Nanohydroxyapatite Loaded with 5-Fluorouracil and Calendula officinalis L. Plant Extract Rich in Myo-Inositols for Treatment of Ovarian Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Determining the Morphology, Hydrodynamic Diameter, and Zeta Potential
2.3. Investigating Chemical Composition and Crystallinity
2.4. Stability Studies
2.5. Determining the Composition of Calendula officinalis L. Plant Extract
2.6. Determining the Radical Scavenging Activity
2.7. Stastistical Analysis
2.8. In Vitro Studies
2.9. Studying Interactions between Nanoparticles and Biomimetic Membranes
2.10. DTF Studies
2.11. Procedures
3. Results
3.1. Chemical Composition Studies of Calendula officinalis L. Extract
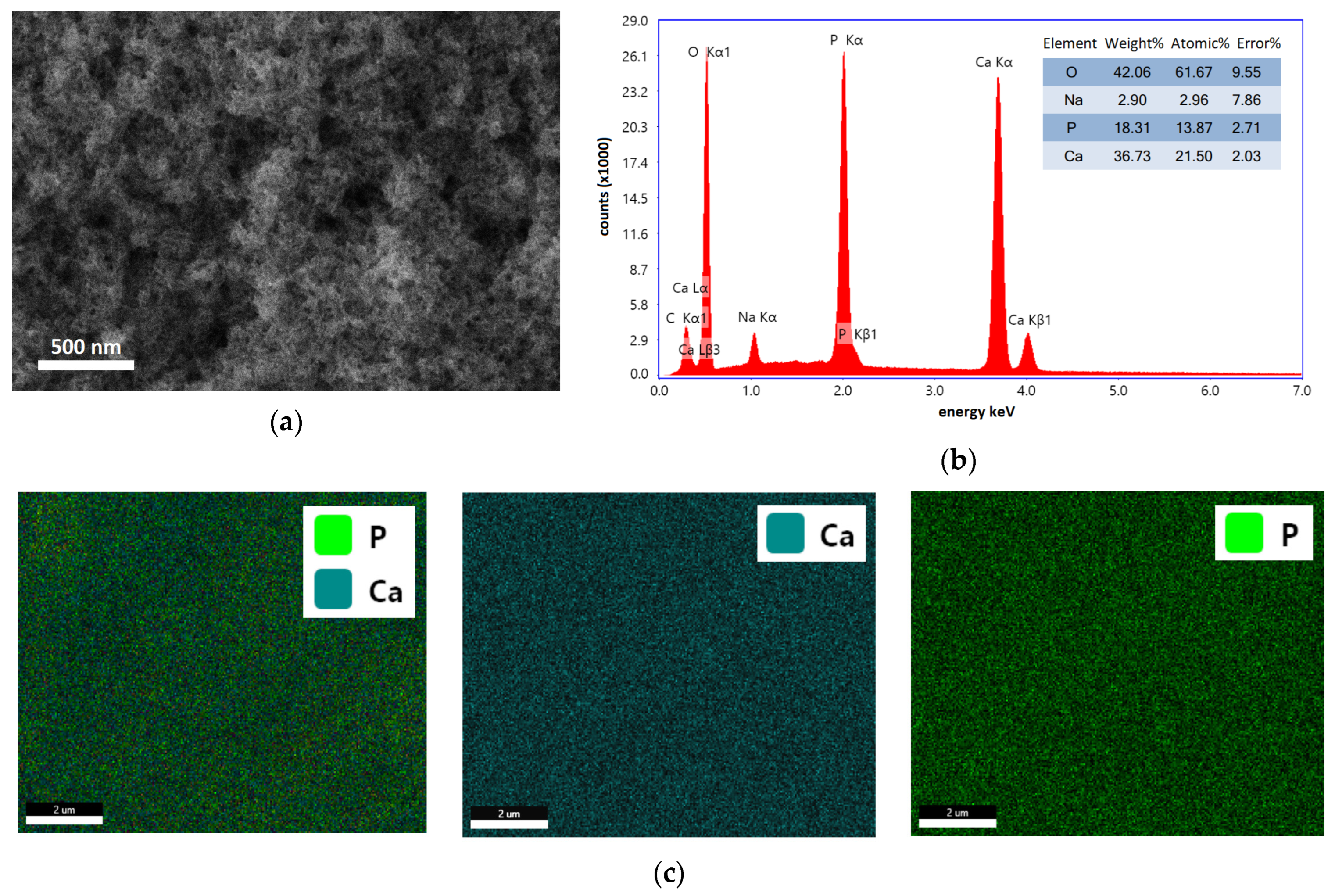
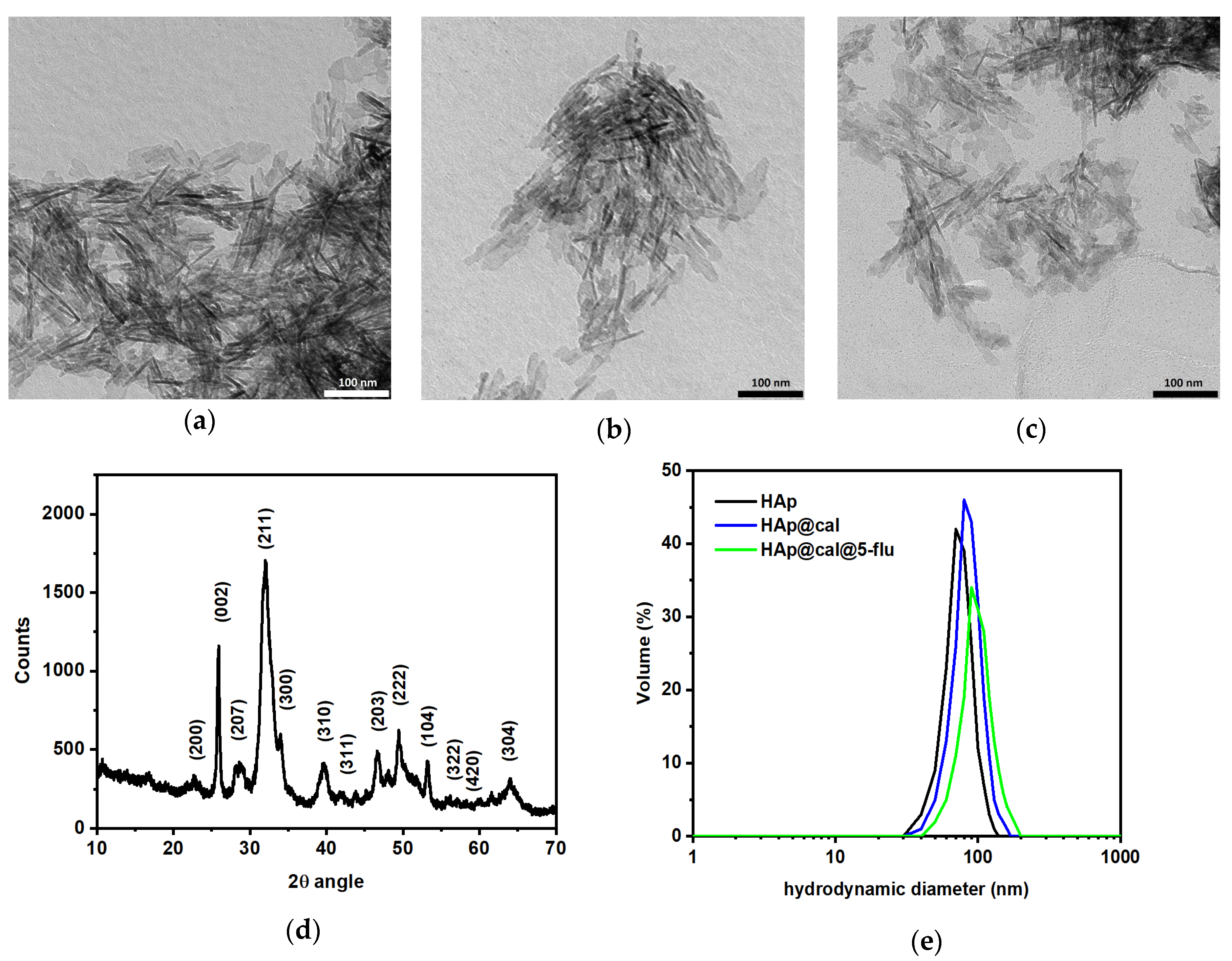
3.2. Morphology, Composition, and Crystallinity Studies
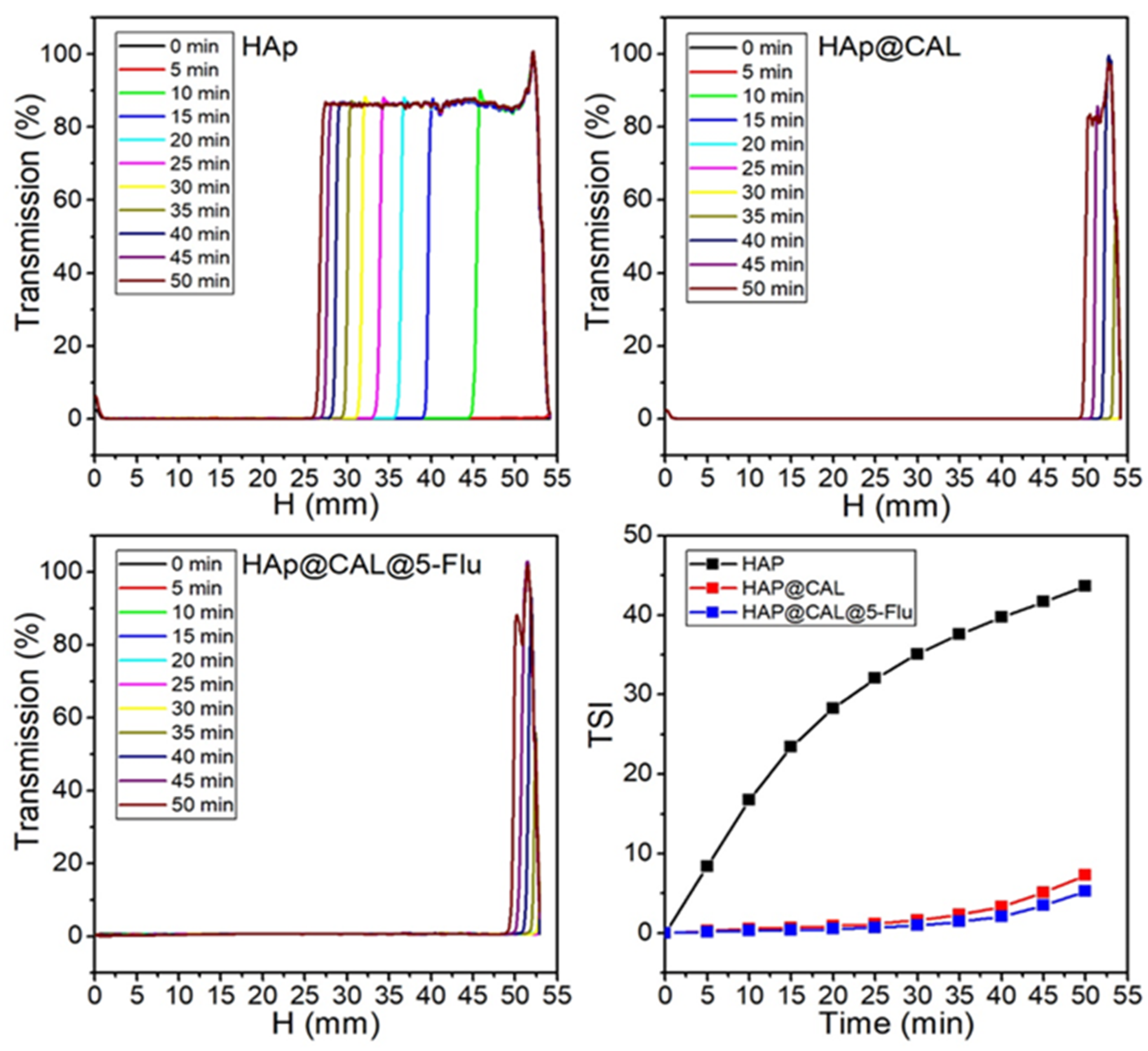
3.3. Turbiscan Analysis
3.4. Antioxidant Properties
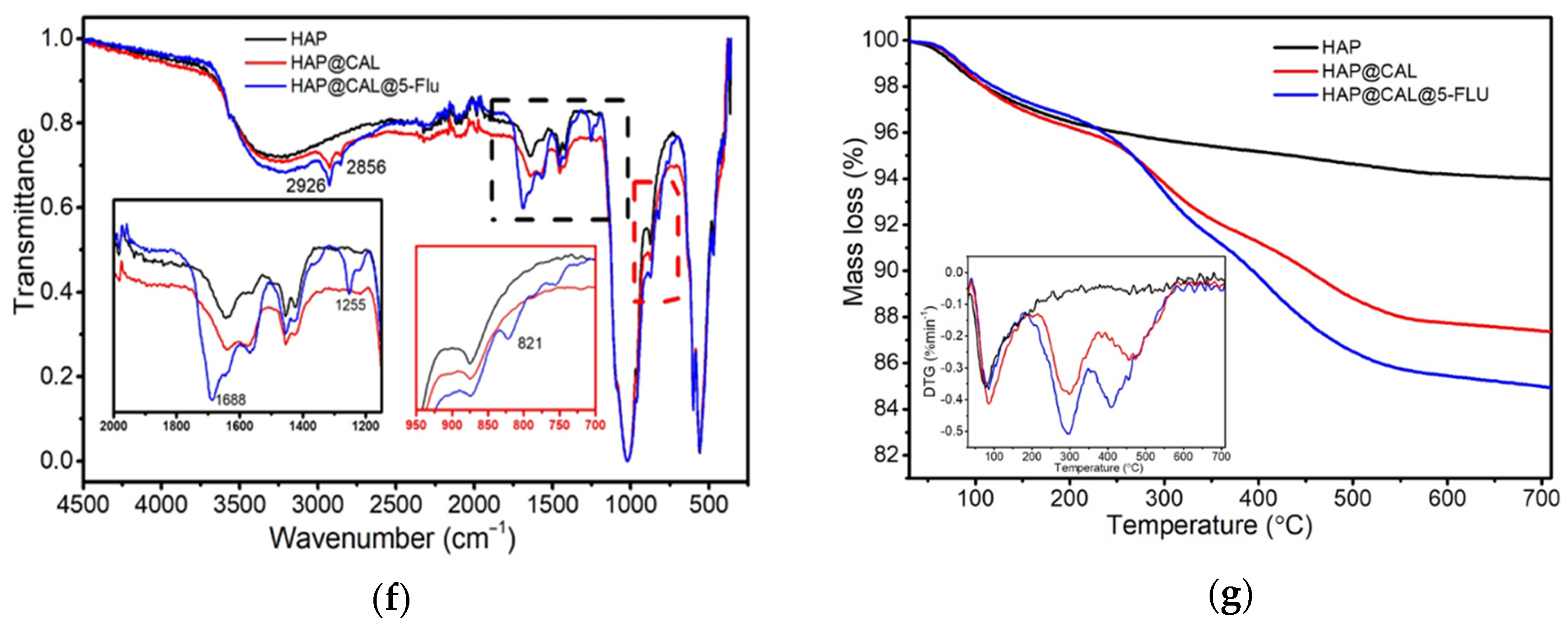
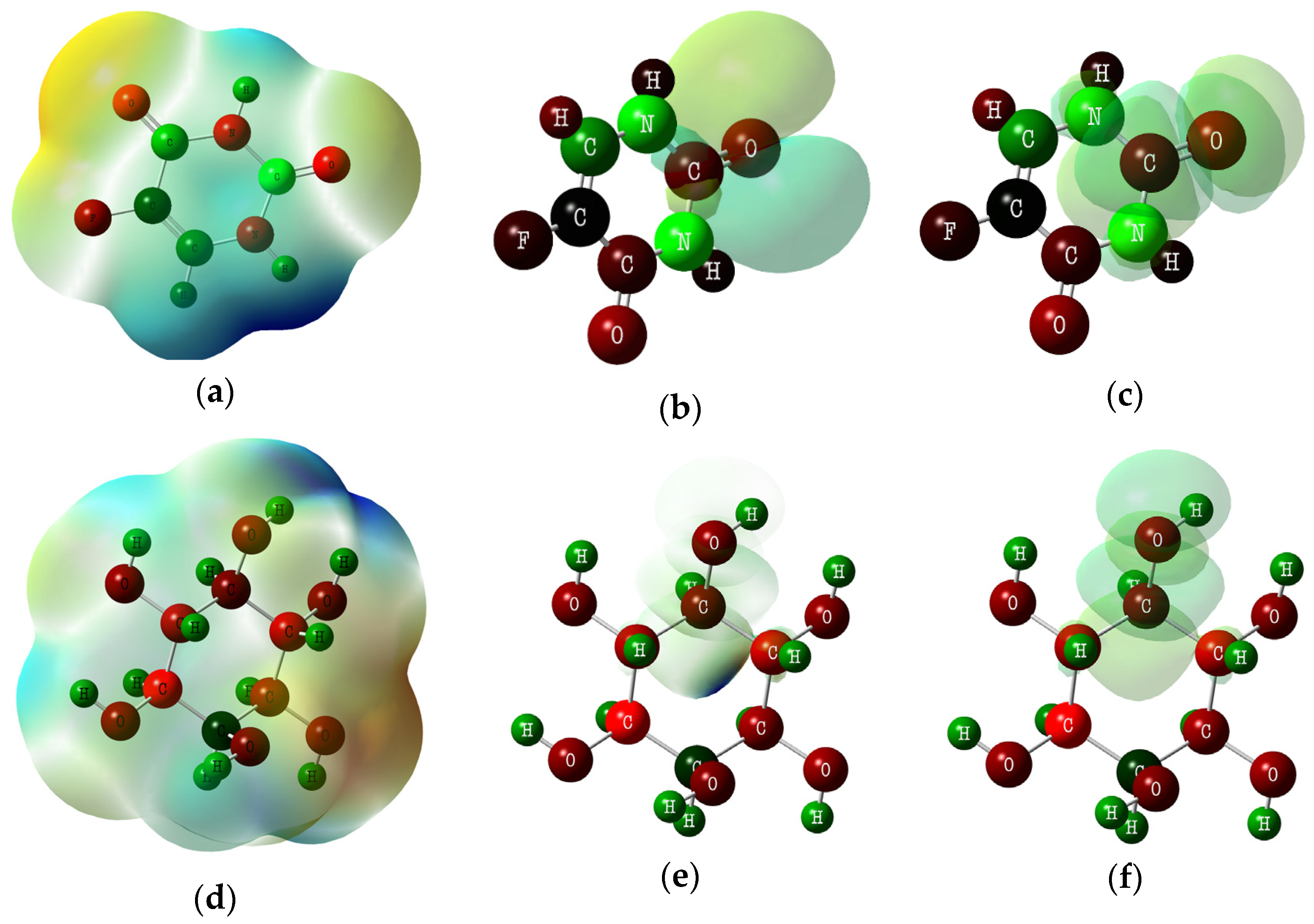
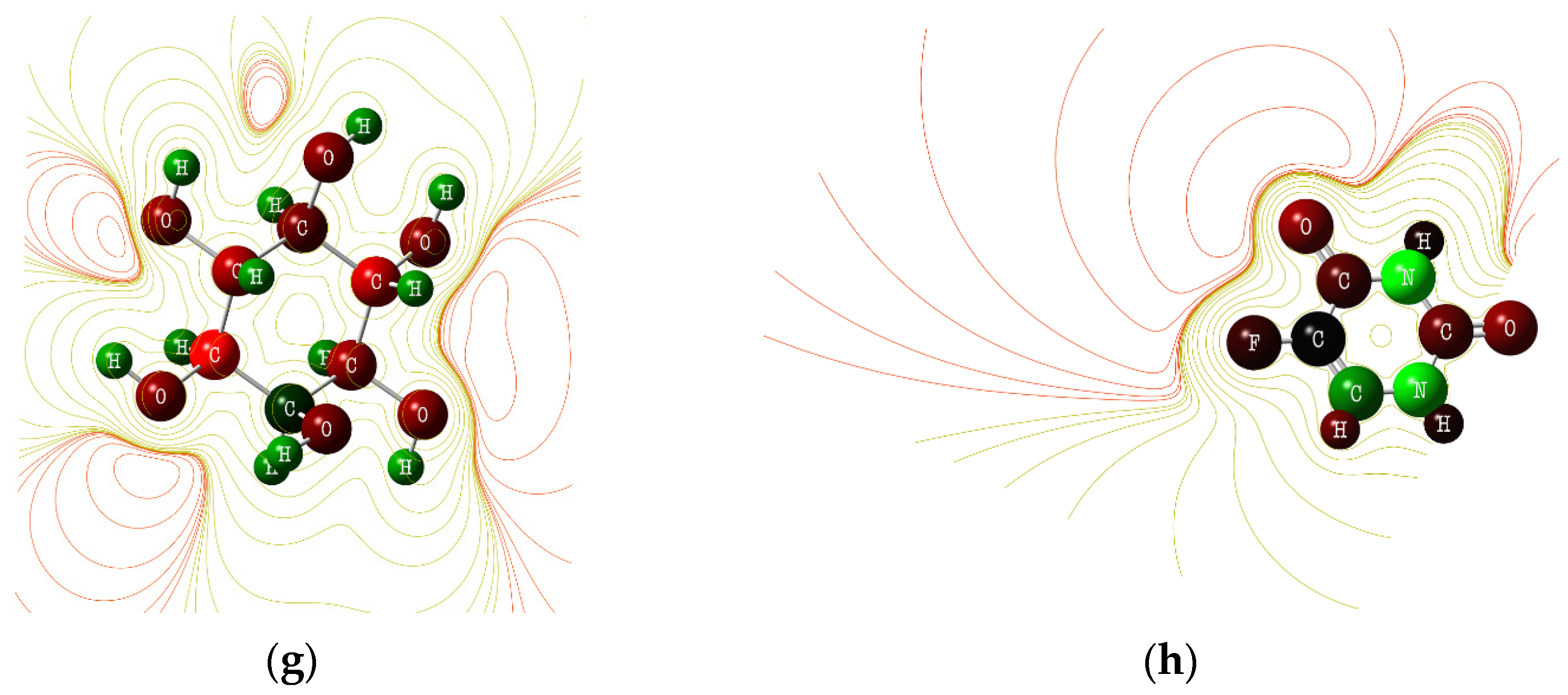
3.5. DTF Analysis
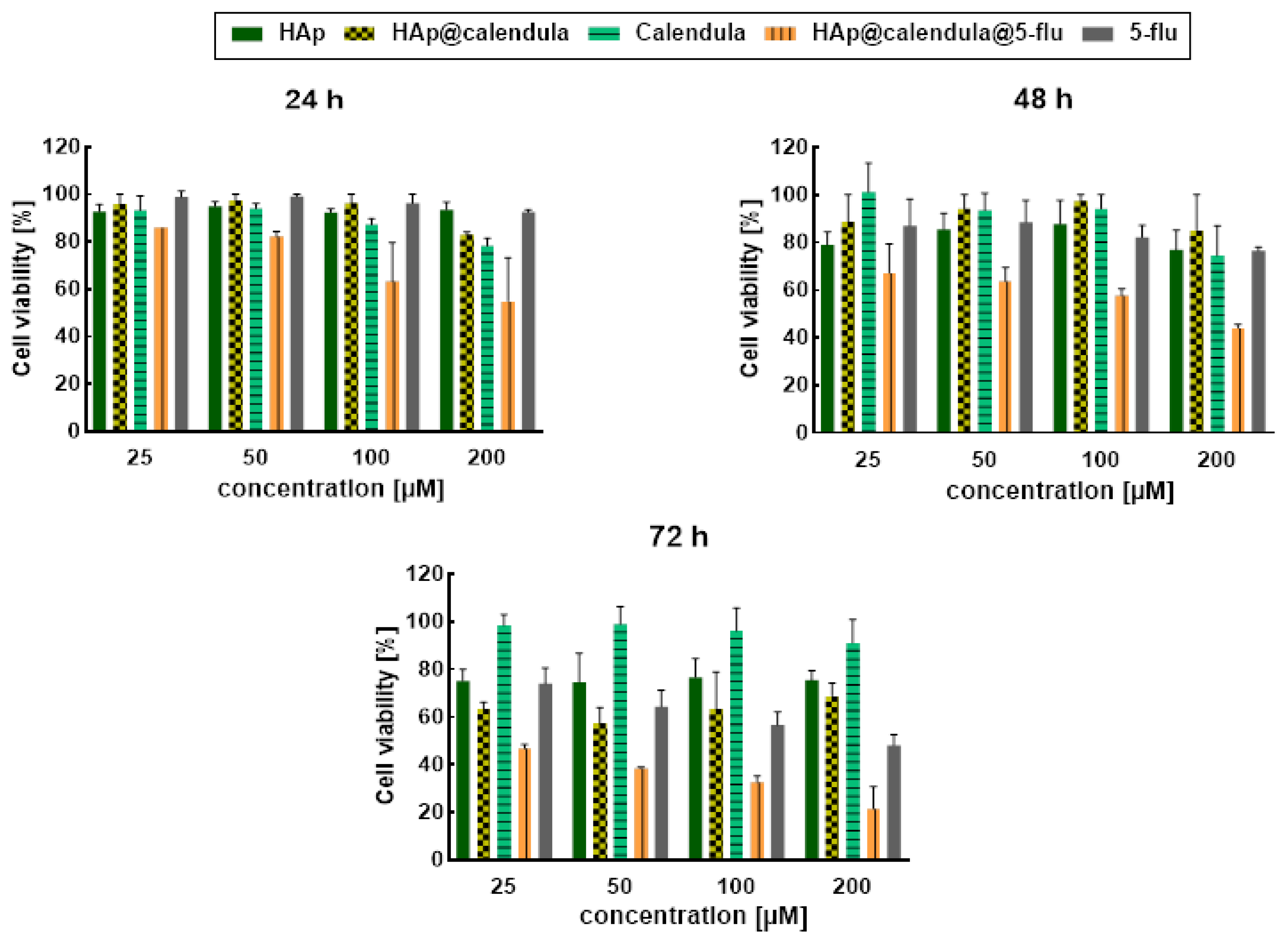
3.6. In Vitro Cytotoxicity Results
3.7. Interaction of Conjugate with Model Membrane System
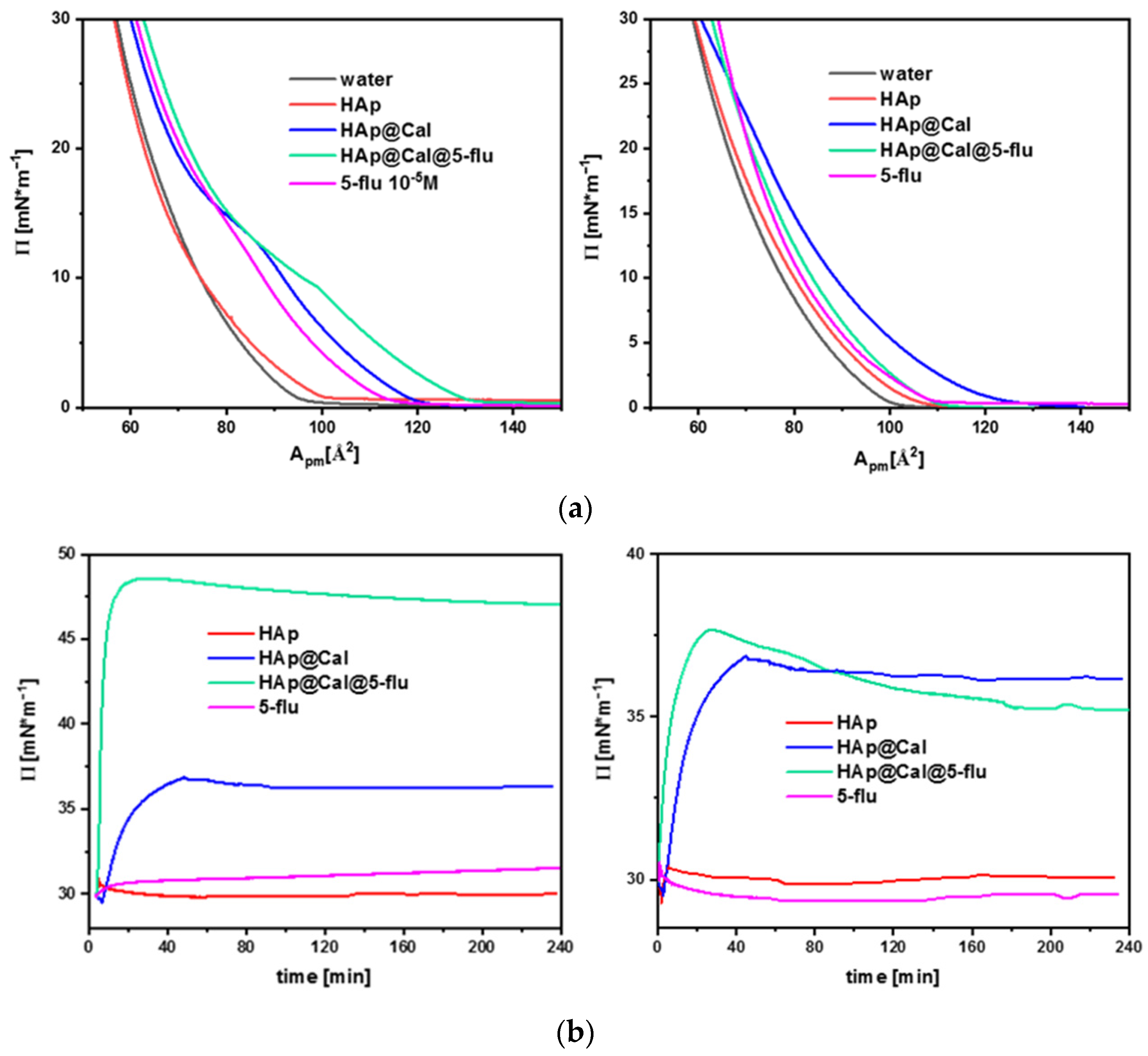
3.7.1. Langmuir Studies
3.7.2. Surface Pressure–Time Dependence
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramawat, K.; Dass, S.; Mathur, M. The chemical diversity of bioactive molecules and therapeutic potential of medicinal plants. In Herbal Drugs: Ethnomedicine to Modern Medicine; Ramawat, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 7–30. [Google Scholar]
- Abdelwahab, S.I.; Taha, M.M.E.; Taha, S.M.E.; Alsayegh, A.A. Fifty-year of Global Research in Calendula Officinalis L. (1971−2021): A Bibliometric Study. Clin. Complement. Med. Pharmacol. 2022, 2, 100059. [Google Scholar] [CrossRef]
- Rogowska, A.; Stpiczyńska, M.; Pączkowski, C.; Szakiel, A. The Influence of Exogenous Jasmonic Acid on the Biosynthesis of Steroids and Triterpenoids in Calendula officinalis Plants and Hairy Root Culture. Int. J. Mol. Sci. 2022, 23, 12173. [Google Scholar] [CrossRef]
- Akhtar, G.; Faried, H.N.; Razzaq, K.; Ullah, S.; Wattoo, F.M.; Shehzad, M.A.; Sajjad, Y.; Ahsan, M.; Javed, T.; Dessoky, E.S.; et al. Chitosan-Induced Physiological and Biochemical Regulations Confer Drought Tolerance in Pot Marigold (Calendula officinalis L.). Agronomy 2022, 12, 474. [Google Scholar] [CrossRef]
- Raal, A.; Kirsipuu, K.; Must, R.; Tenno, S. Content of Total Carotenoids in Calendula officinalis L. from Different Countries Cultivated in Estonia. Nat. Prod. Commun. 2009, 4, 35–38. [Google Scholar] [CrossRef]
- Joly, R.; Forcella, F.; Peterson, D.; Eklund, J. Planting depth for oilseed calendula. Ind. Crop. Prod. 2013, 42, 133–136. [Google Scholar] [CrossRef]
- Siracusa, L.; Napoli, E.; Ruberto, G. Review Novel Chemical and Biological Insights of Inositol Derivatives in Mediterranean Plants. Molecules 2022, 27, 1525. [Google Scholar] [CrossRef] [PubMed]
- Chhetri, D.R. Myo-Inositol and Its Derivatives: Their Emerging Role in the Treatment of Human Diseases. Front. Pharmacol. 2019, 10, 1172. [Google Scholar] [CrossRef] [PubMed]
- Loewus, F.A.; Murthy, P.P.N. myo-Inositol metabolism in plants. Plant Sci. 2000, 150, 1–19. [Google Scholar] [CrossRef]
- Papaleo, E.; Unfer, V.; Baillargeon, J.P.; Chiu, T. Contribution of myo-inositol to reproduction. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 147, 120–123. [Google Scholar] [CrossRef]
- Haris, M.; Cai, K.; Singh, A.; Hariharan, H.; Reddy, R. In vivo mapping of brain myo-inositol. NeuroImage 2011, 54, 2079–2085. [Google Scholar] [CrossRef]
