Abstract
Oral instant membranes can be quickly wetted by the patient’s saliva and dissolved/disintegrated in the mouth without the need for drinking water and chewing, exhibiting great promise for patients from children to the elderly who have difficulties with swallowing. However, the reported instant oral membranes can load and release only one single drug, which greatly hinders their potential applications. Herein, we employ a sequential electrospinning approach to fabricate dual drug-loaded bilayered gelatin oral instant membranes. The results indicate that a gelatin membrane with a uniform nanofibrous structure can be successfully prepared, and that both the hydrophilic model drug and hydrophobic model drug can be embedded into the gelatin nanofibers. X-ray diffraction results verify that the two drugs are well distributed in the nanofibrous matrix in an amorphous state. Owing to the excellent water solubility and large surface area of gelatin nanofibers, the hydrophilic model drug can be quickly dissolved in 101 s, while the hydrophobic model drug can be completely released in 100 s. The bilayered gelatin nanofibrous membrane shows promise for simultaneous loading and release of two drugs for fast-dissolving delivery applications.
1. Introduction
Recently, oral instant membranes have gained great attention in the pharmaceutical industry due to their convenience, fast drug release, high bioavailability, and no risk of asphyxia [1,2,3,4]. Unlike conventional solid drug formulations, oral instant membranes are a kind of thin and flexible dosage form with one or multiple drugs in it, which can be quickly wetted by patient’s saliva and further dissolved/disintegrated in the mouth without the need for drinking water and chewing [2,5,6,7,8,9]. Their unique fast-dissolving performance makes them very promising for patients with swallowing difficulties, such as children and the elderly [10,11,12,13,14]. Generally, patients who have physical problems with swallowing may need to take dual drugs or even multiple drugs to treat different diseases [15,16,17,18,19]. Therefore, the design and preparation of multiple drug-loaded oral instant membranes is highly desirable.
To date, various approaches have been reported to fabricate oral instant membranes, including solvent casting, hot melt extrusion, and electrospinning. Among these, solvent casting is the most convenient methods, as oral instant membranes can be quickly prepared after pouring and molding the drug/polymer mixed solution into a customized mould [20,21,22]. Rashid et al. used this method to fabricate an orally disintegrating membrane containing ranitidine hydrochloride (RHCl). The membrane could be disintegrated within 15 s and release 81% of RHCl in 120 s [23]. Unfortunately, it possesses the disadvantage of brittleness in the resulted membrane. In addition, only one drug can be encapsulated into the membrane. Hot melt extrusion is another frequently used approach [24]. Pimparade et al. produced an oral anti-allergic hot-melt extruded instant membrane. This membrane was disintegrated in 6–10 s, and the dissolution process was completed in 300 s [25]. However, this technique is only suited for encapsulation of thermostable drugs, and only one drug can be loaded during the preparation process. Compared with the above-mentioned two methods, oral instant membranes prepared by the novel electrospinning approach have many unique characteristics, including large surface area, flexibility, and high drug encapsulation amount [26,27,28]. Qin et al. reported that aspirin was encapsulated within the chitosan/pullulan nanofibers by electrospinning, and the membrane was completely dissolved into water within 60 s [29]. Similarly, Németh and coworkers presented an electrospun polyaspartamide fast-dissolving membrane incorporating with vitamin B12, finding that vitamin B12 was dissolved within 60 s [30]. Ponrasu et al. reported that novel jelly fig/pullulan nanofibers were rapidly soluble in water within 60 s [31]. Abbaspour’s group prepared a polyvinylpyrrolidone instant membrane to load valsartan, and found that 90% of the drug was released within 120 s [32]. From the reported publications, it is apparent that the conventional electrospinning method only results in fast-dissolving membranes for loading and releasing only a single drug, which cannot meet the requirements of patients with swallowing difficulties. Thus, it is important to develop a novel method with the capacity to load and release two different drugs from oral instant membranes.
