Abstract
Currently, frequent oil spill accidents caused by transportation, storage, and usage may lead to extensive damage to marine ecosystems. Effective methods for oil spillage recovery from offshore environments are still urgently in demand. A superhydrophobic sponge (MS@PVC@SiO2) was obtained via a facile two-step method for rapid oil adsorption, and a piece of novel collection equipment loaded with MS@PVC@SiO2 was developed for in situ continuous oil/seawater separation. The results showed that MS@PVC@SiO2 exhibits excellent water repellence, compressibility, and durability. Furthermore, the obtained MS@PVC@SiO2 shows high diesel oil adsorption capacity (32 g/g), and excellent recyclability (up to 200 times). The collection equipment demonstrates highly selective oil adsorption capacity and good stability in real seawater. The maximum possible recovery capacity of collection equipment was 557.784 L/h with 98% efficiency, which was much higher than that of commercial disc oil collectors (119.8 L/h). The recovery performance was effectively improved by introducing MS@PVC@SiO2, due to its large specific area and enough storage space. Moreover, even after continuous operation for 58 h in seawater, the collection equipment remained at a high recovery capacity. The results indicate that both MS@PVC@SiO2 and the collection equipment have great application perspectives in practical marine oil spillage recovery.
1. Introduction
With the development of industrialization, there is an increasing demand for oils and organic chemicals. Oil spillage and chemical leak accidents occur frequently worldwide for offshore oil and gas production and transportation [1,2,3]. A typical example is the 2010 Gulf of Mexico oil spill, which released an estimated 4.9 million barrels of crude oil into the ocean, resulting in severe damage to marine life and coastal wetlands [4]. The 2018 gas condensate leak accident in the East China Sea was caused by a tanker collision. An estimated 58 square kilometers of condensate was released into the ocean, which had serious effects on the water quality and marine life in the area. The long-term effects of environmental pollution by such accidents remain a matter of major concern [5,6,7]. Oils, such as light diesel oil, have low viscosity, volatility, flammability, and complex forms of movement in water [8]. If no effective measures are taken immediately after spillage, secondary accidents, including explosions, fire, and poisoning may be caused [9]. Currently, several technologies have been developed to clean up oil spillage, including (i) mechanical collection (skimmers or booms), (ii) physical adsorption (adsorbents), (iii) in situ burning, (iv) chemical dispersants, (v) bioremediation [10,11,12,13,14]. However, poor efficiency, tedious operation process, and high energy consumption make them not the ideal way to realize practical oil/water separation [15,16]. Thus, it is imperative to develop effective methods or equipment for chemical recovery.
It should be noted that adsorbents and device combination oil recovery technology can play a key role in reducing the impact of oil spill accidents, as well as protecting the environment [17,18]. Physical adsorption [19,20,21] and mechanical collection [22,23] have demonstrated the potential for continuous oil/water separation. Adsorbents with superhydrophobic surfaces are the most attractive candidate for chemical adsorption [24,25]. However, traditional adsorbents experience various problems such as high materials cost, low oil sorption capacity, and unsatisfactory reusability [26]. Recently, three-dimensional (3D) porous adsorbents with special wettability, such as melamine sponge [27], polyurethane foam [28,29], carbon aerogels [30,31], and silicone sponges [32] have attracted attention due to their high porosity, selectivity, and absorption capacity [33,34]. Among them, commercialized melamine sponge (MS) with low cost, excellent absorption, hot stability, thermal insulation, and flame retarding properties, is highly desired, since it provides an industrial-scale, economically viable option [35,36,37]. Stolz et al. [38] synthesized a carbonized MS via a single-step pyrolysis treatment with temperatures of 500–600 without further chemical treatment for the clean-up of oil-polluted water. Li et al. [39] applied 2,2-azoisobutyronitrile (AIBN) as initiator, polydimethylsiloxane (PDMS) as prepolymer, and divinylbenzene (DVB) as a monomer to obtain PDVB-PDMS coated superhydrophobic MS, which shows robust superhydrophobicity and high adsorption capability under harsh conditions. Lei et al. [29] fabricated a hydrophobic and catalytic MS via the solvothermal growth of hydrophobic ZIF-8 on its strut surface, which showed excellent oil/water separation performance. However, the carbonized MS usually has a low specific area with sacrificed mechanical properties, which failed to realize long-term stability. Hydrophobic modification of MS via polymerization or gelation normally needs toxic agents or expensive monomers, and sometimes complex preparation processes such as microwave treatment and UV irradiation are involved. By coating nanoparticles, the nanoparticles might peel off from MS in the long term. Thus, to meet the needs of continuous oil/water separation under harsh environments, preparation of economically viable, easy preparation, eco-friendly, robust mechanical properties, and long-term stability superhydrophobic MS is very desirable. When modified MS was used as absorbent, the separation process was non-continuous. Each absorption must be followed by desorption via squeezing or burning. In consequence, saturated adsorbents need to be collected by fishermen manually, which is time- and labor-consuming. Hence, it is difficult for adsorbents to achieve continuous oil/water separation in real-world applications. To solve this problem, the idea of designing a mechanical recovery device based on oil-sorbent materials came up, for continuous oil/water separation with high efficiency. At present, scholars have studied oil-collecting devices. For example, a vessel-type collector was constructed by superhydrophilic–underwater superoleophobic filtration adsorbents [40]. The floating oil can selectively penetrate through the vessel wall and then be collected in the vessel. In another case, an external pumping technique was introduced to realize the continuous collection of oil in situ from the water surface. The oil sorption capacity can be no longer limited to the volume and weight of the adsorbents. However, these technologies were still at the theoretical stage in the laboratory. In the future, the combination of adsorbents and devices might be the most promising technology for practical oil spillage recovery. To realize continuous oil/water separation by 3D porous absorbents in real oil spillage accidents, the novel idea of designing a mechanical recovery device based on oil-sorbent materials came up. For application in the real world, the superhydrophobic 3D porous absorbents must be low-cost, eco-friendly, have good elasticity, high stability, and are easy to prepare on a large-scale. In this work, commercialized MS was selected as a substrate for its excellent absorption, hot stability, thermal insulation, and flame retarding properties. To realize high oil selectivity adsorption, the commercially available inexpensive Poly (vinyl chloride) (PVC) was applied to form a water-repellent polymer film, due to its intrinsic hydrophobicity and chemical stability. However, the polymer surface cannot meet the requirements of the superhydrophobicity. Modified SiO2 nanoparticles was added to create a rough structure on the hydrophobic polymer surface for superhydrophobic coating. Superhydrophobic modified MS was then fabricated by simply soaking MS in the silica-based polymer coating solution. The preparation process is easy and simple, which meets the needs of large-scale production. The modified MS shows an excellent oil selective adsorption performance toward corrosive liquids, including basic and acidic solutions. Additionally, the modified MS demonstrates good mechanical durability, even more than 200 cycles of absorption-desorption could not diminish the material’s superhydrophobicity. Based on the property of the modified MS, explosion-proof type collection equipment was invented by combining the superhydrophobic modified MS with a self-designed mechanical device, which realizes continuous oil/water separation from offshore environments. The collection equipment exhibits a high recovery capacity (557.784 L/h with 98% efficiency), which was much higher than that of commercial disc oil collectors (119.8 L/h). Importantly, the collection equipment also shows good stability even after continuous operation for 58 h in real seawater.
2. Materials and Methods
2.1. Materials
Melamine sponges (MS, density 0.018 g/cm3, porosity 95.5%, pore size 120–170 μm) were purchased from a local supermarket. Poly-vinyl chloride (PVC, K-value 59–55), hydrophobic SiO2 particles (R972), and 3-Aminopropyltriethoxy silane coupling agent (KH550) were received from Aladdin Reagent Co., Ltd. (Shanghai, China). Ethanol (analytically pure) and tetrahydrofuran (THF, analytically pure) were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Diesel oil (0#) was purchased from the Sinopec gas station. Seawater (density 11.6 × 103 g/cm3) was collected from Jiaozhou Bay. Deionized water was prepared by Exo-Pure Water Machine Exceed -Ba in a laboratory.