- Ratiu, I.A.; Al-Suod, H.; Ligor, M.; Ligor, T.; Krakowska, A.; Górecki, R.; Buszewski, B. Simultaneous Determination of Cyclitols and Sugars Following a Comprehensive Investigation of 40 Plants. Food Anal. Methods 2019, 12, 1466–1478. [Google Scholar] [CrossRef]
- Pintaudi, B.; Di Vieste, G.; Bonomo, M. The Effectiveness of Myo-Inositol and D-Chiro Inositol Treatment in Type 2 Diabetes. Int. J. Endocrinol. 2016, 2016, 9132052. [Google Scholar] [CrossRef]
- Amabile, M.I.; De Luca, A.; Tripodi, D.; D’alberti, E.; Melcarne, R.; Imbimbo, G.; Picconi, O.; D’andrea, V.; Vergine, M.; Sorrenti, S.; et al. Personalized Medicine Effects of Inositol Hexaphosphate and Myo-Inositol Administration in Breast Cancer Patients during Adjuvant Chemotherapy. J. Pers. Med. 2021, 11, 756. [Google Scholar] [CrossRef]
- El Hafny-Rahbi, B.; Brodaczewska, K.; Collet, G.; Majewska, A.; Klimkiewicz, K.; Delalande, A.; Grillon, C.; Kieda, C. Tumour angiogenesis normalized by myo-inositol trispyrophosphate alleviates hypoxia in the microenvironment and promotes antitumor immune response. J. Cell. Mol. Med. 2021, 25, 3284–3299. [Google Scholar] [CrossRef]
- Naahidi, S.; Jafari, M.; Edalat, F.; Raymond, K.; Khademhosseini, A.; Chen, P. Biocompatibility of engineered nanoparticles for drug delivery. J. Control. Release 2013, 166, 182–194. [Google Scholar] [CrossRef]
- Sebastiammal, S.; Fathima, A.S.L.; Henry, J.; Wadaan, M.A.; Mahboob, S.; Wadaan, A.M.; Manzoor, I.; Gopinath, K.; Rajeswary, M.; Govindarajan, M. Synthesis, Characterization, Antibacterial, Antifungal, Antioxidant, and Anticancer Activities of Nickel-Doped Hydroxyapatite Nanoparticles. Fermentation 2022, 8, 677. [Google Scholar] [CrossRef]
- Panneerselvam, R.; Anandhan, N.; Gopu, G.; Ganesan, K.P.; Marimuthu, T. Impact of different transition metal ions in the structural, mechanical, optical, chemico-physical and biological properties of nanohydroxyapatite. Appl. Surf. Sci. 2020, 506, 144802. [Google Scholar] [CrossRef]
- Sobierajska, P.; Dorotkiewicz-Jach, A.; Zawisza, K.; Okal, J.; Olszak, T.; Drulis-Kawa, Z.; Wiglusz, R.J. Preparation and antimicrobial activity of the porous hydroxyapatite nanoceramics. J. Alloys Compd. 2018, 748, 179–187. [Google Scholar] [CrossRef]
- Zawisza, K.; Sobierajska, P.; Nowak, N.; Kedziora, A.; Korzekwa, K.; Pozniak, B.; Tikhomirov, M.; Miller, J.; Mrowczynska, L.; Wiglusz, R.J. Preparation and preliminary evaluation of bio-nanocomposites based on hydroxyapatites with antibacterial properties against anaerobic bacteria. Mater. Sci. Eng. C 2020, 106, 110295. [Google Scholar] [CrossRef]
- Mondal, S.; Dorozhkin, S.V.; Pal, U. Recent progress on fabrication and drug delivery applications of nanostructured hydroxyapatite. WIREs Nanomed. Nanobiotechnology 2018, 10, 1504. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Cui, N.; Mao, X.; Huang, Q.; Lee, E.-S.; Jiang, H. Sorption Studies of Tetracycline Antibiotics on Hydroxyapatite (001) Surface—A First-Principles Insight. Materials 2022, 15, 797. [Google Scholar] [CrossRef] [PubMed]
- Vechasilp, J.; Tangtrakulwanich, B.; Oungbho, K.; Yuenyongsawad, S. The efficacy of methotrexate-impregnated hydroxyapatite composites on human mammary carcinoma cells. J. Orthop. Surg. 2007, 15, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Nishio, Y.; Makiura, R.; Nakahira, A.; Kojima, C. Paclitaxel-loaded hydroxyapatite/collagen hybrid gels as drug delivery systems for metastatic cancer cells. Int. J. Pharm. 2013, 446, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Sobierajska, P.; Serwotka-Suszczak, A.; Targonska, S.; Szymanski, D.; Marycz, K.; Wiglusz, R.J. Synergistic Effect of Toceranib and Nanohydroxyapatite as a Drug Delivery Platform—Physicochemical Properties and In Vitro Studies on Mastocytoma Cells. Int. J. Mol. Sci. 2022, 23, 1944. