Abstract
Increasing photon absorption by capturing light is an important way to increase the efficiency of photovoltaic devices. In this regard, the small optical band gap (Eg) and high absorption coefficient of Se-containing thin nanofilms make them ideal for next generation photovoltaic devices based on selenides. Amorphous selenium was introduced into polyamide-6 (PA 6) via a chemical synthesis in a bath and the influence of the products of its reaction with Cd2+ and Ag+ ions on the film phase composition, topographic and optical properties were evaluated. AFM data have revealed that the surface roughness of the a-Se/PA 6 composite noticeably increases compared to that of unreacted PA 6. However, at later stages of film deposition, the roughness decreases, and the thin film becomes smoother and uniform. The incorporation of solid inorganic nanoparticles into flexible polyamide network causes chain stretching, which has been confirmed by ATR-FTIR spectroscopy data. The data of X-ray diffraction analysis, depending on the stage of synthesis, showed the crystalline composition of the film with peaks of Se8, CdSe, Ag2Se and Ag, which may explain the observed optical properties. The optical properties of the composites indicate a shift in the band gap from 4.46 eV for PA 6 to 2.23–1.64 eV upon the stepwise deposition of amorphous Se, CdSe and Ag2Se. Eg is conveniently located in the visible region of solar energy, making the obtained nanofilms ideal for solar energy harvesting.
Keywords:
polyamide-6; selenium; cadmium; silver; selenides; nanofilms; optical properties; light-absorbing materials 1. Introduction
Nanoscale-sized inorganic materials within the polymers are interesting in optoelectronics as light-emitting devices [1] or photovoltaics [2], separation-catalysis [3] and bioelectronics [4]. The semiconductor nanoparticles embedded in the polymer film are easy to process [5]. These composites are usually made by mixing inorganic and organic components, and the obtained structure possesses physical and chemical properties that are not present in the individual components [1].
Selenium and selenium-containing materials have become the subject of many scientific studies due to their exceptional physical–chemical properties and photoelectric characteristics [6,7,8]. As an important inorganic material, selenium has also received a lot of attention due to its good semiconductor properties. For an a-Se film, the value of the band gap is 2.0–2.3 eV [9,10]. Due to their high photoconductivity, various selenides are some of the key materials widely used in optoelectronic devices such as photocopiers, light emitting diodes, xerography and solar cells [8,11,12,13].
Doping with impurity atoms is an effective strategy for optimising the properties required for the development of semiconductor technologies. Newly developed aliovalent doping provides extra electrons (n-type doping) or extra holes (p-type doping) to redefine their doping type for electronic requirements. For CdSe, the most studied nanocrystalline system, it has been proven that additional carriers from heterovalent dopants such as Ag, Cu and In [14,15] can enhance the electronic conductivity in films when they are excited by external energy. Incorporating aliovalent metal cations into binary semiconductors, such as CdSe, change the optoelectronic properties of the newly formed base material, providing greater performance in a number of applications, including solar cells, batteries and LEDs [16,17,18].
Polyamides are widely used as technical polymers with applications ranging from synthetic fibres to industrial and structural ones. Typically, optoelectronic devices use a combination of highly conductive semiconductor components due to their electrical pumping capability and the strong interaction of light and matter of the organic component [19]. The efficient inorganic materials used include cadmium selenide (CdSe) [11] and silver selenide (Ag2Se) [12,13]. CdSe and Ag2Se are direct band gap semiconducting materials with corresponding band gaps of 1.9 eV [20] and 1.8 eV [21], respectively.
Due to the high reduction potential of Se(IV)/Se(0), various reagents for the reduction of selenium (IV) compounds in several types of aqueous solutions have been evaluated. N2H4 [6,22], (NH2)2CS [23], metallic Fe or Zn [24,25,26,27], CuCl [28], TiCl3 [29], SnCl2 [30], Na2S2O4 [31], D-fructose [32] and ascorbic acid [33] have been proposed as quantitative reducing agents for Se(IV) in acidic solutions. In industrial applications, SO2 is the preferred reagent for the reduction of Se(IV) to its elemental state. According to the literature data [10,28], when working with hot (>80 °C) and relatively concentrated solutions of H2SO4 or HCl (pH < 1), the complete reduction of selenium occurs very quickly. However, high toxicity (hydrazine and thiourea), low efficiency (zinc, D-fructose, ascorbic acid) and high cost (CuCl, TiCl3, Na2S2O4 and SnCl2) limit the applicability of these reagents. Sodium sulphite (Na2SO3) is a much better candidate as a strong reducing agent owing to its fast reaction rate, low cost and relatively low toxicity.
Solution-based chemical methods provide an excellent way to produce nano selenium and nano selenides. Each synthesis method has its advantages in obtaining the appropriate structures and properties for each system, and hence the right combinations of processing along with suitable systems must be found to achieve the desired goal.
This work is aimed at studying the change in the properties of polyamide-6 upon the stepwise introduction of amorphous Se, Cd2+ and Ag+ ions and understanding the factors that determine the properties of these light-absorbing materials. These composites were prepared on the PA 6 using various chemical synthesis methods as follows: Se was deposited via chemical bath deposition (CBD), Cd2+ and Ag+ selenides were synthesized via the successive ionic layer adsorption and reaction (SILAR) method and mixed Ag+-Cd2+ selenide composites were obtained using cation exchange (CE). For a deeper understanding of the relationship between the composition and consumer properties of polyamide-6-based selenium nanocomposites, most attention was paid to the evaluation of the microstructure, topography and physicochemical properties.
2. Materials and Methods
Semicrystalline flexible TECAMID®6 (polyamide-6, PA 6) produced by Ensinger GmbH (Nufringen, Germany) was used as a thermoplastic matrix. Nominal characteristics provided by the manufacturer were: opaque PA 6 film, 0.5 mm thick, density 1.13 g/cm3, moisture absorption 3% and water absorption to equilibrium 9.5%. Experiments were performed on 2 × 6 cm2 film samples. Prior to the experiments, PA 6 film samples were washed with NaHCO3 and heated in distilled water for 2 h at 100 °C to remove surface contamination. The criterion for the quality treatment of the PA 6 substrate surface was its uniform wetting with distilled water. After treatment, the substrates were stored in a desiccator.
Selenious acid (H2SeO3, 99.0%), sodium sulphite heptahydrate (Na2SO3∙7H2O, 99.0%), sulphuric acid (H2SO4, 96%), cadmium nitrate tetrahydrate (Cd(NO3)2∙4H2O, 99.997% trace metals basis) and silver nitrate (AgNO3, 99.0%) were purchased from Sigma-Aldrich Chemie GmbH (Munich, Germany) and used as received. All solutions were prepared using distilled water.
The first step involved the synthesis of Se via the CBD process. For Se deposition/insertion, the cleaned PA 6 film cuts were vertically immersed into a beaker with 0.1 M H2SeO3 and 0.15 M Na2SO3 solutions and exposed in a thermostatic vessel for 24 h at 20 ± 1 °C. The concentrations of the H2SeO3 and Na2SO3 solutions (pH 2 with the addition of H2SO4), the deposition temperature and exposure time were chosen to give excellent composites in terms of continuity, smoothness and adhesion to the substrate. The solution was clear at first, became slightly turbid after two hours and turned into a light red liquid after a few hours. The Se precipitate was very fine, but it aggregated into large clusters within ~24 h. This proposed mechanism for the formation of Se films is based on nucleation and the Ostwald ripening process [34]. The process is simple, controllable and reproducible. After the deposition/insertion of Se, the upper layer (Figure 1) was removed from the PA 6 surface with filter paper and samples were thoroughly washed with distilled water and stored in a desiccator between the individual processing steps. Then, obtained composites (referred to in the text as a-Se/PA 6) were used as the initial composite for the stepwise insertion of Cd2+ and Ag+ ions. For this, half of the a-Se/PA6 composites were treated for 3 h with 0.1 M Cd(NO3)2 solution (pH 5.4) at 80 ± 1 °C. The second half of a-Se/PA6 composites were treated for 2 h with 0.1 M AgNO3 solution (pH 6.35) at 20 ± 1 °C. The obtained composites were labelled as Cd-Se/PA 6 and Ag-Se/PA 6, respectively. Then, one part of the Cd-Se/PA 6 composites were treated for 10 min with 0.05 M AgNO3 solution (pH 6.45, 20 ± 1 °C) and washed well, as described above. After immersion into AgNO3 solution, the resulting composites (throughout the text labelled as Ag-Cd-Se/PA 6) were uniformly black and highly reflective with good adhesion. In all cases, the samples were treated in a thermostated vessel. During the deposition process, the beaker was kept undisturbed. All deposited films were evaluated for adhesion by subjecting them to a steady stream of distilled water. After each deposition step, the samples were washed first with hot and then cold distilled water, dried at ambient temperature and stored in a desiccator until analysis. Figure 1 shows a schematic outline of the approach and optical micrograph images of the PA 6 and obtained composites.