2.2. Fabrication of Superhydrophobic Sponge
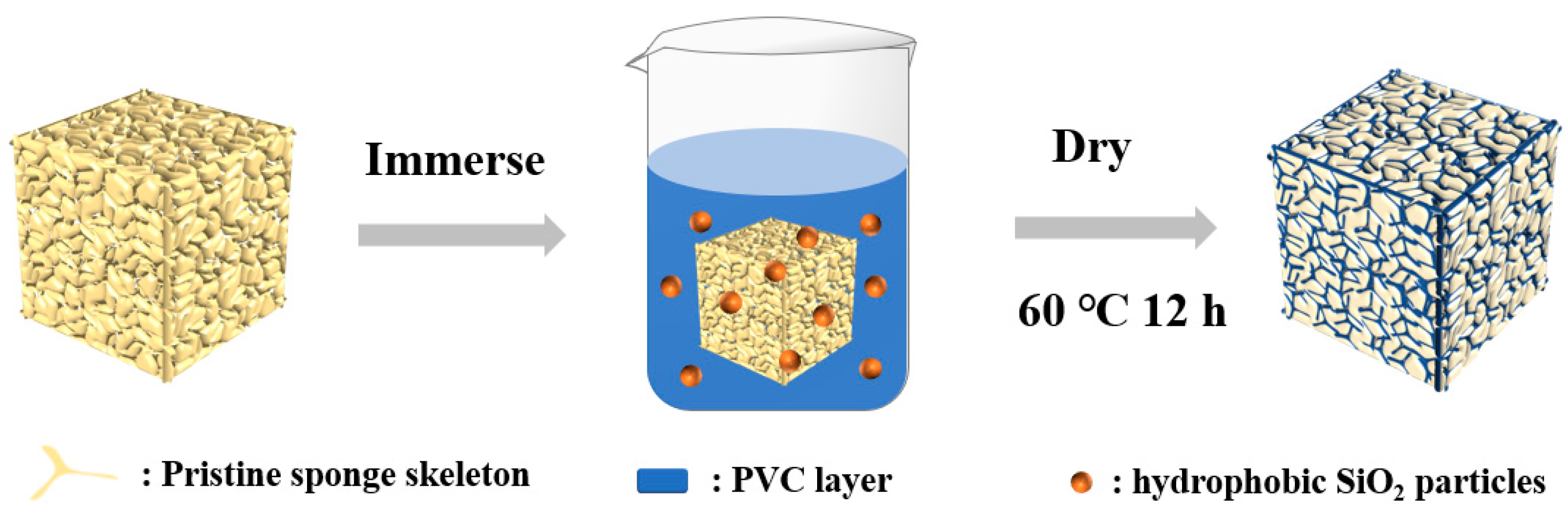
Before use, MS were ultrasonically cleaned by pure ethanol and deionized water for at least 30 min each. In a typical experiment, PVC (1 g) was dissolved in THF (100 mL) [41], followed by the addition of R972 (0.5 g). The suspension was then stirred vigorously for 30 min. PVC (1 wt.%) dissolved in KH550 was then added dropwise under magnetic stirring. The drying melamine sponge was immersed into the mixture solution for 12 h. Finally, the superhydrophobic sponge (MS@PVC@SiO2) was dried at 60 °C. Schematic illustration for the fabrication of MS@PVC@SiO2 sponge was shown in Figure 1.
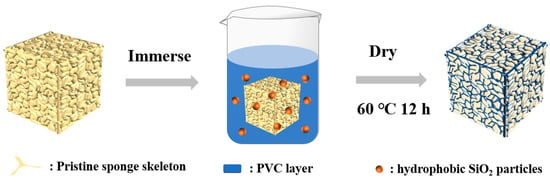
Figure 1.
Schematic illustration for the fabrication of MS@PVC@SiO2 sponge.
2.3. Characterization
The surface morphologies of MS and MS@PVC@SiO2 were observed by scanning electron microscopy (SEM, S4800, Tokyo, Japan). The contact angle measurement was acquired using a contact angle meter (OCA20, DataPhysics, Stuttgart, Germany) at room temperature (22 °C). The average value was determined by measuring five different positions. Fourier transform infrared spectroscopy (FT-IR, NEXUS, Madison, America) was conducted to analyze the chemical compositions of samples. Instron 5967-E2 was applied to characterize the mechanical properties of the samples. The porosity of MS was measured in our laboratory. The pristine MS was first cut into cubes, then soaked in pure water. The sponge was then squeezed by several times to extrude air bubbles. The porosity was calculated as:
where M1 (g) and M2 (g) were the mass of MS before and after adsorption, respectively, p (g/cm3) represents the water density, and V is the sample volume.
2.4. Collection Equipment
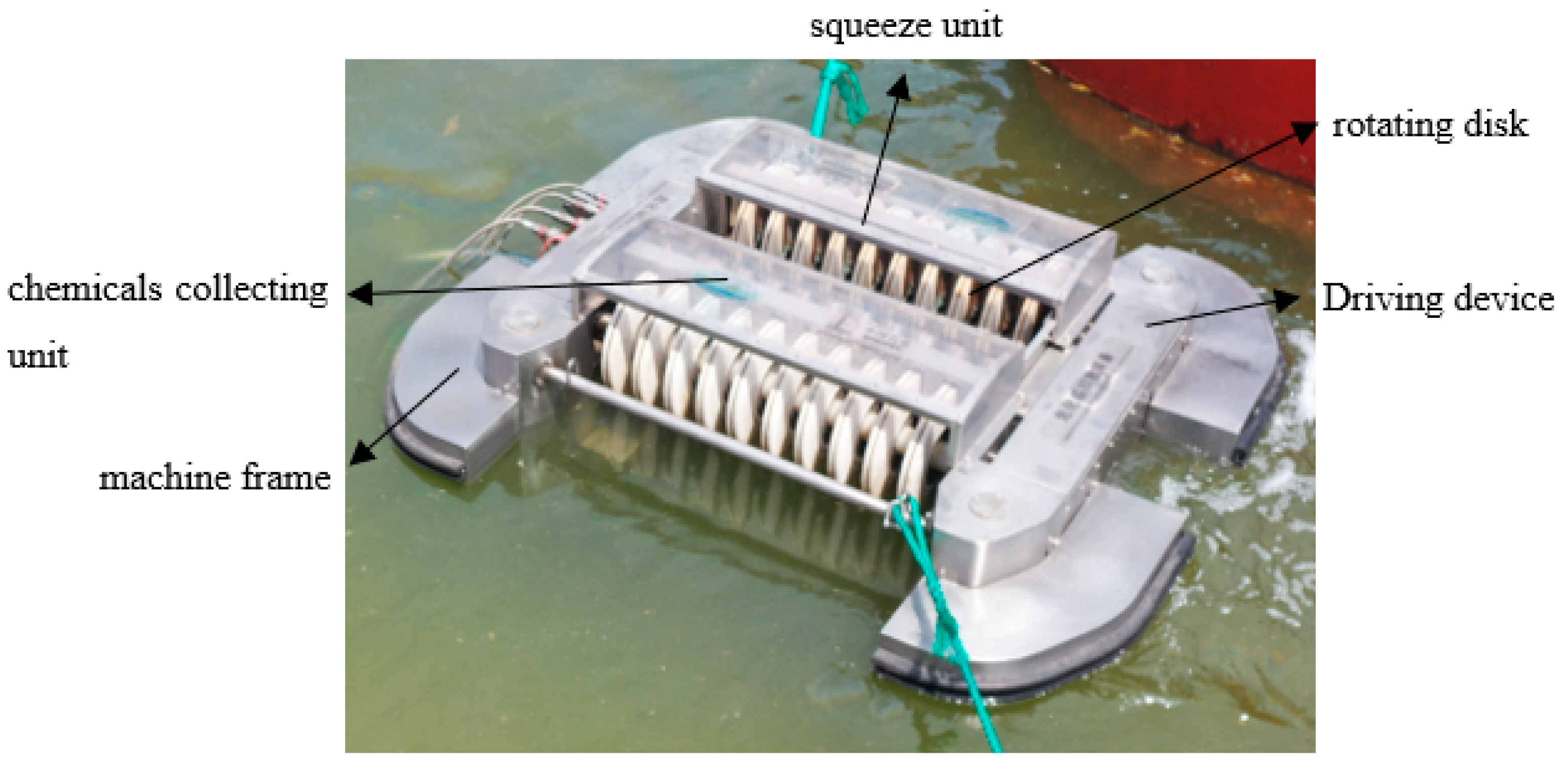
An explosion-proof type of collection equipment based on MS@PVC@SiO2 was designed and made of 316 L. As shown in Figure 2, the collection equipment is composed of a machine frame, chemicals collecting unit, rotating disk coated with MS@PVC@SiO2, squeeze unit, and driving device. All the components are explosion-proof or anti-static. In the operation process, MS@PVC@SiO2 is on the rotating disk immediately and selectively adsorbs oils or water-insoluble organic solvents on the surface of the water. During rotation, oils adsorbed in MS@PVC@SiO2 were then squeezed out by the squeezing unit above. Then, the oils recovered will be collected and transported into the chemical collecting unit. Hence, the automated collection equipment can ensure the recyclability and recoverability of superhydrophobic sponges, which avoids labor and time-consuming oils-soaked adsorbents collection [42].
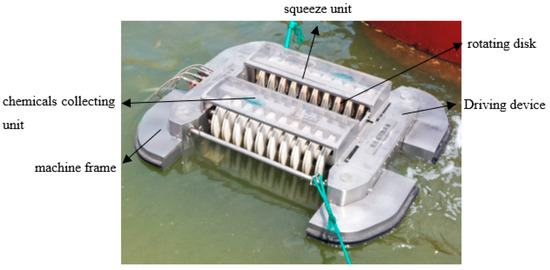
Figure 2.
Image of the chemical collector.
2.5. Adsorption Performance
The adsorption capacity (Q) of MS@PVC@SiO2 for diesel oil was measured. The MS@PVC@SiO2 were first put into diesel oil. After saturation, the weights of the sample before (M1) and after adsorption (M2) were measured. The adsorption capacity was calculated as:
The cycling stability of MS@PVC@SiO2 in real seawater was evaluated by cyclic absorption-squeezing measurement. MS@PVC@SiO2 was compressed for 200 cycles. The mass of MS@PVC@SiO2 before and after adsorption was recorded and calculated by Equation (2).
2.6. Continuous Oil/Water Separation Performance
To study the maximum possible recovery capacity of collection equipment, experiments were conducted referring to the ASTM standard [43]. The chemical collector was placed into a test tank, which is three times the length and width of the chemical collector, and the recovery tank was placed by the waterside. During the process of experiments, the chemical collector must stay in the center of the test area and remain free-floating. The start thickness of diesel oil was approximately 75 mm, after operation for at least 30 s, then end experiments when the thickness approaches 50 mm. Each experiment was repeated three times.
