Nanostructured Silicon Derived from an Agricultural Residue Bagasse Ash via Magnesiothermic Reduction Method
Abstract
1. Introduction
2. Experimental
2.1. Materials Used
2.2. Magnesiothermic Reduction of Silica to Silicon
3. Results and Discussion
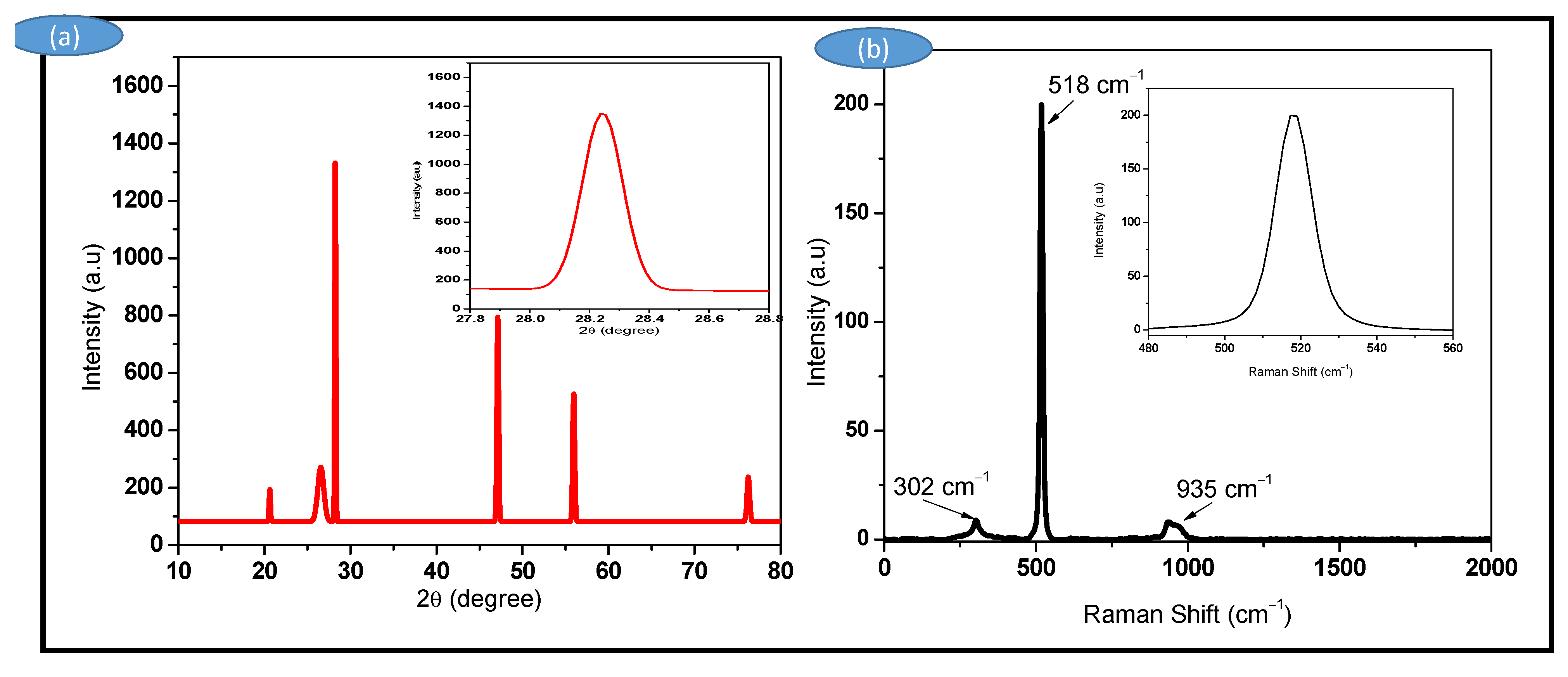
3.1. X-ray Diffraction (XRD) and Raman Spectroscopy
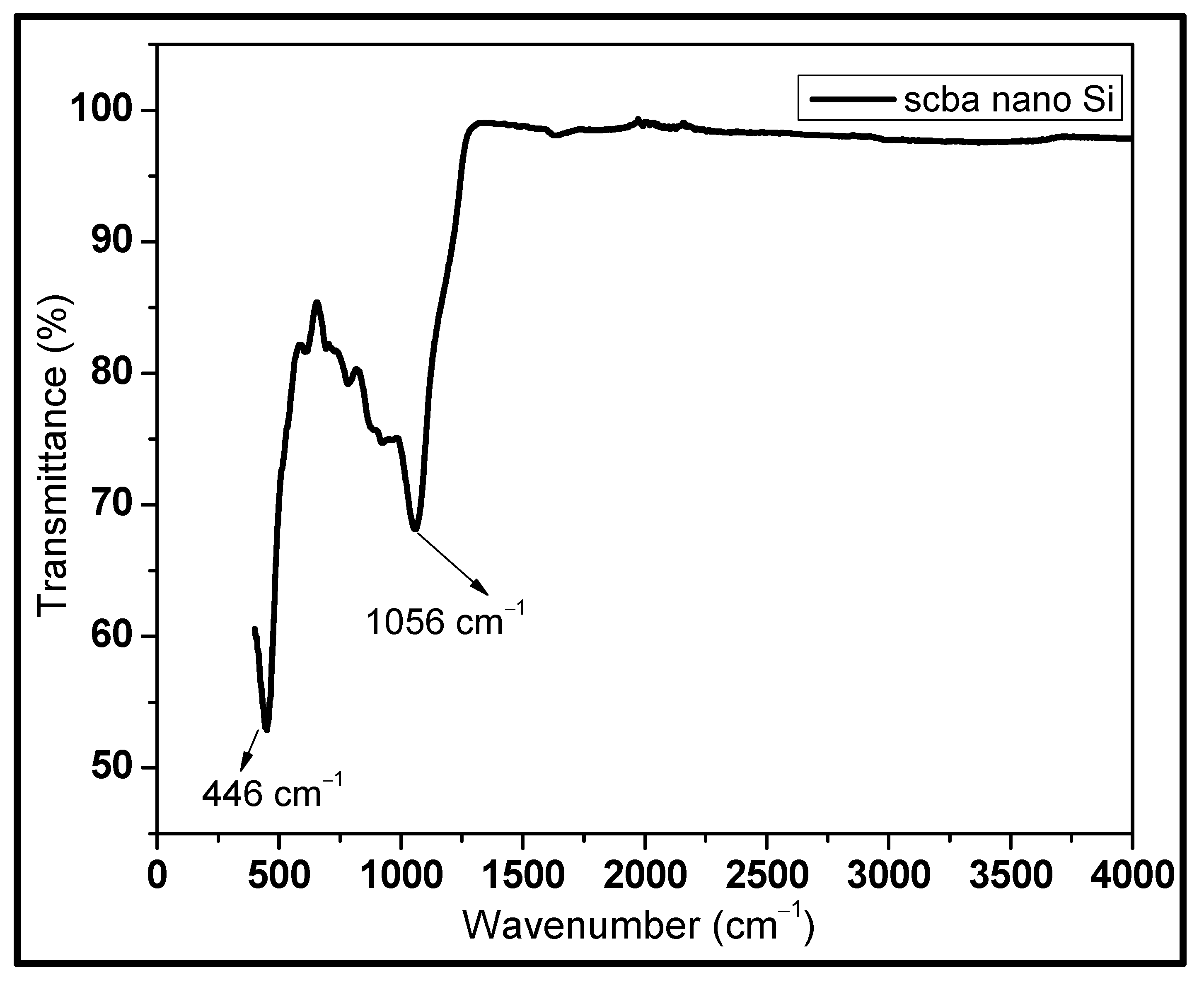
3.2. FTIR Spectroscopy
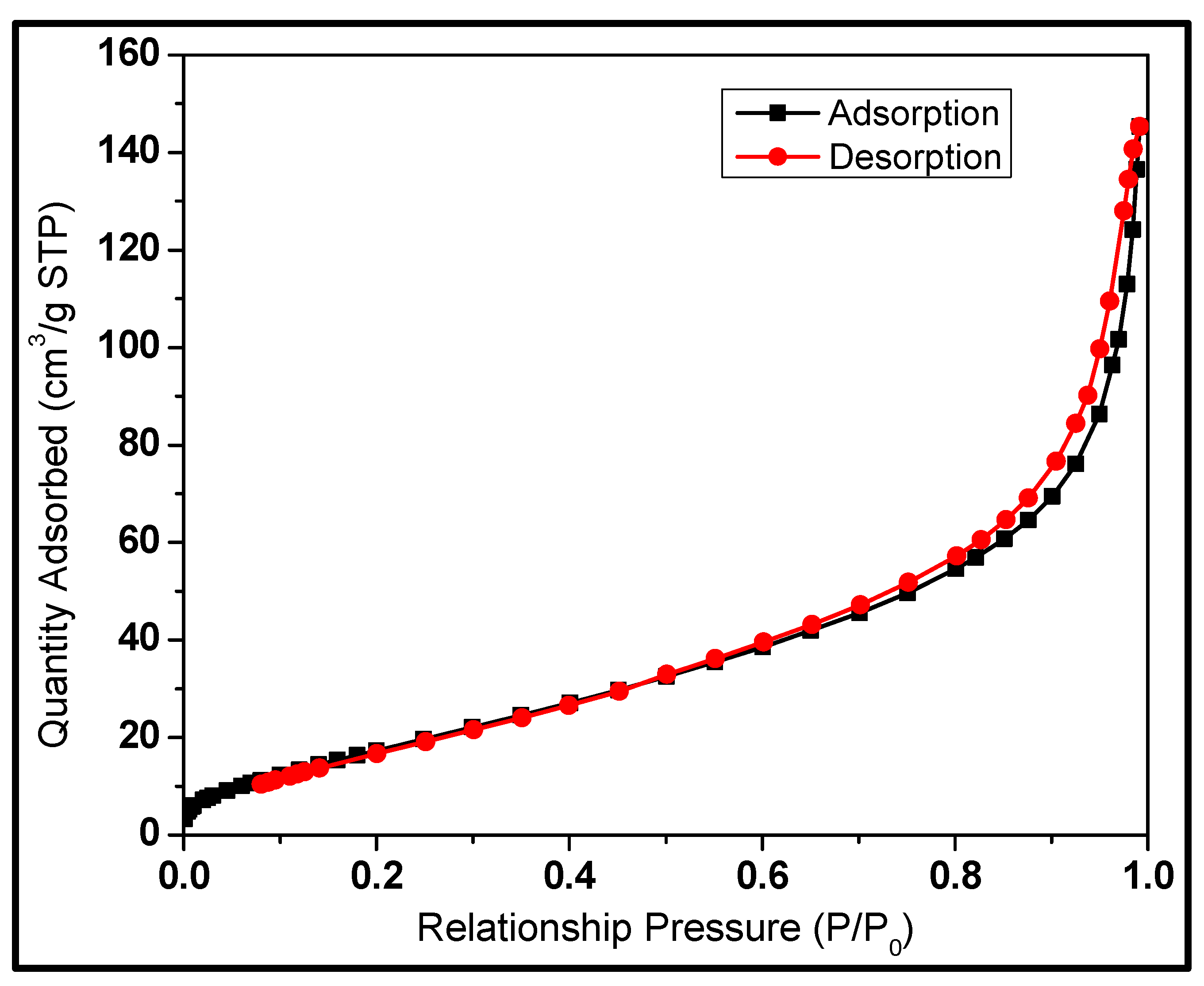
3.3. Nitrogen Physisorption Studies
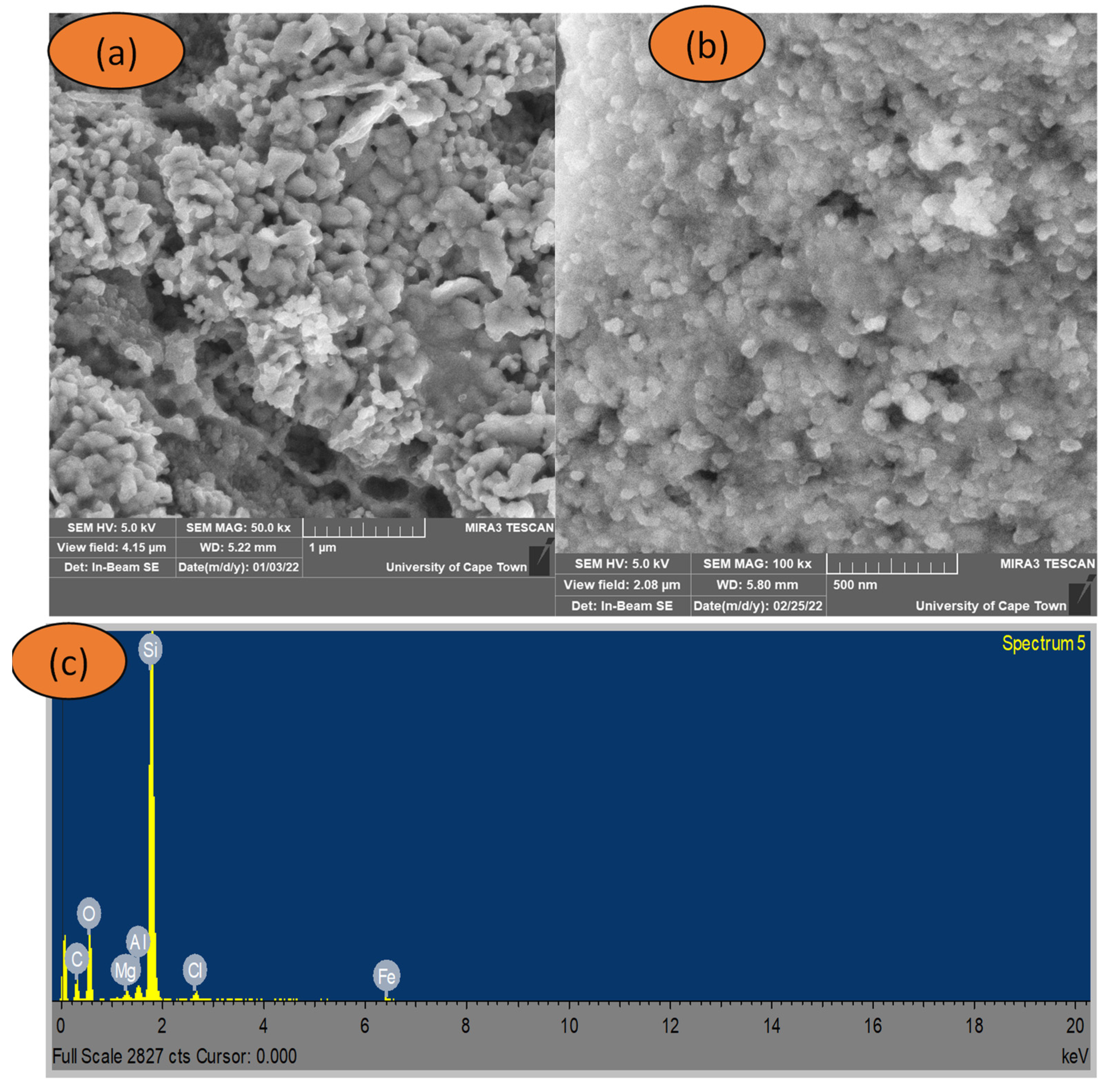
3.4. Scanning Electron Microscopy (SEM) Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yadav, M.; Dwibedi, V.; Sharma, S.; George, N. Biogenic silica nanoparticles from agro-waste: Properties, mechanism of extraction and applications in environmental sustainability. J. Environ. Chem. Eng. 2022, 10, 108550. [Google Scholar] [CrossRef]
- Praneetha, S.; Murugan, A.V. Development of sustainable rapid microwave assisted process for extracting nanoporous Si from earth abundant agricultural residues and their carbon-based nanohybrids for lithium energy storage. ACS Sustain. Chem. Eng. 2015, 3, 224–236. [Google Scholar] [CrossRef]