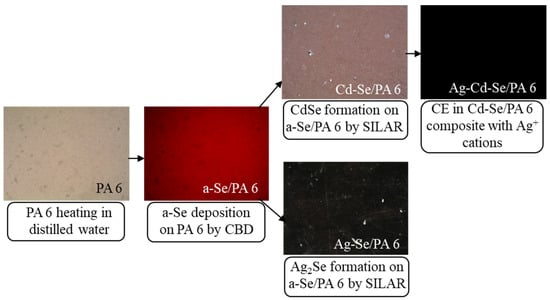
Figure 1.
Optical micrograph images of the PA 6 and obtained composites showing the principal route of combined CBD, SILAR and CE methods for the Se, CdSe and Ag2Se deposition on PA 6. Magnification ×100.
Solution pH was measured using a pH-meter WTW330 (WTW GmbH, Weilheim, Germany). Optical microscopy images were obtained using an Olympus CX31 optical microscope and a C-5050 camera (Tokyo, Japan).
The structure of the composites was analysed using X-ray diffraction method (XRD) on a Bruker D8 Advance diffractometer (Bruker AXS, Karlsruhe, Germany). The diffractometer was supplied together with the software package DIFFRAC.SUITE. (Diffract. EVA. v. 4.5, Bruker AXS, Karlsruhe, Germany). The XRD patterns were produced with CuKα (λ = 0.154178 nm) radiation in the 2θ scanning mode. The XRD data were analysed by observing characteristic peaks in the X-ray diffraction patterns using software package Crystallographica Search Match v.2.1 and the PDF-2 2022 and PDF-4/Minerals 2022 database. Crystallite size (Dc) values were calculated from XRD patterns based on the Bragg and Scherrer equation [35]:
where K is the constant with a normal value of 0.89, λ is the wavelength of CuKα radiation, β(2θ) is the full width at the half-maximum and θ is the Bragg angle. The dislocation density (δ) was evaluated by using the equation [36]:
The strain values (ε) were calculated from the following relation:
Surface topography imaging was conducted using atomic force microscopy (AFM) using a NanoWizard®3 NanoScience microscope (JPK Instruments, Bruker Nano GmbH, Berlin, Germany). Obtained data were analysed using JPKSPM Data Processing software (Version spm-4.3.13). The AFM images (scanning area 5 × 5 μm2) were collected using an AppNano production pyramidal-shaped i-type silicon cantilever (0.01–0.025 ohm/cm, spring constant of 2 N/m) working in contact mode.
Ultraviolet–visible (UV–Vis) absorption spectra (200 to 1100 nm) were recorded on a SpectronicR GenesysTM 8 UV–Vis spectrophotometer (Spectronic instruments, Runcorn, UK) with compensation for PA6 absorption. The energy optical band gaps (Eg) were determined by applying the absorption spectrum fitting (ASF) method [37]. The fundamental absorption edge (λg) can be found using the following plot:
where A is the absorption, λ is the wavelength, and m is the index that takes values 0.5, 1.5, 2 and 3 for direct allowed, direct forbidden, indirect allowed and indirect forbidden transitions, respectively. The extrapolation of the straight-line portion to cut the energy axis gives λg. Then, employing the obtained value of λg the Eg can be directly calculated from the following relationship [37]:
The Urbach energy (EU) is related to the absorption coefficient through the following exponential equation [38]:
where is a constant. In ASF procedure, Equation (6) can be written as: , where D is (d/2.303) [38]. Therefore, the values of EU in eV, were calculated using the slope determined from the linear part of the plot of between ln(A) versus 1/λ according to the following relationship [38]:
The steepness parameter is related to EU the via following equation:
where k is the Boltzmann constant and T is the absolute temperature assumed to be 298 K. The following equation is applied for calculating the interaction strength of electron–phonon (Ee-p) [39]:
The optical electronegativity χ is related to Eg through following equation:
The changes in the chemical structure and the binding configuration of the samples were analysed using attenuated total reflectance (ATR) Fourier transform infrared spectroscopy (FTIR). The ATR-FTIR spectra were recorded on a Perkin Elmer FT-IR Spectrum GX system (Perkin Elmer, Waltham, MA, USA) within the wavenumber range 4000–600 cm−1. Spectra were recorded by averaging 64 scans with a wavenumber resolution of 1 cm−1 at room temperature.
3. Results and Discussions
Adsorption membranes are some of the best ways to obtain semiconductor composites from aqueous solutions. According to the BET method (abbreviated from the Brunauer–Emmett–Teller theory), the determined pore size of less than 2 nm [40] indicates that the PA 6 film is microporous. Therefore, it can adsorb Se micro-particles and Cd2+ and Ag+ cations followed by their diffusion into the polymer. In most cases, adsorption matrixes are charged. Ions and the surface of the polymer attract each other due to opposite charges.
3.1. XRD Analysis
To determine the structural properties of the PA 6 and the obtained composites, XRD patterns were analysed. The XRD pattern of PA 6 sample heated in distilled water is shown in Figure 2, the XRD pattern of the a-Se upper layer scrub is shown in Figure 3, while the XDR pattern of the obtained composites is shown in Figure 4.
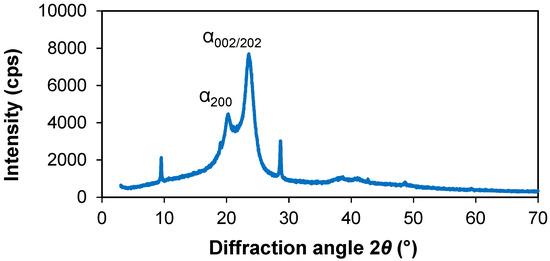
Figure 2.
XRD pattern of the PA 6 sample heated in distilled water.
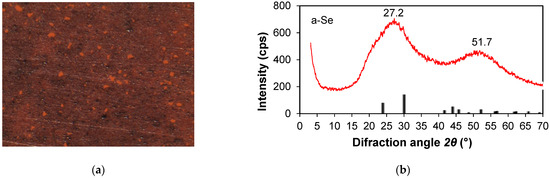
Figure 3.
(a) Optical micrograph of the red upper a-Se layer on a PA 6 (×100 magnification); (b) XRD pattern of the a-Se upper layer scrub. The red line is the experimental pattern, and the black pillars label the peaks from crystalline Se.
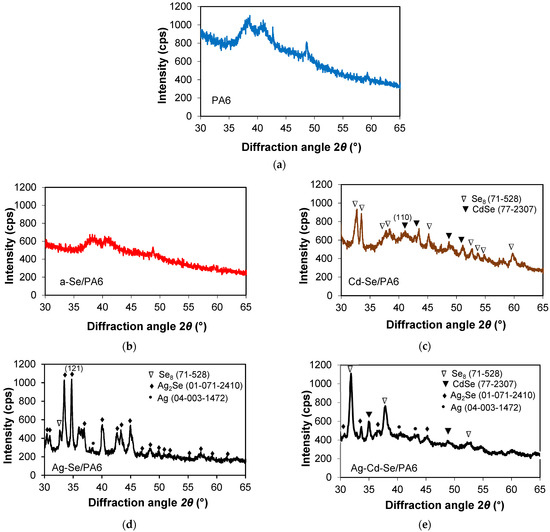
Figure 4.
XRD diffractograms of (a) heated PA 6 and obtained composites: (b) a-Se/PA 6, (c) Cd-Se/PA 6, (d) Ag-Se/PA 6, (e) Ag-Cd-Se/PA 6. PA 6 peaks are not shown.
PA 6 crystallizes into stable α- and γ-, as well as unstable β-forms [41]. XRD studies of the PA 6 sample revealed the presence of main peaks at 2θ 9.52°, 20.26°, 23.56° and 28.64°. Two peaks at 2θ = 20.26° and 23.56° were assigned to the (200) reflection and (002)/(202) reflections of the α crystalline form of PA 6 [41]. XRD observations in [42] provided new information for the secondarily quenched PA 6. The diffraction patterns exhibit three sharp unknown diffraction peaks at 2θ = 9.4°, 18.95° and 28.5°, which are derived from a new long-range order structure. The locations of these peaks do not correspond with a series of calculated 2θ values for the α-, γ-, β- and pleated α- form crystals of PA 6 [42]. It is likely that the diffraction peaks at 2θ = 9.52° and 28.64° also indicate the formation of such an ordered structure after heating the PA 6 samples in distilled water and cooling. Otherwise, the small diffraction peak at 2θ = 9.52° could be attributed to the (020) reflection of the γ crystalline form [41], while the sharp peak at 28.64° could be caused by impurities or instrumental mistakes.
Mixing H2SeO3 and Na2SO3 or sulphur dioxide solutions leads to the release of Se according to the following reaction [28]:
H2SeO3(aq) + 2Na2SO3(aq) → Se(s) + 2Na2SO4(aq) + H2O(l).
A red film was observed on PA 6 (Figure 3). The precipitate, characterised via XRD analysis (Figure 3 and Figure 4), was identified as red amorphous selenium (a-Se).
Because the intensities of the diffraction peaks of PA 6 are very high and overlap with the main peaks of the materials inserted/deposited on the polymer, the XRD patterns of the obtained composites were given in the 2θ assortment of 30–65°. As can be seen from Figure 4b, in the a-Se/PA 6 composite, Se formed as a result of reaction (11) is in the amorphous form, i.e., not detected via XRD analysis.