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zhao, Y.; Wang, C.; Sun, R.; Tang, Y. Effects of physico-chemical properties of ions-doped hydroxyapatite on adsorption and release performance of doxorubicin as a model anticancer drug. Mater. Chem. Phys. 2022, 276, 125440. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, D.; Sun, R.; Shang, S.; Wang, H.; Yang, Y.; Wang, L.; Liu, X.; Sun, T.; Chen, K. Adsorption behavior of drugs on hydroxyapatite with different morphologies: A combined experimental and molecular dynamics simulation study. Ceram. Int. 2019, 45, 19522–19527. [Google Scholar] [CrossRef]
- Kundu, B.; Ghosh, D.; Sinha, M.K.; Sen, P.S.; Balla, V.K.; Das, N.; Basu, D. Doxorubicin-intercalated nano-hydroxyapatite drug-delivery system for liver cancer: An animal model. Ceram. Int. 2013, 39, 9557–9566. [Google Scholar] [CrossRef]
- Luo, H.; Ji, D.; Li, C.; Zhu, Y.; Xiong, G.; Wan, Y. Layered nanohydroxyapatite as a novel nanocarrier for controlled delivery of 5-fluorouracil. Int. J. Pharm. 2016, 513, 17–25. [Google Scholar] [CrossRef]
- Grocholewicz, K.; Matkowska-Cichocka, G.; Makowiecki, P.; Droździk, A.; Ey-Chmielewska, H.; Dziewulska, A.; Tomasik, M.; Trybek, G.; Janiszewska-Olszowska, J. Effect of nano-hydroxyapatite and ozone on approximal initial caries: A randomized clinical trial. Sci. Rep. 2020, 10, 11192. [Google Scholar] [CrossRef]
- Saif, M.W.; Choma, A.; Salamone, S.J.; Chu, E. Pharmacokinetically guided dose adjustment of 5-fluorouracil: A rational approach to improving therapeutic outcomes. J. Natl. Cancer Inst. 2009, 101, 1543–1552. [Google Scholar] [CrossRef]
- Bocci, G.; Barbara, C.; Vannozzi, F.; Dipaolo, A.; Melosi, A.; Barsanti, G.; Allegrini, G.; Falcone, A.; Deltacca, M.; Danesi, R. A pharmacokinetic-based test to prevent severe 5-fluorouracil toxicity. Clin. Pharmacol. Ther. 2006, 80, 384–395. [Google Scholar] [CrossRef]
- Gamelin, E.; Delva, R.; Jacob, J.; Merrouche, Y.; Raoul, J.L.; Pezet, D.; Dorval, E.; Piot, G.; Morel, A.; Boisdron-Celle, M. Individual fluorouracil dose adjustment based on pharmacokinetic follow-up compared with conventional dosage: Results of a multicenter randomized trial of patients with metastatic colorectal cancer. J. Clin. Oncol. 2008, 26, 2099–2105. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Jiang, Y.; Yang, Y.; Zheng, Y.; Zheng, J.; Jiang, H.; Zhang, S.; Lin, L.; Zheng, J.; Zhang, S.; et al. Determining the optimal 5-FU therapeutic dosage in the treatment of colorectal cancer patients. Oncotarget 2016, 7, 81880–81887. [Google Scholar] [CrossRef] [PubMed]
- Chavani, O.P. 5-Fluorouracil Response Prediction and Blood Level–Guided Therapy in Oncology: Existing Evidence Fundamentally Supports Instigation. Ther. Drug Monit. 2020, 42, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R. Gaussian 09, Revision C.02.; Gaussian Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Alghamdi, S.; Abbas, F.; Hussein, R.; Alhamzani, A.; El-Shamy, N. Spectroscopic characterization (IR, UV-Vis), and HOMO-LUMO, MEP, NLO, NBO Analysis and the Antifungal Activity for 4-Bromo-N-(2-nitrophenyl) benzamide; Using DFT Modeling and In silico Molecular Docking. J. Mol. Struct. 2023, 1271, 134001. [Google Scholar] [CrossRef]
- Raghavachari, K. Perspective on density functional thermochemistry. III. The role of exact exchange. Theor. Chem. Acc. 2000, 103, 361–363. [Google Scholar] [CrossRef]
- Elzupir, A.O.; Hussein, R.K.; Ibnaouf, K.H. Intermolecular CH-π Electrons Interaction in Poly (9,9-dioctylfluorenyl-2,7-diyl) (PFO): An Experimental and Theoretical Study. Molecules 2022, 27, 1488. [Google Scholar] [CrossRef]
