Abstract
Y2O3-stabilized ZrO2 (YSZ) has been the material of choice for thermal barrier coatings (TBCs) in the past decades, yet its phase decomposition limits its application above 1200 °C. In this study, Gd2O3 and Yb2O3 co-doped YSZ powders were produced, in which some amounts of monoclinic (m) phase were introduced into the cubic (c) phase matrix. XRD results showed that the fabricated powders obtained by a solid phase synthesis were composed of m and c phases, and hat the m phase content decreased in a sequence of 4Gd-2Yb-4Y, 2Gd-2Yb-6Y and 2Gd-4Yb-4Y powders. This indicated that Yb3+ is an excellent stabilizer in the ZrO2-based lattice, which could largely suppress the formation of the m phase. The m phase content in the powders was almost kept unchanged with heating at 1300 °C, which could provide a toughening effect to the ceramic. All the powders exhibited no obvious sintering at 1300 °C for 150 h. As compared to YSZ, the three fabricated ceramics had lower thermal conductivities, and they increased in a sequence of 4Gd-2Yb-4Y, 2Gd-4Yb-4Y and 2Gd-2Yb-6Y.
1. Introduction
ZrO2 stabilized by oxides has been extensively studied, and one of the representative stabilizers is Y2O3 [1,2,3]. Due to its excellent high-temperature mechanical and thermal–physical properties, Y2O3 partially stabilized ZrO2 (YSZ) has been practically applied in electronics [4,5], atomic energy, the aviation industry, and especially to the thermal barrier coatings (TBC) which offer thermal insulation to the hot components of aero-engines and thus effectively improve their combustion efficiency [6,7,8]. YSZ TBCs have been successfully used for several decades [9,10], but they have become less and less reliable with the development of aero-engines [11,12,13,14]. One crucial aspect in this is issue of the phase decomposition and phase transformation [1,15,16]. As is known to all, YSZ TBCs are usually prepared either by electron beam physical vapor deposition (EB-PVD) [17,18] or by air plasma spraying (APS) [19,20,21]. Additionally, they exhibit a non-transformable metastable tetragonal (t’) phase, which is desirable for thermal insulation yet. At temperatures above 1200 °C [22], the t’-YSZ decomposes into cubic (c)-YSZ and t YSZ, the latter transforming into a monoclinic (m) phase accompanied by some volume expansion [23,24], which is fast and uncontrollable [25]. When the phase-transformed part reaches a critical value, the resulting volume expansions lead to crack initiation, propagation, and even coating spallation.
Many efforts have been made to enhance the high-temperature phase instability of the t’-YSZ, such as selecting alternate stabilizers to Y2O3 for ZrO2-based systems. Cairney et al. reported that the phase stability of ZrO2, stabilized by Yb2O3, is better than that of YSZ at 1300 °C [26]. Er2O3, Sm2O3, Nd2O3, Sc2O3 and Gd2O3 have also been studied as stabilizers for ZrO2, and Er2O3-ZrO2 has the best phase stability [27,28,29,30,31,32]. It is worthy noted that the Gd2O3 dopant improves the sintering resistance of both bulk Gd2O3-ZrO2 (4 mol% stabilizer) and YSZ TBCs, although it decreases the t’ phase stability, as reported by Rahaman [27]. Er2O3, Sm2O3, and Nd2O3 have also been studied as stabilizers for ZrO2, and the t’ phase stability of Er2O3-ZrO2 is the best [28]. Additionally, some reports have revealed that co-doping two or more rare earth oxides (RE2O3) into ZrO2 has an effective function in improving the t’ phase stability. Guo et al. found better t’ phase stability in Sc2O3-Gd2O3-ZrO2 ceramics with increasing Sc2O3 content [33]. Moreover, Guo et al. has analyzed the t’ phase stability of RE2O3 (RE = La, Nd, Gd, Yb)-Yb2O3-YSZ at 1400 °C [34].
Although the modified t’ phase stability has improved at high temperatures, it still inevitably undergoes phase decomposition with long-term annealing. Despite its excellent thermal conductivity and high-temperature phase stability, C-ZrO2 is unfortunately not tough enough for use in TBCs because of its low toughness [15]. The volume expansion, resulting from the t to m phases transformation, can absorb or consume the energy at the crack tip and generate compressive stresses in the region of the main crack action, something which improves the strength and fracture toughness of the coating [20]. Therefore, it is reasonable to believe that setting an appropriate amount of m phase in the c-ZrO2 and maintaining the m phase content during service can effectively improve the performance of ZrO2 ceramics.
In this study, we prepared YSZ co-doped with Gd2O3 and Yb2O3 via a solid-state reaction, which consisted of c and m phases. An investigation was conducted into the effect of co-doped RE2O3 on the monoclinic. The phase stability of the fabricated powders which were heat-treated at 1300 °C was evaluated. In addition, the sintering behavior and thermal conductivity of the co-doped ZrO2 ceramics were investigated and compared with those of YSZ. Finally, the mechanisms of the reduced thermal conductivity, resulting from the rare earth co-doping, were discussed.
2. Experimental Procedure
The co-doped ZrO2 were prepared by solid-state reaction at 1400 °C for 12 h, using Gd2O3, Yb2O3, Y2O3 (99.99% purity) and ZrO2 (99.9% purity) as raw materials. The total stabilizer content of each doped zirconia is 10 mol%, and the specific components are expressed in mol% as follows: 2Gd2O3-2Yb2O3-6Y2O3-90ZrO2 (2Gd-2Yb-6Y), 2Gd2O3-Yb2O3-Y2O3-90ZrO2 (2Gd-4Yb-4Y), and 4Gd2O3-2Yb2O3-4Y2O3-90ZrO2 (4Gd-2Yb-4Y). The raw powers were mixed in a planetary ball mill with absolute ethanol for more than 12 h to obtain a homogeneous mixture. The homogeneously mixed solution was dried, placed in a zirconia crucible at 1400 °C and sintered for 12 h to produce RE2O3-stabilized ZrO2 ceramics. Meanwhile, some mixed powder was cold-pressed at 250 MPa and then sintered at 1400 °C for 10 h to obtain the bulk material for thermal conductivity measurements. A scanning electron microscope (SEM; JEOL 7900F) was used to characterize the morphologies of ceramic powders after calcination and annealing at 1300 °C for 50h, 100, and 150 h. Phase stability was characterized by X-ray diffraction (X′Pert3 MRD, Malvern Panalytical, Cu Kα radiation). The scanning frequency was 0.05°/s. The molar content of the m phase was calculated using Miller’s formula [35], given in Equations (1) and (2):
where Mm and Mc,t are the mole fractions of the m and c/t phases, respectively, and I is the integrated intensity corresponding to the peaks concerned.