The obtained positions of the diffraction peaks at 2θ 42.74°, 43.46°, 48.68° and 51.09° in the Cd-Se/PA 6 composite (Figure 4c) were compared with standard values and mainly indexed as CdSe (PDF No. 77-2307). The peaks at 2θ 32.66°, 33.49°, 37.64°, 38.46°, 45.16°, 52.66°, 53.77°, 54.83° and 59.73° are indexed as Se8 (a cyclic molecule with a ring of eight Se atoms, PDF No. 71-528) with hexagonal and monoclinic unit cells, respectively (Figure 4c, Cd-Se/PA 6 composite). It was found that CdSe has a polycrystalline nature with a predominant (110) orientation plane. These experimental results agree well with the literature data. It is well known [43] that in the case of hexagonal lattices, the strongest chemical bonds are formed by atoms located in a semi-crystalline configuration on crystallographic planes (110) or (101).
Based on the analysis of the XRD results, it can be concluded that the immersion of the a-Se/PA 6 nanocomposite into Cd(NO3)2 solution at 80 ± 1 °C contributes to the gradual crystallisation of red a-Se into grey crystalline selenium (cr-Se), which is very reactive and reacts with various metal cations [44,45]. The solubility of a-Se (2.2 × 10−8 mol/dm3 [46]) was approximately one order of magnitude higher than the solubility of cr-Se (1.5 × 10−9 mol/dm3 [46]). The formation of CdSe can be described using the following reaction [47]:
3Se(s) + 3Cd2+(aq) + 3H2O(l) → 2CdSe(s) + CdSeO3(s) + 6H+(aq).
The formation of the Cd-Se/PA 6 composite leads to a colour change from red to brown (Figure 1).
The XRD diffractogram of Ag-Se/PA 6 nanocomposite (Figure 4d) shows diffraction peaks corresponding to the orthorhombic phase of Ag2Se naumannite (PDF No. 01-071-2410) with two sharp lines of approximately the same intensity along the (112) and (121) planes, respectively. As can be seen, the dominant peak (121) of the orthorhombic system represents the preferred orientation along this plane. The identified peak positions correspond with those indicated in the literature for Ag2Se nanowires [48] and nanoparticles [49]. The reaction involved in this process can be summarised as the following reaction equation [50,51]:
3Se(s) + 6Ag+(aq) + 3H2O(l) → 2Ag2Se(s) + Ag2SeO3(s) + 6H+(aq).
The formation of Ag2Se leads to a colour change from red to black. Along the Ag2Se phase, a minor amount of monoclinic Se8 (PDF No. 71-528) at 2θ 32.66° may remain unreacted in the deposited film phase. Ag-Se/PA 6 composite XRD analysis showed that not only Ag2Se but also the metallic Ag phase (PDF No. 04-003-1472) was identified in the diffractogram. As discussed in [52], excess Ag can be introduced in several ways: in the form of adsorbed metal chains, in the form of point defects or in the form of three-dimensional nano- or micro-inhomogeneities. Typically, the metallic structure of Ag is depicted by a sharp XRD peak at 2θ 38.12°, corresponding to the preferred (111) texture. Metallic Ag is the most probable impurity in chemically deposited Ag2Se layers [48], as shown in the reaction equation as follows:
Se(s) + 4AgNO3(aq) + 3H2O(l) → 4Ag(s) + H2SeO3(aq) + 4HNO3(aq).
The XRD pattern of the Ag-Cd-Se/PA 6 composite (Figure 4e) shows the multiphase crystalline composition of the film. Compared to the XRD pattern of the Cd-Se/PA 6 composite, Se8 and CdSe phases are retained. However, after the treatment of the Cd-Se/PA 6 composite in AgNO3 solution, not only did selenium react with Ag+ ions but CdSe was also partially converted into orthorhombic Ag2Se. The XRD peak at 48.71°, corresponding to hexagonal CdSe, is the only one that did not shift. The remaining peaks disappeared, and a new peak appeared at 35.94°. Two peaks also appeared at 38.29° and 43.50°, corresponding to the metallic Ag phase. Figure 4e also shows that the intensity of the Se8 peaks of the Ag-Cd-Se/PA 6 composite compared to those of the Cd-Se/PA 6 composite (Figure 4c) increases with the appearance of a new phase, which additionally indicates an increase in crystallinity. The broadening of the diffraction peaks is associated primarily with the finite sizes of the crystallites. The formation of Ag2Se in the Cd-Se/PA 6 composite leads to a colour change from brown to black.
The structural parameters of PA 6 and the prepared composites calculated from XRD data are listed in Table 1.

Table 1.
Structural parameters (phase, angle (2θ), crystallite size (Dc), dislocation density (δ), strain values (ε)) of PA 6 and prepared composites calculated from XRD data.
Since the other phases were not found in Cd-Se/PA 6, Ag-Se/PA 6 and Ag-Cd-Se/PA 6 nanocomposites, it could be assumed that SeO32–, SO32– and SO42– ions diffused from the a-Se/PA 6 reacted with the Cd2+ and Ag+ ions to form these compounds in the solution near the sample area. In another case, the by-products of Equations (12) and (13), due to the sufficiently high solubility (Table 2, CdSeO3 (KSP = 5.0 × 10−9 (mol/dm3)2) and Ag2SeO3 (KSP = 1.35 × 10−16 (mol/dm3)3), were removed from the surface of the samples by rinsing the as-synthesized composites with excess hot water.

Table 2.
Standard Gibbs free energy (G) (298.15 K, 1 bar) [53], bond dissociation energies (BDE) [54], solubility product constants (KSP) [46] and the calculated solubility (S) of CdSe and Ag2Se phases.
A theoretical framework provides a general background to support our investigation. The sequence of phase formation in multicomponent systems depends on the structure of the starting material (SM). It is common knowledge that the exchange of cations can be achieved through simple mutual diffusion with other cations [55]. In addition, considering phase formation, the driving force of the solid-state interaction is also determined by the change in the Gibbs free energy, since the system always tends to the lowest possible free energy state [53]. The stoichiometric reactions at an interface between the Cd-Se/PA 6 and Ag+ salt aqueous solution can be written as follows:
The negative values of ΔG°reac,298 indicate that thermodynamic conditions are provided for the proceeding of this reaction. The feasibilities of possible CE reactions were studied via thermodynamic deduction based on the thermodynamic law. CE reactions are based on significant difference in solubility product (KSP) between the SM and the final product (FP). For insoluble materials, the relationship between the Gibbs free energy (ΔG) and the solubility product is expressed according to Equation (16):
where R is the universal gas constant (8.319 J/mol·K), T is the temperature (K), KSP is the solubility product of the SM (in our case CdSe) and ICP is the product of the concentration of FP ions (in our case Ag2Se). ICP can be calculated according to Equation (17):
where and are the concentration of ions (mol/dm3). In this way, the transition reaction from a material with a higher KSP (CdSe) to a material with a lower one (Ag2Se) can proceed spontaneously. However, it is not easy to judge whether the CE reaction can proceed or not since this process is very complex and is associated with many factors, such as a barrier to the activation energy of the phase transition, change in surface free energy, temperature, the active concentrations of ions in the solution, etc. [56]. Therefore, a more correct criterion should be the difference in solubility (S) of the SM and the FP (see Table 2).
However, it should be emphasised that in the case of the solid-phase interaction in multicomponent systems, phase formation at the precursor/solution interface is a dynamic non-equilibrium process that requires the careful consideration of the mechanistic pathways along which they proceed [57]. Apparently, the determining criterion for the possibility of a thermodynamic reaction in hybrid materials is the solubility of CdSe in the starting Cd-Se/PA6 material. The higher solubility of CdSe (Table 2) favours the transformation into Ag2Se. When Cd-Se/PA 6 is immersed in AgNO3 solution, Ag+ reacts with Se2− ions formed from the ionisation of dissolved CdSe, leading to the formation of Ag2Se particles, which results in the reduction of Cd2+ ions in the solution. Before explaining the formation of the Ag2Se phase, it is necessary to discuss the active species that might be involved in the reaction path. Based on the discussion presented above, we assumed that the formation of Ag2Se phases in the Cd-Se/PA 6 composite could be explained through complex mechanism reactions: Ag+ ions were attracted to the surfaces of metal chalcogenides particles due to the large specific area and then adsorbed Ag+ replaced Cd2+ ions from the CdSe lattice and formed Ag2Se. In parallel, some Ag+ ions featuring relatively high ion diffusivity [58,59] propagated into the interior of the polymer and reacted with cr-Se to form Ag2Se according to the reaction (13). The obtained composites (denoted in the text as a-Se/PA 6, Cd-Se/PA 6, Ag-Se/PA 6 and Ag-Cd-Se/PA 6) were homogeneous with good adhesion.
