Abstract
Wet electrostatic precipitators (WESPs) are increasingly used in iron and steel plants and coal-fired power plants due to their higher efficiency at capturing fine particles than conventional dry-type precipitators. In order to achieve ultra-pure purification of dust in steel plant gas, we propose an improved method that involves adding a chemical coagulant and a surfactant to a WESP. The effects of the type and concentration of chemical coagulant and surfactant on the agglomeration effect and dust removal efficiency of blast furnace dust were investigated. The results show that the addition of a chemical coagulant could promote the agglomeration of blast furnace dust particles, and the D50 of dust particles increased from 5.8 to 15.0 μm after the addition of xanthan gum (XTG). The best increase in the blast furnace’s dust particle removal occurred at a concentration of 10 mg/L of XTG, and the dust removal efficiency reached 97.59%. The surfactant dodecyl trimethyl ammonium chloride (DTAC) improved the dust removal efficiency of blast furnace dust when added alone. The dust removal efficiency reached 97.82% when 10 mg/L of XTG and 9 mg/L of DTAC were added synergistically. The addition of a chemical coagulant and surfactant promoted the agglomeration of blast furnace dust and enhanced the dust capture effect of a WESP. We thus demonstrated that we can improve the efficiency of WESP in the future via chemical coagulation. The authors will further study the effect of multi-factor synergistic coupling on the chemical coagulation method in WESPs.
1. Introduction
The iron and steel industry is the second most polluting industry in China after the electric power industry, and it is an important source of fine particle pollution in the air in China [1,2,3]. With the continuous growth of steel production every year, the total emitted amount of pollutants has been increasing, which has had a great impact on the air quality [4,5,6,7,8]. According to statistical data, in the first half of 2021, the national crude steel production reached a total of 563 million tons, which represented an 11.80% year-on-year increase. Pig iron production reached 456 million tons, which represented a 4.0% year-on-year increase. Steel production reached 698 million tons, representing a cumulative year-on-year increase of 13.90% [9]. The total amount of waste gas emission in the first half of 2021 also increased compared with the same period of the previous year. Blast furnace gas generation reached 523,805 billion m3, which represented a 6.80% year-on-year increase, and its recycling rate reached 97.95%. Converter gas generation reached 40.704 billion m3, representing a 13.70% year-on-year increase, and its utilization rate reached 98.40%. Both blast furnaces and converters have improved their gas utilization rate compared with the same period of last year [10]. Although the gas recovery rate has increased in recent years with the improvement of emission standards, some of the gas that is not recovered and is discharged still has a negative impact on the environment [11]. Blast furnace gas is a by-product of blast furnace ironmaking. Blast furnace gas has the characteristics of high dust concentration, high pollution, low calorific value, and difficult recovery, and the poor hydrophilicity of the dust particles in the gas affects the recovery of the gas [12]. When blast furnace gas is used for power generation and other purposes, the ultra-pure purification of gas is required to reduce the dust content in the gas to less than 1 mg/m3 (standard state). Therefore, this study proposes to enhance the hydrophilicity of blast furnace dust by adding a chemical coagulant and a surfactant to a wet electrostatic precipitator to improve the collection efficiency of blast furnace dust and to achieve the ultra-pure purification of gas [13].
The use of a chemical coagulant and a surfactant in wet electrostatic precipitators (WESPs) is based on adding one or more agents to the original dust removal equipment to enhance the agglomeration of fine dust particles to achieve a higher removal efficiency. In the 1990s, Durham et al. [14] sprayed a mixture containing atomized binder into an ESP gas stream and showed that the binder and fine particles effectively collected on the dust collection poles. In 2000, the U.S. Department of Energy and others developed new chemical conditioners for controlling fly ash resistivity and cohesion. In 2001, Johansen et al. [15] compared the morphology and particle size of agglomerates formed by PEG 20000 and PEG 3000 and concluded that PEG 20000 in powder form is the most effective agglomerate. Earlier research on the effect of chemical coagulants on the growth of ultrafine particles is described below. In 2003, Wei et al. [16] summarized the research on chemical agglomeration technology by foreign scientific researchers and proposed the use of a chemical coagulant to promote ultrafine particulate matter growth. In 2007, Zhao et al. [17] used coagulant spraying in a coal-fired flue to promote ultrafine particle growth. Later, researchers began to focus on the effects of temperature, pH, addition point, and coagulant aid when adding chemical coagulants. Rajniak et al. [18] investigated the effect of HPC in combination with other auxiliaries on the agglomeration of particles. In 2009, Li et al. [19] investigated the agglomeration effect of four chemical coagulants including PAM at different concentrations and under different pH conditions. In the same year, Carvalh et al. [20] investigated the physicochemical effects of using carrageenan as a coagulant. In 2011, Forbes et al. [21] investigated the effect of chemical coagulant droplets on the wetting and agglomeration of fine particulate matter at different temperatures. In 2013, Zhao et al. [22] analyzed the variation in dust concentration and particle size when using different coagulant addition points, temperatures, etc.
More and more researchers have begun to focus on the synergistic effect of chemical coagulation and electrocoagulation, the synergistic effect of chemical coagulation and turbulent coagulation, and the synergistic effect of chemical coagulants on the capture efficiency of fine particles. In 2014, Thonglek et al. [23] controlled fine particle agglomeration by pulsing the ESP to generate plasma inside the ESP. In the same year, Liu et al. [24] achieved a 30% increase in PM2.5 removal when adding chemical coagulants to a wet FGD system. In 2015, Balakin et al. [25] investigated the mechanism of agglomeration using capillary bridging in a multiphase flow agglomeration experiment. In 2016, Liu et al. [26] selected six chemical coagulants, including XTG, to study the growth of fine particles. In 2017, Guo et al. [27] evaluated the chemical agglomeration phenomenon by simulating the pressure, temperature, and velocity distributions in a chamber flow field. In 2018, Hu et al. [28] used water, pectin, and sodium alginate solution as coagulants to improve the fine particulate removal efficiency by more than 20%. In 2019, Sun et al. [29] proposed and investigated the effect of different chemical agglomeration and turbulent agglomeration coupling methods on the agglomeration effect and removal performance of ESP particles. Zhou et al. [30] investigated the agglomeration effect of sesbania gum (SBG) and styrene butadiene emulsion (SBE) on fine particulate matter using phase Doppler anemometry. In 2020, Li et al. [31] found that a carrageenan/Tween-80/NH4Cl (KC/TW/NH4Cl) three-component coagulant increased the average particle size of fine particulate matter from 2.8 μm to more than 10.0 μm. In 2021, Gao et al. [32] used chemical agglomeration and turbulent mixing to synergistically promote chemical agglomeration and greatly improve the effectiveness of electrostatic precipitation. Yang et al. [33] applied a new passivation coagulant to a 300 MW unit experimentally, and the exit dust concentration was reduced by about 32%. Therefore, the chemical agglomeration method has good prospects for application in electrostatic precipitators.
