The Oxidation Properties of a NiCrAlY Coating Fabricated by Arc Ion Plating
Abstract
1. Introduction
2. Experimental Procedures
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caron, P.; Khan, T. Improvement of Creep strength in a nickel-base single-crystal superalloy by heat treatment. Mater. Sci. Eng. 1983, 61, 173–184. [Google Scholar] [CrossRef]
- Göbel, M.; Rahmel, A.; Schütze, M. The isothermal-oxidation behavior of several nickel-base single-crystal superalloys with and without coatings. Oxid. Met. 1993, 39, 231–261. [Google Scholar] [CrossRef]
- Guo, D.; Zhao, L.; Jodoin, B. Cold spray for production of in-situ nanocrystalline MCrAlY coatings—Part II: Isothermal oxidation performance. Surf. Coat. Technol. 2021, 409, 126828. [Google Scholar] [CrossRef]
- Bezencon, C.; Schnell, A.; Kurz, W. Epitaxial deposition of MCrAlY coatings on a Ni-base superalloy by laser cladding. Scr. Mater. 2003, 49, 705–709. [Google Scholar] [CrossRef]
- Shi, L.; Xin, L.; Wang, X.; Wang, X.; Wei, H.; Zhu, S.L.; Wang, F.H. Influences of MCrAlY coatings on oxidation resistance of single crystal superalloy DD98M and their inter-diffusion behaviors. J. Alloys Compd. 2015, 649, 515–530. [Google Scholar] [CrossRef]
- Drajewicz, M.; Kubaszek, T.; Kościelniak, B.; Góral, M.; Dziadosz, D. The isothermal oxidation of mcraly protective coatings. Mater. Sci. Forum 2021, 1016, 407–412. [Google Scholar] [CrossRef]
- Demir, H.; Cosgun, A.E. The effect on energy efficiency of yttria-stabilized zirconia on brass, copper and hardensed steel nozzle in addictive manufacturing. Coatings 2022, 12, 690. [Google Scholar] [CrossRef]