3.2. AFM Analysis
Surface roughness is an important indicator of the quantitative characteristics of the sample surface. Uniform numerical parameters are useful for classifying surfaces from the same type of materials produced by the same method [60]. On a selected small scan area of 5 µm × 5 µm, 2D height data (Figure 5A) and 3D topographic (Figure 5B) AFM images of the respective PA 6 and a-Se/PA 6, Cd-Se/PA 6, Ag-Se/PA 6 and Ag-Cd-Se/PA 6 composites are shown. In particular, 3D images can better visualize the spatial characteristics of PA 6 and obtained composites. Since the composites did not have a pronounced texture but were characterized via complex relief topography, the roughness parameters were calculated from profilograms obtained for different surface areas (Figure 5C). The length of the scan areas was approximately 4.5–6.5 μm. The corresponding measured topographic parameters are collected in Table 3. Z is the maximum height of the surface. Surface roughness (Ra) is defined as the average value of the surface height relative to the central plane, and root mean square (RMS) is defined as the standard deviation of the surface height within a given area. Peak to valley roughness Rt is the vertical height distance between the highest peak and lowest valley of the roughness profile within the overall measuring distance [61].
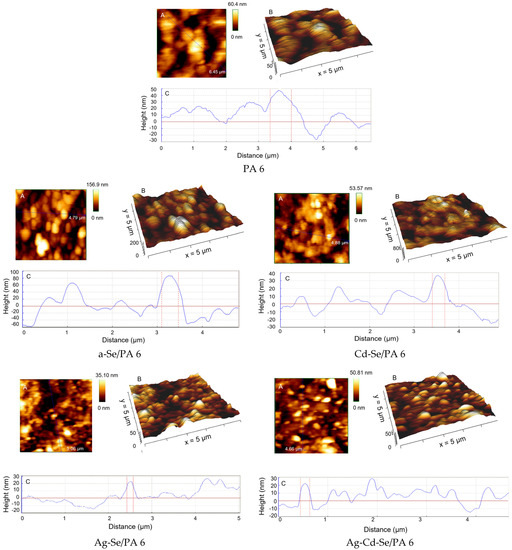
Figure 5.
Sequential 2D height data (A), 3D AFM topographic images (B) and the height profile (of the blue line in (A)) (C) of PA 6 and the obtained nanocomposites.

Table 3.
Surface topographical parameters Ra, RMS and Rt obtained via AFM analysis.
Surface roughness is a component of surface texture and plays a significant role in determining how an object will interact with its environment since surface irregularities can act as nucleation centres for film growth [62]. Therefore, the surface texture of the substrate is very essential for obtaining a high-quality coating. Figure 5B shows an irregular and rough texture of PA 6 with a deep valley. At the initial stage of the a-Se film growth process, the particles may form islands, caused by the high diffusivity of adsorbed particles on a larger substrate. Additionally, the mobility and diffusion of the SO32– and SeO32– ions into the sub-surface space of PA 6 may enhance or inhibit the grain growth and affect the surface topography and roughness of the deposited films. The surface roughness is high. From the roughness values presented in Table 3, it can be seen that all measured parameters of the surface roughness of the a-Se/PA 6 composite increased noticeably compared to the analogous parameters of unreacted PA 6. However, at later stages of film deposition, the roughness decreased, since, apparently, the growth of CdSe and Ag2Se occurred between the islands of the a-Se layer. These islands merged, and the thin film became smoother and uniform. Average roughness (Ra) and root mean square (RMS) correlate well between the obtained nanocomposites, as can be seen from Table 3, and are significantly reduced compared to the a-Se/PA 6 composite. An analysis of the surface topography has shown that the resulting Cd-Se/PA 6, Ag-Se/PA 6 and Ag-Cd-Se/PA 6 nanocomposites are relatively smooth with a Ra value of 7.93–9.82 nm and an RMS value of 10.17–12.17 nm. In addition, Rt varies from 41.32 to 61.81 nm.
The non-Gaussian roughness profile distribution Rsk is sensitive to occasional deep valleys or high peaks, as it measures the symmetry of the profile distribution arond its midline (Figure 5C). According to the literature [63], a symmetrical distribution is reflected in zero skewness, dominant profiles at peaks above a flatter average reflect positive skewness and negative skewness refers to profiles predominant in deep valleys. For the PA 6 film sample, in the height distribution, surface peaks dominate over valleys with a skewness value Rsk equal to 6.24. For the a-Se/PA 6 composite, Rsk is −0.014, and for the Cd-Se/PA 6 composite, Rsk is −0.0098, i.e., an almost symmetrical distribution. For the Ag-Se/PA 6 and Ag-Cd-Se/PA 6 composites, Rsk is 3.82 and 5.42, respectively, i.e., the surface peaks again dominate over the valleys. Film roughness has a sensitive effect on various physical properties, including optical ones.
3.3. ATR-FTIR Analysis
The FTIR study allows us to discover the conformational structural changes of the polymer chain, as well as the complexation and interaction between the polymeric matrices and inorganic nanoparticles. The ATR-FTIR spectra of the PA 6 and obtained nanocomposites within a wave number range from 650 to 1650 cm−1 are displayed in Figure 6. PA 6 consists of (CH2)5 segments separated by a parallel or antiparallel arrangement of secondary amide groups [64]. The bands related to NH–CO fragments are mainly affected by the mutual arrangement of H–bonds, while the vibrations from CH2 groups reflect the effect of the chain conformation [65]. In the PA 6 spectrum, the absorption bands at 834 cm−1, 929 cm−1, 960 cm−1, 1031 cm−1 and 1200 cm−1 (Table 4) confirm predominantly the α-crystalline phase. The spectral features at 1476 cm−1 and 1417 cm−1 represent CH2 scissoring vibrations next to −NH and >C=O groups, respectively. The band at 1463 cm−1 corresponds to scissoring vibrations of CH2. Two typical absorption bands at 1633 and 1535 cm−1 represents the amide I and amide II group, respectively.
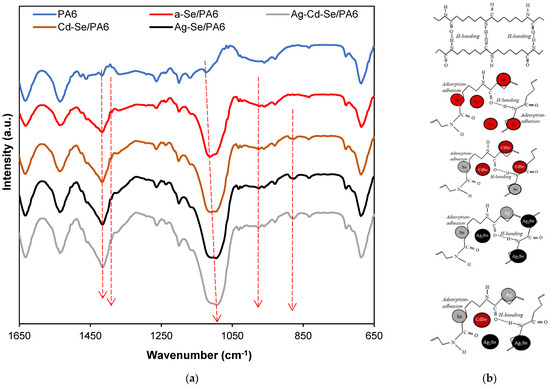
Figure 6.
(a) FTIR spectra in the region of 1650–650 cm−1 of the PA 6 matrix and the obtained nanocomposites; (b) schematic diagram of the internal amide network in the PA 6 and possible network in the obtained composites.

Table 4.
Band location and assignments of PA 6 and obtained nanocomposites spectra over wavenumber 1650–650 cm−1.
The FTIR vibration bands of Se8 rings are reported at 487.9 cm−1 and 737.7 cm−1 [66]. These peaks are absent in the FTIR spectrum of a-Se/PA 6, which confirms the XRD data that the inserted selenium is amorphous. The incorporation of solid inorganic nanoparticles into a flexible polyamide network causes chain stretching [65,67]. It can be seen that most of the characteristic vibration bands of PA 6 in the wavenumber region from 650 to 1650 cm−1 are retained in the FTIR spectrum of a-Se/PA 6; however, the intensity of the peaks changes: some of them become more intense and some of them expand. It can be concluded that as a-Se nanoparticles are introduced between the polymer chains and stretched, the position and intensity of the peaks change. Thus, the peak of neat PA 6 at 1124 cm−1 in a-Se/PA 6 spectrum shifts to 1112 cm−1, and two new small peaks are recorded at 974 cm−1 and 1390 cm−1. C–C symmetry vibration causes IR absorption at the 1390 cm−1 [68].
As reported in [20,69], the vibrational mode of CdSe is between 400 and 700 cm−1. The IR absorption spectrum of selenium-rich CdSe nanocrystals shows a peak at 800 cm−1 [70]. The infrared spectra for Ag2Se nanoparticles of various sizes (from 5 nm to 28 nm) display a strong absorbing peak in the mid-IR range from 2100 cm−1 to 650 cm−1. [71]. Thus, it is possible to avoid the problem of overlapping characteristic peaks of metal selenides and PA 6. The IR spectra of the Cd-Se/PA 6, Ag-Se/PA 6 and Ag-Cd-Se/PA 6 composites are very similar to the spectrum of a-Se/PA 6 but show an even greater shift of the peak at 1112 cm−1 to lower frequencies (Table 4) and the presence of another new peak at 877 cm−1. In analogy with data [65], those weak vibrations can be attributed to the CdSe or Ag2Se compounds. Another dimension of the functionality and complexity of inorganic–organic materials is introduced if the selenides are combined with each other. For example, the negative energies of chemical bond formation of CdSe and Ag2Se promote the creation of Ag–Cd–Se interfacial bonds, which lead to non-selective nucleation in Ag2Se/CdSe hetero-nanostructures [72]. Thus, the peak at 1108 cm−1 in the IR spectrum of the Ag-Se/PA 6 composite shifts to 1094 cm−1 in comparison with the IR spectrum of the Ag-Cd-Se/PA 6 composite.