All of the above studies show that chemical coagulants have a good effect on the growth of fine particles of dust and can significantly improve dust removal efficiency. However, most of the particles selected in these studies were coal-fired dust, and the problems of high water consumption and high cost of coagulant dosage still exist, making it difficult to widely promote chemical agglomeration technology in dust removal applications. In this study, we focus on blast furnace dust with poor agglomeration performance. By adding a chemical coagulant to the original spraying system of a wet electrostatic precipitator, the dust removal efficiency can be improved without modifying the original equipment. In addition, this study applies a surfactant and a chemical coagulant simultaneously to investigate their synergistic effects and to identify a suitable concentration of chemical coagulant to promote dust agglomeration. This method could reduce economic costs and enhance the development of chemical agglomeration technology.
The purpose of this study is to investigate the effects of a chemical coagulant and a surfactant on the removal of blast furnace dust in wet scrubbers. The effects of chemical type and concentration on the agglomeration effect of blast furnace dust were systematically investigated to optimize the chemical agent type and concentration to improve the efficiency of WESP at capturing blast furnace dust. Our results may help improve the efficiency of WESPs in the future via chemical coagulation. The authors will further study the effect of multi-factor synergistic coupling on the chemical coagulation method in WESPs.
2. Materials and Methods
2.1. Experimental Setup
2.1.1. Wet Electrostatic Precipitators System
The wet type electric dedusting system used in this study is shown in Figure 1 and includes a low voltage control system, a high voltage power supply control system, a spraying system, and a fan system. The spraying system includes a circulating pool and a water pump. The electrode setup used a BS mansard cathode line and a 480C anode plate, and the dust collector size was 2.8 × 1.4 × 5.8 (L × W × H) (m). The dust inlet concentration was set to 80 mg/m3, the power supply voltage was 72 kV, and the current was 0.3 mA. When the wet-type electric dedusting system was in operation, the water pump sent water from the circulating pool to the inside of the dedusting system through nozzle atomization.
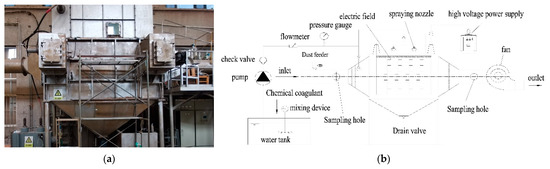
Figure 1.
Wet electrostatic precipitator system. (a) is the field physical diagram of WESP. (b) is the internal structure of WESP.
2.1.2. Sampling System
In this experiment, the isokinetic sampling method was used for the dust at the outlet of the WESP. The dust concentration obtained via this method was more accurate and closer to the dust concentration under real conditions. The following equipment was used in the sampling process: a filter cartridge (filter membrane), a sampling head, a drying bottle, a buffer bottle, a vacuum pump, and a flow meter. A diagram of the sampling system is shown in Figure 2. A high silica glass fiber filter cartridge was selected for dust sampling, and it was placed in the oven for drying before and after sampling to eliminate the influence of flue gas temperature and humidity. The total measurement error of the sampling system is less than 0.1 mg/m3.
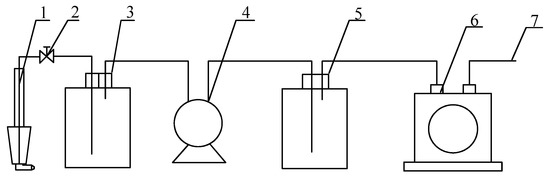
Figure 2.
Sampling system. 1. Sampling tube. 2. Needle valves. 3. Dry bottles. 4. Pumping Pump. 5. Buffer bottles. 6. Wet gas flow meter. 7. Discharge tube.
2.1.3. Particle Size Distribution Measurement Device
A BT-9300H laser particle size analyzer was used to measure the dust particle size distribution. The device includes a sample cell, an ultrasonic disperser, a centrifugal circulation pump, and an electric stirrer.
2.1.4. Representation
In this study, a field emission scanning electron microscope (SEM, S4800-II, Kyoto, Japan) equipped with an energy spectrometer system was used to observe the morphology and elemental composition of blast furnace dust.
2.2. Experimental Materials
The experimental conditions used in this study were as follows: the gas temperature was 18–25 °C and the relative humidity was 20%–40%. The dust used was blast furnace dust, the operating voltage of the WESP system was 40 kV, the field gas velocity was 1 m/s, and the nozzle pressure was 0.5 MPa. The blast furnace dust was dried before entering the WESP, and then a quantity of dust was uniformly transported from the feed inlet to the interior of the precipitator by a fan. Inside the dust collector, dust particles went through the stages of gas ionization, dust charging, dust collection, and spray cleaning. Most of the dust particles were trapped, and the uncaught dust escaped through the pipeline.
The drugs used in this experiment included a chemical coagulant and a surfactant, as is shown in Table 1 below. By changing the concentration and type of chemical coagulant and surfactant, the effects of different variables on the corona discharge effect, dust agglomeration effect, and removal efficiency of the wet-type dust collector were investigated. At the end of the experiment, the dust was collected to measure the particle size distribution, observe the dust particle growth effect, and calculate the dust removal efficiency.

Table 1.
Chemical coagulants and surfactants used in the experiments.