- Liu, N.; Huo, K.; McDowell, M.T.; Zhao, J.; Cui, Y. Rice husks as a sustainable source of nanostructured silicon for high performance Li-ion battery anodes. Sci. Rep. 2013, 3, 1919. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Zou, X.; Ren, H.; Muhammad, F.; Huang, C.; Qiu, S.; Zhu, G. Fabrication of high surface area mesoporous silicon via magnesiothermic reduction for drug delivery. Microporous Mesoporous Mater. 2011, 142, 194–201. [Google Scholar] [CrossRef]
- Jeong, J.H.; Kim, K.H.; Jung, D.W.; Kim, K.; Lee, S.M.; Oh, E.S. High-performance characteristics of silicon inverse opal synthesized by the simple magnesium reduction as anodes for lithium-ion batteries. J. Power Sources 2015, 300, 182–189. [Google Scholar] [CrossRef]
- Zhou, Z.; Han, G.; Lu, X.; Wang, G.; Zhou, X. High-performance magnesium-based thermoelectric materials: Progress and challenges. J. Magnes. Alloys 2022, 10, 1719–1736. [Google Scholar] [CrossRef]
- Zhong, H.; Zhan, H.; Zhou, Y.H. Synthesis of nanosized mesoporous silicon by magnesium-thermal method used as anode material for lithium ion battery. J. Power Sources 2014, 262, 10–14. [Google Scholar] [CrossRef]
- Huang, Z.G.; Gao, K.; Wang, X.G.; Xu, C.; Song, X.M.; Shi, L.X.; Zhang, Y.; Hoex, B.; Shen, W.Z. Large-area MACE Si nano-inverted-pyramids for PERC solar cell application. Sol. Energy 2019, 188, 300–304. [Google Scholar] [CrossRef]
- Li, M.; Dai, Y.; Ma, W.; Yang, B.; Chu, Q. Review of new technology for preparing crystalline Silicon solar cell materials by metallurgical method. IOP Conf. Ser. Earth Environ. Sci. 2017, 94, 012016. [Google Scholar] [CrossRef]