- Demir, H. The effects on thermal efficiency of yttria-stabilized zirconia and lanthanum zirconate-based thermal barrier coatings on aluminum heating black for 3D printer. Coatings 2021, 11, 792. [Google Scholar] [CrossRef]
- Yuan, K.; Peng, R.L.; Li, X.H.; Johansson, S.; Wang, Y.D. Some aspects of elemental behaviour in HVOF MCrAlY coatings in high-temperature oxidation. Surf. Coat. Technol. 2015, 261, 86–101. [Google Scholar] [CrossRef]
- Hua, C.; Song, P.; Huang, T.; He, X.; Li, C.; Zhai, R.; Li, Q.; Huang, W.; Lu, J. The growth mechanism of oxide scale with Pt on NiCoCrAlY coating in water vapor at 1050 °C. Mod. Phys. Lett. B 2021, 35, 2150111. [Google Scholar] [CrossRef]
- Ghadami, F.; Aghdam, A.S.R.; Ghadami, S. Microstructural characteristics and oxidation behavior of the modified MCrAlX coatings: A critical review. Vacuum 2021, 185, 109980. [Google Scholar] [CrossRef]
- Feuerstein, A.; Knapp, J.; Taylor, T.; Ashary, A.; Bolcavage, A.; Hitchman, N. Technical and economical aspects of current thermal barrier coating systems for gas turbine engines by thermal spray and EBPVD: A Review. J. Therm. Spray Technol. 2008, 17, 199–213. [Google Scholar] [CrossRef]
- Bobzin, K.; Wietheger, W.; Knoch, M.A. Development of thermal spray processes for depositing coatings on thermoplastics. J. Therm. Spray Technol. 2021, 30, 157–167. [Google Scholar] [CrossRef]
- Kellai, A.; Kahla, S.; Dehimi, S.; Babes, B. Microstructural and mechanical properties of welding and thermal spraying coatings on ductile cast iron. Defect Diffus. Forum 2021, 406, 300–311. [Google Scholar] [CrossRef]