3. Analysis and Discussion
3.1. Blast Furnace Dust Particle Size Distribution and Morphology
3.1.1. Particle Size Distribution Analysis
The particle size distribution of the blast furnace dust is shown in Figure 3. D10, D50, and D90 indicate the particle size when the cumulative particle size distribution number reached 10%, 50%, and 90%, respectively. D50 is also called xdmedian size or median particle size. It can be seen that the particle size of xdblast furnace dust is small, with a particle size distribution in the range of 0.2–40 μm, and D50 was 6.754 μm. We found that, 10% of the dust was less than 2.010 μm, 50% was less than 6.754 μm, and 90% was less than 17.33 μm. In addition, particles with a size below 2.5 μm accounted for about 20.14% of the total, and particles with a size below 10 μm accounted for about 79.77% of the total. These dust particles below the particle size can cause serious air pollution, which is why they are the key target of blast furnace dust capture.
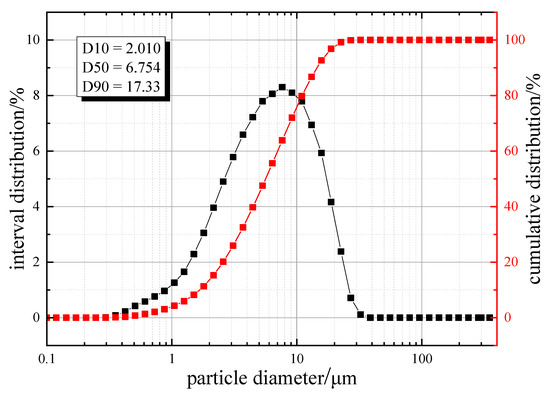
Figure 3.
Particle size distribution of blast furnace dust.
3.1.2. Morphological Analysis
Figure 4a shows that the blast furnace dust particles are varied in size and showed an irregular shape and a porous structure. The particles were either connected or dispersed with each other, which may be due to the various chemical changes accompanying the combustion process, resulting in the particles bonding together to form larger particle clusters during cooling. Figure 4b shows that the metallic elements in the blast furnace dust were mainly Ca, Na, Fe, and Al, and the inorganic elements Si and C were the most abundant.
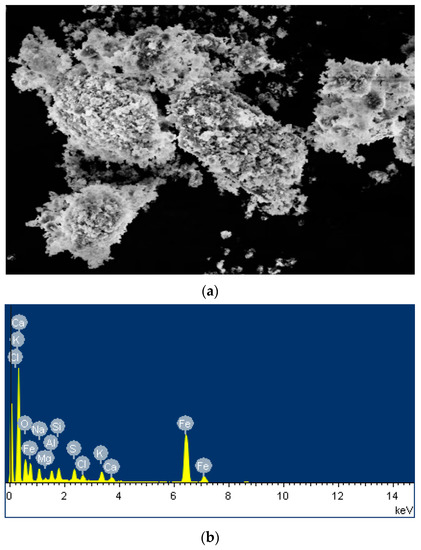
Figure 4.
SEM morphology of blast furnace dust.
3.2. Influence of Chemical Coagulant on the Effect of Blast Furnace Dust Capture
3.2.1. Effect of Coagulant Type
Coagulation Effect
Different types of chemical coagulants have different effects on particle agglomeration. In this study, five coagulants, PAM, KGM, KC, XTG, and kieselguhr, were selected at a concentration of 15 mg/L, and the experimental results are shown in Figure 5. The D10 particle sizes of the five coagulants showed no significant differences, but the differences were more obvious for D50 and D90. The sequence of the corresponding particle sizes of D90 were XTG, kieselguhr, PAM, KGM, and KC in descending order. XTG is a polymeric compound with strong hydrophilic properties, which dissolves in water and can form a highly viscous solution. XTG was added to the recirculation basin and sprayed into the dust collector through the nozzle as droplets, which collided with fine particles and bound together in a “bridging” manner to form larger particle clusters. The addition of a chemical coagulant makes the fine particles grow, increasing the probability of particle capture and improving the dust removal efficiency. Regarding the consumption of reagents, a simple calculation showed that less than 1.5 kg of agent was required to treat 100,000 m3 of gas. Therefore, the cost of the reagents was low.
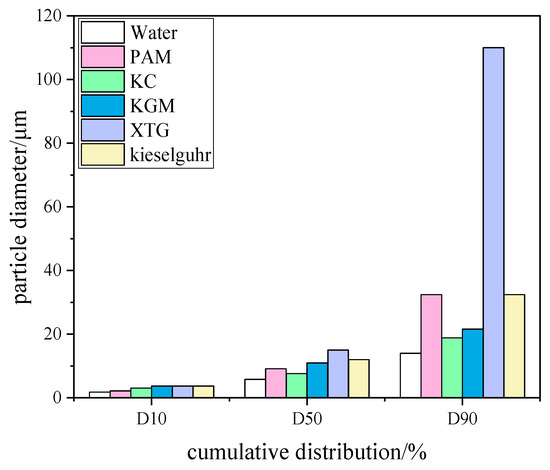
Figure 5.
Histogram of particle size cumulative distribution of coagulant types.
Dust Removal Efficiency
To further investigate the effect of chemical coagulants on the removal of blast furnace dust in WESPs, this study applied sampling tubes to sample the inlet and outlet locations of the dust collector to calculate the dust removal efficiency of the WESP with different coagulants. The electric field wind speed was 1.0 m/s, the nozzle pressure was 0.5 MPa, the operating voltage was 40 kV, and the concentration of all five coagulants was 15 mg/L. Figure 6 shows that the dust removal efficiency increased after the addition of a coagulant. When the coagulant was XTG, the highest dust removal efficiency reached 97.59%. When the coagulant was KC, the dust removal efficiency was the lowest (95.68%). Among the five coagulants, XTG had the best agglomeration effect but the worst corona discharge performance and the highest dust removal efficiency. Similarly, the agglomeration effect of PAM was slightly worse than that of kieselguhr, but its corona discharge performance was better than that of kieselguhr. Therefore, it is assumed that the dust removal efficiency was more influenced by the agglomeration effect of particulate matter when the difference in corona discharge performance was not significant.
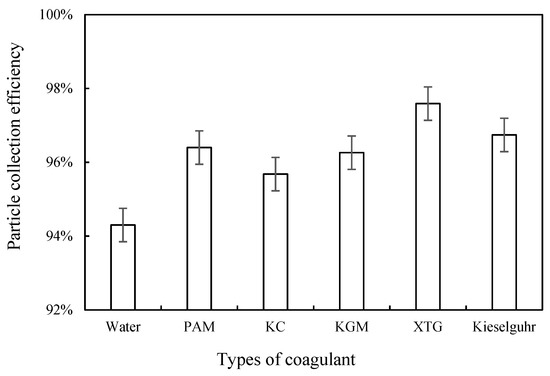
Figure 6.