- Croze, M.L.; Soulage, C.O. Potential role and therapeutic interests of myo-inositol in metabolic diseases. Biochimie 2013, 95, 1811–1827. [Google Scholar] [CrossRef]
- Chau, J.F.L.; Lee, M.K.; Law, J.W.S.; Chung, S.K.; Chung, S.S.M. Sodium/myo-inositol cotransporter-1 is essential for the development and function of the peripheral nerves. FASEB J. 2005, 19, 1887–1889. [Google Scholar] [CrossRef]
- Dai, Z.; Chung, S.K.; Miao, D.; Lau, K.S.; Chan, A.W.; Kung, A.W. Sodium/myo-Inositol Cotransporter 1 and myo-Inositol Are Essential for Osteogenesis and Bone Formation. J. Bone Miner. Res. 2011, 26, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.; McWilliams, A.; Leriche, J.; MacAulay, C.; Wattenberg, L.; Szabo, E. A phase I study of myo-inositol for lung cancer chemoprevention. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1526–1531. [Google Scholar] [CrossRef] [PubMed]
- Eagle, H.; Oyama, V.I.; Levy, M.; Freeman, A.E. myo-Inositol as an essential growth factor for normal and malignant human cells in tissue culture. J. Biol. Chem. 1957, 226, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, M.; Dinicola, S.; Bevilacqua, A.; Cucina, A. Broad Spectrum Anticancer Activity of Myo-Inositol and Inositol Hexakisphosphate. Int. J. Endocrinol. 2016, 2016, 5616807. [Google Scholar] [CrossRef]
- Graf, E.; Eaton, J.W. Antioxidant functions of phytic acid. Free Radic. Biol. Med. 1990, 8, 61–69. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, Y.; Wang, X.-L. Inositol hexaphosphate-induced enhancement of natural killer cell activity correlates with suppression of colon carcinogenesis in rats. World J. Gastroenterol. 2005, 11, 5044–5046. [Google Scholar] [CrossRef]
- Chang, L.; Huang, S.; Zhao, X.; Hu, Y.; Ren, X.; Mei, X.; Chen, Z. Preparation of ROS active and photothermal responsive hydroxyapatite nanoplatforms for anticancer therapy. Mater. Sci. Eng. C 2021, 125, 112098. [Google Scholar] [CrossRef]
- Barbosa, A.A.; Júnior, S.A.; Mendes, R.L.; de Lima, R.S.; Ferraz, A.D.V. Multifunctional hydroxyapatite with potential for application in theranostic nanomedicine. Mater. Sci. Eng. C 2020, 116, 111227. [Google Scholar] [CrossRef]
- Thi Thom, N.; Thi Mai Thanh, D.; Thi Nam, P.; Phuong, N.T.; Buess-Herman, C. Adsorption behavior of Cd2+ ions using hydroxyapatite (HAp) powder. Green Process. Synth. 2018, 7, 409–416. [Google Scholar] [CrossRef]
- Thanh, D.T.M.; Trang, P.T.T.; Huong, H.T.; Nam, P.T.; Phuong, N.T.; Trang, N.T.T.; Hoang, T.; Lam, T.D.; Park, J.S. Fabrication of poly (lactic acid)/hydroxyapatite (PLA/HAp) porous nanocomposite for bone regeneration. Int. J. Nanotechnol. 2015, 12, 391–404. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, Y.; Guo, J.; Yuan, J. Features of impinging streams intensifying processes and their applications. Int. J. Chem. Eng. 2010, 2010, 681501. [Google Scholar] [CrossRef]
- Zebarjad, S.M.; Sajjadi, S.A.; Sdrabadi, T.E.; Yaghmaei, A.; Naderi, B. A Study on Mechanical Properties of PMMA/Hydroxyapatite Nanocomposite. Engineering 2011, 3, 795–801. [Google Scholar] [CrossRef]
- Rout, A.; Agrawal, S. Structural, morphological and electrical properties of new type Dy doped Ca6−xNa2Y2(SiO4)6(OH)2 hydroxyapatite compound synthesized by co-precipitation method. J. Electroceramics 2022, 48, 74–94. [Google Scholar] [CrossRef]
- Serhiienko, A.; Dontsova, T.; Yanushevska, O.; Lapinskyi, A.; Krymets, G. Synthesis and characterization of hydroxyapatite and composite based on it with collagen/alginate. Chem. Pap. 2022, 76, 385–392. [Google Scholar] [CrossRef]
- Ragunath, C.; Kousalya, L.; Venkatachalam, R.; Anitha, S. Green Synthesis of Hydroxyapatite Nanoparticles from Wrightia tinctoria and Its Antibacterial Activity. BioNanoScience 2022, 12, 723–730. [Google Scholar] [CrossRef]