The thermal diffusivity (α) of RE2O3 co-doped ZrO2 was measured between 20 °C and 1200 °C using a laser flash meter (LFA 427; Netzsch, Selb, Germany), with an interval temperature of 200 °C. Before measuring the thermal diffusivity, the front and back surface of the samples were coated with a graphite film to allow thermal absorption of the laser pulse. Each sample was measured three times at selected temperatures. The specific heat capacity (Cp) is calculated from the heat capacity values of the individual constituent oxide based on the Neumann-Kopp rule [36]. The density (ρ) was measured by the Archimedes’ method. The thermal conductivity (λ) was calculated by Equation (3):
Since the sintered sample cannot be completely dense, the influence () on the thermal conductivity of the material can be fixed using the following equation, in which λ0 expresses the full density of the sample (λ0) [37]:
3. Results and Discussion
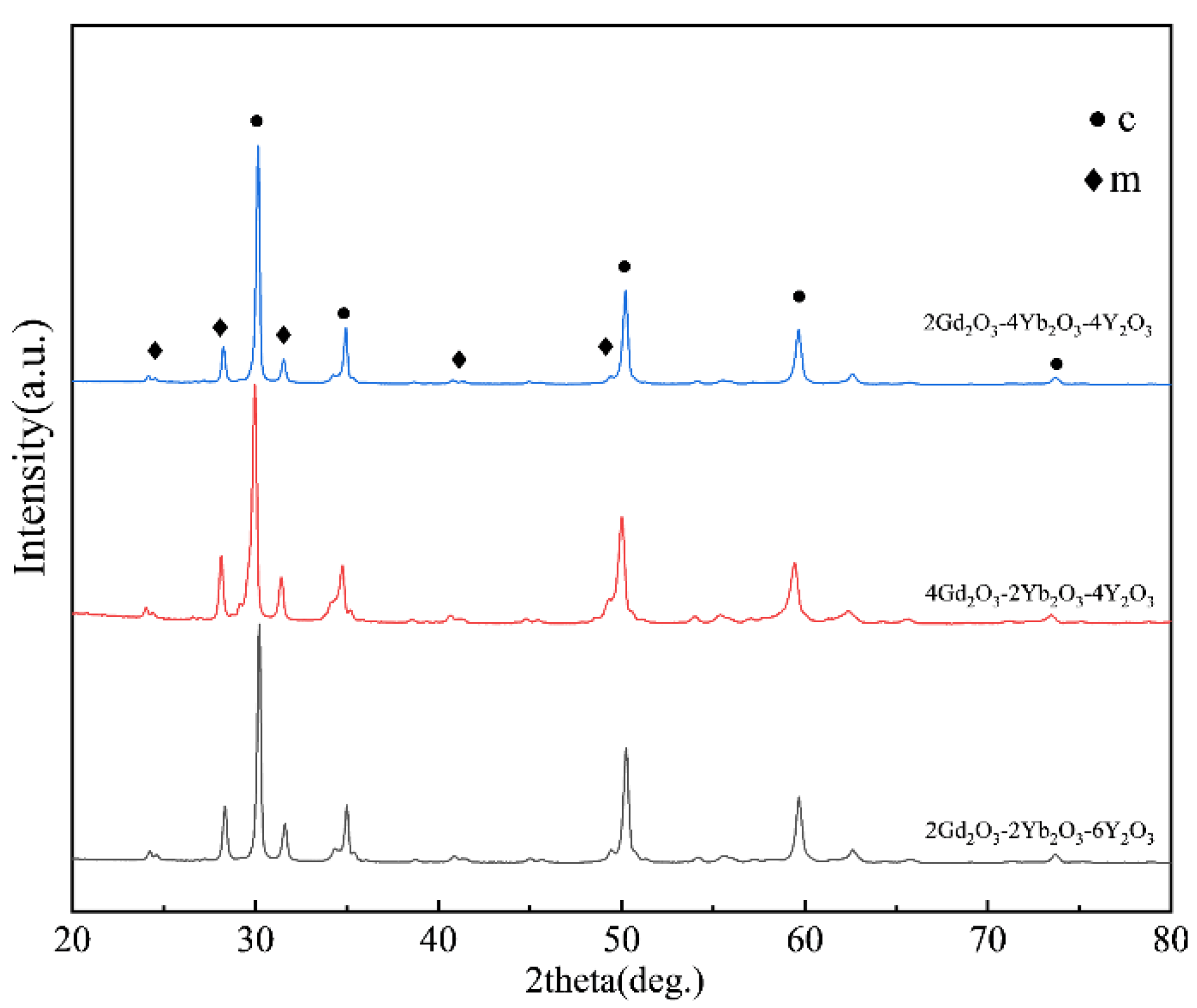
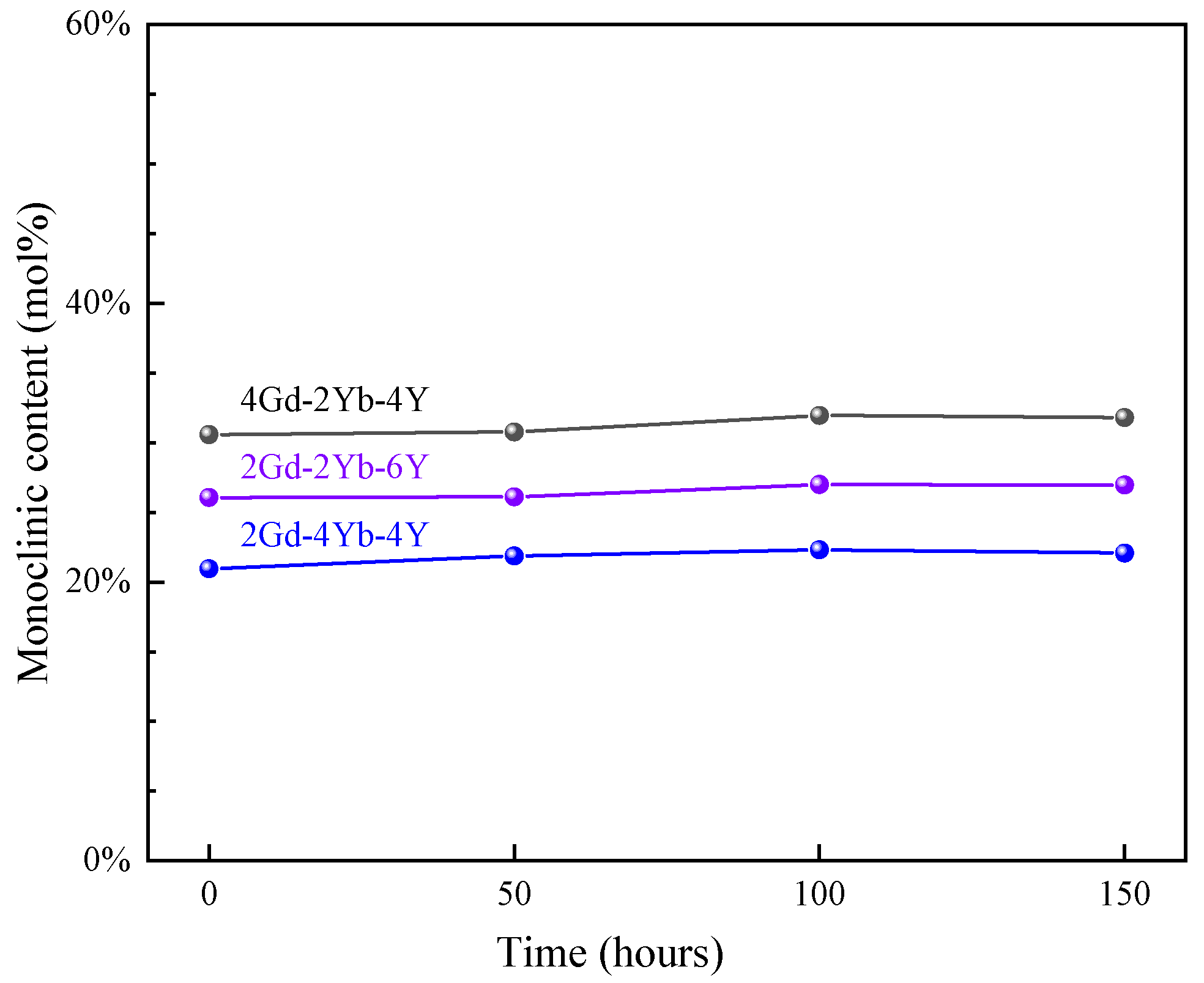
Figure 1 shows the XRD patterns of the as-fabricated 2Gd-2Yb-6Y, 4Gd-2Yb-4Y and 2Gd-4Yb-4Y powders. For all samples, the primary phase is the c phase. In the range between 2θ ≈ 28.3° and 31.5° of all the patterns the diffraction peaks were found, which are characteristic peaks of the m phase. Hence, it could be identified that the fabricated powders are composed of c and m phases. The m phase contents in the three types of co-doped ZrO2 were calculated and illustrated in Figure 2. It could be found that the m phase content in the samples increases in a sequence of 2Gd-4Yb-4Y, 2Gd-2Yb-6Y and 4Gd-2Yb-4Y powders.
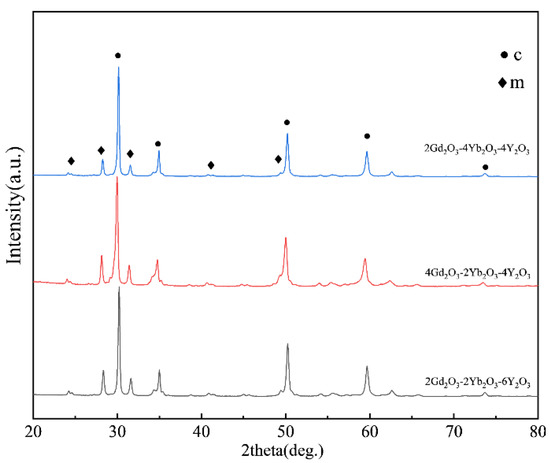
Figure 1.
XRD patterns of as-fabricated 2Gd-2Yb-6Y, 4Gd-2Yb-4Y and 2Gd-4Yb-4Y powders.
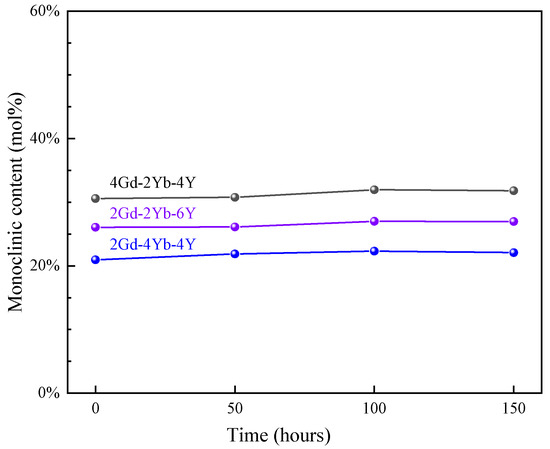
Figure 2.
