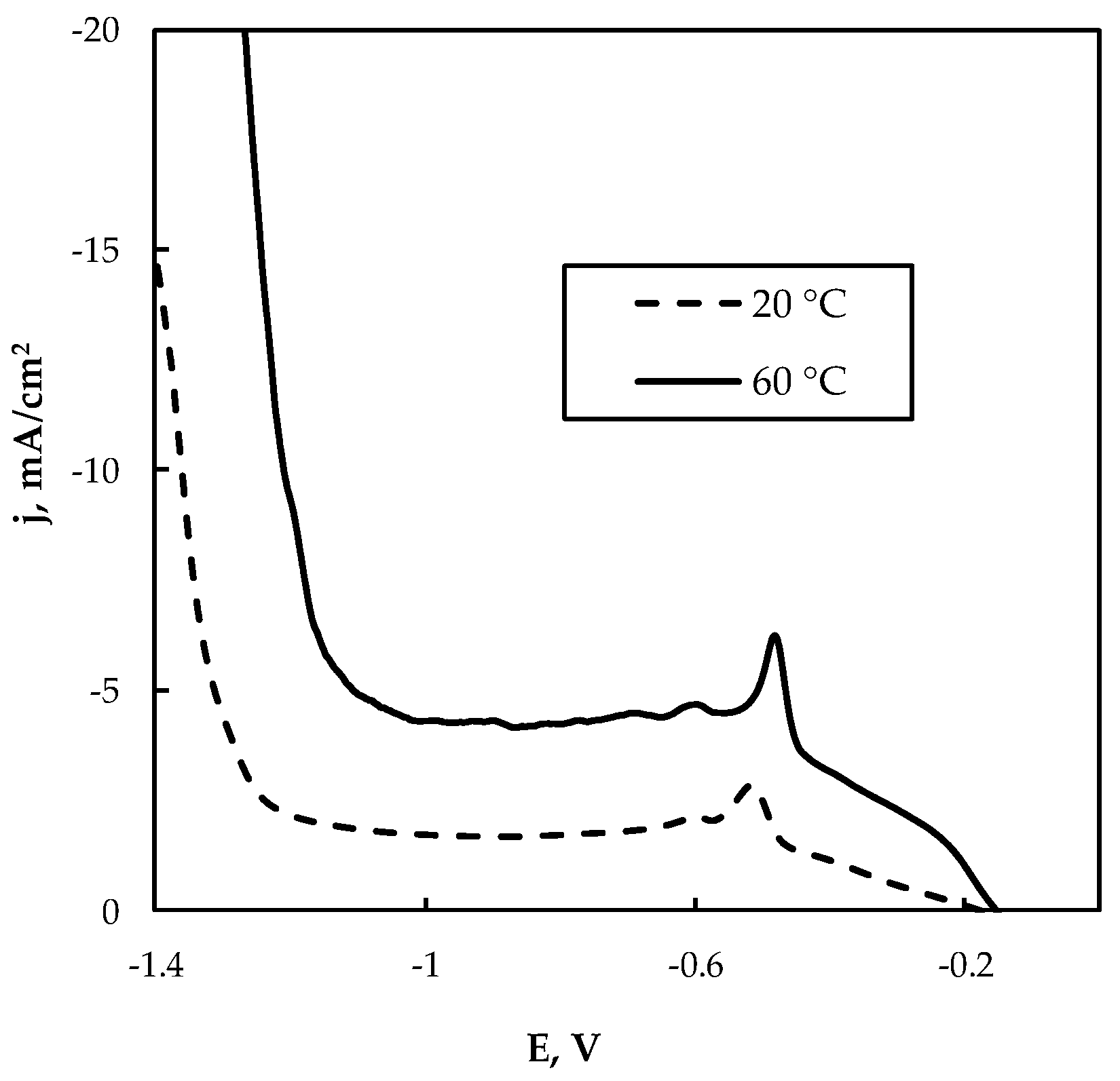
The leachate “L2” was obtained from the selected leaching of WEEE, namely from the waste of printed circuit boards, and therefore it contains essential contents of Cu(II) and Sn(II), and the content of Sn(II) is higher than Cu(II) (see Table 1). As shown in Figure 8, in this case, the cathodic current strongly depends on temperature, and at 60 °C the currents were 2–3 times higher than those obtained at 20 °C. Therefore, performing electrowinning at elevated temperatures will be beneficial for the process. The potentiostatic conditions were also applied in this case. The deposition at higher cathodic potentials (−0.85 V and higher) led to the formation of crumble powder from the electrode, which is an unacceptable result from a practical point of view.