- Yang, L.L.; Gao, F.Y.; Zhou, Z.H.; Jia, Y.X.; Du, Y.; Wang, J.L.; Qiao, Y.X.; Zhu, S.L.; Wang, F.H. Oxidation behavior of the AlN coatings on the TiAl alloy at 900 °C. Corros. Sci. 2022; in press. [Google Scholar] [CrossRef]
- Guo, M.H.; Wang, Q.M.; Ke, P.L.; Gong, J.; Sun, C.; Huang, R.F.; Wen, L.S. The preparation and hot corrosion resistance of gradient NiCoCrAlYSiB coatings. Surf. Coat. Technol. 2006, 200, 3942–3949. [Google Scholar] [CrossRef]
- Yang, L.L.; Chen, M.H.; Wang, J.L.; Qiao, Y.X.; Guo, P.Y.; Zhu, S.L.; Wang, F.H. Microstructure and composition evolution of a single-crystal superalloy caused by elements interdiffusion with an overlay NiCrAlY coating on oxidation. J. Mater. Sci. Technol. 2020, 45, 49–58. [Google Scholar] [CrossRef]
- Xie, S.; Lin, S.; Shi, Q.; Wang, W.; Song, C.; Xu, W.; Dai, M. A study on the mechanical and thermal shock properties of MCrAlY coating prepared by arc ion plating. Surf. Coat. Technol. 2021, 413, 127092. [Google Scholar] [CrossRef]
- Meng, B.; Wang, J.L.; Yang, L.L.; Chen, M.H.; Zhu, S.L.; Wang, F.H. On the rumpling mechanism in nanocrystalline coatings: Improved by reactive magnetron sputtering with oxygen. J. Mater. Sci.Technol. 2023, 132, 69–80. [Google Scholar] [CrossRef]
- Meng, B.; Wang, J.L.; Yang, L.L.; Zhu, L.J.; Chen, M.H.; Zhu, S.L.; Wang, F.H. Influence of fuel combustion on the corrosion behavior of pipeline steels in fire flooding technology. NPJ Mater. Degrad. 2022, 21, 6. [Google Scholar] [CrossRef]
- Ren, P.; Yang, Y.F.; Li, W.; Zhu, S.L.; Wang, F.H. Cyclic oxidation behavior of a multilayer composite coating for single crystal superalloys. Corros. Sci. 2018, 145, 26–34. [Google Scholar] [CrossRef]