- Inasawa, S.; Ono, Y.; Mizuguchi, T.; Sunairi, A.; Nakamura, S.I.; Tsuji, Y.; Yamaguchi, Y. Cross-sectional analysis of the core of silicon microparticles formed via zinc reduction of SiCl4. CrystEngComm 2017, 19, 2681–2686. [Google Scholar] [CrossRef]
- Shen, P.; Uesawa, N.; Inasawa, S.; Yamaguchi, Y. Characterization of flowerlike silicon particles obtained from chemical etching: Visible fluorescence and superhydrophobicity. Langmuir 2010, 26, 13522–13527. [Google Scholar] [CrossRef]
- Bao, Z.; Weatherspoon, M.R.; Shian, S.; Cai, Y.; Graham, P.D.; Allan, S.M.; Ahmad, G.; Dickerson, M.B.; Church, B.C.; Kang, Z.; et al. Chemical reduction of three-dimensional silica micro-assemblies into microporous silicon replicas. Nature 2007, 446, 172–175. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, D.J.; Cho, K.M.; Kim, S.J.; Park, J.K.; Jung, H.T. Complete magnesiothermic reduction reaction of vertically aligned mesoporous silica channels to form pure silicon nanoparticles. Sci. Rep. 2015, 5, 9014. [Google Scholar] [CrossRef]
- Xing, A.; Tian, S.; Tang, H.; Losic, D.; Bao, Z. Mesoporous silicon engineered by the reduction of biosilica from rice husk as a high-performance anode for lithium-ion batteries. RSC Adv. 2013, 3, 10145–10149. [Google Scholar] [CrossRef]