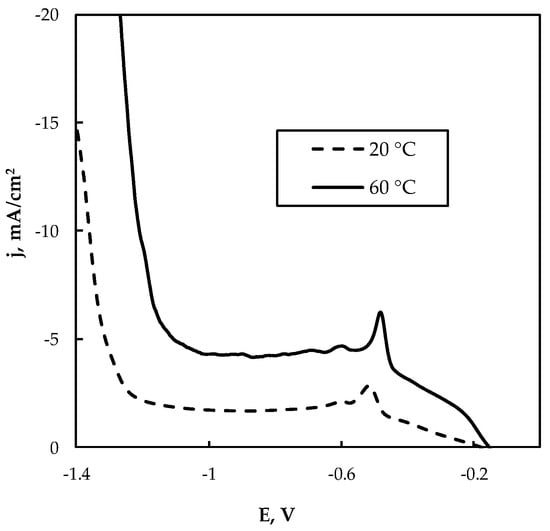
Figure 8.
Cathodic polarization curve on Cu electrode at 20 °C and 60 °C from the leachate “L2”; potential scan rate 5 mV/s, pH 6.5.
Meanwhile, a compact coating formed on the steel cathode (see Figure 9) when the electrodeposition was carried out at lower cathodic potentials (−0.6 V) with a deposition rate of 1.27 × 10−4 g·cm−2·min−1.

Figure 9.
SEM image of electrodeposit obtained at E = −0.6 V and 60 °C from the solution “L2”.
However, in this case, the ratio of metals in the electrodeposits differed from the ones in the solutions (see Table 3), i.e., under the given deposition conditions Cu was the dominant element in the electrodeposit, while Sn(II) was dominant in the solution. Therefore, future studies will be directed to found the optimal conditions of electrowinning from such leachates in order to obtain electrodeposits with a higher content of Sn.

Table 3.
The composition of deposits obtained at −0.60 V and 60 °C from leachate “L2” at pH 6.5.
4. Conclusions
The electrowinning of various metals from the leachates obtained in the course of WEEE chemical treatment by environmentally friendly approaches was reviewed and discussed, including essentials on the configuration of cathodes and solutions for technological processes.
The perspectives of tin electrowinning from citrate-based solutions were assessed as a case study. The tin electrodeposition from a citric acid solution is a rather effective process: the deposition rate of 0.0035 g·cm−2·min−1 and the current efficiency of 86% can be achieved under potentiostatic conditions (E = −0.85 V) and a temperature of 60 °C.
However, the presence of Cu(II) and Pb(II) inhibited the deposition rate of tin. Hence, Sn content decreased significantly in the deposits compared to the ratio of Sn(II) concentration to concentrations of Cu(II) and Pb(II) in the solution. Therefore, further studies would be directed to find the optimal conditions for electrowinning from the leachates in order to achieve the ratio of Sn content to Cu and Pb contents in the deposits closer to their concentration ratios in the solution.
Author Contributions
Investigation, N.T. and H.C.; Methodology and planning of experiments, H.C.; Visualization, N.T.; Writing—Original Draft Preparation, H.C. and N.T.; Writing—Review and Editing, H.C. and N.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research has received funding from Horizon 2020 research and innovation program under MSCA-RISE-2017, project SMARTELECTRODES No. 778357, and partially was supported by the National Agency for Research and Development Moldova, within the project No. 20.80009.5007.18
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Waste from Electrical and Electronic Equipment (WEEE). Available online: https://environment.ec.europa.eu/topics/waste-and-recycling/waste-electrical-and-electronic-equipment-weee_en (accessed on 27 December 2022).