The shift and broadening of the C–C stretching vibration peaks, accompanied by the appearance of new small peaks of low intensity, allowed us to conclude that intermediate structures inside the polyamide chains were formed. The peak at 1124 cm−1, arising from the all-trans C–C stretching vibrations, loses its spectral position. In addition, the peak was broadened, and its intensity noticeably increases in all spectra of the obtained composites (Figure 6).
3.4. UV–Vis Analysis
The experimental absorption spectra are presented in Figure 7.
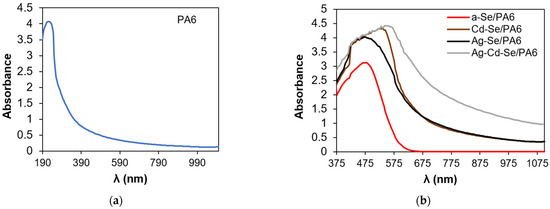
Figure 7.
(a) Absorption spectra of PA 6 and (b) obtained composites.
The absorption spectrum of PA 6 (Figure 7a) displays one absorption peak at around 225 nm. The colour of the obtained a-Se/PA 6 nanocomposite is red, suggesting a change in optical properties. As reported in [73,74], Se nanoparticles spectra, depending on their size, have a number of absorption peaks in the UV–visible region from 250 to 700 nm. In the UV–Vis absorption spectrum of the a-Se/PA 6 nanocomposite, a peak appeared at 475 nm (Figure 7b).
In the UV–Vis spectrum of the Cd-Se/PA 6 nanocomposite, a peak appeared at 535 nm. According to the literature data, CdSe quantum dots have a wide absorption band in the spectral range of 410–545 nm [75], and the absorption maximum of CdSe nanocrystals, depending on the particle size, is in the range from 492 to 578 nm [76]. Therefore, the peak at 535 nm can be attributed to CdSe.
In the Ag-Se/PA 6 UV–Vis spectrum, a peak appeared at 475 nm. Ag2Se nanoparticles absorb in a wide spectral region from 300 to 600 nm [21], while two sharp peaks are observed in the spectrum of quantum dots at 440 and 607 nm, respectively [77]. Monodisperse silver nanoparticles, depending on the size, shape, and distribution in nanostructures, have two absorption bands: one broad peak in the range of 420–430 nm and a shoulder at 580–590 nm [78]. Therefore, a broad peak from 380 to 590 nm with a maximum at 475 nm can be attributed to the Ag2Se phase with a small amount of Ag nanoparticles.
In the UV–Vis spectrum of the Ag-Cd-Se/PA 6 nanocomposite, the peak appeared at 555 nm. According to the literature data [79,80,81], the introduction of Ag+ ions into CdSe nanocrystals via the CE reaction does not lead to noticeable changes in the absorption spectra of CdSe in the UV–visible region. In contrast, other authors have suggested that the formation of the CdSe-Ag2Se core–shell leads to the complete disappearance of the CdSe absorption peak in the UV–Vis spectra [82]. Thus, it can be stated that the absorption spectrum of the Ag-Cd-Se/PA 6 composite is a superposition of the individual absorption spectra of the CdSe, Ag2Se and Ag phases. Its absorption edge was shifted to the longer wavelength region, and its absorption increases in the range of 375–555 nm compared to the region (375–535 nm) of Cd-Se/PA 6 and Ag-Se/PA 6 composites. This red shift of the λg may be related to the common complex alignment of the valence and conduction bands, as well as to the presence of multiple defects and vacancies. The determination of the phase composition of the obtained composites according to the data of optical studies is in good agreement with the XRD data.
The experimental UV–Vis spectra correspond better to the rectilinear section on the graphs A2·λ−2 = (λ−1) (Figure 8), which confirm the direct transitions of the obtained nanocomposites.
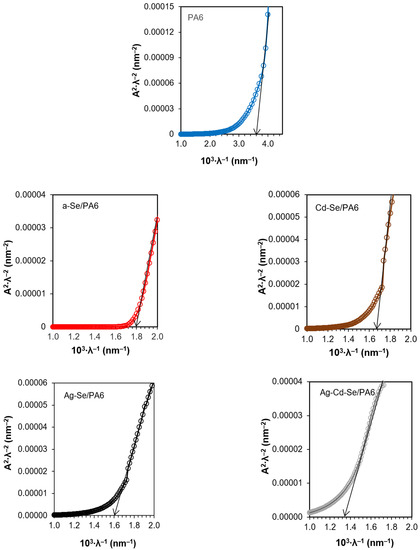
Figure 8.
Dependence A2∙λ−2 on λ−1 for the PA 6 and obtained nanocomposites.
All the calculated optical parameters are provided in Table 5 for comparison.

Table 5.
The optical parameters of PA 6 and studied nanocomposites.
The decrease in Eg of each subsequent nanocomposite compared to PA 6 is due to a change in the optical properties. Eg of nanocomposites can be affected by many factors, such as composition, particle size and distribution, shape, agglomeration/aggregation state, solubility, surface morphology/topography, structure crystallinity and many different structural defects or deformations [83,84]. The wavelength at which the signal becomes saturated is interpreted as being the value of the absorption edge or the Eg of the semiconductor. For a-Se/PA 6 composite Eg is 2.23 ± 0.02 eV. Eg measured for bulk a-Se is 1.99 ± 0.02 eV [85]. The introduction of Cd2+ ions occurred at 80 °C, and amorphous Se crystallized under heat treatment. Eg measured for cr-Se is 1.85–1.92 eV [85]. An increase in the crystallinity of the obtained composites (Figure 4) and the carrier concentration was accompanied by a decrease in Eg from 2.05 ± 0.02 eV for the Cd-Se/PA 6 composite and to 1.64 ± 0.02 eV for the Ag-Cd-Se/PA 6 composite (Table 5). The obtained values correspond with the literature data: CdSe and Ag2Se have a band gap of 1.9 eV [20] and 1.8 eV [21], respectively.
One of the sources of unfavourable recombination is the sub-band absorption tail, which was characterized by the Urbach energy. Absorption tails are defined as densities of states that extend from the bands into the band gap of the photoactive layer. Tail states inside the band gap act as traps and recombination centres and trap charge carriers. The EU is influenced by thermal and structural disturbances in semiconductor materials [86,87,88]. In this work, we analysed structure-dependent EU. The Urbach energy plots were constructed and are shown in Figure 9, and the calculated values are given in Table 5. EU reflects the density of states in the band tails and, hence, the local microstructural disorder [89]. As can be seen from the values presented in Table 5, the EU of PA 6 is 1.31 eV and is higher than those of the obtained nanocomposites. The relatively high value of EU indicates a greater tendency for the transformation of weak structural bonds into defects [90]. The a-Se/PA 6 nanocomposite (amorphous phase) is more structurally disordered. With the stepwise incorporation of Cd2+ and Ag+ ions, EU decreased. That may be due to an increase in the structural ordering of nanocomposites, which has been confirmed by an increase in crystallinity detected via XRD analysis. In addition, it was previously reported [91] that thicker selenide films have less structural randomness, since with an increase in the thickness and density of the layers, structural defects are minimized and subsequently the EU is also minimized.
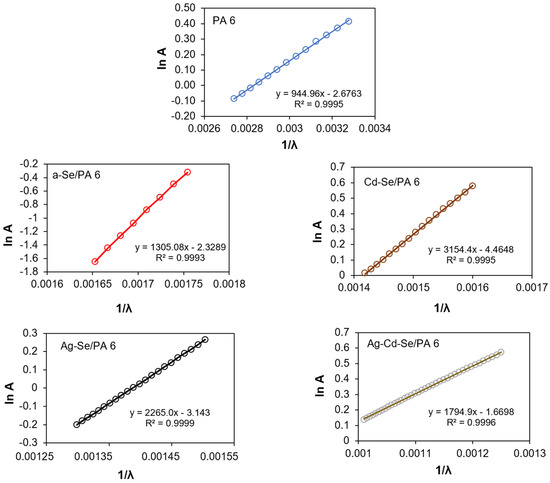
Figure 9.
Urbach plots for PA 6 and obtained nanocomposites.
The σ characterizes the broadening of the optical absorption edge arising from the electron-phonon interactions [39,92]. The steepness parameter of PA 6 is lower than that of the obtained nanocomposites at the same temperature (Table 5). The lower differences in electronegativity decrease the orbital overlap and the band gap.
An optical study of the formed coatings of Se, silver and cadmium selenides has revealed a decrease in the band gap in the range of 2.23–1.64 eV compared to the pristine PA 6 polymer (4.46 eV). It can be assumed that composites with such variations in Eg with the desired composition, crystallinity and surface topography can be considered potential candidates for new light and flexible solar cell technologies for sustainable energy harvesting. From a coating standpoint, such composites often contribute to significant cost savings by using inexpensive substrates while maintaining the properties of the coating material.