- Scharnweber, T.; Santos, C.; Franke, R.-P.; Almeida, M.M.; Costa, M.E.V. Influence of Spray-dried Hydroxyapatite-5-Fluorouracil Granules on Cell Lines Derived from Tissues of Mesenchymal Origin. Molecules 2008, 13, 2729–2739. [Google Scholar] [CrossRef] [PubMed]
- Holzwarth, U.; Gibson, N. The Scherrer equation versus the ‘Debye-Scherrer equation’. Nat. Nanotechnol. 2011, 6, 534. [Google Scholar] [CrossRef] [PubMed]
- Balu, S.; Sundaradoss, M.V.; Andra, S.; Jeevanandam, J. Facile biogenic fabrication of hydroxyapatite nanorods using cuttlefish bone and their bactericidal and biocompatibility study. Beilstein J. Nanotechnol. 2020, 11, 285–295. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, M.; Ji, S.; Zhang, L.; Cao, W.; Wang, H.; Wang, S. Preparation and application of hydroxyapatite extracted from fish scale waste using deep eutectic solvents. Ceram. Int. 2021, 47, 9366–9372. [Google Scholar] [CrossRef]
- Wilson, O.C.; Hull, J.R. Surface modification of nanophase hydroxyapatite with chitosan. Mater. Sci. Eng. C 2008, 28, 434–437. [Google Scholar] [CrossRef]
- Rajapakse, H.D.; Adikary, S.U. Synthesis and characterization of chitosan/hydroxyapatite nanocomposite for bone tissue engineering applications. In Proceedings of the 2021 Moratuwa Engineering Research Conference (MERCon), Moratuwa, Sri Lanka, 27–29 July 2021; IEEE: New York, NY, USA, 2021. [Google Scholar]
- Slavov, A.; Panchev, I.; Kovacheva, D.; Vasileva, I. Physico-chemical characterization of water-soluble pectic extracts from Rosa damascena, Calendula officinalis and Matricaria chamomilla wastes. Food Hydrocoll. 2016, 61, 469–476. [Google Scholar] [CrossRef]
- Gupta, A.; Tiwari, G.; Tiwari, R.; Srivastava, R. Factorial designed 5-fluorouracil-loaded microsponges and calcium pectinate beads plugged in hydroxypropyl methylcellulose capsules for colorectal cancer. Int. J. Pharm. Investig. 2015, 5, 234. [Google Scholar] [CrossRef]
- Phuong, N.T.; Nam, N.H.; Hong, C.T.; Thi Hai, D.; Osial, M.; Giersig, M. Apatite ore-based nanostructures: Novel and eco- friendly sorbent for e cient removal of wastewater containing Pb2+ and Fe3+. Res. Sq. 2022, 8, 1–16. [Google Scholar] [CrossRef]
- Manoj, M.; Mangalaraj, D.; Ponpandian, N.; Viswanathan, C. Core–shell hydroxyapatite/Mg nanostructures: Surfactant free facile synthesis, characterization and their in vitro cell viability studies against leukaemia cancer cells (K562). RSC Adv. 2015, 5, 48705–48711. [Google Scholar] [CrossRef]
- Mir, M.; Leite, F.L.; Herrmann, P.S.D.P.; Pissetti, F.L.; Rossi, A.M.; Moreira, E.L.; Mascarenhas, Y.P. XRD, AFM, IR and TGA study of nanostructured hydroxyapatite. Mater. Res. 2012, 15, 622–627. [Google Scholar] [CrossRef]
- Sobierajska, P.; Wiglusz, R.J. Influence of Li+ ions on the physicochemical properties of nanocrystalline calcium–strontium hydroxyapatite doped with Eu3+ ions. New J. Chem. 2019, 43, 14908–14916. [Google Scholar] [CrossRef]
- Chandrasekar, A.; Sagadevan, S.; Dakshnamoorthy, A. Synthesis and characterization of nano-hydroxyapatite (n-HAP) using the wet chemical technique. Int. J. Phys. Sci. 2013, 8, 1639–1645. [Google Scholar] [CrossRef]
- Santos, C.; Rovath, C.F.; Franke, R.P.; Almeida, M.; Costa, M. Spray-dried hydroxyapatite-5-Fluorouracil granules as a chemotherapeutic delivery system. Ceram. Int. 2009, 35, 509–513. [Google Scholar] [CrossRef]
- Fierascu, I.; Bunghez, I.-R.; Fierascu, R.-C.; Ion, R.M.; Dinu Pîrvu, C.E.; Nuta, D. Characterization and Antioxidant Activity of Phytosynthesised Silver Na-noparticles Using Calendula. Farmacja 2014, 62, 129–136. [Google Scholar]