The recovery capacity was calculated as follows:
The recovery efficiency can be calculated by the following formula:
where V0 (mL) and V (mL) were the volumes of oils recovered and total liquid, respectively, and t (s) represents the elapsed time.
With floating and volatile characteristics, oils tend to spread rapidly on the water surface under the influence of wind or waves. Thus, the experiments under different diesel oil film thicknesses (0.1, 1, 5, 10, 20, 50, and 75 mm) were carried out to further evaluate the recovery performance of collection equipment.
To investigate continuous oil/water separation performance in real seawater, experiments were conducted at Sinopec Qingdao petrochemical co., LTD, with seawater directly pumped out from Jiaozhou Bay. The equipment was set to automatic mode with a rotating speed of 25 r/min. The diesel oil thickness shall be maintained at 50 mm, during the 7 days (56 h) of operation. The operation process was recorded, and the recovery capacity and efficiency were calculated by Equation (3), and Equation (4), respectively.
3. Results
3.1. Characterization
Figure 1 depicts the preparation process of the MS@PVC@SiO2 sponge. When the cleaned MS was fully immersed into a mixture solution, the skeleton of the MS wrapped the PVC layer with hydrophobic SiO2 microparticles. Upon drying, the “glue” PVC layer and the micro-particles were firmly adhered to the MS skeleton, resulting in the superhydrophobic MS@PVC@SiO2 sponge.
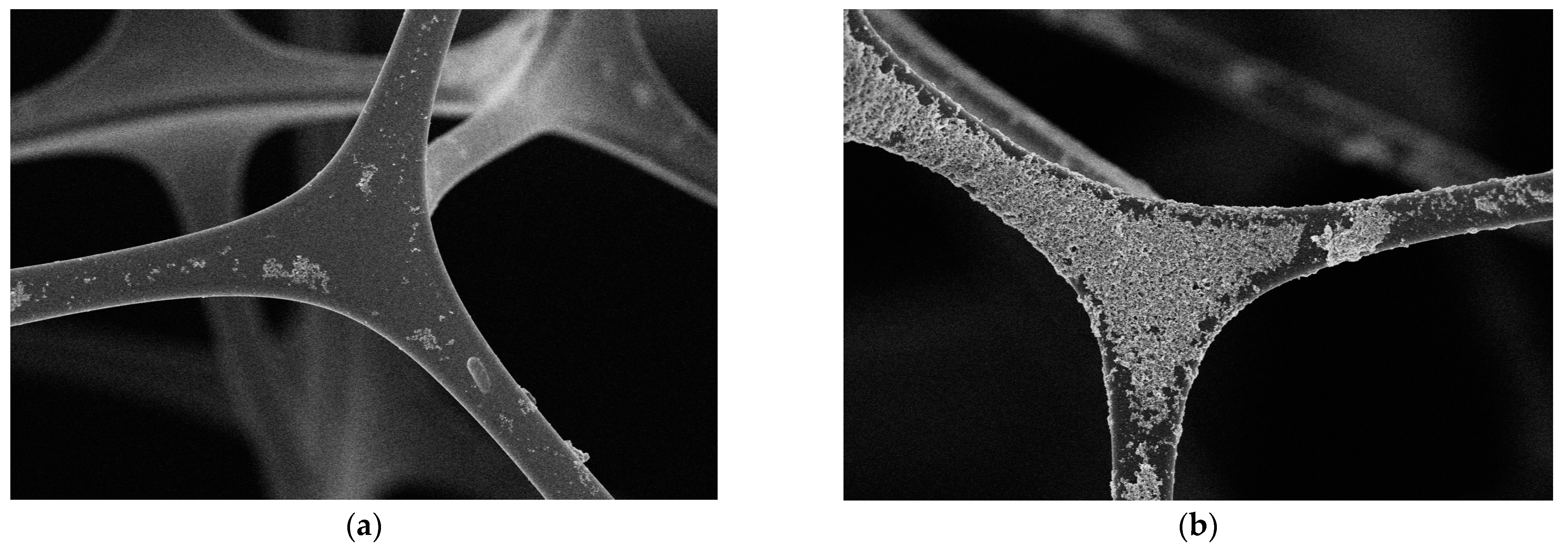
Figure 3 shows the microscopic morphology photo of MS and MS@PVC@SiO2. The image reveals the structure of MS to be a highly porous 3D intercrossed network (Figure 3a), hence being beneficial for oil adsorption. It shows the surface of MS is relatively smooth. After modification, MS@PVC@SiO2 maintained the 3D porous structure. However, the surface of MS@PVC@SiO2 became rough with a dense coating. Figure 3b shows the hydrophobic SiO2 nanoparticles unevenly located on the surface of the sponge skeleton, which increases the roughness of the surface. The randomly rough surface of MS@PVC@SiO2, due to the construction of micro/nano hierarchical structures, is beneficial to form super-hydrophobic surfaces. The observation demonstrated hydrophobic SiO2 nanoparticles can be firmly immobilized by PVC onto the skeleton surface of MS@PVC@SiO2.
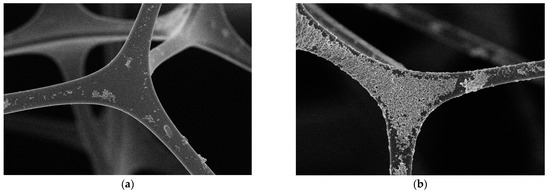
Figure 3.
SEM images of (a) MS; (b) MS@PVC@SiO2.
The surface wettability of MS and MS@PVC@SiO2 was investigated by water contact angle (WCA) measurement at room temperature. The WCA of MS (Figure 4a) is 0° due to its intrinsic hydrophilic. Figure 4b shows the water droplets deposited on the surface of MS@PVC@SiO2 to form almost perfect spheres, with a WCA 149.8°. Moreover, the water droplets can be easily rolled off from the surface of MS@PVC@SiO2, indicating well water repellency. It can be inferred that the water–repelling film was successfully fabricated on the surface of MS@PVC@SiO2. This may be because the “glue” PVC layer and SiO2 microparticles were firmly adhered to the MS skeleton, which gives rise to a nano-scaled roughness that results in superhydrophobicity [44,45]. The temperature effect on WCA is a topic for future works.

Figure 4.
The water contact angle of (a) MS; (b) MS@PVC@SiO2.
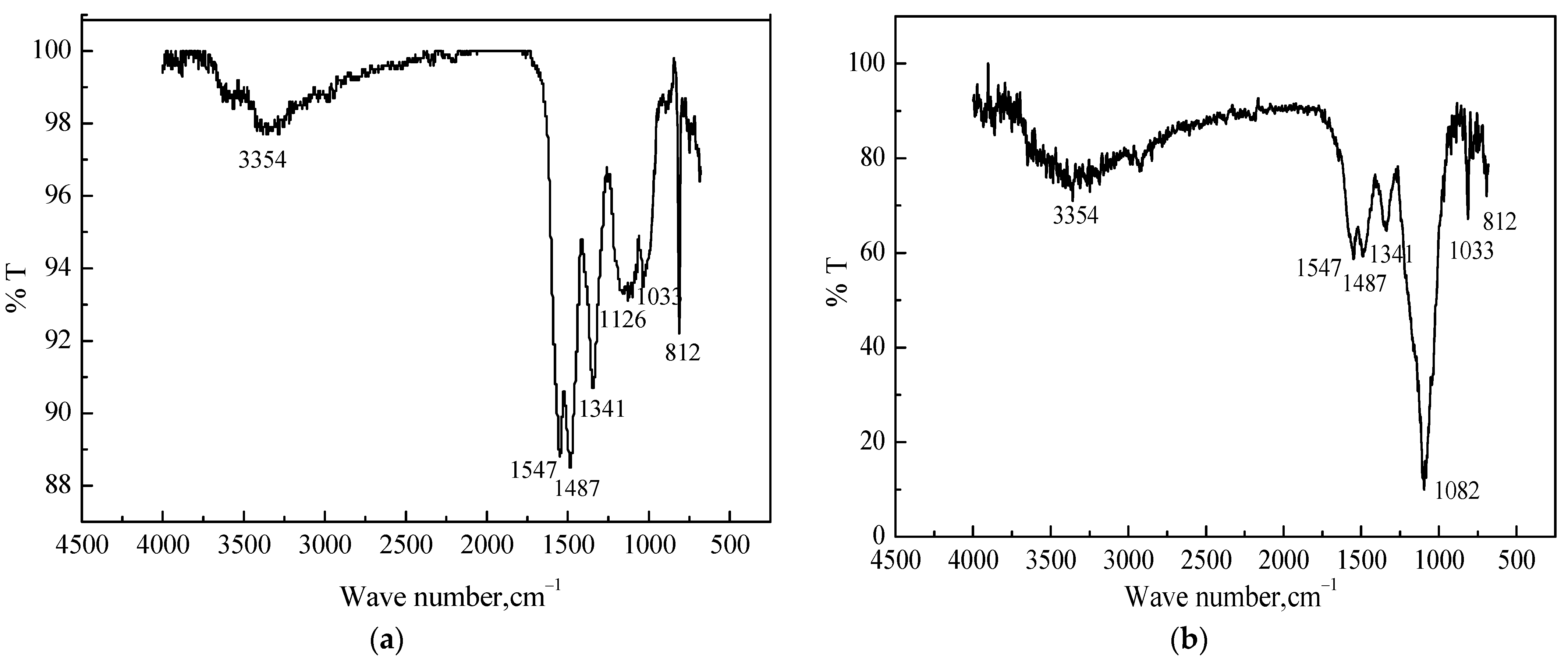
To confirm the composition of MS@PVC@SiO2, FTIR spectra were conducted. Figure 5a shows strong characteristic peaks of MS at 3354, 1487, 1341, and 812 cm−1, which can be ascribed to N–H, C=N, and thiotriazinone vibration [46], respectively. The absorption bands at 1547 cm−1 can be attributed to O–H bending vibration. The peaks located at 1126 and 1033 cm−1 can be attributed to C–O–C stretching. In Figure 5b, compared to MS, the peaks located at 1487, 1341 and 812 cm−1 became less pronounced owing to the coating of PVC. The emergence of a new peak (1082 cm−1) assigning O–Si–O stretching indicated the coating of SiO2 particles on the surface of MS@PVC@SiO2 [47,48]. Moreover, the intensity of O–H stretching peaks at 1547 cm−1 was reduced significantly, implying less hydrophilic oxygen-containing groups on the surface of MS@PVC@SiO2.