M phase contents of 2Gd-2Yb-6Y, 4Gd-2Yb-4Y and 2Gd-4Yb-4Y powders with time at 1300 °C.
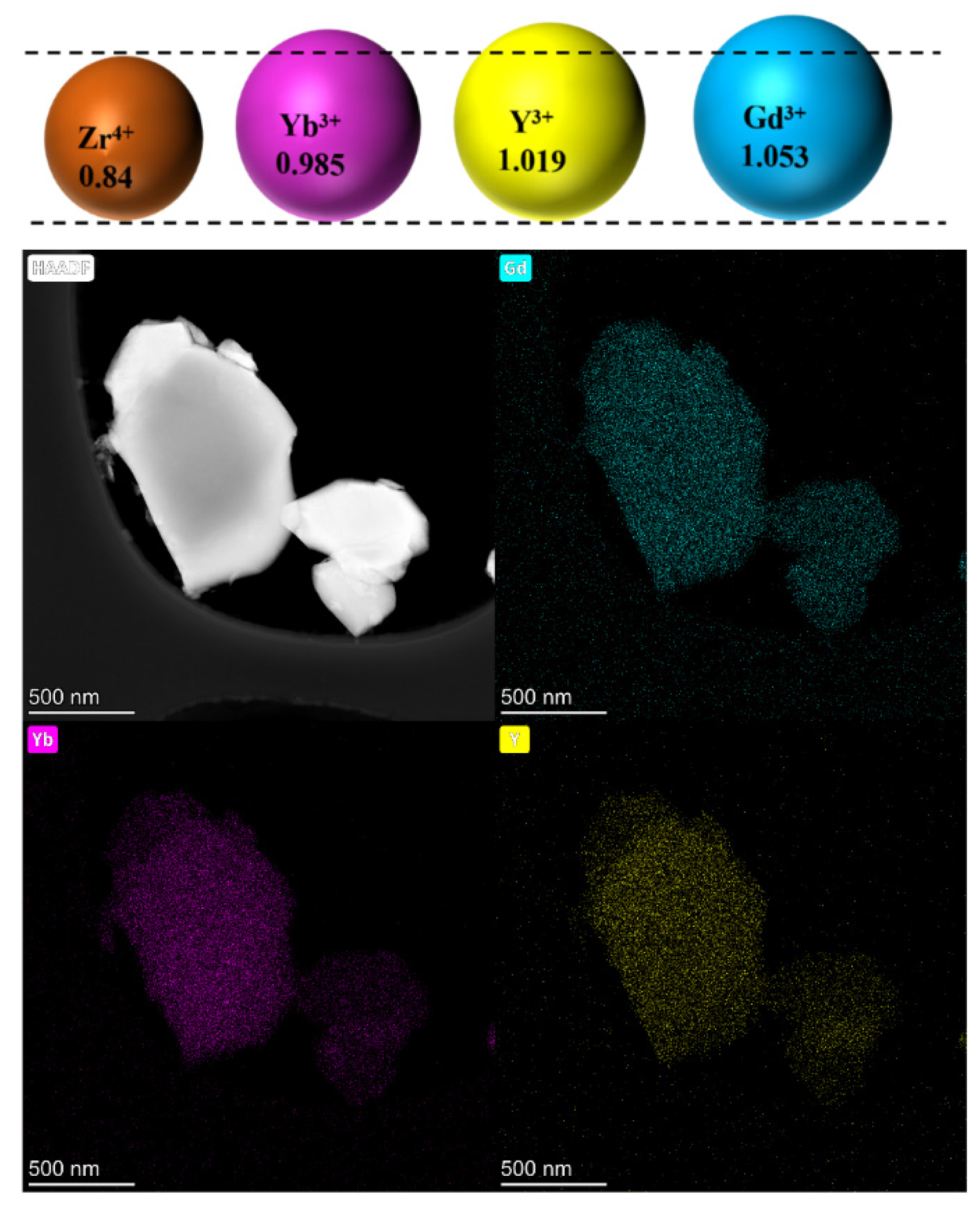
The different m contents in the three samples could be attributed to the difference in the stabilization ability of Yb, Y and Gd. When rare earth cations are used to stabilize ZrO2, a smaller size misfit between RE3+ and Zr4+ ions in the ZrO2 lattice is desirable. The cation radii of Yb3+, Y3+, Gd3+ and Zr4+ are 0.985 Å, 1.019 Å, 1.053 Å and 0.84 Å in the 8-coordination, respectively. Thus, doping Yb3+ into the ZrO2 lattice is considered to have the most ability to obtain a stable c phase [38]. Based on this consideration, the variation tendency of the m phase content in the samples could be well explained. Comparing samples 2Gd-4Yb-4Y and 2Gd-2Yb-6Y, they have the same range of Gd, but the first sample is rich in Yb, while the second is rich in Y. Since Yb3+ is smaller than Y3+, it is reasonable that the 2Gd-4Yb-4Y sample has a higher content of c phase content than the 2Gd-2Yb-6Y sample. Thus, the content of m phase in the 2Gd-4Yb-4Y sample is lower than that in the 2Gd-2Yb-6Y sample. The Yb contents of the 2Gd-2Yb-6Y and 4Gd-2Yb-4Y samples are identical, but the former contains lower Y3+ levels. Hence, the 2Gd-2Yb-6Y sample has a higher ability to produce the c phase, and its m phase content is lower. Therefore, the m phase content follows the sequence m2Gd-4Yb-4Y ˂ m2Gd-2Yb-6Y ˂ m4Gd-2Yb-4Y, further evidence that Yb3+ is an excellent stabilizer in the ZrO2 lattice that can largely suppress the formation of the m phase.
The lattice parameters of the c phase in the powders 2Gd-2Yb-6Y, 4Gd-2Yb-4Y and 2Gd-4Yb-4Y were calculated, and the results are listed in Table 1. It was found that the lattice parameters of the samples increase in a sequence of 2Gd-4Yb-4Y, 2Gd-2Yb-6Y and 4Gd-2Yb-4Y. This could also be explained based upon