- Wang, J.L.; Chen, M.H.; Zhu, S.L.; Wang, F.H. Ta effect on oxidation of a nickel-based single-crystal superalloy and its sputtered nanocrystalline coating at 900–1100 °C. Appl. Surf. Sci. 2015, 345, 194–203. [Google Scholar] [CrossRef]
- Wang, J.L.; Chen, M.H.; Yang, L.L.; Zhu, S.L.; Wang, F.H. Comparative study of oxidation and interdiffusion behavior of AIP NiCrAlY and sputtered nanocrystalline coatings on a nickel-based single-crystal superalloy. Corros. Sci. 2015, 98, 530–540. [Google Scholar] [CrossRef]
- Yang, L.L.; Zhou, Z.H.; Yang, R.Z.; Wang, J.L.; Chen, M.H.; Qiao, Y.X.; Zhu, S.L.; Wang, F.H. Effect of Al and Cr on the oxidation behavior of nanocrystalline coatings at 1050 °C. Corros. Sci. 2022, 200, 110191. [Google Scholar] [CrossRef]
- Xiao, Q.; Huang, Q.Y.; Yang, L.L.; Ren, P.; Wang, Q.W.; Yang, Y.F.; Li, W.; Zhu, S.L.; Wang, F.H. The cyclic oxidation behavior of a Pt modified γ’ nanocrystalline coating at 1150 °C. Corros. Sci. 2022, 208, 110638. [Google Scholar] [CrossRef]
- Yang, S.S.; Li, S.; Wang, J.L.; Bao, Z.; Yang, L.L.; Chen, M.H.; Zhu, S.L.; Wang, F.H. A novel single-phase γ’ nanocrystalline coating with high resistance to oxidation and scale rumpling yet mitigated interdiffusion with the alloy substrate. Corros. Sci. 2021, 192, 109866. [Google Scholar] [CrossRef]
- Yang, L.L.; Wang, J.L.; Yang, R.; Yang, S.; Jia, Y.; Chen, M.; Qiao, Y.X.; Guo, P.; Zhu, S.L.; Wang, F.H. Oxidation behavior of a nanocrystalline coating with low Ta content at high temperature. Corros. Sci. 2021, 180, 109182. [Google Scholar] [CrossRef]
- Huang, D.; Qiao, Y.X.; Yang, L.L.; Wang, J.L.; Chen, M.H.; Zhu, S.L.; Wang, F.H. Effect of shot peening of substrate surface on cyclic oxidation behavior of sputtered nanocrystalline coating. Acta Metall. Sin. 2022; in press. [Google Scholar] [CrossRef]