- Seroka, N.S.; Taziwa, R.; Khotseng, L. Green Synthesis of Crystalline Silica from Sugarcane Bagasse Ash: Physico-Chemical Properties. Nanomaterials 2022, 12, 2184. [Google Scholar] [CrossRef]
- Atkins, P.; Overton, T. Shriver and Atkins’ Inorganic Chemistry; Oxford University Press: New York, NY, USA, 2010. [Google Scholar]
- Li, B.; Yu, D.; Zhang, S.L. Raman spectral study of silicon nanowires. Phys. Rev. B 1999, 59, 1645. [Google Scholar] [CrossRef]
- Xia, H.; He, Y.L.; Wang, L.C.; Zhang, W.; Liu, X.N.; Zhang, X.K.; Feng, D.; Jackson, H.E. Phonon mode study of Si nanocrystals using micro-Raman spectroscopy. J. Appl. Phys. 1995, 78, 6705–6708. [Google Scholar] [CrossRef]
- Holzapfel, M.; Buqa, H.; Hardwick, L.J.; Hahn, M.; Würsig, A.; Scheifele, W.; Novák, P.; Kötz, R.; Veit, C.; Petrat, F.M. Nano silicon for lithium-ion batteries. Electrochim. Acta 2006, 52, 973–978. [Google Scholar] [CrossRef]
- Richter, H.; Wang, Z.P.; Ley, L. The one phonon Raman spectrum in microcrystalline silicon. Solid State Commun. 1981, 39, 625–629. [Google Scholar] [CrossRef]
- Zhang, S.L.; Wang, X.; Ho, K.S.; Li, J.; Diao, P.; Cai, S. Raman spectra in a broad frequency region of p-type porous silicon. J. Appl. Phys. 1994, 76, 3016–3019. [Google Scholar] [CrossRef]