Effect of coagulant type on dust removal efficiency.
3.2.2. Effect of Coagulant Concentration
Coagulation Effect
The agglomeration effect of adding different concentrations of chemical coagulants was different. XTG, which had a better agglomeration effect, was selected to investigate the effect of concentration. Four different concentrations of XTG, 0 mg/L, 5 mg/L, 10 mg/L, and 15 mg/L, were added to the circulating pool for the experiment. As can be seen in Figure 7, the content of large-size particles gradually increased with an increase in coagulant concentration. Without XTG, 20.14% of PM2.5, 79.77% of PM10, and 20.23% of particles within 10–100 μm were found. When XTG was added, the PM2.5 content was significantly reduced. A concentration of 5 mg/L of XTG can reduce the PM2.5 content to 7.9% and the PM10 content to 62.33%, and the particulate matter content within 10–100 μm increased significantly to 37.67%. When the XTG concentration was 10 mg/L, the PM2.5 content remained basically unchanged, the PM10 content continued to decrease, and the content of particulate matter within 10–100 μm was dominant. There was particulate matter with a particle size larger than 100 μm, and the content of particulate matter with a particle size larger than 100 μm was 0.2%. When the XTG concentration was 15 mg/L, the PM2.5 content was reduced to 6.32% and the PM10 content was reduced to 41.9%. The dust particle size was concentrated between 10 μm and 100 μm, and the dust content in this particle size range was 45.85%. The proportion of particles larger than 100 μm increased to 12.25%. In general, chemical coagulant molecules contain chemical groups that can interact with the particle surface. When a chemical coagulant molecule collides with a particle, some groups in the chemical coagulant molecule will be adsorbed on the surface of the particle, while the rest of the particle can be in contact with other collision particles, resulting in secondary adsorption. In this way, the particles formed aggregates with each other by the action of chemical coagulant molecules [18,34]. Therefore, the particle growth was better with an increased concentration of the coagulant.
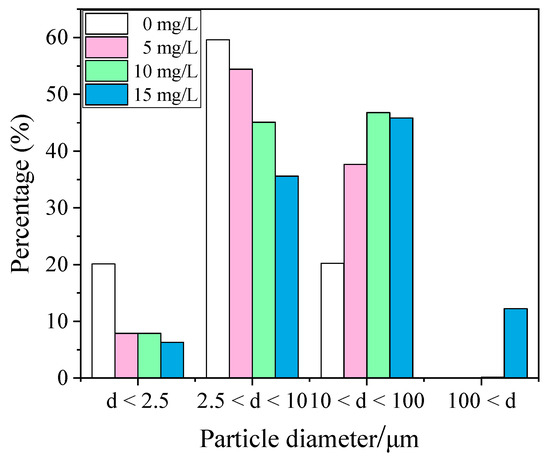
Figure 7.
Histogram of particle size interval content of coagulant concentration.
Dust Removal Efficiency
In order to investigate the effect of chemical coagulant concentration on the removal of blast furnace dust in WESPs, dust at the inlet and outlet of the dust collector was collected through the sampling tube, and the dust removal efficiency of the five coagulants at different concentrations was calculated. As can be seen in Figure 8, the dust removal efficiency of the five chemical coagulants at different concentrations were XTG, kieselguhr, PAM, KGM, and KC in descending order, and the dust removal efficiency increases gradually with the coagulant concentration. The maximum efficiency of XTG was achieved at a concentration of 10 mg/L, and the efficiency did not change significantly when the concentration of XTG was increased. XTG has excellent physical and chemical properties. Compared with the several other agglomerating agents used, it has good dust coagulation and dust removal effects. XTG helical molecules can agglomerate fine particles into larger particles under the action of van der Waals forces, hydrogen bonds, hydrophobic interactions, etc. However, it has the disadvantage of poor solubility, and it can easily form a non-conductive coating layer on the electrode surface. When the concentration is high, the electrostatic precipitator cannot work normally. When the chemical coagulant is added in excess, the particle surface is saturated by the chemical coagulant molecules and there are no adsorption vacancies on the particle surface, so the chemical coagulant loses its bridging effect. At the same time, due to the effect of polymer adsorption membrane vacancy resistance or mutual repulsion between the particles, the particles were again in a stable state of dispersion. In addition, continuing to increase the concentration of the coagulant leads to corona discharge performance degradation, resulting in a decrease in the capture efficiency of blast furnace dust. Therefore, the amount of chemical coagulant should be moderate, as more does not necessarily mean better.
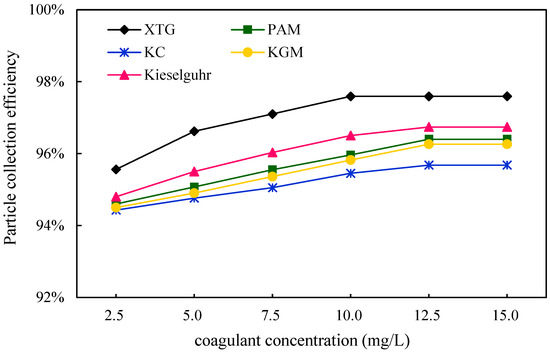
Figure 8.
Effect of coagulant concentration on dust removal efficiency.