the difference in the RE3+ radius. By comparing 2Gd-2Yb-6Y and 4Gd-2Yb-4Y, their Yb contents are identical, but the latter contains more Gd3+. Thus, 4Gd-2Yb-4Y has a more significant lattice parameter than 2Gd-2Yb-6Y. For the case of 2Gd-2Yb-6Y and 4Gd-2Yb-4Y, the Yb contents are the same, but 2Gd-2Yb-6Y contains more Y and thus shows a smaller lattice parameter.

Table 1.
Lattice parameters in different ceramic samples.
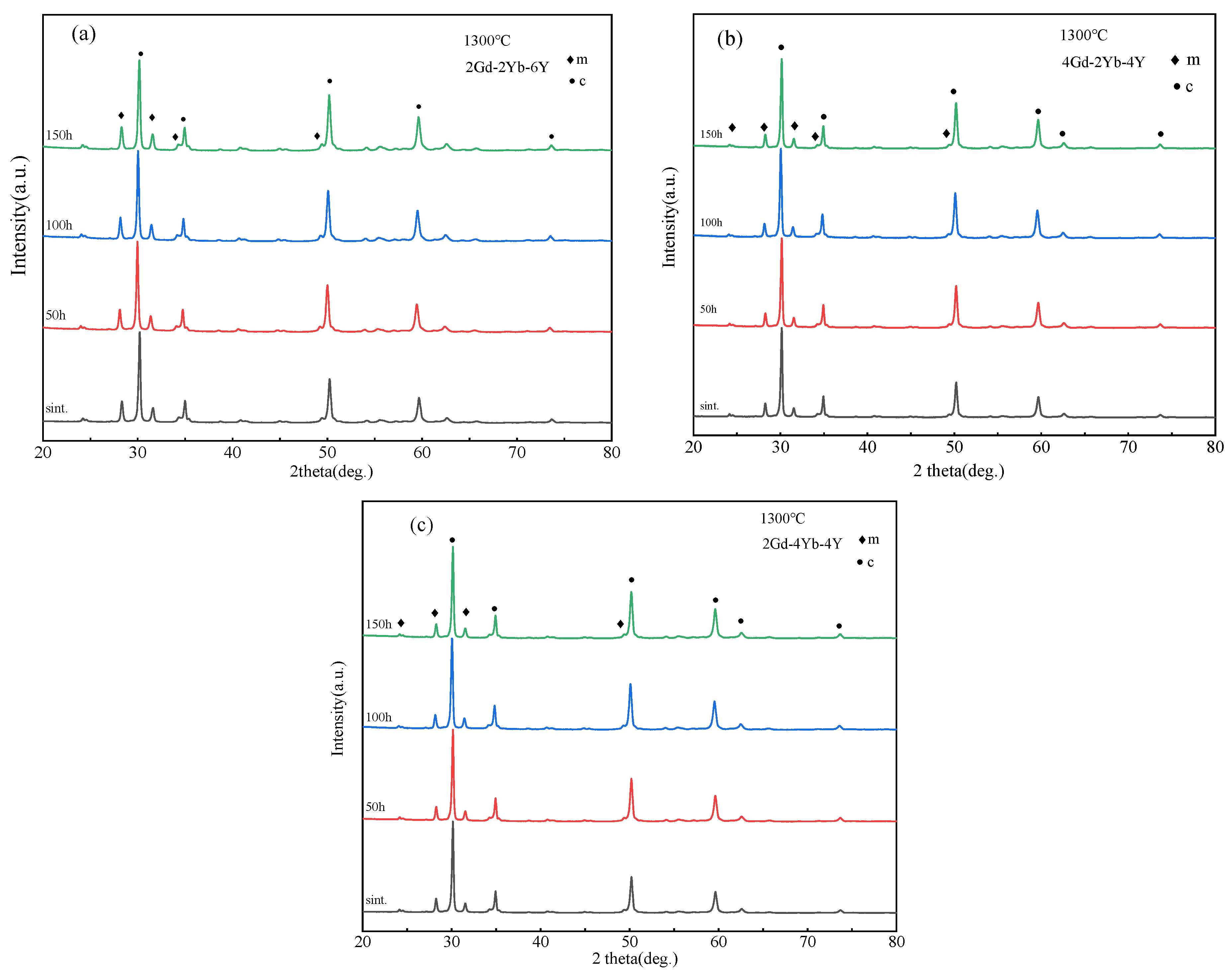
To investigate the changes in the phase structure of the co-doped zirconia powder at 1300 °C, heat treatment tests were performed at different times. Figure 3 shows the XRD patterns of 2Gd-2Yb-6Y, 4Gd-2Yb-4Y and 2Gd-4Yb-4Y powders at different heat treatment times. The c and m phases are still present at each time point, and the changes in m phase content in the samples are shown in Figure 2. It can be seen that the m phase contents in all samples remain almost unchanged with the heating time. 2Gd-2Yb-6Y, 4Gd-2Yb-4Y and 2Gd-4Yb-4Y are composed of c and m phases. It is known that the c phase does not undergo any transformation during heat treatments. Therefore, it is reasonable to keep the content of m phase constant after heat treatment. However, the m phase undergoes transformation during heat treatment. It transforms into a t phase, and the t→m phase transition occurs during the subsequent cooling process. This process belongs to the martensitic transformation, a phenomenon which can provide a toughening effect to the ceramic. As a result, 2Gd-2Yb-6Y, 4Gd-2Yb-4Y and 2Gd-4Yb-4Y are expected to have higher toughness than those with a pure c phase. Even though, it should be noted that the proportion of m phase in the sample should not be too high as this would be detrimental to the ceramics for TBC applications [39,40].
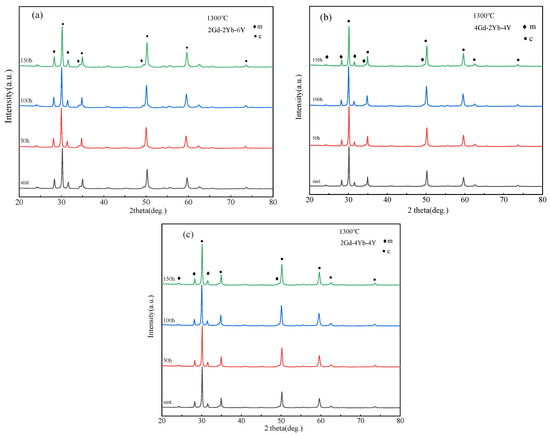