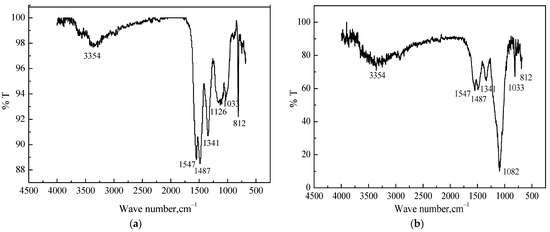
Figure 5.
The FTIR spectra of (a) MS; (b) MS@PVC@SiO2.
The mechanical parameters of MS and MS@PVC@SiO2 are shown in Table 1. It can be inferred that the mechanical properties of MS@PVC@SiO2 have been improved effectively. MS@PVC@SiO2 exhibits satisfying mechanical properties such as high mechanical strength and high ductility. This may be because microcracks or plastic deformation can be formed with the existence of PVC/SiO2 agglomerates, which demonstrates high ductility.

Table 1.
Mechanical properties of the sponge samples.
3.2. Adsorption Performance
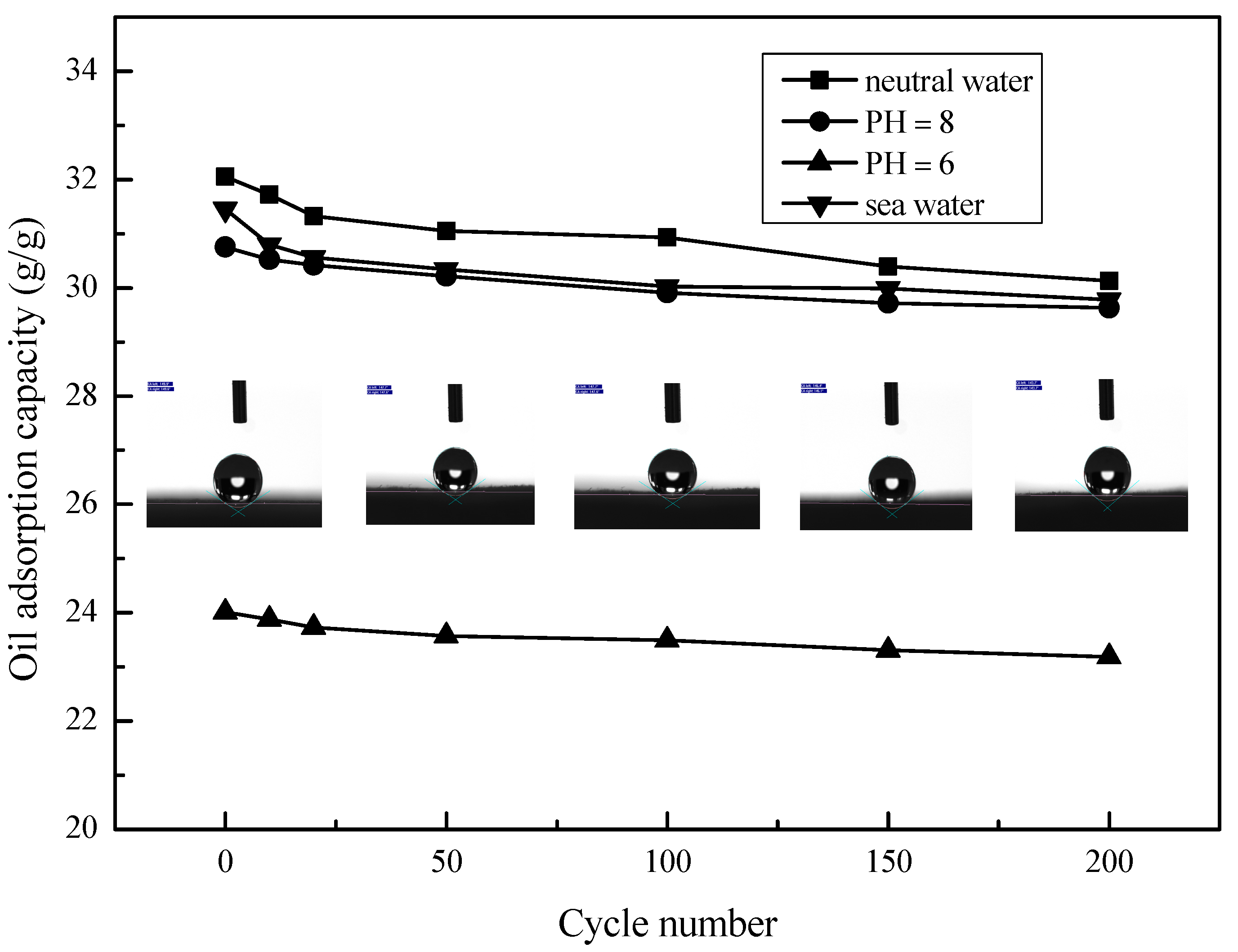
The selective sorption capacity of oil and water by MS and MS@PVC@SiO2 was studied. Table 2 shows that the oil adsorption capacity of MS@PVC@SiO2 was 32.05 g/g with 98.6% efficiency. While the solution absorbed by the pristine MS was mainly water with only 4.9% diesel oil contained. The adsorption capacity for oil of MS@PVC@SiO2 (32.05 g/g) was much higher than that of the pristine MS (2.45 g/g). In addition, the value is higher than most of the absorption capacities of other absorbent materials outlined in the literature (Table 3). Long-term durability against corrosive liquids is essential for adsorbents used in oil/water separation. To investigate the potential of MS@PVC@SiO2 for continuous oil/water separation, the oil adsorption capacity and recyclability under harsh conditions were examined. Figure 6 shows that the adsorption capacities of MS@PVC@SiO2 for diesel oil from water and real seawater were 32.05 and 31.45 g/g, respectively. In addition, the absorbed diesel oil in the MS@PVC@SiO2 matrix can be easily recovered by a simple squeezing method in real seawater. The diesel oil adsorption capacity of MS@PVC@SiO2 remained at 94.7% of its initial adsorption capacity in seawater even after 200 cycles. Moreover, after 200 cycles of absorption and desorption, the contact angle of MS@PVC@SiO2 was still in the range of 143°–147°. From Figure 6, the maximum oil adsorption capacity is typical in neutral water (PH = 7). The oil adsorption capacity decreases in water with a decrease in pH to 6. This may be attributed to the protonation of amine groups under acidic conditions [49,50]. The chains of amine groups changed from a dense state into a loose state, which leads to low diesel oil adsorption of MS@PVC@SiO2 from water. The oil adsorption capacity also decreases in seawater. This may be because amine groups chelated with metal ions in seawater, which causes low diesel oil adsorption of MS@PVC@SiO2 [51,52]. The excellent mechanical and environmental durability demonstrates the great potential of MS@PVC@SiO2 to achieve continuous oil/water separation in real-world applications, due to its high porosity, strong mechanical strength, and high elasticity [53].

Table 2.
The selective sorption capacity of oil and water by MS and MS@PVC@SiO2.

Table 3.
Summary of the adsorption capacities of oils by various sponge-based adsorbents.
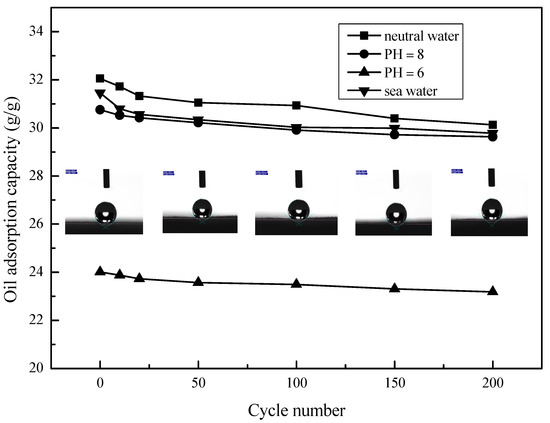
Figure 6.
Adsorption performance of MS@PVC@SiO2 for diesel oil from different water solutions.