3.3. Effect of Surfactants on Blast Furnace Dust Capture
3.3.1. Influence of Surfactant Type
Coagulation Effect
In order to investigate the effect of surfactants on the efficiency of WESPs at trapping blast furnace dust, three different surfactants, dodecyl trimethyl ammonium chloride (DTAC), sodium dodecyl sulfate (SDS), and octylphenyl polyoxyethylene ether (TX-100), were selected. The surfactants, in line with the experimental method of chemical coagulants, were injected into the recirculation basin and entered the dust collector through the nozzle. After the experiment, the particle size distribution was measured by collecting the particles in the recirculation basin. The results are shown in Figure 9. The more obvious agglomeration effect was shown by DTAC, followed by SDS, and TX-100 basically had no agglomeration effect. There was no significant change in D10 after the addition of a surfactant compared with no agent. When no agent was added, the particle D50 and D90 were 5.8 and 14 μm, respectively. When the surfactant TX-100 was added, the D50 and D90 increased to 6.382 and 15.75 μm, respectively. When SDS was added, the D50 and D90 increased to 13.14 and 18.87 μm, respectively. When DTAC was added, the D50 continued to increase. Therefore, among the three surfactants, the best effect on the agglomeration of blast furnace dust was exhibited by DTAC. The surfactant molecules completed the adsorption of fine dust and water molecules through hydrophobic bonding and electrostatic interactions, forming an adsorption configuration in which hydrophobic groups were adsorbed on the surface of fine particles and hydrophilic groups were extended to water; thus, the wetting of the dust was achieved. At the particle/water interface, the surfactant molecules were adsorbed on the particle surface due to the influence of van der Waals forces. However, when the surfactant molecules were adsorbed on the surface of the particles, their oxygen-containing groups tended to form hydrogen bonds with water molecules, and such hydrogen bonds had a lifting effect on the surfactant molecules, causing the surfactant molecules to aggregate at the interface. Multiple such particles wrapped by water film collide with each other to achieve particle aggregation [35].
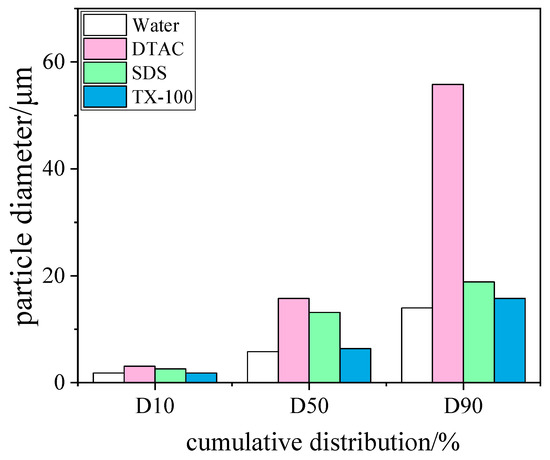
Figure 9.
Histogram of particle size cumulative distribution of surfactant types.
Dust Removal Efficiency
We measured the inlet and outlet dust concentration after adding DTAC, SDS, and TX-100 and calculated the dust removal efficiency. As can be seen in Figure 10, the dust removal efficiencies were all improved with the addition of surfactants. When DTAC was added, the highest dust removal efficiency of 97.08% was achieved. When the surfactant was TX-100, the dust removal efficiency was the lowest at 96.17%. The dust removal efficiency was between that of DTAC and TX-100 when SDS was added (96.78%). Among the three surfactants, the addition of DTAC resulted in the best particle growth, the best corona discharge performance, and the highest dust removal efficiency. We find that the effects of the three selected surfactants on dust growth and corona discharge performance remained consistent, which was different from the effect of chemical coagulants, and this phenomenon is likely related to their different solubilities in water.
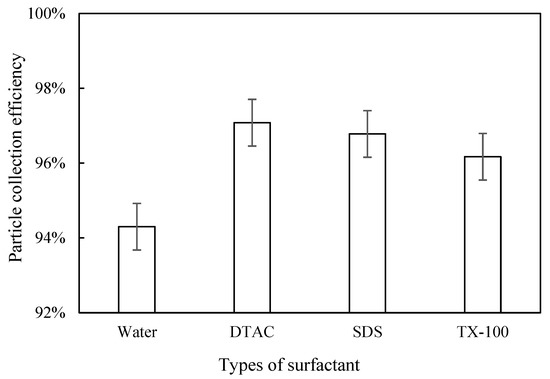
Figure 10.
Effect of surfactant type on dust removal efficiency.
3.3.2. Effect of Surfactant Concentration
Coagulation Effect
In order to continue to investigate the effect of surfactants on the growth effects of particles, DTAC, which had the best agglomeration effect among the three surfactants, was selected to continue the blast furnace dust agglomeration experiment in this study with concentrations of 0, 5, 7, and 9 mg/L, and the results are shown in Figure 11. The content of large-size particles gradually increased with an increase in the surfactant concentration. At the DTAC concentration of 0 mg/L, 20.14% of PM2.5, 79.77% of PM10, and 20.23% of particles within 10–100 μm were found. After adding DTAC, the PM2.5 content was significantly reduced. When the DTAC concentration was 5 mg/L, the PM2.5 content was reduced to 8.64%, the PM10 content was reduced to 48.81%, and the content of particulate matter within 10–100 μm was significantly increased to 50.56%. There were particulate matter particle sizes larger than 100 μm, and the content of particulate matter particle sizes larger than 100 μm was 1.26%. When the DTAC concentration was 7 mg/L, the PM2.5 content remained basically unchanged, the PM10 content decreases slightly, and the particulate matter content within 10–100 μm was slightly higher than when DTAC was 5 mg/L. When the DTAC concentration was 9 mg/L, the PM10 content decreased to 41.76%, the dust content in the range of 10–100 μm was 54.59%, and the content of particulate matter with particle size larger than 100 μm increased to 3.65%. Therefore, with an increase in the surfactant concentration, the particulate matter grew better. DTAC, as a cationic surfactant, had the effect of reducing the surface charge and compressing the double electric layer for the adsorption of particulate matter, which was in a mosaic, chain-sequence fixation model on the surface of the particulate matter. As a result of this fixation, the surface charge of the particles was not uniformly distributed, and they easily attracted each other because they had opposite charges, and this caused flocculation.
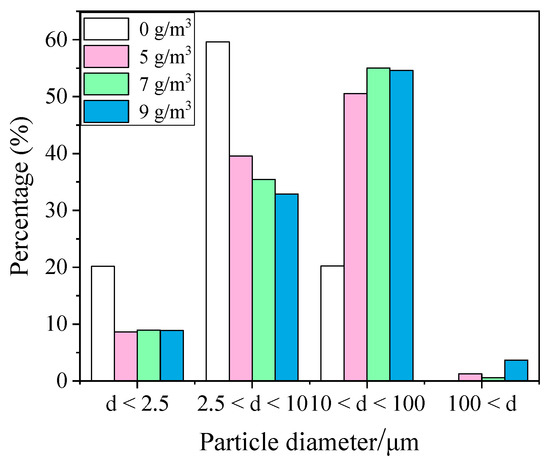
Figure 11.