Figure 3.
XRD patterns of (a) 2Gd-2Yb-6Y, (b) 4Gd-2Yb-4Y and (c) 2Gd-4Yb-4Y powders after 50, 100 h and 150 h at 1300 °C.
Figure 4 shows the microstructure of the powders after 150 h of heat treatment. All samples showed good sintering resistance, with most powders maintaining an independent grain shape. In the 2Gd-2Yb-6Y powder, many separated grains were connected by a neck structure, the neck between the grains grew significantly, the deformation of the grains was more significant, and a clear trend of grain boundary movement was found. Conversely, in the 4Gd-2Yb-4Y and 2Gd-4Yb-4Y powders, most of the grains still maintained an independent polygonal structure with fewer and smaller necks, indicating a lower degree of sintering in these two ceramic samples. The microscopic mechanism of the initial sintering of ceramic powders is the lattice diffusion from the inner grain boundary to the neck. The low sintering rate of polyoxides-doped ZrO2 is reasonable, Gd3+ has a large ionic radius, and its distribution requires higher energy. In contrast, Yb3+ has a smaller ionic radius, but its larger atomic mass also limits the grain boundary diffusion, a factor which improves the sintering resistance of ceramics. This is consistent with the cation distribution in Figure 5 over 150 h. Therefore, the degree of sintering is also lower. Since the ceramic samples in this study are a mixture of c and m phases, which increases the energy required for grain boundary movement, and thus doping with Gd2O3, Yb2O3, and, Y2O3 helps to improve the resistance of the ZrO2-based ceramics.
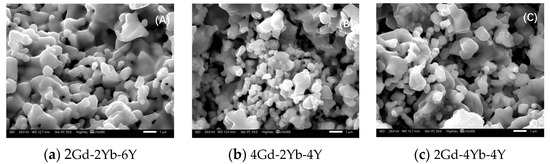
Figure 4.
Microstructure of different component powders after 150 h at 1300 °C.
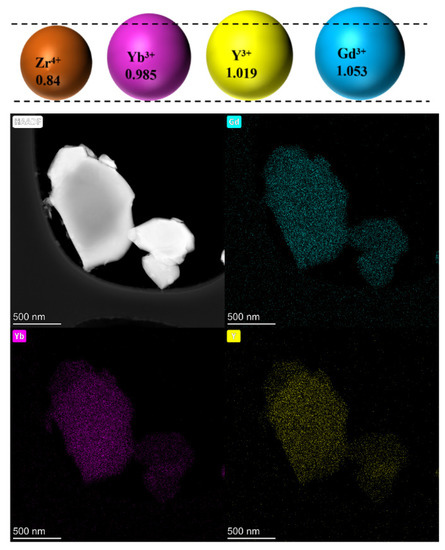
Figure 5.
Elemental distribution of the 2Gd-4Yb-4Y powder after heat treatment for 150 h at 1300 °C.