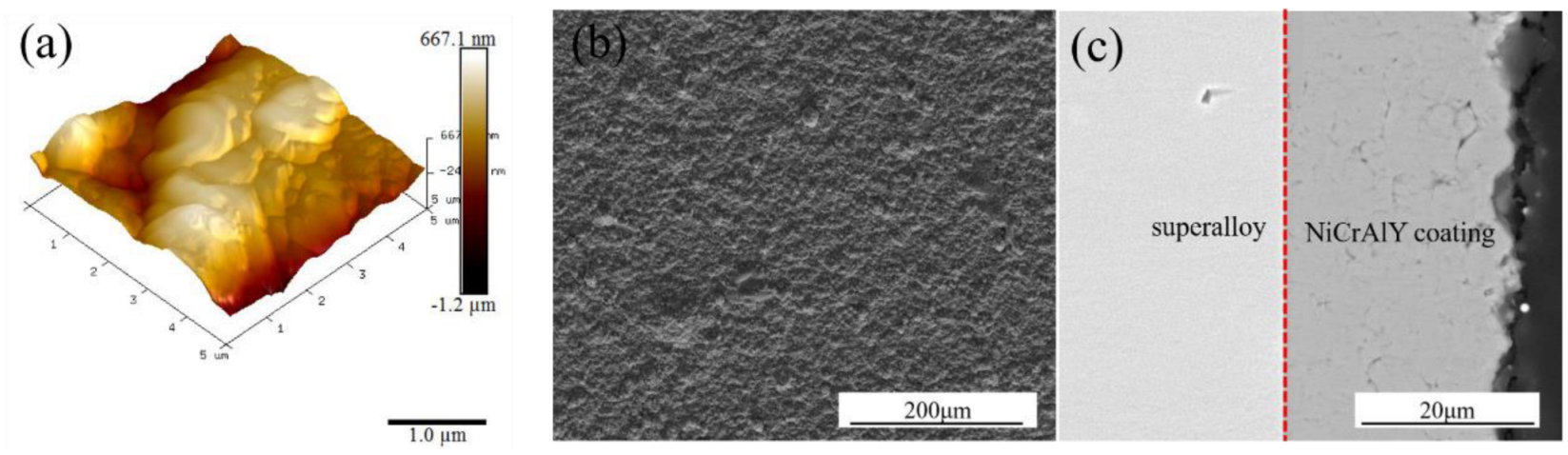

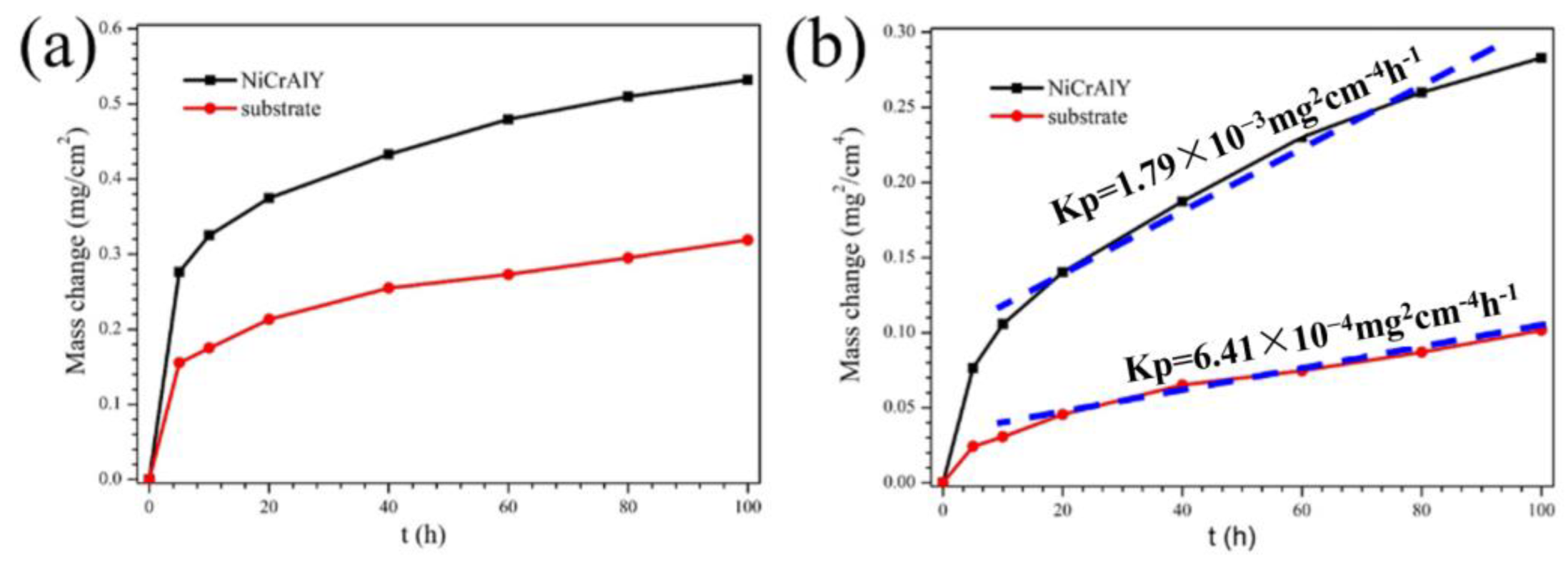

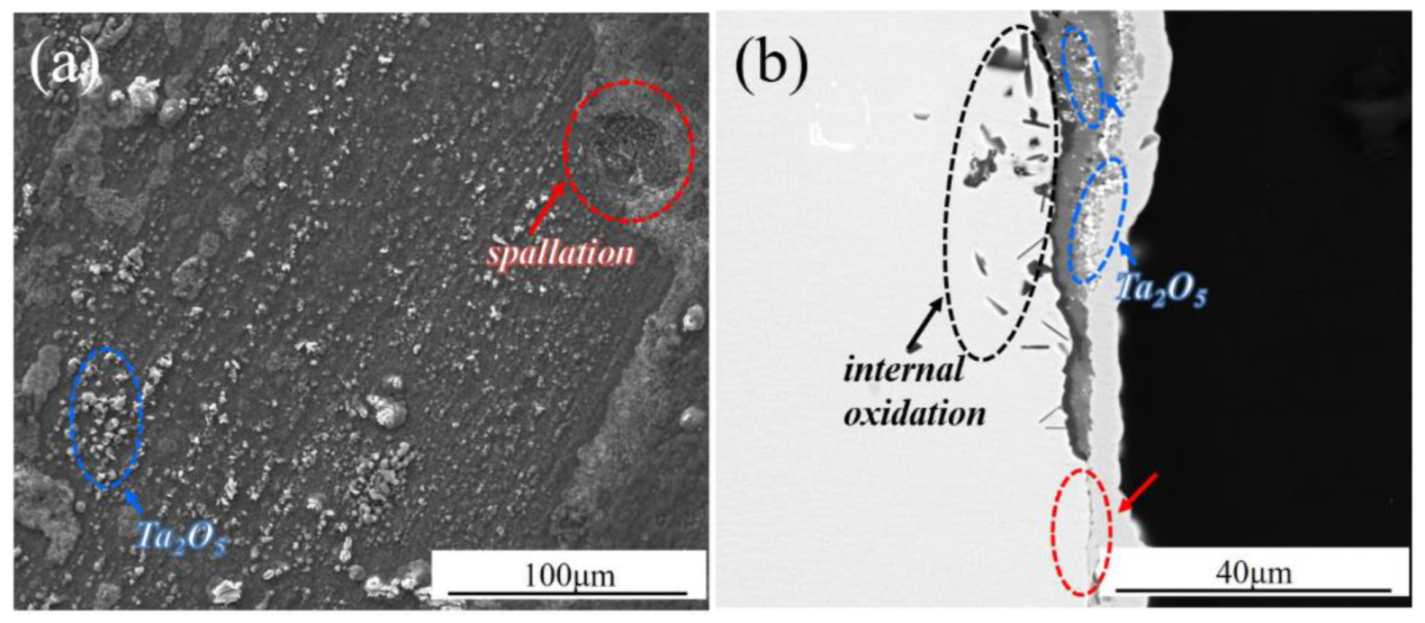
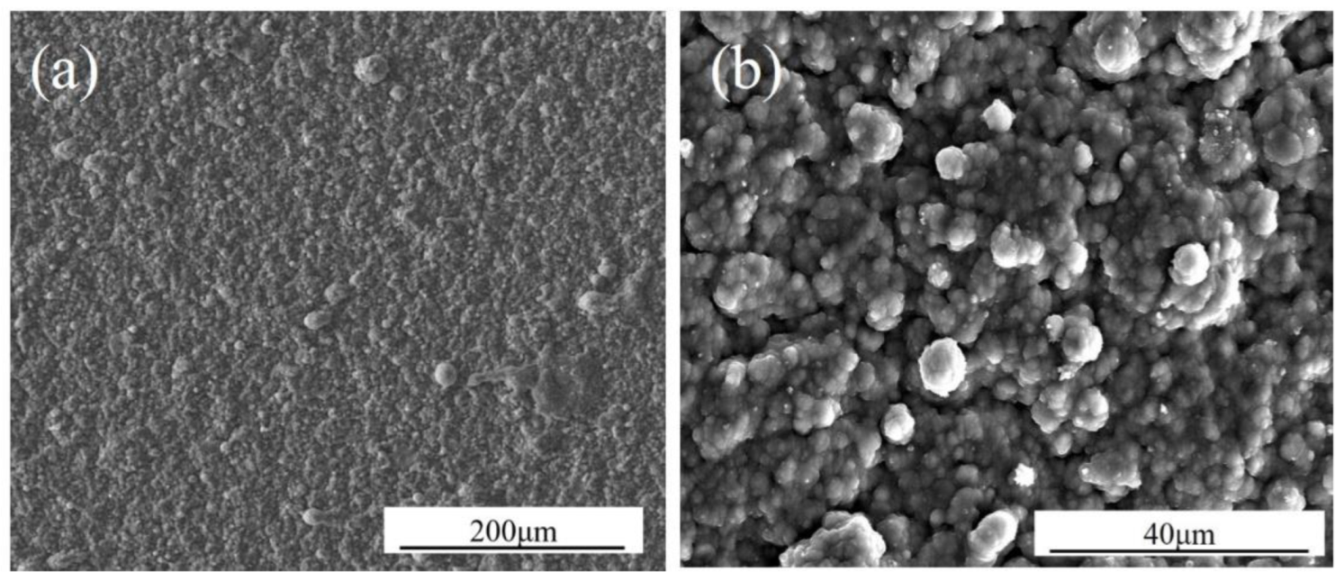

| Ni | Co | Cr | Al | Ta | Mo | W | Re | Hf | C | B | Y | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N5 | Bal. | 7.5 | 7 | 6.2 | 6.5 | 1.5 | 5 | 3 | 0.15 | 0.05 | 0.004 | 0.01 |
| NiCrAlY | Bal. | 27 | 11 | 0.5 |
| Ni | Co | Cr | Al | Ta | Mo | W | Re | O | Y | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 16.75 | 4.62 | 47.21 | 20.19 | 0.86 | 1.35 | 2.92 | 4.10 | 0 | 0 |
| 2 | 52.01 | 6.56 | 6.67 | 14.37 | 16.54 | 0.71 | 3.49 | 0 | 0 | 0 |
| 3 | 34.62 | 7.12 | 8.45 | 4.12 | 3.05 | 6.98 | 14.96 | 20.7 | 0 | 0 |
| 4 | 1.12 | 1.05 | 0.98 | 2.04 | 0 | 0 | 0 | 0 | 47.22 | 48.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.; Zhou, Z.; Yang, L.; Qiao, Y. The Oxidation Properties of a NiCrAlY Coating Fabricated by Arc Ion Plating. Coatings 2023, 13, 22. https://doi.org/10.3390/coatings13010022
Huang L, Zhou Z, Yang L, Qiao Y. The Oxidation Properties of a NiCrAlY Coating Fabricated by Arc Ion Plating. Coatings. 2023; 13(1):22. https://doi.org/10.3390/coatings13010022
Chicago/Turabian StyleHuang, Lei, Zhaohui Zhou, Lanlan Yang, and Yanxin Qiao. 2023. "The Oxidation Properties of a NiCrAlY Coating Fabricated by Arc Ion Plating" Coatings 13, no. 1: 22. https://doi.org/10.3390/coatings13010022
APA StyleHuang, L., Zhou, Z., Yang, L., & Qiao, Y. (2023). The Oxidation Properties of a NiCrAlY Coating Fabricated by Arc Ion Plating. Coatings, 13(1), 22. https://doi.org/10.3390/coatings13010022