- Hagelüken, C.; Goldmann, D. Recycling and circular economy—Towards a closed loop for metals in emerging clean technologies. Miner Econ. 2022, 35, 539–562. [Google Scholar] [CrossRef]
- Talens Peiró, L.; Nuss, P.; Mathieux, F.; Blengini, G.A. Towards Recycling Indicators Based on EU Flows and Raw Materials System Analysis Data; JRC Technical Report; EUR 29435 EN; Publications Office of the European Union: Luxembourg, 2018. [Google Scholar]
- Žiūkaitė, S.; Ivanauskas, R.; Tatariants, M.; Denafas, G. Feasibilities for hydrometallurgical recovery of precious metals from waste printed circuit boards in Lithuania. Chemija 2017, 28, 109–116. [Google Scholar]
- Tunsu, C.; Retegan, T. Hydrometallurgical processes for the recovery of metals from WEEE. In WEEE Recycling; Chagnes, A., Cote, G., Ekberg, C., Nilsson, M., Retegan, T., Eds.; Elsevier Science BV: Amsterdam, The Netherlands, 2016; pp. 139–175. [Google Scholar]
- Kamarska, K. Citric acid as an eco-friendly inhibitor for the EN AW-2024 aluminum alloy corrosion in acidic medium. J. Ecol. Eng. 2023, 24, 307–311. [Google Scholar] [CrossRef]
- Beukes, N.T.; Badenhorst, J. Copper electrowinning: Theoretical and practical design. In Proceedings of the hydrometallurgy conference SAIMM, Gauteng, South Africa, 24–26 February 2009; pp. 213–240. [Google Scholar]
- Haeger, A.; Forrestal, C.; Xu, P.; Ren, Z.J. High performance spiral wound microbial fuel cell with hydraulic characterization. Bioresour. Technol. 2014, 174, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Ramachandra Rao, S. Hydrometallurgical processes. In Waste Management Series; Ramachandra Rao, S., Ed.; Elsevier Science BV: Amsterdam, The Netherlands, 2006; Volume 7, pp. 71–108. [Google Scholar]
- Vainoris, M.; Cesiulis, H.; Tsyntsaru, N. Metal foam electrode as a cathode for copper electrowinning. Coatings 2020, 10, 822. [Google Scholar] [CrossRef]
- Liu, C.K.; Li, C.W. Simultaneous recovery of copper and surfactant by an electrolytic process from synthetic solution prepared to simulate a concentrate waste stream of a micellar-enhanced ultrafiltration process. Desalination 2004, 169, 185–192. [Google Scholar] [CrossRef]
- Almeida, L.C.; Gasparotto, L.H.S.; Bocchi, N.; Rocha-Filho, R.C.; Biaggio, S.R. Galvanostatic Pb(II) removal from a simulated wastewater by using a stainless-steel wool cathode in a flow-through cell: A factorial-design study. J. Appl. Electrochem. 2008, 38, 167–173. [Google Scholar] [CrossRef]
- Chang, S.H.; Wang, K.S.; Hu, P.I.; Lui, I.C. Rapid recovery of dilute copper from a simulated Cu–SDS solution with low-cost steel wool cathode reactor. J. Hazard. Mater. 2009, 163, 544–549. [Google Scholar] [CrossRef]
- Naumov, K.D.; Lobanov, V.G.; Zelyakh, Y.D. Gold Electrowinning from cyanide solutions using three-dimensional cathodes. Metallurgist 2017, 61, 249–253. [Google Scholar] [CrossRef]
- Lister, T.E.; Wang, P.; Anderko, A. Recovery of critical and value metals from mobile electronics enabled by electrochemical processing. Hydrometallurgy 2014, 149, 228–237. [Google Scholar] [CrossRef]
- Pilone, D.; Kelsall, G.H. Metal recovery from electronic scrap by leaching and electrowinning IV. In Electrometallurgy and environmental hydrometallurgy; Young, C., Alfantazi, A., Anderson, C., James, A., Dreisinger, D., Harris, B., Eds.; Wiley: Hoboken, NJ, USA, 2013; Volume 2, pp. 1565–1575. [Google Scholar]
- Vegliò, F.; Quaresima, R.; Fornari, P.; Ubaldini, S. Recovery of valuable metals from electronic and galvanic industrial wastes by leaching and electrowinning. Waste Manag. 2003, 23, 245–252. [Google Scholar] [CrossRef]
- Preetam, A.; Modak, A.; Jadhao, P.R.; Naik, S.N.; Pant, K.K.; Kumara, V. A comprehensive study on the extraction of transition metals from waste random access memory using acetic acid as a chelating solvent. J. Environ. Chem. Eng. 2022, 10, 108761. [Google Scholar] [CrossRef]
- Belfqueh, S.; Seron, A.; Chapron, S.; Arrachart, G.; Menad, N. Evaluating organic acids as alternative leaching reagents for rare earth elements recovery from NdFeB magnets. J. Rare Earths 2022, in press. [Google Scholar] [CrossRef]
- Musariri, B.; Akdogan, G.; Dorfling, C.; Bradshaw, S. Evaluating organic acids as alternative leaching reagents for metal recovery from lithium ion batteries. Miner. Eng. 2019, 137, 108–117. [Google Scholar]
- Mohammadi, M.; Nakhaie, D.; Asselin, E.; Alfantazi, A. Failure investigation of stainless steel anodes used in gold electrowinning. Eng. Fail. Anal. 2019, 106, 104183. [Google Scholar] [CrossRef]
- Soleymani, M.; Sadri, F.; Zhang, S.; Ghahreman, A. The role of thiosulfate and sulfite in gold thiosulfate electrowinning process: An electrochemical view. Process. Saf. Environ. Prot. 2022, 166, 232–240. [Google Scholar] [CrossRef]
- Kasper, A.C.; Carrillo, A.J.; García Gabaldón, M.; Veit, H.M.; Pérez, H.V. Determination of the potential gold electrowinning from an ammoniacal thiosulphate solution applied to recycling of printed circuit board scraps. Waste Manag. Res. 2016, 34, 47–57. [Google Scholar] [CrossRef]
- Urbanski, T.S.; Fornari, P.; Abbruzzese, C. Gold electrowinning from aqueous–alcoholic thiourea solutions. Hydrometallurgy 2000, 55, 137–152. [Google Scholar] [CrossRef]
- Ippolito, N.M.; Birloaga, I.; Ferella, F.; Centofanti, M.; Vegliò, F. Preliminary study on gold recovery from high grade e-waste by thiourea leaching and electrowinning. Minerals 2021, 11, 235. [Google Scholar] [CrossRef]
- Wang, Z.; Peng, C.; Yliniemi, K.; Lundström, M. Recovery of high-purity silver from spent silver oxide batteries by sulfuric acid leaching and electrowinning. ACS Sustain. Chem. Eng. 2020, 8, 15573–15583. [Google Scholar] [CrossRef]
- Safarzadeh, M.S.; Bafghi, M.S.; Moradkhani, D.; Ilkhchi, M.O. A review on hydrometallurgical extraction and recovery of cadmium from various resources. Miner. Eng. 2007, 20, 211–220. [Google Scholar] [CrossRef]
- Takemoto, K.; Imori, T.; Ouchi, T.; Takahashi, H. Method For Manufacturing High Purity Tin, Electrowinning Apparatus for High Purity Tin and High Purity Tin. U.S. Patent 20160097139, 2016. Available online: https://www.freepatentsonline.com/y2016/0097139.html (accessed on 27 December 2022).