4. Conclusions
To sum up, the amorphous Se films conjugated with PA 6 were successfully obtained by exposing the PA 6 sample to an acidified aqueous solution of H2SeO3 and Na2SO3. The surface topography of the a-Se/PA 6 consists of granular amorphous Se nanoparticles. This texture ensures the optimal deposition of the next phase. The use of prepared a-Se/PA 6 composites opens the way for the stepwise deposition of CdSe and Ag2Se films using chemical methods. The positions of the diffraction peaks in the Cd-Se/PA 6 composite were mainly indexed as hexagonal CdSe and monoclinic Se8. XRD analysis has clearly shown that the Cd-Se/PA 6 and Ag-Se/PA 6 films are polycrystalline and exhibit a phase transformation from a-Se to a Se8 monoclinic structure. The XRD pattern of the Ag-Cd-Se/PA 6 composite obtained via the CE method showed a complex crystalline composition of the film with peaks of monoclinic Se8, hexagonal CdSe, orthorhombic Ag2Se and metallic Ag. As indicated by the AFM results, the surface roughness of the a-Se/PA 6 composite noticeably increased compared to unreacted PA 6. However, at later stages of film deposition, the roughness decreased, and the thin film became smoother and uniform. ATR-FTIR spectroscopy data have revealed the formation of intermediate structures within PA 6. The resulting composites differed in colour, which affected their optical properties. The optical spectra show that the nanostructured films have a high absorption coefficient in the visible region. The studied composites are direct band gap semiconductors, which exhibit a redshift of the band gap from 4.46 eV for PA 6 to 2.23–1.64 eV upon the stepwise deposition of CdSe and Ag2Se films due to a change in chemical composition and could be promising candidates for use in implantable micro-devices, as well as for solar electronics. Studies on EU and σ describe the broadening of solar absorption edges around optical band gaps, which correlates with the polycrystalline composition and heterogeneous surface topography.
Author Contributions
Conceptualization, V.K.; methodology, V.K.; software, V.K.; validation, E.S. and V.K.; formal analysis, E.S.; investigation, E.S.; resources, V.K.; data curation, V.K.; writing—original draft preparation, E.S.; writing—review and editing, V.K.; visualization, E.S.; supervision, V.K.; funding acquisition, V.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Doctoral Fund of Kaunas University of Technology, No. A-410, approved 26 June 2019.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Park, Y.; Advincula, R.C. Hybrid semiconductor nanoparticles: π-conjugated ligands and nanostructured films. Chem. Mater. 2011, 23, 4273–4294. [Google Scholar] [CrossRef]
- Liu, R. Hybrid organic/inorganic nanocomposites for photovoltaic cells. Materials 2014, 7, 2747–2771. [Google Scholar] [CrossRef] [PubMed]
- Soler-Illia, G.J.; Azzaroni, O. Multifunctional hybrids by combining ordered mesoporous materials and macromolecular building blocks. Chem. Soc. Rev. 2011, 40, 1107–1150. [Google Scholar] [CrossRef] [PubMed]
- Willner, I.; Willner, B. Functional nanoparticle architectures for sensoric, optoelectronic, and bioelectronic applications. Pure Appl. Chem. 2002, 74, 1773–1783. [Google Scholar] [CrossRef]
- Di Luccio, T.; Piscopiello, E.; Laera, A.M.; Antisari, M.V. Structural studies of thin films of semiconducting nanoparticles in polymer matrices. Mater. Sci. Eng. C 2007, 27, 1372–1376. [Google Scholar] [CrossRef]
- Gates, B.; Mayers, B.; Cattle, B.; Xia, Y. Synthesis and characterization of uniform nanowires of trigonal selenium. Adv. Funct. Mater. 2002, 12, 219–227. [Google Scholar] [CrossRef]
- Jiang, F.; Cai, W.; Tan, G. Facile Synthesis and optical properties of small selenium nanocrystals and nanorods. Nanoscale Res. Lett. 2017, 12, 401. [Google Scholar] [CrossRef]
- Barik, A.R.; Adarsh, K.V.; Naik, R.; Ganesan, R.; Yang, G.; Zhao, D.; Jain, H.; Shimakawa, K. Role of rigidity and temperature in the kinetics of photodarkening in GexAs(45−x)Se55 thin films. Opt. Express 2011, 19, 13158–13163. [Google Scholar] [CrossRef]
- Udachan, S.; Ayachit, N.; Udachan, L.; Halembre, R. Impact of thickness on the optical properties of selenium thin films. Ing. Univ. 2020, 25, 1–24. [Google Scholar] [CrossRef]
- Lu, D.K.; Chang, Y.F.; Yang, H.Y.; Xie, F. Sequential removal of selenium and tellurium from copper anode slime with high nickel content. Trans. Nonferrous Met. Soc. China 2015, 25, 1307–1314. [Google Scholar] [CrossRef]
- Afzaal, M.; O’Brien, P. Recent developments in II–VI and III–VI semiconductors and their applications in solar cells. J. Mater. Chem. 2006, 16, 1597–1602. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, W.; Lu, Y.; Cai, K.; Li, Y.; Wang, Z.; Wu, M.; Huang, C.; He, J. High power factor Ag/Ag2Se composite films for flexible thermoelectric generators. ACS Appl. Mater. Interfaces 2021, 13, 14327–14333. [Google Scholar] [CrossRef]
- Wu, M.; Cai, K.; Li, X.; Li, Y.; Liu, Y.; Lu, Y.; Wang, Z.; Zhao, W.; Wei, P. Ultraflexible and high-thermoelectric-performance sulfur-doped Ag2Se film on nylon for power generators. ACS Appl. Mater. Interfaces 2022, 14, 4307–4315. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Tuinenga, C.; Fungura, F.; Dagtepe, P.; Chikan, V.; Jasinski, J. Progress toward producing n-type CdSe quantum dots: Tin and indium doped CdSe quantum dots. J. Phys. Chem. C 2009, 113, 13008–13015. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Q.; Liu, J.L.; Wu, Y.S.; Cheng, Y.; Ji, M.W.; Qian, M.H.; Hao, W.C.; Zhang, L.J.; Wei, X.J.; et al. Heterovalent-doping-enabled efficient dopant luminescence and controllable electronic impurity via a new strategy of preparing II–VI nanocrystals. Adv. Mater. 2015, 27, 2753–2761. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.D.; Dong, A.G.; Buhro, W.E. Solution–liquid–solid synthesis, properties, and applications of one-dimensional colloidal semiconductor nanorods and nanowires. Chem. Rev. 2016, 116, 10888–10933. [Google Scholar] [CrossRef]
- Coughlan, C.; Ibanez, M.; Dobrozhan, O.; Singh, A.; Cabot, A.; Ryan, K.M. Compound copper chalcogenide nanocrystals. Chem. Rev. 2017, 117, 5865–6109. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J. Nanointerface chemistry: Lattice-mismatch-directed synthesis and application of hybrid nanocrystals. Chem. Rev. 2020, 120, 2123–2170. [Google Scholar] [CrossRef]
- Agranovich, V.M.; Gartstein, Y.N.; Litinskaya, M. Hybrid resonant organic–inorganic nanostructures for optoelectronic applications. Chem. Rev. 2011, 111, 5179–5214. [Google Scholar] [CrossRef]
- Mehta, C.; Abbas, J.M.; Saini, G.S.S.; Tripathi, S.K. Effect of deposition parameters on the optical and electrical properties of nanocrystalline CdSe. Chalcogenide Lett. 2007, 4, 133–138. [Google Scholar]
- Pejova, B.; Najdoski, M.; Grozdanov, I.; Dey, S.K. Chemical bath deposition of nanocrystalline (111) textured Ag2Se thin films. Mater. Lett. 2000, 43, 269–273. [Google Scholar] [CrossRef]
- Gates, B.; Yin, Y.; Xia, Y. A Solution-phase approach to the synthesis of uniform nanowires of crystalline selenium with lateral dimensions in the range of 10–30 nm. J. Am. Chem. Soc. 2000, 122, 12582–12583. [Google Scholar] [CrossRef]
- Lee, J.C.; Kurniawan, K.; Chung, K.W.; Kim, S. Metallurgical process for total recovery of all constituent metals from copper anode slimes: A review of established technologies and current progress. Met. Mater. Int. 2021, 27, 2160–2187. [Google Scholar] [CrossRef]