- Kale, P.G.; Solanki, C.S. Synthesis of si nanoparticles from freestanding porous silicon (PS) film using ultrasonication. In Proceedings of the 2010 35th IEEE Photovoltaic Specialists Conference, Honolulu, HI, USA, 20–25 June 2010; pp. 003692–003697. [Google Scholar]
- Lee, J.H.; Kwon, J.H.; Lee, J.W.; Lee, H.S.; Chang, J.H.; Sang, B.I. Preparation of high purity silica originated from rice husks by chemically removing metallic impurities. J. Ind. Eng. Chem. 2017, 50, 79–85. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W.; Salzberg, H.W. Adsorption surface area and porosity. J. Electrochem. Soc. 1967, 114, 279Ca. [Google Scholar] [CrossRef]
- Falk, G.; Shinhe, G.P.; Teixeira, L.B.; Moraes, E.G.; de Oliveira, A.N. Synthesis of silica nanoparticles from sugarcane bagasse ash and nano-silicon via magnesiothermic reactions. Ceram. Int. 2019, 45, 21618–21624. [Google Scholar] [CrossRef]
| Precursor | Reaction Conditions | Properties of As-Produced Silicon |
|---|---|---|
| Algae | T = 650 °C | Nanocrystal silicon SSA-500 m2/g, Pore Size Diameter-2 nm. [11] |
| (Stober Method) | T = 800 °C t = 12 h Argon atm | Porous nanocrystalline Si [12] |
| Rice Husk (RH), Bamboo Culm (BC), and Sugarcane Bagasse (SCB) | T = 650 °C t = 30 min (MW-MR) | Porous Silicon, Pore diameter (50–80 nm) SSA [13] |
| RH | T = 650 °C t = 7 h | Porous silicon SSA 150 m2/g [14] |
| Component | Wt% |
|---|---|
| 79.40 | |