- Kanazawa, T.; Morimoto, K.; Yamaguchi, I. Tin Electrowinning Method. Japan Patent JP2020117798A, 6 August 2020. [Google Scholar]
- Ishiwatari, M. Method and apparatus for electrolytic purification of high purity tin. Japan Patent JP3882608B2, 21 February 2020. [Google Scholar]
- Guo, X.; Qin, H.; Tian, Q.; Li, D. Recovery of metals from waste printed circuit boards by selective leaching combined with cy-clone electrowinning process. J. Hazard. Mater. 2020, 384, 121355. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Li, W.Q.; Wang, Z.Y.; Yang, Y.J.-Q.; Zheng, A.-N.; Yang, F.-Z.; Zhan, D.; Wu, D.-Y.; Tian, Z.-Q. Novel, simple, and green citrate-based copper electronic electroplating bath in microvia void-free filling for printed circuit board application. ACS Sustain. Chem. Eng. 2022, 10, 14204–14211. [Google Scholar] [CrossRef]
- Bigos, A.; Wolowicz, M.; Janusz-Skuza, M.; Starowicz, Z.; Szczerba, M.J.; Bogucki, R.; Beltowska-Lehman, E. Citrate-based baths for electrodeposition of nanocrystalline nickel coatings with enhanced hardness. J. Alloys Compd. 2021, 850, 156857. [Google Scholar] [CrossRef]
- Sharma, A.; Min Park, Y.; Lee, S.; Ahn, B. Morphology, resistivity and corrosion behavior of tin coatings plated from citric acid bath. Mater. Res. Express 2019, 6, 116589. [Google Scholar] [CrossRef]
- Halli, P.; Agarwal, V.; Partinen, J.; Lundström, M. Recovery of Pb and Zn from a citrate leach liquor of a roasted EAF dust using precipitation and solvent extraction. Sep. Purif. Technol. 2020, 236, 116264. [Google Scholar] [CrossRef]
- Tsurusaki, T.; Ohgai, T. Mechanical properties of solder-jointed copper rods with electrodeposited Sn-Zn alloy films. Materials 2020, 13, 1330. [Google Scholar] [CrossRef]
- Ding, L.; Chen, C.; Li, Q.; Yuan, J.; Li, H.; Xue, Y.; Dong, H.; Li, B.; Niu, Y. Process and theoretical research on electroplating Cu–Sn alloys of low Sn. J. Appl. Electrochem. 2021, 51, 1287–1299. [Google Scholar] [CrossRef]
- Guo, F.Y.; Yu, J.K.; Xiao, J.J.; Qiao, Q.; Yang, H.B.; Guo, Y.Q. Preparation of FeCoNiCr high entropy alloy coatings and optimization of process parameters. Rare Metal. Mat. Eng. 2021, 50, 2337–2342. [Google Scholar]
- Deng, D.; Deng, C.; Liu, T.; Xue, D.; Gong, J.; Tan, R.; Mi, X.; Gong, B.; Wang, Z.; Liu, C.; et al. Selective recovery of copper from electroplating sludge by integrated EDTA mixed with citric acid leaching and electrodeposition. Sep. Purif. Technol. 2022, 301, 121917. [Google Scholar] [CrossRef]
- Jadhav, U.; Su, C.; Hocheng, H. Leaching of metals from large pieces of printed circuit boards using citric acid and hydrogen peroxide. Environ. Sci. Pollut. Res. 2016, 23, 24384–24392. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).