- Li, Z.; Huang, D.; McDonald, L.M. Heterogeneous selenite reduction by zero valent iron steel wool. Water Sci. Technol. 2017, 75, 908–915. [Google Scholar] [CrossRef]
- Liang, L.; Yang, W.; Guan, X.; Li, J.; Xu, Z.; Wu, J.; Huang, Y.; Zhang, X. Kinetics and mechanisms of pH-dependent selenite removal by zero valent iron. Water Res. 2013, 47, 5846–5855. [Google Scholar] [CrossRef]
- Liang, L.P.; Jiang, X.; Yang, W.J.; Huang, Y.Y.; Guan, X.H.; Li, L.N. Kinetics of selenite reduction by zero-valent iron. Desalin. Water Treat. 2015, 53, 2540–2548. [Google Scholar] [CrossRef]
- Jain, R.; Jordan, N.; Schild, D.; van Hullebusch, E.D.; Weiss, S.; Franzen, C.; Farges, F.; Hübner, R.; Lens, P.N.L. Adsorption of zinc by biogenic elemental selenium nanoparticles. Chem. Eng. J. 2015, 260, 855–863. [Google Scholar] [CrossRef]
- Baeshov, A.; Baeshova, A. A reduction phenomenon on the anode surface: Selenate and copper (II) ions reduction to their elemental state on the anode surface. J. Chem. Technol. Metall. 2020, 55, 1105–1110. [Google Scholar]
- Kalaparthi, R.; Korapu, S.; Gandam, H.; Kurimella, V.R. Synthesis of selenium nanoparticles using sodium selenite [Se(IV)] as a precursor and titanium(III) chloride as a reducing agent. Int. J. Eng. Res. 2020, 9, 359–363. [Google Scholar] [CrossRef]
- Geoffroy, N.; Demopoulos, G.P. Stannous chloride—An effective reducing agent for the removal of selenium(IV) from acidic solution. J. Chem. Technol. Biotechnol. 2012, 87, 983–989. [Google Scholar] [CrossRef]
- Geoffroy, N.; Demopoulos, G.P. Reductive precipitation of elemental selenium from selenious acidic solutions using sodium dithionite. Ind. Eng. Chem. Res. 2009, 48, 10240–10246. [Google Scholar] [CrossRef]
- Vieira, A.P.; Stein, E.M.; Andreguetti, D.X.; Cebrián-Torrejón, G.; Doménech-Carbó, A.; Colepicolod, P.; Ferreira, A.M.D.C. “Sweet Chemistry”: A green way for obtaining selenium nanoparticles active against cancer cells. J. Braz. Chem. Soc. 2017, 28, 2021–2027. [Google Scholar] [CrossRef]
- Vahdati, M.; Moghadam, T.T. Synthesis and characterization of selenium nanoparticles lysozyme nanohybrid system with synergistic antibacterial properties. Sci. Rep. 2020, 10, 510. [Google Scholar] [CrossRef] [PubMed]
- Kunita, M.H.; Girotto, E.M.; Radovanovic, E.; Gonçalves, M.C.; Ferreira, O.P.; Muniz, E.C.; Rubira, A.F. Deposition of copper sulfide on modified low-density polyethylene surface: Morphology and electrical characterization. Appl. Surf. Sci. 2002, 202, 223–231. [Google Scholar] [CrossRef]
- Essaidia, H.; Gantassi, A.; Touihria, S.; Ouerfelli, J. Tuning the structural, optical and electrical properties of AgInSe2 thin films prepared by sequentially deposited silver and indium nano-films under vacuum. Optik 2019, 182, 866–875. [Google Scholar] [CrossRef]
- Sutapa, I.W.; Wahab, A.W.; Taba, P.; Nafie, N.L. Dislocation, crystallite size distribution and lattice strain of magnesium oxide nanoparticles. J. Phys. Conf. Ser. 2018, 979, 012021. [Google Scholar] [CrossRef]
- Souri, D.; Tahan, Z.E. A new method for the determination of optical band gap and the nature of optical transitions in semiconductors. Appl. Phys. B Lasers Opt. 2015, 119, 273–279. [Google Scholar] [CrossRef]
- Souri, D.; Shomalian, K. Band gap determination by absorption spectrum fitting method (ASF) and structural properties of different compositions of (60 − x) V2O5–40TeO2–xSb2O3 glasses. J. Non Cryst. Solids 2009, 355, 1597–1601. [Google Scholar] [CrossRef]
- Solaymani, S.; Talu, S.; Nezafat, N.B.; Dejam, L.; Shafiekhani, A.; Ghaderi, A.; Zelati, A. Optical properties and surface dynamics analyses of homojunction and hetrojunction Q/ITO/ZnO/NZO and Q/ITO/ZnO/NiO thin films. Results Phys. 2021, 29, 104679. [Google Scholar] [CrossRef]
- Krylova, V. The study of silver sulfide layers formed on the polyamide film surface. Chemija 2012, 23, 203–209. [Google Scholar]
- Krylova, V.; Dukštienė, N.; Žalenkienė, S.; Baltrusaitis, J. Chemical and structural changes in polyamide based organic–inorganic hybrid materials upon incorporation of SeS2O62− precursor. Appl. Surf. Sci. 2017, 392, 634–641. [Google Scholar] [CrossRef]
- Zhao, Z.; Zheng, W.; Tian, H.; Yu, W.; Han, D.; Li, B. Crystallization behaviors of secondarily quenched Nylon 6. Mater. Lett. 2007, 61, 925–928. [Google Scholar] [CrossRef]
- Du, J.L.; Fang, Y.; Fu, E.G.; Ding, X.; Yu, K.Y.; Wang, Y.G.; Wang, Y.Q.; Baldwin, J.K.; Wang, P.P.; Bai, Q. What determines the interfacial configuration of Nb/Al2O3 and Nb/MgO interface. Sci. Rep. 2016, 6, 33931. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Choi, J.Y.; Lee, Y.H.; Park, J.T.; Song, H. Formation of metal selenide and metal-selenium nanoparticles using distinct reactivity between selenium and noble metals. Chem. Asian J. 2015, 10, 1452–1456. [Google Scholar] [CrossRef]
- Yanhui, Z.; Xu, M.; Liu, Y.; Bai, Y.; Deng, Y.; Liu, J.; Chen, L. Green synthesis of Se/Ru alloy nanoparticles using gallic acid and evaluation of their anti-invasive effects in HeLa cells. Colloids Surf. B 2016, 44, 118–124. [Google Scholar] [CrossRef]
- Olin, Å.; Noläng, B.; Osadchii, E.G.; Öhman, L.-O.; Rosén, E. Chemical Thermodynamics of Selenium, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 2005; 894p, Available online: https://www.oecd-nea.org/dbtdb/pubs/vol7-selenium.pdf (accessed on 16 August 2023).
- Jiang, X.; Mayers, B.; Herricks, T.; Xia, Y. Direct synthesis of Se@CdSe nanocables and CdSe nanotubes by reacting cadmium salts with Se nanowires. Adv. Mater. 2003, 15, 1740–1743. [Google Scholar] [CrossRef]
- Gates, B.; Mayers, B.; Wu, Y.; Sun, Y.; Cattle, B.; Yang, P.; Xia, Y. Synthesis and characterization of crystalline Ag2Se nanowires through a template-engaged reaction at room temperature. Adv. Funct. Mater. 2002, 12, 679–686. [Google Scholar] [CrossRef]
- Ayele, D.W. A facile one-pot synthesis and characterization of Ag2Se nanoparticles at low temperature. Egypt. J. Basic Appl. Sci. 2016, 3, 149–154. [Google Scholar] [CrossRef]
- Gates, B.; Wu, Y.; Yin, Y.; Yang, P.; Xia, Y. Single-crystalline nanowires of Ag2Se can be synthesized by templating against nanowires of trigonal Se. J. Am. Chem. Soc. 2001, 123, 11500–11501. [Google Scholar] [CrossRef]
- Jiang, Z.-Y.; Xie, Z.-X.; Zhang, X.-H.; Huang, R.-B.; Zheng, L.-S. Conversion of Se nanowires to Se/Ag2Se nanocables and Ag2Se nanotubes. Chem. Phys. Lett. 2003, 378, 313–316. [Google Scholar] [CrossRef]
- Kienle, L.; Duppel, V.; Mogwitz, B.; Janek, J.; Kreutzbruck, M.V.; Leineweber, A.; Simon, A. Synthesis–real structure–property: The showcase of silver-rich Ag2Se. Cryst. Growth Des. 2011, 11, 2412–2421. [Google Scholar] [CrossRef]
- Porter, D.A.; Easterling, K.E. Phase Transformations in Metals and Alloys, 4th ed.; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Luo, Y.R. Comprehensive Handbook of Chemical Bond Energies, 1st ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Saruyama, M.; Sato, R.; Teranishi, T. Transformations of ionic nanocrystals via full and partial ion exchange reactions. Acc. Chem. Res. 2021, 54, 765–775. [Google Scholar] [CrossRef]
- Thiel, F.; Palencia, C.; Weller, H. Kinetic analysis of the cation exchange in nanorods from Cu2−xS to CuInS2: Influence of djurleite’s phase transition temperature on the mechanism. ACS Nano 2023, 17, 3676–3685. [Google Scholar] [CrossRef]
- Beberwyck, B.J.; Surendranath, Y.; Alivisatos, A.P. Cation exchange: A versatile tool for nanomaterials synthesis. J. Phys. Chem. C 2013, 117, 19759–19770. [Google Scholar] [CrossRef]
- Liu, G.; Liu, Y.; Zhao, J.; Pan, A.; Wang, B.; Zhu, T. Controllable Ag migration to form one-dimensional Ag/Ag2S@ZnS for bifunctional catalysis. ACS Appl. Energy Mater. 2020, 3, 6146–6154. [Google Scholar] [CrossRef]