3.3. Continuous Oil/Water Separation Performance
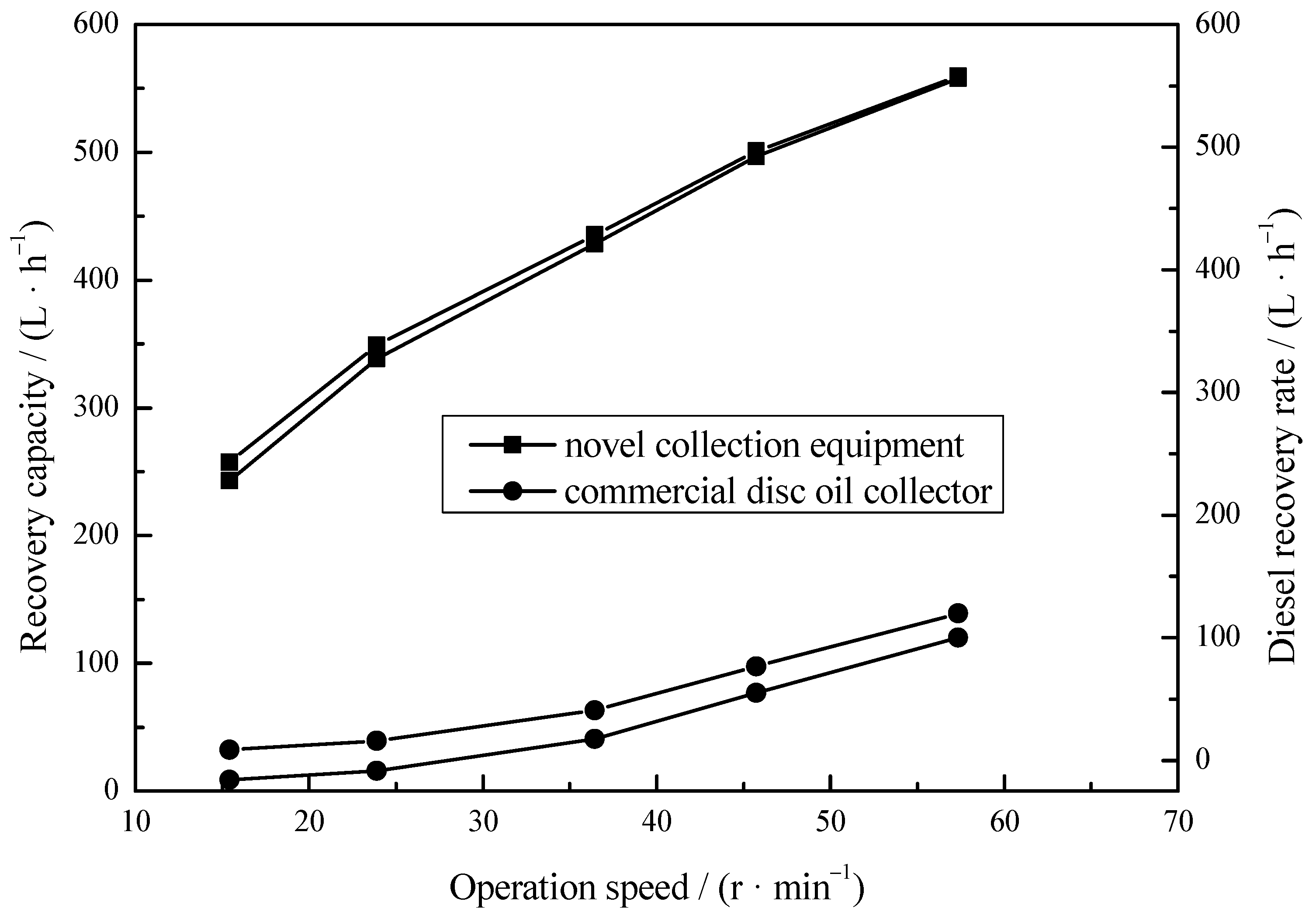
To apply MS@PVC@SiO2 in the field of continuous oil/water separation, the collection equipment was designed (Figure 2). The novel collection equipment can save the time- and labor-consuming process of adsorbent recovery and compression, and oil/water separation can be operated continuously and simultaneously. The maximum possible recovery capacities of the collection equipment at different operation speeds were studied. Figure 7 shows that the recovery capacity of this collection equipment (557.784 L/h) was much higher than that of the commercial disc oil collectors (119.8 L/h), which indicates that oil/water separation performance is improved after modification by MS@PVC@SiO2. This may be due to the fact that MS@PVC@SiO2 with a 3D porous structure provides large storage space for a chemical skimmer. The diesel oil recovery rate (NRR) of the collection equipment was comparable to the total recovery rate (TR). The recovery efficiency (RE) of the developed equipment can be reached as high as 98% under ideal conditions. This may be because MS@PVC@SiO2 can adsorb diesel oil selectively and continuously without adsorbing water, owing to its superhydrophobic characteristic and robust stability. The results suggest that the oils floated on the water surface can be collected by the equipment easily, rapidly, and continuously. As mentioned above, the combination of superhydrophobic 3D porous materials and an adapted device is therefore a promising solution for continuous oil/water separation in situ from the sea.
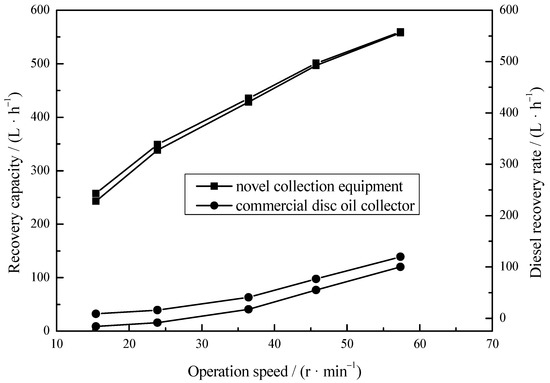
Figure 7.
The maximum possible recovery capacity of the collection equipment.
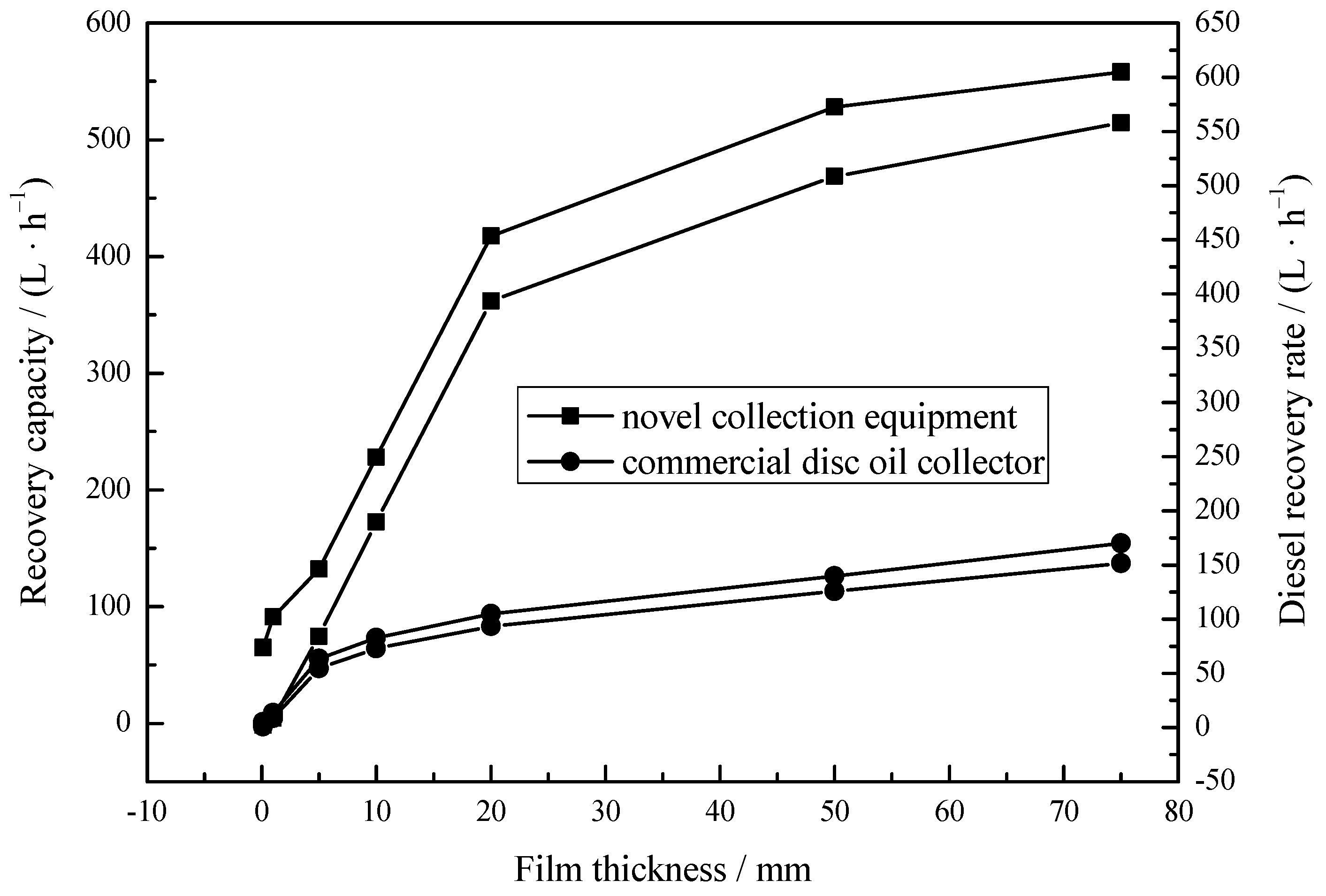
When the film thickness is low, most oils spilled on the sea spread so rapidly that slicks become fragmented. The encounter rate between traditional oil skimmers and oil slicks is invariably low even at low operating speeds. Thus, recovery of significant quantities of oils is impossible. Experiments under different diesel oil film thicknesses were carried out to evaluate recovery performance in practical oil/water separation situations. Figure 8 depicts that the NRR of the collection equipment (1.92 L/h) was higher than that of commercial disc oil collectors (0.648 L/h) even at 1 mm film thickness. The recovery efficiency of the collection equipment was better than commercial disc oil collector at any film thickness. This may be related to the spontaneous infiltration process of MS@PVC@SiO2. The superhydrophobic porous MS@PVC@SiO2 spontaneously and immediately inhales oils by capillary force [61], which prevents oils from spreading and concentrates oils during recovery. This indicates the combination of superhydrophobic 3D porous materials and an adapted device can overcome the basic limitation of traditional oil skimmer.