- Al-Mussawi, Z.K.; Al-Hussani, I.M. Phytochemical study of Calendula officinalis plant by used GC-MS and FTIR techniques. Plant Arch. 2019, 19, 845–851. [Google Scholar]
- Almeida, M.O.; Barros, D.A.S.; Araujo, S.C.; Faria, S.H.; Maltarollo, V.G.; Honorio, K.M. Study on molecular structure, spectroscopic properties (FTIR and UV–Vis), NBO, QTAIM, HOMO-LUMO energies and docking studies of 5-fluorouracil, a substance used to treat cancer. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 184, 169–176. [Google Scholar] [CrossRef]
- Jubeen, F.; Liaqat, A.; Amjad, F.; Sultan, M.; Iqbal, S.Z.; Sajid, I.; Niazi, M.B.K.; Sher, F. Synthesis of 5-Fluorouracil Cocrystals with Novel Organic Acids as Coformers and Anticancer Evaluation against HCT-116 Colorectal Cell Lines. Cryst. Growth Des. 2020, 20, 2406–2414. [Google Scholar] [CrossRef]
- Lin, F.H.; Lee, Y.H.; Jian, C.; Wong, J.-M.; Shieh, M.-J.; Wang, C.-Y. A study of purified montmorillonite intercalated with 5-fluorouracil as drug carrier. Biomaterials 2002, 23, 1981–1987. [Google Scholar] [CrossRef] [PubMed]
- Delforce, L.; Hofmann, E.; Nardello-Rataj, V.; Aubry, J.M. TiO2 nanoparticle dispersions in water and nonaqueous solvents studied by gravitational sedimentation analysis: Complementarity of Hansen Parameters and DLVO interpretations. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 628, 127333. [Google Scholar] [CrossRef]
- Yue, M.; Huang, M.; Zhu, Z.; Huang, T.; Huang, M. Effect of ultrasound assisted emulsification in the production of Pickering emulsion formulated with chitosan self-assembled particles: Stability, macro, and micro rheological properties. LWT 2022, 154, 112595. [Google Scholar] [CrossRef]
- Santos, J.; Trujillo-Cayado, L.A.; Carrillo, F.; López-Castejón, M.L.; Alfaro-Rodríguez, M.C. Relation between Droplet Size Distributions and Physical Stability for Zein Microfluidized Emulsions. Polymers 2022, 14, 2195. [Google Scholar] [CrossRef]
- Pascaud, K.; Mercé, M.; Roucher, A.; Destribats, M.; Backov, R.; Schmitt, V.; Sescousse, R.; Brouillet, F.; Sarda, S.; Ré, M. Pickering emulsion as template for porous bioceramics in the perspective of bone regeneration. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 643, 128748. [Google Scholar] [CrossRef]
- Navarrete-Segado, P.; Tourbin, M.; Frances, C.; Grossin, D. Masked stereolithography of hydroxyapatite bioceramic scaffolds: From powder tailoring to evaluation of 3D printed parts properties. Open Ceram. 2022, 9, 100235. [Google Scholar] [CrossRef]
- Dai, J.; Wang, G.; Ma, L.; Wu, C. Study on the surface energies and dispersibility of graphene oxide and its derivatives. J. Mater. Sci. 2015, 50, 3895–3907. [Google Scholar] [CrossRef]
- Preethi, K.C.; Kuttan, G.; Kuttan, R. Antioxidant Potential of an Extract of Calendula officinalis. Flowers in Vitro. and in Vivo. Pharm. Biol. 2008, 44, 691–697. [Google Scholar] [CrossRef]
- Braga, P.C.; Dal Sasso, M.; Culici, M.; Spallino, A.; Falchi, M.; Bertelli, A.; Morelli, R.; Scalzo, R.L. Antioxidant activity of calendula officinalis extract: Inhibitory effects on chemiluminescence of human neutrophil bursts and electron paramagnetic resonance spectroscopy. Pharmacology 2009, 83, 348–355. [Google Scholar] [CrossRef]
- Martin, D.; Navarro Del Hierro, J.; Villanueva Bermejo, D.; Fernández-Ruiz, R.; Fornari, T.; Reglero, G. Bioaccessibility and Antioxidant Activity of Calendula officinalis Supercritical Extract as Affected by in Vitro Codigestion with Olive Oil. J. Agric. Food Chem. 2016, 64, 8828–8837. [Google Scholar] [CrossRef] [PubMed]