- Stehlik, S.; Shimakawa, K.; Wagner, T.; Frumar, M. Diffusion of Ag ions under random potential barriers in silver-containing chalcogenide glasses. J. Phys. D Appl. Phys. 2012, 45, 205304. [Google Scholar] [CrossRef]
- Bhushan, B. Surface Roughness Analysis and Measurement Techniques; Modern Tribology Handbook; CRC Press LLC: Boca Raton, FL, USA, 2001; pp. 48–114. [Google Scholar]
- Gadelmawla, E.S.; Koura, M.M.; Maksoud, T.M.A.; Elewa, I.M.; Soliman, H.H. Roughness parameters. J. Mater. Process. Technol. 2002, 123, 133–145. [Google Scholar] [CrossRef]
- Song, G.; Wang, Y.; Tan, D.Q. A review of surface roughness impact on dielectric film properties. IET Nanodielectr. 2022, 5, 1–23. [Google Scholar] [CrossRef]
- He, X.; Liao, W.; Wang, G.; Zhong, L.; Li, M. Evaluation of hydrodynamic lubrication performance of textured surface from the perspective of skewness and kurtosis. Ind. Lubr. Tribol. 2018, 70, 829–837. [Google Scholar] [CrossRef]
- Caban, R.; Gnatowski, A. Structural and thermal examinations of polyamide modified with fly ash from biomass combustion. Materials 2023, 16, 5277. [Google Scholar] [CrossRef]
- Krylova, V.; Dukštienė, N. The structure of PA-Se-S-Cd composite materials probed with FTIR spectroscopy. Appl. Surf. Sci. 2019, 470, 462–471. [Google Scholar] [CrossRef]
- Sun, H.; Zhu, X.; Yang, D.; Wangyang, P.; Gao, X.; Tian, H. An economical method for amorphous selenium thick films preparation: E-beam evaporation. Mater. Lett. 2016, 183, 94–96. [Google Scholar] [CrossRef]
- Tüzüner, S.; Demir, M.M. Dispersion of organophilic Ag nanoparticles in PS-PMMA blends. Mater. Chem. Phys. 2015, 162, 692–699. [Google Scholar] [CrossRef][Green Version]
- Ji, Y.; Yang, X.; Ji, Z.; Zhu, L.; Ma, N.; Chen, D.; Jia, X.; Tang, J.; Cao, Y. DFT-calculated IR spectrum amide I, II, and III band contributions of N-methylacetamide fine components. ACS Omega 2020, 5, 8572–8578. [Google Scholar] [CrossRef] [PubMed]
- Kotkata, M.F.; Masoud, A.E.; Mohamed, M.B.; Mahmoud, E.A. Structural characterization of chemically synthesized CdSe nanoparticles. Phys. E Low-Dimens. 2009, 41, 640–645. [Google Scholar] [CrossRef]
- Zeng, Y.; Kelley, D.F. Surface charging in CdSe quantum dots: Infrared and transient absorption spectroscopy. J. Phys. Chem. C 2017, 121, 16657–16664. [Google Scholar] [CrossRef]
- Qu, J.; Goubet, N.; Livache, C.; Martinez, B.; Amelot, D.; Gréboval, C.; Chu, A.; Ramade, J.; Cruguel, H.; Ithurria, S.; et al. Intraband mid-Infrared transitions in Ag2Se nanocrystals: Potential and limitations for Hg free low cost photodetection. J. Phys. Chem. C 2018, 122, 18161–18167. [Google Scholar] [CrossRef]
- Sadtler, B.; Demchenko, D.O.; Zheng, H.; Hughes, S.M.; Merkle, M.G.; Dahmen, U.; Wang, L.-W.; Alivisatos, A.P. Selective facet reactivity during cation exchange in cadmium sulfide nanorods. J. Am. Chem. Soc. 2009, 131, 5285–5293. [Google Scholar] [CrossRef]
- Lin, Z.H.; Wang, C.R.C. Evidence on the size-dependent absorption spectral evolution of selenium nanoparticles. Mater. Chem. Phys. 2005, 92, 591–594. [Google Scholar] [CrossRef]
- Panahi-Kalamuei, M.; Mousavi-Kamazani, M.; Salavati-Niasari, M.; Mostafa Hosseinpour-Mashkani, S. A simple sonochemical approach for synthesis of selenium nanostructures and investigation of its light harvesting application. Ultrason. Sonochem. 2015, 23, 246–256. [Google Scholar] [CrossRef]
- Surana, K.; Salisu, I.T.; Mehra, R.M.; Bhattacharya, B. A simple synthesis route of low temperature CdSe-CdS core-shell quantum dots and its application in solar cell. Opt. Mater. 2018, 82, 135–140. [Google Scholar] [CrossRef]
- Hegazy, M.A.; Abd El-Hameed, A.M. Characterization of CdSe-nanocrystals used in semiconductors for aerospace applications: Production and optical properties. NRIAG J. Astron. Geophys. 2014, 3, 82–87. [Google Scholar] [CrossRef]
- Ramezanloo, B.; Molaei, M.; Karimipour, M. Red emissive Ag2Se quantum dots (QDs) with room-temperature synthesis of both orthorhombic and superionic cubic phases via stirring approach. J. Lumin. 2018, 204, 419–423. [Google Scholar] [CrossRef]
- Singh, S.; Bharti, A.; Meena, V.K. Green synthesis of multi-shaped silver nanoparticles: Optical, morphological and antibacterial properties. J. Mater. Sci. Mater. Electron. 2015, 26, 3638–3648. [Google Scholar] [CrossRef]
- Xu, X.; Wang, X.; Zhang, Y.; Li, P. Ion-exchange synthesis and improved photovoltaic performance of CdS/Ag2S heterostructures for inorganic-organic hybrid solar cells. Solid State Sci. 2016, 61, 195–200. [Google Scholar] [CrossRef]
- Di, T.; Cheng, B.; Ho, W.; Yu, J.; Tang, H. Hierarchically CdS–Ag2S nanocomposites for efficient photocatalytic H2 production. Appl. Surf. Sci. 2019, 470, 196–204. [Google Scholar] [CrossRef]
- Bubenov, S.S.; Dorofeev, S.G.; Kotin, P.A.; Znamenkov, K.O.; Kuznetsova, T.A. Oleic capped CdSe nanocrystals silver doped in the course of synthesis. Mendeleev Commun. 2014, 24, 250–252. [Google Scholar] [CrossRef]
- Asadpour-Zeynali, K.; Mollarasouli, F. A novel and facile synthesis of TGA-capped CdSe@Ag2Se core-shell quantum dots as a new substrate for high sensitive and selective methyldopa sensor. Sens. Actuator B Chem. 2016, 237, 387–399. [Google Scholar] [CrossRef]
- Ansari, M.Z.; Khare, N. Effect of intrinsic strain on the optical band gap of single phase nanostructured Cu2ZnSnS4. Mater. Sci. Semicond. Process 2017, 63, 220–226. [Google Scholar] [CrossRef]
- Purushotham, E.; Krishna, N.G. Effect of particle size and lattice strain on Debye-Waller factors of Fe3C nanoparticles. Bull. Mater. Sci. 2014, 37, 773–778. [Google Scholar] [CrossRef][Green Version]
- Li, J.; Zhu, X.; Yang, D.; Gu, P.; Wu, H. Investigations on structural, optical and X-radiation responsive properties of a-Se thin films fabricated by thermal evaporation method at low vacuum degree. Materials 2018, 11, 368. [Google Scholar] [CrossRef] [PubMed]
- Shportko, K.V. Disorder and compositional dependences in Urbach-Martienssen tails in amorphous (GeTe)x(Sb2Te3)1−x alloys. Sci. Rep. 2019, 9, 6030. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, C.; Sandberg, O.J.; Zarrabi, N.; Li, W.; Meredith, P.; Armin, A. A universal Urbach rule for disordered organic semiconductors. Nat. Commun. 2021, 12, 3988. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.J.; Mahadevan, S.; Yuan, J.; Ho, J.K.W.; Gao, Y.X.; Liu, W.; Zhong, H.; Yan, H.; Zou, Y.P.; Tsang, S.W.; et al. Unraveling Urbach tail effects in high performance organic photovoltaics: Dynamic vs static disorder. ACS Energy Lett. 2022, 7, 1971–1979. [Google Scholar] [CrossRef]
- Ambrosone, G.; Basa, D.K.; Coscia, U.; Fathallah, M. Study on the microstructural and overall disorder in hydrogenated amorphous silicon carbon films. J. Appl. Phys. 2008, 104, 123520. [Google Scholar] [CrossRef]
- Rani, S.; Sanghi, S.; Agarwal, A.; Seth, V.P. Study of optical band gap and FTIR spectroscopy of Li2O·Bi2O3·P2O5 glasses. Spectrochim. Acta A 2009, 74, 673–677. [Google Scholar] [CrossRef]
- Ananth Kumar, R.T.; Chithra Lekha, P.; Sanjeeviraja, C.; Pathinettam Padiyan, D. Evolution of structural disorder using Raman spectra and Urbach energy in GeSe0.5S1.5 thin films. J. Non-Cryst. 2014, 405, 21–26. [Google Scholar] [CrossRef]
- Kabir, H.; Rahman, M.M.; Uddin, K.M.; Bhuiyand, A.H. Structural, morphological, compositional and optical studies of plasma polymerized 2-furaldehyde amorphous thin films. Appl. Surf. Sci. 2017, 423, 983–994. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).