Histogram of particle size interval content of surfactant concentration.
Dust Removal Efficiency
To investigate the effect of surfactant concentration on the efficiency of WESPs at capturing blast furnace dust, the dust concentrations at the inlet and outlet of the dust collector were collected using sampling tubes, and the dust removal efficiency of the three surfactants at the different selected concentrations was calculated. The results are shown in Figure 12. The dust removal efficiency of the three surfactants at different concentrations was DTAC, SDS, and TX-100 in descending order, and it is obvious that the dust removal efficiency tends to gradually climb upward with an increase in the surfactant concentration in the figure. At low concentrations, the dust removal efficiency of DTAC and SDS remained the same, and the dust removal efficiency of SDS increased slowly as the concentration increased. Among several surfactants, DTAC has the best agglomeration effect and the highest dust removal efficiency. DTAC has a strong hydrophobic association effect, which can reduce the liquid film tension, improve the wettability of dust, and ensure the agglomeration of dust, thus improving the dust removal efficiency. Although higher concentrations of surfactants were not further investigated in this experiment, the growth rate of the dust removal efficiency gradually slows down as the concentration increases in Figure 13, especially for DTAC and SDS, and the curve tends to level off. Therefore, as with chemical coagulants, the amount of surfactant should be kept moderate and should be determined according to the concentration of dust to be removed. In addition, the dosage of both chemical coagulants and surfactants should be considered in terms of economic factors as well as dust removal efficiency, as excessive doses may lead to high costs.
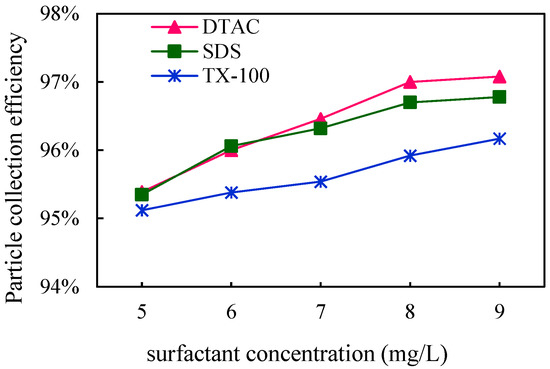
Figure 12.
Effect of surfactant concentration on dust removal efficiency.
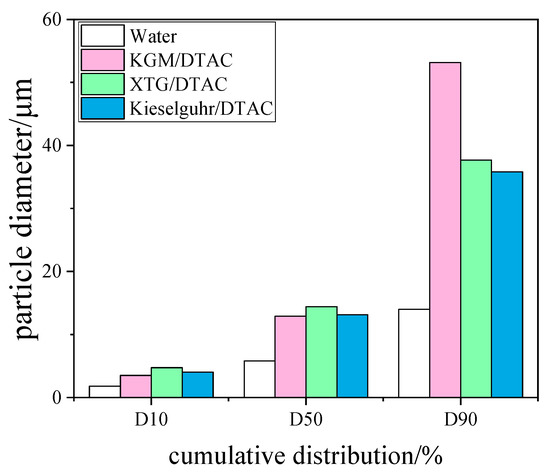
Figure 13.
Histogram of cumulative particle size distribution of the synergistic effect of surfactant and coagulant.
3.4. Effect of Synergy on Blast Furnace Dust Capture
3.4.1. Impact of Synergistic Species
According to Nsengiyumva [36], when two different chemical coagulation solutions are mixed together, they may exhibit synergistic effects. DTAC at 9 mg/L was chosen as the surfactant to study the effect of synergistic interaction between surfactant and chemical coagulant on blast furnace dust capture. The chemical coagulants involved in the synergistic effect were changed to XTG, kieselguhr and KGM at 10 mg/L, and the effects of the synergistic effect of XTG/DTAC, kieselguhr/DTAC, and KGM/DTAC on the agglomeration effect of blast furnace dust were investigated. The results are shown in Figure 13. Compared with Figure 9 and Figure 13, it was found that the D10 particle size increased slightly under the synergistic effect compared with the addition of DTAC only, but the D50 and D90 particle sizes decreased. When comparing Figure 5 and Figure 13, it was found that the D10 particle size increased slightly under the synergistic effect of XTG/DTAC compared to the addition of XTG only, and the D10 particle size was 4.738 μm. However, the D50 and D90 particle sizes were smaller than that of XTG only. Similarly, the D10 particle size increased slightly with kieselguhr/DTAC synergy compared to that with kieselguhr only, and the D10 particle size was 4.031 μm. Unlike the XTG/DTAC synergy, the D50 and D90 particle sizes with kieselguhr/DTAC synergy were smaller than those with kieselguhr only. The particle size of D10 did not change under KGM/DTAC synergism compared with that of KGM only, but the particle sizes of D50 and D90 increased significantly, corresponding to 12.9 μm and 53.16 μm, respectively. At this time, the addition of polymeric chemical coagulants can make the small flocs become large flocs through adsorption and bridging effects. Therefore, the addition of a DTAC cationic surfactant increased the possibility of bridging with other polymeric chemical coagulants. In addition, polymer molecular chains tended to intertwine and form a mesh structure, thus increasing the possibility of particle capture and aggregation [37].
3.4.2. Dust Removal Efficiency
The inlet and outlet dust concentrations after adding KGM/DTAC, XTG/DTAC, and kieselguhr/DTAC were measured, and the dust removal efficiency was calculated separately. As can be seen in Figure 14, the highest dust removal efficiency of 97.82% was achieved with the synergistic effect of XTG/DTAC. Compared with the highest dust removal efficiency when only XTG or DTAC was added, both increased, but the increase was smaller. The dust removal efficiency reached 97.60% with the synergistic effect of kieselguhr/DTAC, which was a smaller increase than when only kieselguhr or DTAC was added. The dust removal efficiency with KGM/DTAC synergy was the lowest among the three, at 97.10%. The efficiency was significantly higher compared to when only KGM was added, but the dust removal efficiency was almost unchanged compared to when only DTAC was added. KGM is a gel with a stable cross-linking structure, which has hydrophobic and strong hydrogen bonding effects, and DTAC also has hydrophobic association effects. Under the synergistic effect of two chemical coagulants, the surface of blast furnace dust will generate a hydrophobic effect, and it will agglomerate into larger particles via the bridging effect of the chemical coagulant, thus improving the dust collection efficiency. Therefore, the addition of a surfactant to the chemical coagulant would improve the dust removal efficiency. The improvement of dust removal efficiency was not obvious, but this increases the economic cost. For a coagulant with a low dust removal efficiency, the addition of a surfactant can significantly improve the dust removal efficiency.