- Kasapović, J.; Pejić, S.; Stojiljković, V.; Todorović, A.; Radošević-Jelić, L.; Saičić, Z.S.; Pajović, S.B. Antioxidant status and lipid peroxidation in the blood of breast cancer patients of different ages after chemotherapy with 5-fluorouracil, doxorubicin and cyclophosphamide. Clin. Biochem. 2010, 43, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Alok, A.; Singh, S.; Kumar, P.; Bhati, K.K. Potential of engineering the myo-inositol oxidation pathway to increase stress resilience in plants. Mol. Biol. Rep. 2022, 49, 8025–8035. [Google Scholar] [CrossRef] [PubMed]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Wielińska, J.; Nowacki, A.; Liberek, B. 5-Fluorouracil—Complete Insight into Its Neutral and Ionised Forms. Molecules 2019, 24, 3683. [Google Scholar] [CrossRef] [PubMed]
- Motskin, M.; Wright, D.M.; Muller, K.; Kyle, N.; Gard, T.; Porter, A.; Skepper, J. Hydroxyapatite nano and microparticles: Correlation of particle properties with cytotoxicity and biostability. Biomaterials 2009, 30, 3307–3317. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.Y.; Sun, X.Y.; Ouyang, J.M. Effects of physical properties of nano-sized hydroxyapatite crystals on cellular toxicity in renal epithelial cells. Mater. Sci. Eng. C 2019, 103, 109807. [Google Scholar] [CrossRef]
- Montazeri, N.; Jahandideh, R.; Biazar, E. Synthesis of fluorapatite–hydroxyapatite nanoparticles and toxicity investigations. Int. J. Nanomed. 2011, 6, 197–201. [Google Scholar] [CrossRef]
- Shi, Z.; Huang, X.; Cai, Y.; Tang, R.; Yang, D. Size effect of hydroxyapatite nanoparticles on proliferation and apoptosis of osteoblast-like cells. Acta Biomater. 2009, 5, 338–345. [Google Scholar] [CrossRef]
- Weinberg, S.E.; Sun, L.Y.; Yang, A.L.; Liao, J.; Yang, G.Y. Overview of Inositol and Inositol Phosphates on Chemoprevention of Colitis-Induced Carcinogenesis. Molecules 2021, 26, 31. [Google Scholar] [CrossRef] [PubMed]
- Kassie, F.; Kalscheuer, S.; Matise, I.; Ma, L.; Melkamu, T.; Upadhyaya, P.; Hecht, S.S. Inhibition of vinyl carbamate-induced pulmonary adenocarcinoma by indole-3-carbinol and myo-inositol in A/J mice. Carcinogenesis 2010, 31, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Wattenberg, L.W. Chemoprevention of pulmonary carcinogenesis by myo-inositol. Anticancer Res. 1999, 19, 3659–3661. [Google Scholar]
- Elderdfi, M.; Sikorski, A.F. Langmuir-monolayer methodologies for characterizing protein-lipid interactions. Chem. Phys. Lipids 2018, 212, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Olusegun, S.; Osial, M.; Majkowska-Pilip, A.; Żelechowska-Matysiak, K.; Nieciecka, D.; Krajewski, M.; Pękała, M.; Krysiński, P. Synthesis and characterization of Sr2+ and Gd3+ doped magnetite nanoparticles for magnetic hyperthermia and drug delivery application. Ceram. Int. 2023, 49, 19851–19860. [Google Scholar] [CrossRef]
- Nieciecka, D.; Rękorajska, A.; Cichy, D.; Konska, P.; Żuk, M.; Krysiński, P. Synthesis and characterization of magnetic drug carriers modified with Tb3+ ions. Nanomaterials 2022, 12, 795. [Google Scholar] [CrossRef]
- Janmey, P.A.; Kinnunen, P.K.J. Biophysical properties of lipids and dynamic membranes. Trends Cell Biol. 2006, 16, 538–546. [Google Scholar] [CrossRef]
- Jabłonowska, E.; Matyszewska, D.; Nazaruk, E.; Godlewska, M.; Gaweł, D.; Bilewicz, R. Lipid membranes exposed to dispersions of phytantriol and monoolein cubosomes: Langmuir monolayer and HeLa cell membrane studies. Biochim. Biophys. Acta (BBA) Gen. Subj. 2021, 1865, 129738. [Google Scholar] [CrossRef]
- Nguyen, P.T.; Wilczewski, S.; Lewandowski, J.; Majkowska-Pilip, A.; Żelechowska-Matysiak, K.; Nieciecka, D.; Studziński, W.; Olusegun, S.J.; Syczewski, M.; Giersig, M.; et al. 5-fluorouracil and curcuminoids extract from Curcuma longa L. loaded into nanohydroxyapatite as a drug delivery carrier for SKOV-3 and HEpG2 cancer cells treatment. Ceram. Int. 2023, 49, 25775–25787. [Google Scholar] [CrossRef]