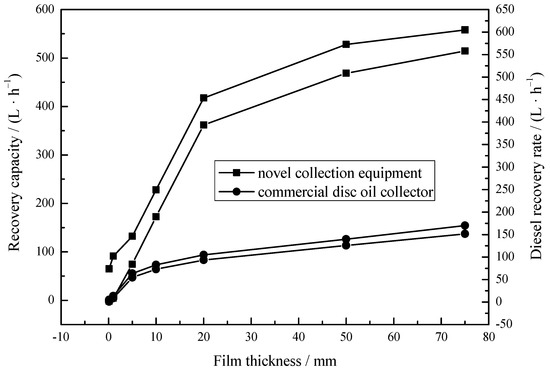
Figure 8.
Recovery capacity of the collection equipment under different diesel film thicknesses.
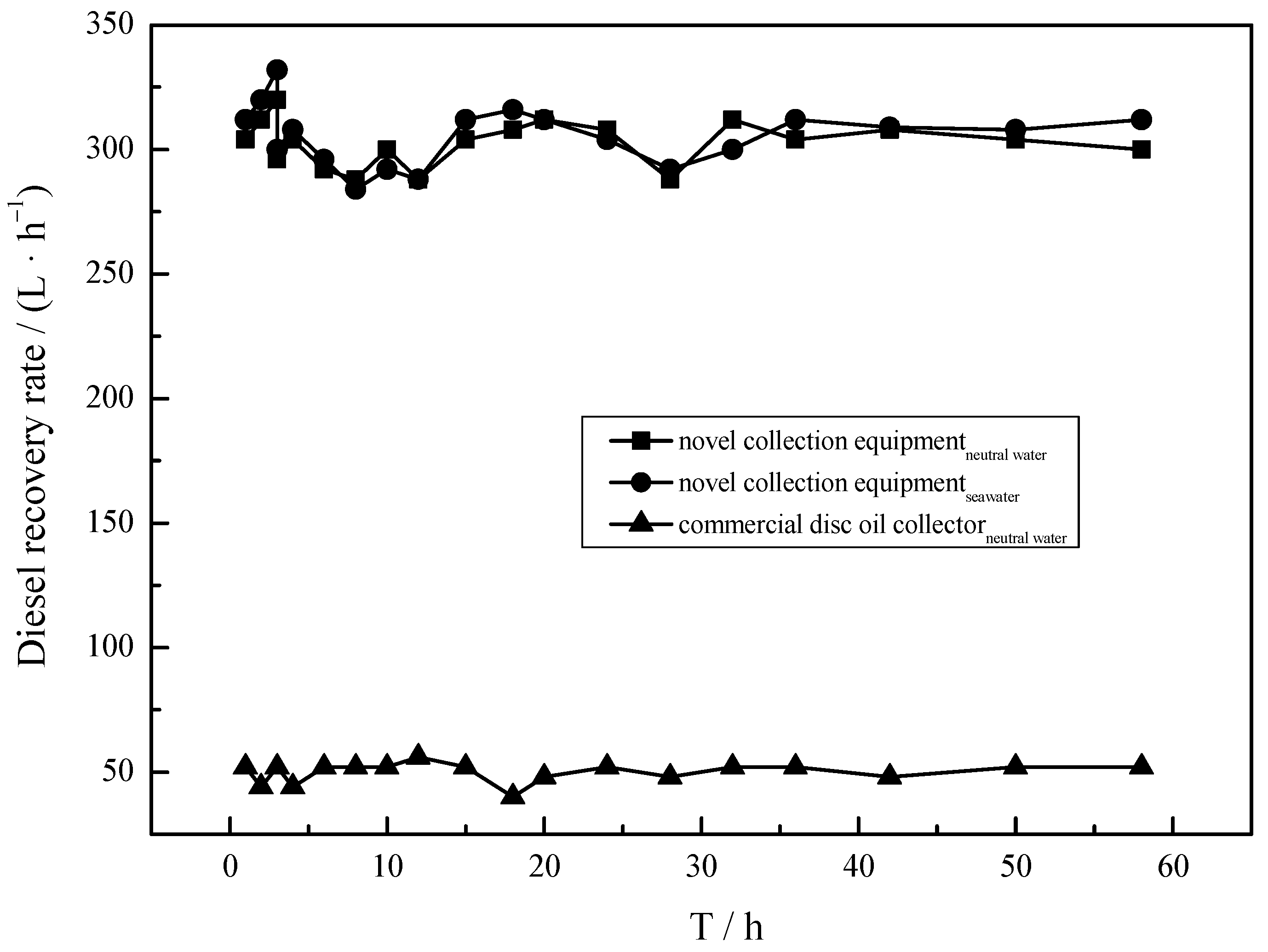
In a real oil spill scenario, operation stability is also one of the most significant targets for developed collection equipment. However, traditional oil skimmers so far developed have the limitation that they will not operate stably in anything but neutral and clean conditions. Figure 9 depicts the NRR of collection equipment under continuous operation in neutral and real seawater. It can be observed that the curves are existed basically as a straight line, implying the collection equipment loaded with MS@PVC@SiO2 remains at a high recovery capacity after running for 58 h. This can be attributed to the strong mechanical strength, high elasticity, and excellent reusability of developed MS@PVC@SiO2 [62]. The recovery capacity in water and seawater environments were almost the same. This may be attributed to the anti-corrosive coating of MS@PVC@SiO2 [63,64]. Thus, it can be inferred that the developed collection equipment has great potential to realize continuous oil/seawater separation in real-world applications.
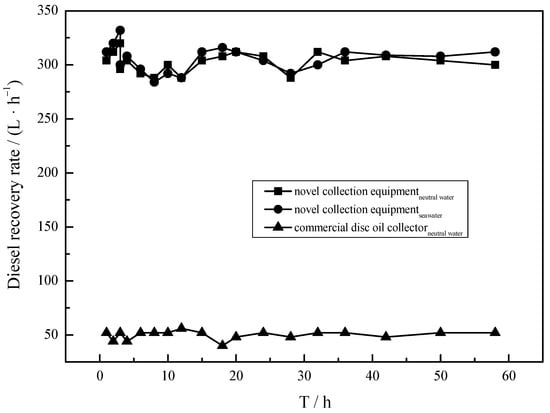
Figure 9.
The operation stability of collection equipment under 58 h continuous operation.
4. Conclusions
In this work, silica-based polymer coating was prepared by a facile and simple method with commercially available inexpensive PVC and SiO2 nanoparticles. MS@PVC@SiO2 was successfully fabricated by simply soaking MS in the coating solution without using toxic and expensive agents. The MS@PVC@SiO2 showed hydrophobicity (WCA 149.8°) due to the adherence of hydrophobic modified SiO2 microparticles “glued” by intrinsic hydrophobicity PVC layer onto the surface of the pristine MS. It is very significant that the hydrophobicity of MS@PVC@SiO2 was well maintained in oil-water/seawater interface. The excellent water repellency of MS@PVC@SiO2 endows its oil adsorption capacity (32.05 g/g) and recyclability (at least 200 cycles). The present work provides a new type of MS for oil recovery in real oil spill scenarios. Inspired by an oil skimmer, the idea of loading a rotating disc with MS@PVC@SiO2 came up. The novel collection equipment loaded with MS@PVC@SiO2 was then developed for in situ rapid oil recovery. With the help of the invented equipment, the oil/water separation process can be operated continuously and saves the trouble of recovering absorbents. The collection equipment exhibits highly selective oil adsorption capacity (98%) and good stability in real seawater, which has great potential in marine oil spillage recovery and other water/oil separating systems.