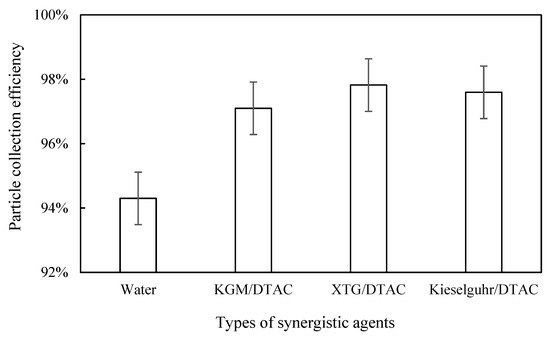
Figure 14.
Effect of synergistic types on dust removal efficiency.
4. Conclusions
In this study, the effect of a chemical coagulant on blast furnace dust capture was investigated by coagulation experiments and dust removal efficiency experiments, and the synergistic effects of a chemical coagulant and a surfactant on the dust removal effect was found.
- (1)
- All five chemical coagulants selected in this study can effectively promote the capture of blast furnace dust. The D50 of blast furnace dust and the proportion of large particles increased after the addition of coagulants. XTG had the best agglomeration effect on blast furnace dust, KC had the worst agglomeration effect, and their corresponding WESP removal efficiencies were 97.59% and 95.68%, respectively. With an increase in the XTG concentration, the agglomeration effect was better, and the dust removal efficiency reached its highest level when the XTG concentration was 10 mg/L.
- (2)
- All three surfactants effectively improved the trapping efficiency of blast furnace dust in a WESP. As far as the agglomeration effect of the blast furnace dust was concerned, DTAC had the best agglomeration effect, and TX-100 had the worst agglomeration effect, corresponding to 97.08% and 96.17% removal efficiency of WESP, respectively; with an increase in the surfactant concentration, the blast furnace dust particles grew better.
- (3)
- The synergistic effect of a chemical coagulant and a surfactant can improve the agglomeration effect and capture efficiency of furnace dust, but this effect was more obvious when the dust removal efficiency of the chemical coagulant was low. When XTG at 10 mg/L and DTAC at 9 mg/L acted synergistically, the number of respirable particles was significantly reduced, and the dust removal efficiency reached its maximum of 97.82%.
Author Contributions
Conceptualization, L.X. and Y.H.; methodology, L.X. and Y.H.; software, Y.H.; validation, L.X., Y.H. and H.C.; formal analysis, Y.H.; investigation, Y.H. and H.C.; resources, L.X.; data curation, L.X. and Y.H.; writing—original draft preparation, Y.H.; writing—review and editing, L.X.; visualization, L.X.; supervision, L.X.; project administration, L.X.; funding acquisition, L.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Natural Science Foundation of Hebei province, China (Grant No. E2015203236). This research was supported by the Qinhuangdao science and technology research and development program (Grant No. 202003B033).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature
| d | Particle diameter |
| D10 | Diameter when the cumulative distribution of dust is 10% |
| D50 | Diameter when the cumulative distribution of dust is 50% |
| D90 | Diameter when the cumulative distribution of dust is 90% |
References
- BP. BP Statistical Review of China Energy 2015. Available online: http://www.bp.com/zh_cn/china/reports-and-publications/bp_20351.html (accessed on 30 October 2022).
- Meij, R.; Te Winkel, B. The emissions and environmental impact of PM10 and trace elements from a modern coal-fired power plant equipped with ESP and wet FGD. Fuel Process. Technol. 2004, 85, 641–656. [Google Scholar] [CrossRef]
- Senior, C.L.; Helble, J.J.; Sarofim, A.F. Emissions of mercury, trace elements, and fine particles from stationary combustion sources. Fuel Process. Technol. 2000, 65, 263–288. [Google Scholar] [CrossRef]
- Pope Iii, C.A.; Burnett, R.T.; Thun, M.J.; Calle, E.E.; Krewski, D.; Ito, K.; Thurston, G.D. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA 2002, 287, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Neas, L.M. Fine particulate matter and cardiovascular disease. Fuel Process. Technol. 2000, 65, 55–67. [Google Scholar] [CrossRef]
- Tai, A.P.K.; Mickley, L.J.; Jacob, D.J. Correlations between fine particulate matter (PM 2.5) and meteorological variables in the United States: Implications for the sensitivity of PM 2.5 to climate change. Atmos. Environ. 2010, 4, 3976–3984. [Google Scholar] [CrossRef]
- Avise, J.; Chen, J.; Lamb, B.; Wiedinmyer, C.; Guenther, A.; Salathe, E.; Mass, C. Attribution of projected changes in summertime US ozone and PM 2.5 concentrations to global changes. Atmos. Chem. Phys. 2009, 9, 1111–1124. [Google Scholar] [CrossRef]
- Ylätalo, S.I.; Hautanen, J. Electrostatic precipitator penetration function for pulverized coal combustion. Aerosol. Sci. Technol. 1998, 29, 17–30. [Google Scholar] [CrossRef]
- China Iron and Steel Industry Association: National Steel Production in June 2021. Available online: www.chinaisa.org.cn (accessed on 15 July 2021). (In Chinese).
- China Iron and Steel Industry Association: Environmental Protection of Member Enterprises in June 2021. Available online: www.chinaisa.org.cn (accessed on 21 July 2021). (In Chinese).