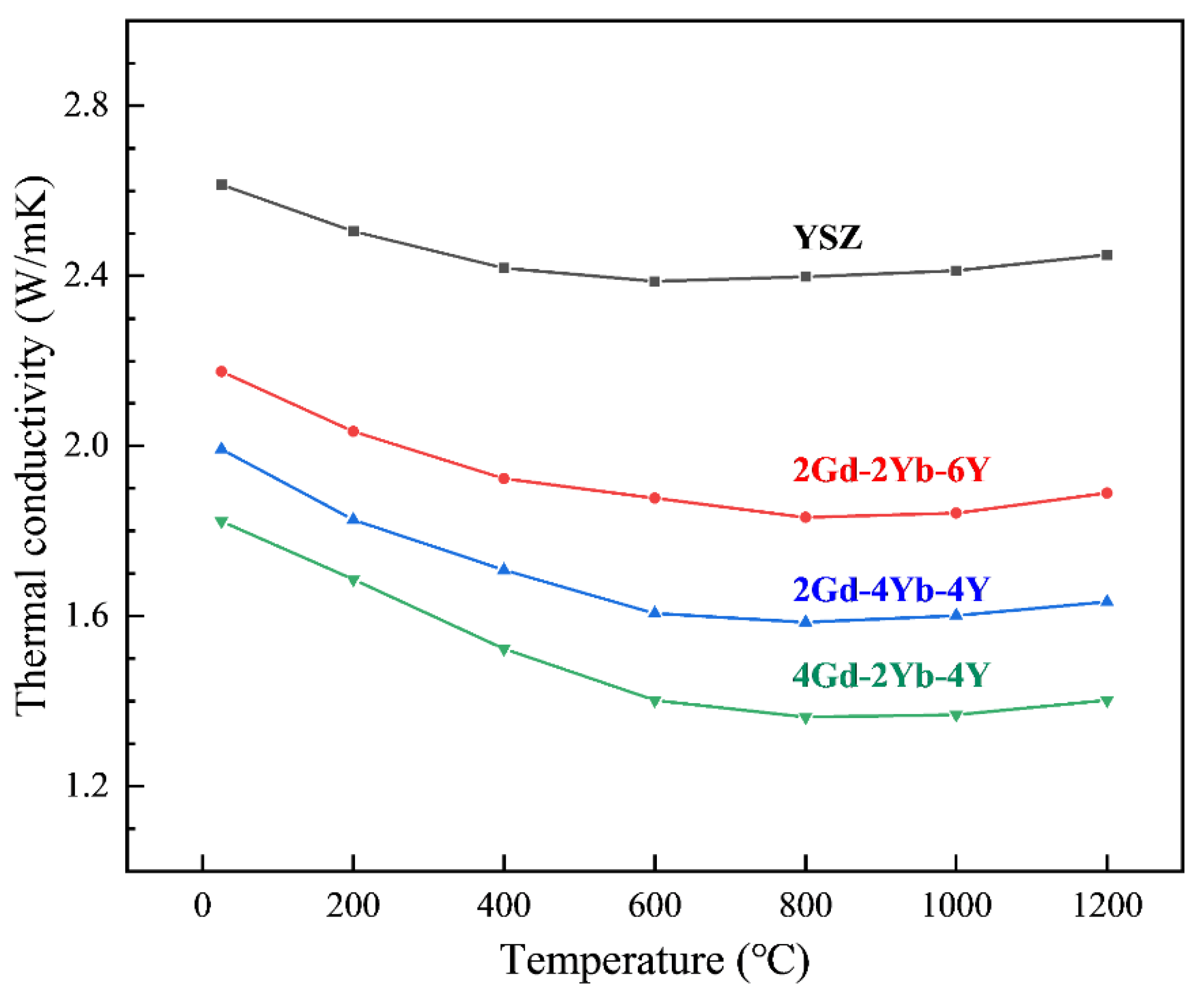
Figure 6 shows the thermal conductivities of 2Gd-2Yb-6Y, 4Gd-2Yb-4Y, 2Gd-4Yb-4Y and YSZ ceramics. The error bars are neglected because they are smaller than the symbols. Firstly, the thermal conductivity of ceramics decreases with increasing temperature in the room temperature range up to 800–1000 °C and increases when the temperature exceeds 1000 °C. Secondly, it is found that all the co-doped ceramics have lower thermal conductivities than YSZ. At 1000 °C, the thermal conductivities of 2Gd-2Yb-6Y, 2Gd-4Yb-4Y, and 4Gd-2Yb-4Y are 1.889 W/mK, 1.663 W/mK and 1.420 W/mK, respectively.
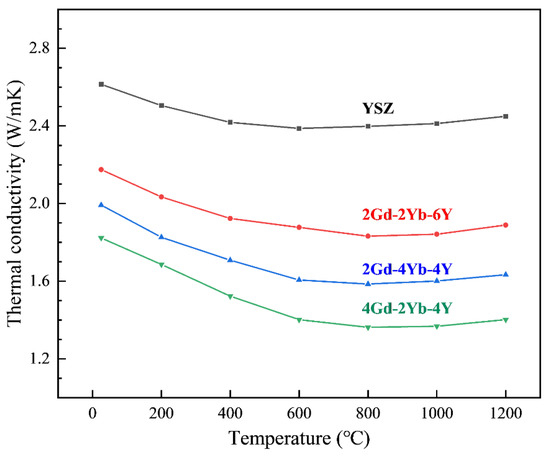
Figure 6.
The thermal conductivity of the specimens as a function of temperature.
It is well known that thermal conductivity is highly dependent on the non-harmonic effects caused by phonon scattering and on the vacancies caused by defects [41,42]. In this study, co-doping with RE2O3 causes more defects and elastic strain fields, reducing phonons average free range and leading to the lower thermal conductivity of ZrO2 co-doped with Gd2O3, Yb2O3, and Y2O3 than YSZ.
For the ZrO2 doped with RE2O3, the phonon scattering rates can be expressed with the following Equation (5) [43] due to the different atomic masses of doped RE3+ and Zr4+:
where τ is the relaxation time, α3 is the atomic volume, νs is the transverse wave velocity, ω is the phonon frequency, c is the ratio of the number of defects per unit volume focus to the number of dot positions, M is the Zr4+ mass of cation, and ∆M is the mass difference between the doped RE3+ and Zr4+. The scattering rate of the phonons is proportional to the square of ∆M. Thus, the larger the mass difference between the doped cations and Zr4+ is, the stronger the phonon scattering will be, resulting in lower thermal conductivity. Therefore, ceramics prepared in this study have lower thermal conductivities.
The doping of RE2O3 to YSZ produces an elastic field due to the difference in ionic radii between RE3+ and Zr4+, dislodging surrounding atoms and altering the phonon frequency.
Due to the difference in ionic radius between RE3+ and Zr4+, doping YSZ with RE2O3 produces an elastic field, which dislodges atoms and affects phonon frequencies, enhancing phonon scattering [43]:
where J is a constant, γ is the Gruneisen’s constant, R is the Zr4+ cation radius, and ∆R is the radius difference between the doped RE3+ and Zr4+. Thus, the larger the radius difference between the doped cations and Zr4+, the stronger the phonon scattering, resulting in lower thermal conductivity. Hence, the fabricated ceramics in this study are shown to have lower thermal conductivities compared to YSZ.