| 1.03 | |
| 8.45 | |
| 2.39 | |
| 0.04 | |
| 0.61 | |
| 1.06 | |
| 1.06 | |
| 3.86 | |
| 0.22 | |
| 0.03 | |
| 0.04 | |
| 0.02 | |
| 0.04 | |
| 1.18 |
| Wavelength (cm−1) | Bond | Type |
|---|---|---|
| 1056 | Si-O | Stretching mode |
| 446 | Si-O | Rocking vibration |
| Sample ID | BETSSA (m2/g) | Vp (cm3) | Dp (nm) |
|---|---|---|---|
| SCBA nano-silicon | 74 | 0.23 | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seroka, N.S.; Taziwa, R.; Khotseng, L. Nanostructured Silicon Derived from an Agricultural Residue Bagasse Ash via Magnesiothermic Reduction Method. Coatings 2023, 13, 221. https://doi.org/10.3390/coatings13020221
Seroka NS, Taziwa R, Khotseng L. Nanostructured Silicon Derived from an Agricultural Residue Bagasse Ash via Magnesiothermic Reduction Method. Coatings. 2023; 13(2):221. https://doi.org/10.3390/coatings13020221
Chicago/Turabian StyleSeroka, Ntalane S., Raymond Taziwa, and Lindiwe Khotseng. 2023. "Nanostructured Silicon Derived from an Agricultural Residue Bagasse Ash via Magnesiothermic Reduction Method" Coatings 13, no. 2: 221. https://doi.org/10.3390/coatings13020221
APA StyleSeroka, N. S., Taziwa, R., & Khotseng, L. (2023). Nanostructured Silicon Derived from an Agricultural Residue Bagasse Ash via Magnesiothermic Reduction Method. Coatings, 13(2), 221. https://doi.org/10.3390/coatings13020221