Author Contributions
Conceptualization, X.Y. and Y.X.; methodology, Y.X.; validation, X.Y. and X.S. (Xuejia Sheng); formal analysis, Y.X., X.S. (Xuejia Sheng) and X.S. (Xiangning Song); visualization, X.Y., X.S. (Xuejia Sheng); writing—original draft preparation, X.Y.; writing—review and editing, S.Z.; supervision, S.Z.; project administration, S.Z.; funding acquisition, S.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Key Project of “Science and Technology Boosting Economy 2020.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data are available within the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, Y.; Ping, H.; Ma, Z.-H.; Pan, L.-G. Statistical analysis of sudden chemical leakage accidents reported in China between 2006 and 2011. Environ. Sci. Pollut. Res. Int. 2014, 21, 5547–5553. [Google Scholar] [CrossRef]
- Hou, J.; Gai, W.-M.; Cheng, W.-Y.; Deng, Y.-F. Hazardous chemical leakage accidents and emergency evacuation response from 2009 to 2018 in China: A review. Saf. Sci. 2021, 135, 105101. [Google Scholar] [CrossRef]
- Hassler, B. Accidental Versus Operational Oil Spills from Shipping in the Baltic Sea: Risk Governance and Management Strategies. Ambio 2011, 40, 170–178. [Google Scholar] [CrossRef] [PubMed]
- McNutt, M.K.; Camilli, R.; Crone, T.J.; Guthrie, G.D.; Hsieh, P.A.; Ryerson, T.B.; Savas, O.; Shaffer, F. Review of flow rate estimates of the Deepwater Horizon oil spill. Proc. Natl. Acad. Sci. USA 2012, 109, 20260–20267. [Google Scholar] [CrossRef]
- Lan, R.; Qiao, B. Construction of Loss Assessment Method of Eco-service Value in Sea Area Polluted by Chemical Leakage Accidents Based on Ecological Impact Prediction Model. IOP Conf. Ser. Earth Environ. Sci. 2019, 310, 052065. [Google Scholar] [CrossRef]
- By, A.; Ysl, B. Designing an effective mitigation system based on the physical barrier for hazardous chemical leakage accidents. J. Ind. Eng. Chem. 2019, 80, 370–375. [Google Scholar]
- Dou, Z.; Mebarki, A.; Cheng, Y.; Zheng, X.; Jiang, J.; Wang, Y.; Li, Y.; Li, J. Review on the emergency evacuation in chemicals-concentrated areas. J. Loss Prev. Process. Ind. 2019, 60, 35–45. [Google Scholar] [CrossRef]
- Radovic, J.; Rial, D.; Lyons, B.; Harman, C.; Viñas, L.; Beiras, R.; Readman, J.W.; Thomas, K.V.; Bayona, J.M. Post-incident monitoring to evaluate environmental damage from shipping incidents: Chemical and biological assessments. J. Environ. Manag. 2012, 109, 136–153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.D.; Zheng, X.P. Characteristics of hazardous chemical accidents in China: A statistical investigation. J. Loss Prevent. Proc. 2012, 25, 686–693. [Google Scholar] [CrossRef]
- Visco, A.; Quattrocchi, A.; Nocita, D.; Montanini, R.; Pistone, A. Polyurethane Foams Loaded with Carbon Nanofibers for Oil Spill Recovery: Mechanical Properties under Fatigue Conditions and Selective Absorption in Oil/Water Mixtures. Nanomaterials 2021, 11, 735. [Google Scholar] [CrossRef]
- Osipov, K.; Mokochunina, T.V.; Panyukova, D.I.; Trukhina, M.V.; Maryutina, T.A. A comparison of standard test methods for determining the laboratory effectiveness of oil spill dispersants: Their benefits and drawbacks. Ind. Lab. Diagn. Mater. 2021, 87, 23–29. [Google Scholar] [CrossRef]
- Yu, P.; Bao, R.-Y.; Shi, X.-J.; Yang, W.; Yang, M.-B. Self-assembled high-strength hydroxyapatite/graphene oxide/chitosan composite hydrogel for bone tissue engineering. Carbohydr. Polym. 2017, 155, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Mapelli, F.; Scoma, A.; Michoud, G.; Aulenta, F.; Boon, N.; Borin, S.; Kalogerakis, N.; Daffonchio, D. Biotechnologies for Marine Oil Spill Cleanup: Indissoluble Ties with Microorganisms. Trends Biotechnol. 2017, 35, 860–870. [Google Scholar] [CrossRef]
- Mahandari, C.P.; Yamin, M.; Asandi, D. Tar Balls Collector for Mechanical Recovery in Combating Oil Spill on the Marine Environment. Appl. Mech. Mater. 2015, 776, 331–336. [Google Scholar] [CrossRef]
- Xia, C.; Li, Y.; Fei, T.; Gong, W. Facile one-pot synthesis of superhydrophobic reduced graphene oxide-coated polyurethane sponge at the presence of ethanol for oil-water separation. Chem. Eng. J. 2018, 345, 648–658. [Google Scholar] [CrossRef]
- Xie, A.; Chen, Y.; Cui, J.; Lang, J.; Li, C.; Yan, Y.; Dai, J. Facile and green fabrication of superhydrophobic sponge for continuous oil/water separation from harsh environments. Colloids Surf. A 2019, 563, 120–129. [Google Scholar] [CrossRef]
- Khormali, A.; Koochi, M.R.; Varfolomeev, M.A.; Ahmadi, S. Experimental study of the low salinity water injection process in the presence of scale inhibitor and various nanoparticles. J. Pet. Explor. Prod. Technol. 2022, 13, 903–916. [Google Scholar] [CrossRef]
- Safari, M.; Rahimi, A.; Lah, R.M.; Gholami, R.; Khur, W.S. Sustaining sulfate ions throughout smart water flooding by nanoparticle based scale inhibitors. J. Mol. Liq. 2020, 310, 113250–113259. [Google Scholar] [CrossRef]
- Yan, X.; Liu, F.; Mu, G.; Zhou, Z.; Xie, Y.; Li, L.; Yang, Y.; Wang, X. Adsorption of toluene vapours on micro–meso hierarchical porous carbon. Micro Nano Lett. 2018, 13, 641–645. [Google Scholar] [CrossRef]
- Ongarbayev, Y.; Belgibayeva, A.; Kudaybergenov, K.; Mansurov, Z. Oil Spill Cleanup from Sea Water by Porous Sorbents. Eurasian Chem.-Technol. J. 2015, 17, 41–45. [Google Scholar] [CrossRef]
- Nyankson, E.; Rodene, D.; Gupta, R.B. Advancements in Crude Oil Spill Remediation Research after the Deepwater Horizon Oil Spill. Water Air Soil Pollut. 2015, 227, 29–51. [Google Scholar] [CrossRef]
- Li, H.; Wu, W.; Bubakir, M.M.; Chen, H.; Zhong, X.; Liu, Z.; Ding, Y.; Yang, W. Polypropylene fibers fabricated via a needleless melt-electrospinning device for marine oil-spill cleanup. J. Appl. Polym. Sci. 2014, 131, 40080–40089. [Google Scholar] [CrossRef]
- Broje, V.; Keller, A.A. Improved Mechanical Oil Spill Recovery Using an Optimized Geometry for the Skimmer Surface. Environ. Sci. Technol. 2006, 40, 7914–7918. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shi, F.; Niu, J.; Jiang, Y.; Wang, Z. Superhydrophobic surfaces: From structural control to functional application. J. Mater. Chem. 2008, 18, 621–633. [Google Scholar] [CrossRef]
- Yan, X.; Xie, Y.; Zhao, S.; Sheng, X.; Zhou, Z. Preparation of modified superhydrophobic sponge and its application in xylene leakage recovery. Desalin. Water Treat. 2020, 1, 187–194. [Google Scholar] [CrossRef]
- Abdelhafeez, I.A.; Zhou, X.; Yao, Q.; Yu, Z.; Gong, Y.; Chen, J. Multifunctional Edge-Activated Carbon Nitride Nanosheet-Wrapped Polydimethylsiloxane Sponge Skeleton for Selective Oil Absorption and Photocatalysis. ACS Omega 2020, 5, 4181–4190. [Google Scholar] [CrossRef]
- Feng, Y.; Yao, J. Design of Melamine Sponge-Based Three-Dimensional Porous Materials toward Applications. Ind. Eng. Chem. Res. 2018, 57, 7322–7330. [Google Scholar] [CrossRef]
- Lv, W.Y.; Mei, Q.; Xiao, J.; Du, M.; Zheng, Q. 3D Multiscale Superhydrophilic Sponges with Delicately Designed Pore Size for Ultrafast Oil/Water Separation. Adv. Funct. Mater. 2017, 27, 1704293. [Google Scholar] [CrossRef]
- Lei, Z.; Deng, Y.; Wang, C. Multiphase surface growth of hydrophobic ZIF-8 on melamine sponge for excellent oil/water separation and effective catalysis in a Knoevenagel reaction. J. Mater. Chem. A 2018, 6, 3258–3263. [Google Scholar] [CrossRef]
- Li, L.; Li, B.; Sun, H.; Zhang, J. Compressible and conductive carbon aerogels from waste paper with exceptional performance for oil/water separation. J. Mater. Chem. A 2017, 5, 14858–14864. [Google Scholar] [CrossRef]
- Zhai, Z.; Zheng, Y.; Du, T.; Tian, Z.; Liu, Z. Green and sustainable carbon aerogels from starch for supercapacitors and oil-water separation. Ceram Int. 2021, 47, 22080–22087. [Google Scholar] [CrossRef]