- Matino, I.; Dettori, S.; Colla, V.; Weber, V.; Salame, S. Forecasting blast furnace gas production and demand through echo state neural network-based models: Pave the way to off-gas optimized management. Appl. Energy 2019, 253, 113578. [Google Scholar] [CrossRef]
- Zhao, Q.E. Trend analysis of sulfur in titanium slag from off-sheet titanium concentrate smelting. Steel Vanadium Titan. 2018, 39, 97–101. (In Chinese) [Google Scholar]
- Guo, Y.H. Current status and future development trend of blast furnace gas purification and quality utilization technology. J. Iron Steel Res. 2020, 32, 525–531. (In Chinese) [Google Scholar]
- Durham, M.D.; Schlager, R.J.; Ebner, T.G.; Stewart, R.M.; Bustard, C.J. Method and Apparatus for Decreased Undesired Particle Emissions in Gas Streams. U.S. Patent 5,893,943, 13 April 1999. [Google Scholar]
- Johansen, A.; Schæfer, T. Effects of physical properties of powder particles on binder liquid requirement and agglomerate growth mechanisms in a high shear mixer. Eur. J. Pharm. Sci. 2001, 14, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Zhang, J.Y.; Wang, C.M.; Zheng, C.G. Research progress of coal combustion ultrafine particle agglomeration promotion technology. Coal Convers. 2003, 26, 27–31. (In Chinese) [Google Scholar]
- Zhao, Y.C.; Zhang, J.Y.; Wei, F.; Chen, J.; Zheng, C.G. Experimental study on the agglomeration promotion mechanism of ultrafine particulate matter from coal combustion. J. Chem. Eng. 2007, 58, 2876–2881. (In Chinese) [Google Scholar]
- Rajniak, P.; Mancinelli, C.; Chern, R.T.; Stepanek, F.; Farber, L.; Hill, B.T. Experimental study of wet granulation in fluidized bed: Impact of the binder properties on the granule morphology-ScienceDirect. Int. J. Pharm. 2007, 334, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L.; Zhang, J.Y.; Zhao, Y.C.; Ding, F.; Zhao, C.G. Experimental study on solid-liquid agglomeration of fine coal combustion particles. Chin. J. Electr. Eng. 2009, 29, 62–66. (In Chinese) [Google Scholar]
- Campo, V.L.; Kawano, D.F.; Campo, V.L.; Kawano, D.F.; Da Silva, D.B., Jr.; Carvalho, I. Carrageenans: Biological properties, chemical modifications, and structural analysis–A review. Carbohydr. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Forbes, E. Shear, selective and temperature responsive flocculation: A comparison of fine particle flotation techniques. Int. J. Miner. Process. 2011, 99, 1–10. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, Y.; Bao, J.J.; Geng, J.F.; Yang, L.J. Experimental study of chemical agglomeration to promote the removal of fine particulate matter from coal combustion. Chin. J. Electr. Eng. 2013, 33, 52–58. (In Chinese) [Google Scholar]
- Thonglek, V.; Kiatsiriroat, T. Agglomeration of sub-micron particles by a non-thermal plasma electrostatic precipitator. J. Electrost. 2013, 72, 33–38. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, L.J.; Pan, D.P.; Huang, R.T. Experiments on the Removal of Fine Particles in Existing Air Pollution Control Devices by Chemical Agglomeration. Adv. Mater. Res. 2014, 3384, 756–760. [Google Scholar]
- Balakin, B.V.; Kutsenko, K.V.; Lavrukhin, A.A.; Kosinski, P. The collision efficiency of liquid bridge agglomeration. Chem. Eng. Sci. 2015, 137, 590–600. [Google Scholar]
- Liu, Y.; Hu, B.; Zhou, L.; Jiang, Y.; Yang, L. Improving the Removal of Fine Particles with an Electrostatic Precipitator by Chemical Agglomeration. Energy Fuels 2016, 30, 8441–8447. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, J.; Zhao, Y.; Wang, S.; Jiang, C.; Zheng, C. Chemical agglomeration of fine particles in coal combustion flue gas: Experimental evaluation. Fuel 2017, 203, 557–569. [Google Scholar] [CrossRef]
- Hu, B.; Yang, Y.; Zhou, L.; Ao, S.; Cai, L.; Linjun, Y.; Roszak, S. Experimental and DFT studies of PM2.5 removal by chemical agglomeration. Fuel 2018, 212, 27–33. [Google Scholar]
- Sun, Z.; Yang, L.; Shen, A.; Zhou, L.; Wu, H. Combined effect of chemical and turbulent agglomeration on improving the removal of fine particles by different coupling mode. Powder Technol. 2019, 344, 242–250. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, W.; Wu, H.; Shen, A.; Yuan, Z.; Yang, L. Investigation on the relationship of droplet atomization performance and fine particle abatement during the chemical agglomeration process. Fuel 2019, 245, 65–77. [Google Scholar] [CrossRef]
- Li, R.; Li, C.; Zhuang, J.; Zhu, H.; Fang, L.; Sun, D. Mechanistic Influence of Chemical Agglomeration Agents on Removal of Inhalable Particles from Coal Combustion. ACS Omega 2020, 5, 25906–25912. [Google Scholar] [CrossRef]
- Gao, S.; Xiao, L.C. Study on Dust Turbulence-Chemical Agglomeration for Electrostatic Precipitation Technology. E3S Web Conf. 2021, 245, 03011. [Google Scholar]
- Yang, G.Z.; Zhao, Y.C.; Xiong, Z.; Gong, B.G.; Gao, T.; Zhang, J.Y. Study on chemical agglomeration enhanced dedusting and co-desulfurization of 300MW coal-fired power plant with zero wastewater discharge. Chin. J. Electr. Eng. 2021, 41, 5274–5282. (In Chinese) [Google Scholar]
- Lyklema, J. Modern Trends of Colloid Science in Chemistry and Biology. In Adsorption of Polyelectrolytes and Their Effect on the Interaction of Colloid Particles; Eicke, H.F., Ed.; Birkhäuser: Basel, Switzerland, 1985; pp. 55–73. [Google Scholar]
- Zhang, J.G.; Liu, Y.T.; Wang, M.; Wang, Y.; Li, H.; Xie, J.; Zhao, W.; Zhou, W.; Ye, S.; Li, L.; et al. Mechanism of non-ionic surfactant effect on coal wettability based on molecular dynamics simulation. Eng. Sci. Technol. 2022, 54, 191–202. (In Chinese) [Google Scholar]
- Nsengiyumva, E.M.; Alexandridis, P. Xanthan gum in aqueous solutions: Fundamentals and applications. Int. J. Biol. Macromol. 2022, 216, 583–604. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.J. (Ed.) Principles of Chemical Flocculant Action; Science Press: Beijing, China, 2005; pp. 86–92. (In Chinese) [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).