As shown in Figure 6, the thermal conductivities of the co-doped samples increase in an order of 4Gd-2Yb-4Y, 2Gd-4Yb-4Y and 2Gd-2Yb-6Y, which could be explained by the basis of the above discussion on the effects of ∆M and ∆R on the phonon scattering. In comparison, the Y2O3 content of 4Gd-2Yb-4Y and 2Gd-4Yb-4Y ceramics is the same. Thus, only the impact of Gd and Yb on the phonon scattering needs to be considered. Compared to Yb3+, Gd3+ is larger and lighter. Thus ∆R (Gd3+-Zr4+) is larger than ∆R (Yb3+-Zr4+), but ∆M (Gd3+-Zr4+) is smaller than ∆M (Yb3+-Zr4+). Since 4Gd-2Yb-4Y has lower thermal conductivities than 2Gd-4Yb-4Y, it could be implied that the ∆R plays a more dominant role in affecting phonon scattering than the ∆M for those two ceramics. For the 2Gd-4Yb-4Y and 2Gd-2Yb-6Y ceramics, ∆M (Yb3+-Zr4+) is larger than ∆M (Y3+-Zr4+), but ∆R (Yb3+-Zr4+) is smaller than ∆R (Y3+-Zr4+). 2Gd-4Yb-4Y has lower thermal conductivities than 2Gd-2Yb-6Y, which implies that in this case, the ∆M plays a more dominant role in affecting the phonon scattering than the ∆R.
4. Conclusions
10 mol% for Gd2O3, Yb2O3, and Y2O3 co-doped ZrO2 was synthesized by the solid-state reaction method:
- Analysis of the phase content analysis of the synthesized powders revealed that the content of m phase gradually decreased for the powders 4Gd-2Yb-4Y, 2Gd-2Yb-6Y, and 2Gd-4Yb-4Y. This suggests that Yb2O3 effectively suppresses the formation of the m phase and is an excellent stabilizer for ZrO2. A subsequent heat treatment test at 1300 °C, conducted for 150 h, showed that m phase remained almost unchanged, indicating that it can significantly improve the toughness of a given material.
- No sintering was observed in any of the powders, suggesting that the addition of Gd2O3 and Yb2O3 improves the sintering resistance of YSZ materials.
- Co-doping Gd2O3 and Yb2O3 can effectively reduce the thermal conductivity of YSZ. The co-doping increases the discrepancy between the radius and atomic mass of RE3+ and Zr4+ ions, which increases the phonon scattering and reduces the thermal conductivity of the material, resulting in increased phonon scattering. The experimental results indicate the Gd2O3 is more effective at decreasing thermal conductivity than co-doped ZrO2.
As a result of these comprehensive properties, 4Gd-2Yb-4Y ceramics may be considered a promising candidate for TBC manufacturing. Additionally, more work will focus on the mechanical properties of 4Gd-2Yb-4Y materials and the performance of coatings with this composition.
Author Contributions
Writing—original draft preparation, data curation, formal analysis, and investigation, H.T.; review and editing, L.W.; funding acquisition, L.W. and L.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded for National Natural Science Foundation of China in 2021, design and functional realization mechanism of high temperature ablation resistant, highly efficient thermal insulation and large strain tolerance protective coatings.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Padture, N.P. Advanced structural ceramics in aerospace propulsion. Nat. Mater. 2016, 15, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.R.; Levi, C.G. Materials Design for the Next Generation Thermal Barrier Coatings. Annu. Rev. Mater. Res. 2003, 33, 383–417. [Google Scholar] [CrossRef]
- Clarke, D.R.; Oechsner, M.; Padture, N.P. Thermal-barrier coatings for more efficient gas-turbine engines. MRS Bull. 2012, 37, 891–898. [Google Scholar] [CrossRef]
- Nomura, M.; Kage, Y.; Müller, D. Electrical and thermal properties of polycrystalline Si thin films with phononic crystal nanopatterning for thermoelectric applications. Appl. Phys. Lett. 2015, 106, 223106. [Google Scholar] [CrossRef]
- Yanagisawa, R.; Tsujii, N.; Mori, T. Nanostructured planar-type uni-leg Si thermoelectric generators. Appl. Phys. Express 2020, 13, 095001. [Google Scholar] [CrossRef]
- Clarke, D.R.; Phillpot, S.R. Thermal barrier coating materials. Mater. Today 2005, 8, 22–29. [Google Scholar] [CrossRef]
- Vassen, R.; Cao, X.Q.; Tietz, F. Zirconates as new materials for thermal barrier coatings. J. Am. Ceram. Soc. 2000, 83, 2023–2028. [Google Scholar] [CrossRef]
- Sun, C.; Hui, R.; Roller, J. Cathode materials for solid oxide fuel cells: A review. J. Solid State Electrochem. 2010, 14, 1125–1144. [Google Scholar] [CrossRef]
- Kakuda, T.R.; Limarga, A.M. Evolution of thermal properties of EB-PVD 7YSZ thermal barrier coatings with thermal cycling. Acta Mater. 2009, 57, 2583–2591. [Google Scholar] [CrossRef]
- Evans, A.G.; Clarke, D.R. The influence of oxides on the performance of advanced gas turbines. J. Eur. Ceram. Soc. 2008, 28, 1405–1419. [Google Scholar] [CrossRef]
- Darolia, R. Thermal barrier coatings technology: Critical review, progress update, remaining challenges and prospects. Int. Mater. Rev. 2013, 58, 315–348. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, C. Hot corrosion behavior of nanostructured Gd2O3 doped YSZ thermal barrier coating in presence of Na2SO4 + V2O5 molten salts. Prog. Nat. Sci. Mater. Int. 2017, 27, 507–513. [Google Scholar] [CrossRef]
- Wang, C.; Zinkevich, M.; Aldinger, F. Phase diagrams and thermodynamics of rare-earth-doped zirconia ceramics. Pure Appl. Chem. 2007, 79, 1731–1753. [Google Scholar] [CrossRef]
- Lu, X.; Xu, P.; Wang, H. Cooling potential and applications prospects of passive radiative cooling in buildings: The current state-of-the-art. Renew. Sustain. Energy Rev. 2016, 65, 1079–1097. [Google Scholar] [CrossRef]