- Cao, J.; Wang, D.; An, P.; Zhang, J.; Feng, S. Highly compression-tolerant and durably hydrophobic macroporous silicone sponges synthesized by a one-pot click reaction for rapid oil/water separation. J. Mater. Chem. A 2018, 6, 18025–18030. [Google Scholar] [CrossRef]
- Shin, J.H.; Heo, J.-H.; Jeon, S.; Park, J.H.; Kim, S.; Kang, H.-W. Bio-inspired hollow PDMS sponge for enhanced oil–water separation. J. Hazard. Mater. 2019, 365, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Cao, R.; Li, M.; Chen, G.; Tian, J. Superhydrophobic and superoleophilic cuttlebone with an inherent lamellar structure for continuous and effective oil spill cleanup. Chem. Eng. J. 2021, 420, 127596–127606. [Google Scholar] [CrossRef]
- Ding, Y.; Xu, W.; Yu, Y.; Hou, H.; Zhu, Z. One-Step Preparation of Highly Hydrophobic and Oleophilic Melamine Sponges via Metal-Ion-Induced Wettability Transition. ACS Appl. Mater. Interfaces 2018, 10, 6652–6660. [Google Scholar] [CrossRef] [PubMed]
- Nemanič, V.; Zajec, B.; Žumer, M.; Figar, N.; Kavšek, M.; Mihelič, I. Synthesis and characterization of melamine–formaldehyde rigid foams for vacuum thermal insulation. Appl. Energy 2014, 114, 320–326. [Google Scholar] [CrossRef]
- Oribayo, O.; Feng, X.; Rempel, G.L.; Pan, Q. Modification of formaldehyde-melamine-sodium bisulfite copolymer foam and its application as effective sorbents for clean up of oil spills. Chem. Eng. Sci. 2017, 160, 384–395. [Google Scholar] [CrossRef]
- Stolz, A.; Le Floch, S.; Reinert, L.; Ramos, S.M.; Tuaillon-Combes, J.; Soneda, Y.; Chaudet, P.; Baillis, D.; Blanchard, N.; Duclaux, L.; et al. Melamine-derived carbon sponges for oil-water separation. Carbon 2016, 107, 198–208. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Wang, M.; Men, X.; Xue, Q. One-pot fabrication of nanoporous polymer decorated materials: From oil-collecting devices to high-efficiency emulsion separation. J. Mater. Chem. A 2017, 5, 5077–5087. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Q. Facile approach in fabricating superhydrophobic coatings from silica-based nanocomposite. Appl. Surf. Sci. 2010, 257, 33–36. [Google Scholar] [CrossRef]
- Liu, L.; Chen, C.; Yang, S.; Xie, H.; Gong, M.; Xu, X. Fabrication of superhydrophilic–underwater superoleophobic inorganic anti-corrosive membranes for high-efficiency oil/water separation. Phys. Chem. Chem. Phys. 2015, 18, 1317–1325. [Google Scholar] [CrossRef]
- Ge, J.; Ye, Y.-D.; Yao, H.-B.; Zhu, X.; Wang, X.; Wu, L.; Wang, J.-L.; Ding, H.; Yong, N.; He, L.-H.; et al. Pumping through Porous Hydrophobic/Oleophilic Materials: An Alternative Technology for Oil Spill Remediation. Angew. Chem. Int. Ed. 2014, 53, 3612–3616. [Google Scholar] [CrossRef] [PubMed]
- ASTM F 2709-15; Standard Test Method for Determining a Measured Nameplate Recovery Rate of Stationary Oil Skimmer Systems. American Society for Testing and Materials: Philadelphia, PA, USA,, 2015.
- Wang, H.; Xue, Y.; Ding, J.; Feng, L.; Wang, X.; Lin, T. Durable, Self-healing superhydrophobic and superoleophobic surfaces from fluorinated-decyl polyhedral oligomeric silses quioxane and hydrolyzed fluorinated alkyl silane. Angew. Angew. Chem. Eeit 2011, 50, 11433–11436. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhi, D.; Zhu, W.; Sathasivam, S.; Parkin, I.P. Facile fabrication of durable superhydrophobic SiO2/polyurethane composite sponge for continuous separation of oil from water. RSC Adv. 2017, 7, 11362–11366. [Google Scholar] [CrossRef]
- Wang, J.; Kong, Q.G.; Zhang, L.; Qian, H.Y. Preparation and properties of fluorinated low surface energy modified superamphiphobic coatings. Surf. Technol. 2018, 47, 76–82. [Google Scholar]
- Kulkarni, S.A.; Ogale, S.B.; Vijayamohanan, K.P. Tuning the hydrophobic properties of silica particles by surface silanization using mixed self-assembled monolayers. J. Colloid Interface Sci. 2008, 318, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Sam, E.K.; Liu, J.; Lv, X. Surface Engineering Materials of Superhydrophobic Sponges for Oil/Water Separation: A Review. Ind. Eng. Chem. Res. 2021, 60, 2353–2364. [Google Scholar] [CrossRef]
- Zhu, Y.; Xie, W.; Xing, T.; Li, J. pH-Induced non-fouling membrane for effective separation of oil-in-water emulsion. J. Membr. Sci. 2015, 477, 131–138. [Google Scholar] [CrossRef]
- Wei, C.; Lin, L.; Zhao, Y.; Zhang, X.Y.; Huang, X.J. Fabrication of pH-Sensitive Superhydrophilic/Underwater Superoleophobic Poly(vinylidene fluoride)-graft-(SiO2 Nanoparticles and PAMAM Dendrimers) Membranes for Oil-Water Separation. ACS Appl. Mater. Int. 2020, 12, 19130–19139. [Google Scholar] [CrossRef]
- Zhou, S.K.; Liu, Y.J.; Jiang, H.Y.; Deng, W.J.; Zeng, G.M. Adsorption of U(VI) from Aqueous Solution by a Novel Chelating Adsorbent Functionalized with Amine Groups: Equilibrium, Kinetic, and Thermodynamic Studies. Environ. Eng. Sci. 2017, 35, 53–61. [Google Scholar] [CrossRef]
- Abbasizadeh, S.; Keshtkar, A.R.; Mousavian, M.A. Sorption of heavy metal ions from aqueous solution by a novel cast PVA/TiO2 nanohybrid adsorbent functionalized with amine groups. J. Ind. Eng. Chem. 2014, 20, 1656–1664. [Google Scholar] [CrossRef]
- Gui, X.; Li, H.; Wang, K.; Wei, J.; Jia, Y.; Li, Z.; Fan, L.; Cao, A.; Zhu, H.; Wu, D. Recyclable carbon nanotube sponges for oil absorption. Acta Mater. 2011, 59, 4798–4804. [Google Scholar] [CrossRef]
- Peng, M.; Chen, G.; Zeng, G.; Chen, A.; He, K.; Huang, Z.; Hu, L.; Shi, J.; Li, H.; Yuan, L.; et al. Superhydrophobic kaolinite modified graphene oxideemelamine sponge with excellent properties for oilewater separation. Appl. Clay Sci. 2018, 163, 63–71. [Google Scholar] [CrossRef]
- Ke, Q.; Jin, Y.; Jiang, P.; Yu, J. Oil/Water Separation Performances of Superhydrophobic and Superoleophilic Sponges. Langmuir 2014, 30, 13137–13142. [Google Scholar] [CrossRef]
- Cao, N.; Yang, B.; Barras, A.; Szunerits, S.; Boukherroub, R. Polyurethane sponge functionalized with superhydrophobic nanodiamond particles for efficient oil/water separation. Chem. Eng. J. 2017, 307, 319–325. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Y. Oil/water mixtures and emulsions separation of stearic acid-functionalized sponge fabricated via a facile one-step coating method. Sep. Purif. Technol. 2017, 181, 183–191. [Google Scholar] [CrossRef]
- Khosravi, M.; Azizian, S. Synthesis of a Novel Highly Oleophilic and Highly Hydrophobic Sponge for Rapid Oil Spill Cleanup. ACS Appl. Mater. Interfaces 2015, 7, 25326–25333. [Google Scholar] [CrossRef]
- Choi, S.-J.; Kwon, T.; Im, H.; Moon, D.-I.; Baek, D.J.; Seol, M.-L.; Duarte, J.P.; Choi, Y.-K. A Polydimethylsiloxane (PDMS) Sponge for the Selective Absorption of Oil from Water. ACS Appl. Mater. Interfaces 2011, 3, 4552–4556. [Google Scholar] [CrossRef]
- Zhao, X.; Li, L.; Li, B.; Zhang, J.; Wang, A. Durable superhydrophobic/superoleophilic PDMS sponges and their applications in selective oil absorption and in plugging oil leakages. J. Mater. Chem. A 2014, 2, 18281–18287. [Google Scholar] [CrossRef]
- Washburn, E.W. The dynamics of capillary flow. Phys. Rev. J. Arch. 1921, 17, 273–283. [Google Scholar] [CrossRef]
- Wang, G.; Zeng, Z.; Wu, X.; Ren, T.; Han, J.; Xue, Q. Three-dimensional structured sponge with high oil wettability for the clean-up of oil contaminations and separation of oil–water mixtures. Polym. Chem. 2014, 5, 5942–5948. [Google Scholar] [CrossRef]
- Wu, Z.-Y.; Li, C.; Liang, H.-W.; Zhang, Y.-N.; Wang, X.; Chen, J.-F.; Yu, S.-H. Carbon nanofiber aerogels for emergent cleanup of oil spillage and chemical leakage under harsh conditions. Sci. Rep. 2014, 4, 4079. [Google Scholar] [CrossRef] [PubMed]
- Sutar, R.S.; Kalel, P.J.; Latthe, S.S.; Kumbhar, D.A.; Mahajan, S.S.; Chikode, P.P.; Patil, S.S.; Kadam, S.S.; Gaikwad, V.H.; Xing, R.; et al. Superhydrophobic PVC/SiO2 Coating for Self-Cleaning Application. Macromol. Symp. 2020, 393, 034–040. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).