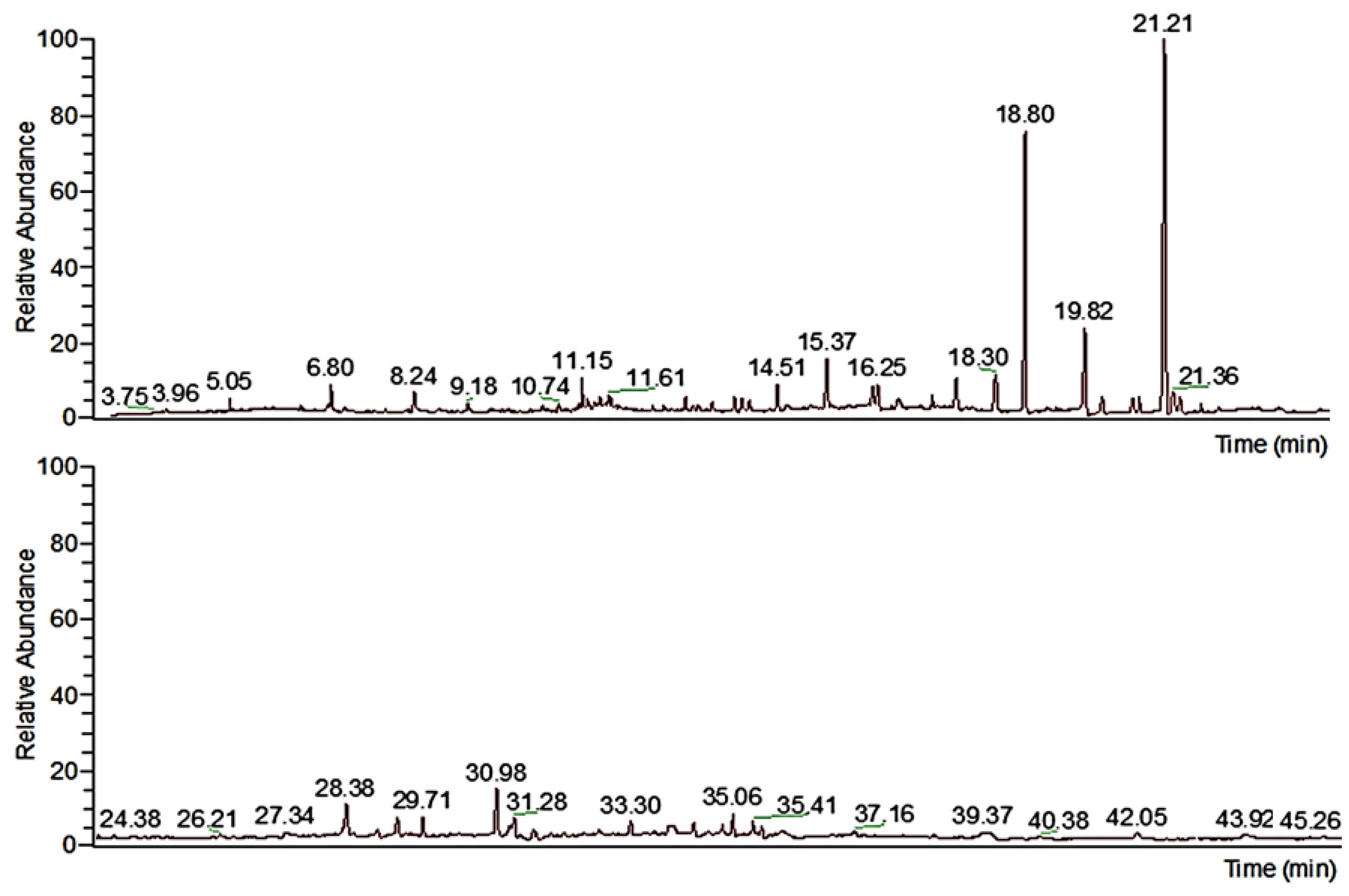
| Retention Time, min | Name of Molecule | Content, % |
|---|---|---|
| 5.06 | lactic acid | 0.6625 |
| 6.80 | Acetin | 0.9923 |
| 9.18 | salicylic acid | 0.3617 |
| 11.15 | Ledene | 1.5297 |
| 11.24 | 2,4-ditert butylphenol | 0.3777 |
| 11.61 | 2(4H) benzofuranone 5,6,7,7a tetrahydro-4,4,7a-trimethyl-R | 0.3285 |
| 11.67 | dodecanoic acid | 0.4141 |
| 12.94 | 2,4-ditert butylphenol | 0.5055 |
| 13.39 | tau-murrolol | 0.5326 |
| 13.78 | ((1R,4S,4aS,8aS)-4-isopropyl-1,6-dimethyl-1,2,3,4,4a,7,8,8a-octahydronaphthalen-1-yl)oxy | 0.8539 |
| 13.91 | tau-cadinol | 0.7169 |
| 14.04 | myristic acid | 0.6400 |
| 14.51 | ((1R,4S,4aS,8aS)-4-isopropyl-1,6-dimethyl-1,2,3,4,4a,7,8,8a-octahydronaphthalen-1-yl)oxy | 1.7623 |
| 15.37 | 3-(2-Hydroxyethyl)-2,2,4-trimethyl-3-cyclohexene-1-carbaldehyde | 3.4663 |
| 16.17 | 7-(3-Hydroxy-propyl)-bicyclo[2.2.1]heptan-2-one | 1.3798 |
| 16.25 | myristic acid TMS | 1.8612 |
| 16.60 | D-fructose | 0.5646 |
| 17.61 | hexadecanoic acid TMS | 1.8856 |
| 18.30 | n-hexadecanoic acid | 3.4895 |
| 18.79 | myo-inositol | 23.6283 |
| 19.82 | palmitic acid | 7.6933 |
| 20.13 | scyllo-inositol | 1.1784 |
| 20.66 | 9,12-octadecadienoic acid methyl ester | 1.0332 |
| 20.78 | 9,12,15-octadecatrienoic acid methyl ester ZZZ | 1.1167 |
| 21.21 | myo-inositol | 33.2834 |
| 21.36 | 9,12-octadecadienoic acid ZZ | 1.5959 |
| 21.49 | 9,12,15-octadecatrienoic acid | 1.2011 |
| 21.85 | octadecanoic acid | 0.3762 |
| 28.38 | hexadecanoic acid | 1.6693 |
| 29.26 | 6βBicyclo[4.3.0]nonane, 5β-iodomethyl-1β-isopropenyl-4α,5α-dimethyl-, | 1.1223 |
| 31.61 | methyl galactoside | 0.8303 |
| 34.38 | 2-methyl-4-octenal | 0.6879 |
| 35.06 | cedranoxide 8,14 | 2.2590 |
| Material | RSA, % (SD) |
|---|---|
| Hydroxyapatite | 1.1 d (0.8) |
| Calendula extract | 20.7 a (0.5) |
| 5-fluorouracil | 4.0 c (1.2) |
| HAp@Cal | 9.9 b (0.7) |
| HAp@Cal@5-flu | 20.0 a (1.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osial, M.; Wilczewski, S.; Szulc, J.; Nguyen, H.D.; Nguyen, T.K.O.; Skórczewska, K.; Majkowska-Pilip, A.; Żelechowska-Matysiak, K.; Nieciecka, D.; Pregowska, A.; et al. Nanohydroxyapatite Loaded with 5-Fluorouracil and Calendula officinalis L. Plant Extract Rich in Myo-Inositols for Treatment of Ovarian Cancer Cells. Coatings 2023, 13, 1944. https://doi.org/10.3390/coatings13111944
Osial M, Wilczewski S, Szulc J, Nguyen HD, Nguyen TKO, Skórczewska K, Majkowska-Pilip A, Żelechowska-Matysiak K, Nieciecka D, Pregowska A, et al. Nanohydroxyapatite Loaded with 5-Fluorouracil and Calendula officinalis L. Plant Extract Rich in Myo-Inositols for Treatment of Ovarian Cancer Cells. Coatings. 2023; 13(11):1944. https://doi.org/10.3390/coatings13111944
Chicago/Turabian StyleOsial, Magdalena, Sławomir Wilczewski, Joanna Szulc, Hai Dang Nguyen, Thi Kieu Oanh Nguyen, Katarzyna Skórczewska, Agnieszka Majkowska-Pilip, Kinga Żelechowska-Matysiak, Dorota Nieciecka, Agnieszka Pregowska, and et al. 2023. "Nanohydroxyapatite Loaded with 5-Fluorouracil and Calendula officinalis L. Plant Extract Rich in Myo-Inositols for Treatment of Ovarian Cancer Cells" Coatings 13, no. 11: 1944. https://doi.org/10.3390/coatings13111944
APA StyleOsial, M., Wilczewski, S., Szulc, J., Nguyen, H. D., Nguyen, T. K. O., Skórczewska, K., Majkowska-Pilip, A., Żelechowska-Matysiak, K., Nieciecka, D., Pregowska, A., Nguyen, T. P., Tymoszuk, A., Kulus, D., & Giersig, M. (2023). Nanohydroxyapatite Loaded with 5-Fluorouracil and Calendula officinalis L. Plant Extract Rich in Myo-Inositols for Treatment of Ovarian Cancer Cells. Coatings, 13(11), 1944. https://doi.org/10.3390/coatings13111944