- Vasanthavel, S.; Kannan, S. Structural investigations on the tetragonal to cubic phase transformations in zirconia induced by progressive yttrium additions. J. Phys. Chem. Solids 2018, 112, 100–105. [Google Scholar] [CrossRef]
- Hurwitz, F.I.; Rogers, R.B.; Guo, H. Phase development and pore stability of yttria- and ytterbia-stabilized zirconia aerogels. J. Am. Ceram. Soc. 2020, 103, 6700–6711. [Google Scholar] [CrossRef]
- Ochrombel, R.; Schneider, J.; Hildmann, B. Thermal expansion of EB-PVD yttria stabilized zirconia. J. Eur. Ceram. Soc. 2010, 30, 2491–2496. [Google Scholar] [CrossRef]
- Naraparaju, R.; Hüttermann, M.; Schulz, U. Tailoring the EB-PVD columnar microstructure to mitigate the infiltration of CMAS in 7YSZ thermal barrier coatings. J. Eur. Ceram. Soc. 2017, 37, 261–270. [Google Scholar] [CrossRef]
- Lashmi, P.G.; Ananthapadmanabhan, P.V.; Unnikrishnan, G. Present status and future prospects of plasma sprayed multilayered thermal barrier coating systems. J. Eur. Ceram. Soc. 2020, 40, 2731–2745. [Google Scholar] [CrossRef]
- Ponnuchamy, M.B.; Gandhi, A.S. Phase and fracture toughness evolution during isothermal annealing of spark plasma sintered zirconia co-doped with Yb, Gd and Nd oxides. J. Eur. Ceram. Soc. 2015, 35, 1879–1887. [Google Scholar] [CrossRef]
- Lima, R.S. Perspectives on Thermal Gradients in Porous ZrO2-7~8 wt.% Y2O3 (YSZ) Thermal Barrier Coatings (TBCs) Manufactured by Air Plasma Spray (APS). Coatings 2020, 10, 812. [Google Scholar] [CrossRef]
- Clarke, D.R.; Levi, C.G. Enhanced zirconia thermal barrier coating systems. Proc. Inst. Mech. Eng. Part A J. Power Energy 2006, 220, 85–92. [Google Scholar] [CrossRef]
- Ren, X.; Pan, W. Mechanical properties of high-temperature-degraded yttria-stabilized zirconia. Acta Mater. 2014, 69, 397–406. [Google Scholar] [CrossRef]
- Fu, Z.; Wu, N.; Long, H. Fabrication of Highly Transparent Y2O3 Ceramics via Colloidal Processing Using ZrO2-Coated Y2O3 Nanoparticles. Coatings 2022, 12, 1077. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, C.; Xu, L. Effects of TiO2 doping on the defect chemistry and thermo-physical properties of Yb2O3 stabilized ZrO2. J. Eur. Ceram. Soc. 2017, 37, 4163–4169. [Google Scholar] [CrossRef]
- Cairney, J.M.; Rebollo, N.R.; Rühle, M. Phase stability of thermal barrier oxides: A comparative study of Y and Yb additions. Int. J. Mater. Res. 2007, 98, 1177–1187. [Google Scholar] [CrossRef]
- Rahaman, M.; Gross, J.; Dutton, R. Phase stability, sintering, and thermal conductivity of plasma-sprayed ZrO2–Gd2O3 compositions for potential thermal barrier coating applications. Acta Mater. 2006, 54, 1615–1621. [Google Scholar] [CrossRef]
- Boissonnet, G.; Chalk, C.; Nicholls, J.R. Phase stability and thermal insulation of YSZ and erbia-yttria co-doped zirconia EB-PVD thermal barrier coating systems. Surf. Coat. Technol. 2020, 389, 125566. [Google Scholar] [CrossRef]
- Jones, R.L.; Mess, D. Improved tetragonal phase stability at 1400 °C with scandia, yttria-stabilized zirconia. Surf. Coat. Technol. 1996, 86, 94–101. [Google Scholar] [CrossRef]
- Leoni, M.; Jones, R.L.; Scardi, P. Phase stability of scandia–yttria-stabilized zirconia TBCs. Surf. Coat. Technol. 1998, 108, 107–113. [Google Scholar] [CrossRef]
- Fan, W.; Wang, Y.; Liu, Y. Mechanical Properties Durability of Sc2O3-Y2O3 Co-Stabilized ZrO2 Thermal Barrier Materials for High Temperature Application. Coatings 2022, 12, 155. [Google Scholar] [CrossRef]
- Yan, Z.; Peng, H.; Yuan, K. Optimization of Yb2O3-Gd2O3-Y2O3 Co-Doped ZrO2 Agglomerated and Calcined Powders for Air Plasma Spraying. Coatings 2021, 11, 373. [Google Scholar] [CrossRef]
- Sun, L. Influence of partial substitution of Sc2O3 with Gd2O3 on the phase stability and thermal conductivity of Sc2O3-doped ZrO2. Ceram. Int. 2013, 39, 3447–3451. [Google Scholar] [CrossRef]
- Guo, L.; Li, M.; Ye, F. Phase stability and thermal conductivity of RE2O3 (RE = La, Nd, Gd, Yb) and Yb2O3 co-doped Y2O3 stabilized ZrO2 ceramics. Ceram. Int. 2016, 42, 7360–7365. [Google Scholar] [CrossRef]
- Miller, R.A. Phase stability in plasma sprayed partially stabilized zirconia-yttria. Am. Ceram. Soc. 1981, 3, 241–253. [Google Scholar]
- Kubaschewski, O. Materials Thermochemistry, 6th ed.; Pergamon Press: Oxford, UK, 1993; pp. 254–326. [Google Scholar]
- Wu, J.; Wei, X.Z.; Padture, N.P. Low-thermal-conductivity rare-earth zirconates for potential thermal-barrier-coating applications. J. Am. Ceram. Soc. 2002, 85, 3031–3035. [Google Scholar] [CrossRef]
- Zacate, M.O.; Minervini, L. Defect cluster formation in M2O3-doped cubic ZrO2. Solid State Ion. 2000, 128, 243–254. [Google Scholar] [CrossRef]
- Xue, M.; Liu, S.; Wang, X. High fracture toughness of 3Y-TZP ceramic over a wide sintering range. Mater. Chem. Phys. 2020, 244, 122693. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, L.; Zhao, X. Toughening effect of Yb2O3 stabilized ZrO2 doped in Gd2Zr2O7 ceramic for thermal barrier coatings. Mater. Sci. Eng. A 2015, 648, 385–391. [Google Scholar] [CrossRef]
- Tian, Z.; Lin, C.; Zheng, L. Defect-mediated multiple-enhancement of phonon scattering and decrement of thermal conductivity in (YxYb1-x)2SiO5 solid solution. Acta Mater. 2018, 144, 292–304. [Google Scholar] [CrossRef]
- Ishibe, T.; Tomeda, A.; Komatsubara, Y. Carrier and phonon transport control by domain engineering for high-performance transparent thin film thermoelectric generator. Appl. Phys. Lett. 2021, 118, 151601. [Google Scholar] [CrossRef]
- Berman, R. Thermal Conduction in Solid; Clarendon Press: Oxford, UK, 1976. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).