In this study, we propose a novel sequential electrospinning approach to fabricate a water-soluble bilayered gelatin membrane with the capacity to be simultaneously loaded with hydrophilic and hydrophobic drugs. The reason for choosing gelatin as the skeletal polymer is that gelatin is a water-soluble biopolymer made from the degradation of collagen in acidic or basic conditions. In addition, gelatin is rich in beneficial amino acids, especially glycine and proline, which can promote wound healing and prevent wrinkles. Previous studies have shown that gelatin can accelerate the elasticity of connective tissue and increase cartilage density, and can additionally help to repair intestinal wall damage and rebuild the protective mucosa of the intestine. The glycine in gelatin has unique anti-inflammatory effect [33,34,35,36,37]. To verify this hypothesis, we first fabricated gelatin nanofibrous membranes by electrospinning and investigated the water-induced dissolution process of the pristine gelatin nanofibrous membranes. Then, a sequential electrospinning approach was employed to fabricate dual (hydrophilic/hydrophobic) drug-loaded bilayered electrospun membranes and the corresponding drug release profiles were carefully measured. To the best of our knowledge, this is the first report of a fast-dissolving dual drug-loaded membrane using the sequential electrospinning technique.
2. Materials and Methods
2.1. Materials
Gelatin (Gel) was purchased from the Rousselot Company (Sonora, TX, USA). Trifluoroethanol (TFEA) was supplied by Shanghai Aladdin Biochemical Technology Co. Ltd. (Shanghai, China). Rhodamine B (Rhb) was obtained from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China). Fluorescein (Flu) was provided by Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China). All reagents were of analytical grade and were used without further purification.
2.2. Preparation of Pristine Gelatin Nanofibrous Membrane, Single Drug-Loaded Gelatin Nanofibrous Membrane, and Dual Drug-Loaded Gelatin Nanofibrous Membrane
Gel (0.5000 g) was dissolved in 4 mL of TFEA with stirring for 6 h. The gelatin solution was poured into a 5 mL medical glass syringe. Gelatin nanofibrous membrane was prepared using a home-made electrospinning setup containing a high voltage DC power supply (DW-P303-1ACF0, Dongwen High Voltage Power Supply Co. Ltd., Tianjin, China), a microinjection pump (LSP01-1A, Baoding Lange Constant Current Pump Co. Ltd., Baoding, China), and a collecting plate. The spinning voltage was controlled at 4–8 kV. The flow velocity of the gelatin solution was fixed at 1.2–1.5 mL/h. The distance from the needle to the collecting plate was 12–15 cm. The obtained gelatin membrane was dried in an oven at 37 °C for 24 h before use.
For the preparation of hydrophilic drug-loaded gelatin nanofibrous membrane, 0.0500 g Rhb powder was dissolved into the gelatin solution with stirring for 2 h. For the preparation of hydrophobic drug-loaded gelatin nanofibrous membrane, 0.0125 g Flu powder was dissolved into the gelatin solution with stirring for 2 h. The detailed processes were the same as before. In order to fabricate dual drug-loaded gelatin nanofibrous membrane, 1500 μL of Gel/Rhb solution was first electrospun and deposited onto the conductive collector. Then, 1500 μL of Gel/Flu solution was processed into nanofibers and stacked above the first layer. The bilayered membrane was dried in the oven at 37 °C for 24 h.
2.3. Characterization
The morphology of the nanofibrous membrane was observed by a fluorescent microscope and a field emission scanning electron microscope (SEM) (S4800, Hitachi, Tokyo, Japan). Water contact angle (WCA) images of the gelatin membrane were taken by a static contact angle tester (DSA25, Kruss, Hamburg, Germany). A 2 μL water droplet was dripped on the surface of the gelatin membrane and the change in WCA was recorded. Functional groups in the samples (three kinds of nanofibrous membranes, drug powder) were obtained using an FTIR spectrometer (TENSOR 27, Bruker, Karlsruhe, Germany). The X-ray diffraction (XRD) patterns of the samples (three kinds of nanofibrous membranes, drug powder) were recorded using an X-ray diffractometer (D8 ADVAHCL*, Bruker, Karlsruhe, Germany) with Cu Kα radiation. The thickness of the membrane was measured using a thickness gauge.
2.4. Fast-Dissolving Performance of the Drug-Loaded Electrospun Gelatin Membrane
In order to investigate the in vitro fast-dissolving performance of the electrospun gelatin membrane, a small piece of the membrane was placed into water. The whole dissolution process was monitored by a camera, and the dissolution time was recorded. To study the dual drug release profiles of the gelatin nanofibrous membrane, the absorption spectra of both Rhb and Flu in the solution were taken using a UV-Vis spectrophotometer (Jinghua Instruments, Shanghai, China). Experiments were run in triplicate per sample.
3. Results and Discussion
3.1. Characterization and Fast-Dissolving Performance of the Gelatin Nanofibrous Membrane
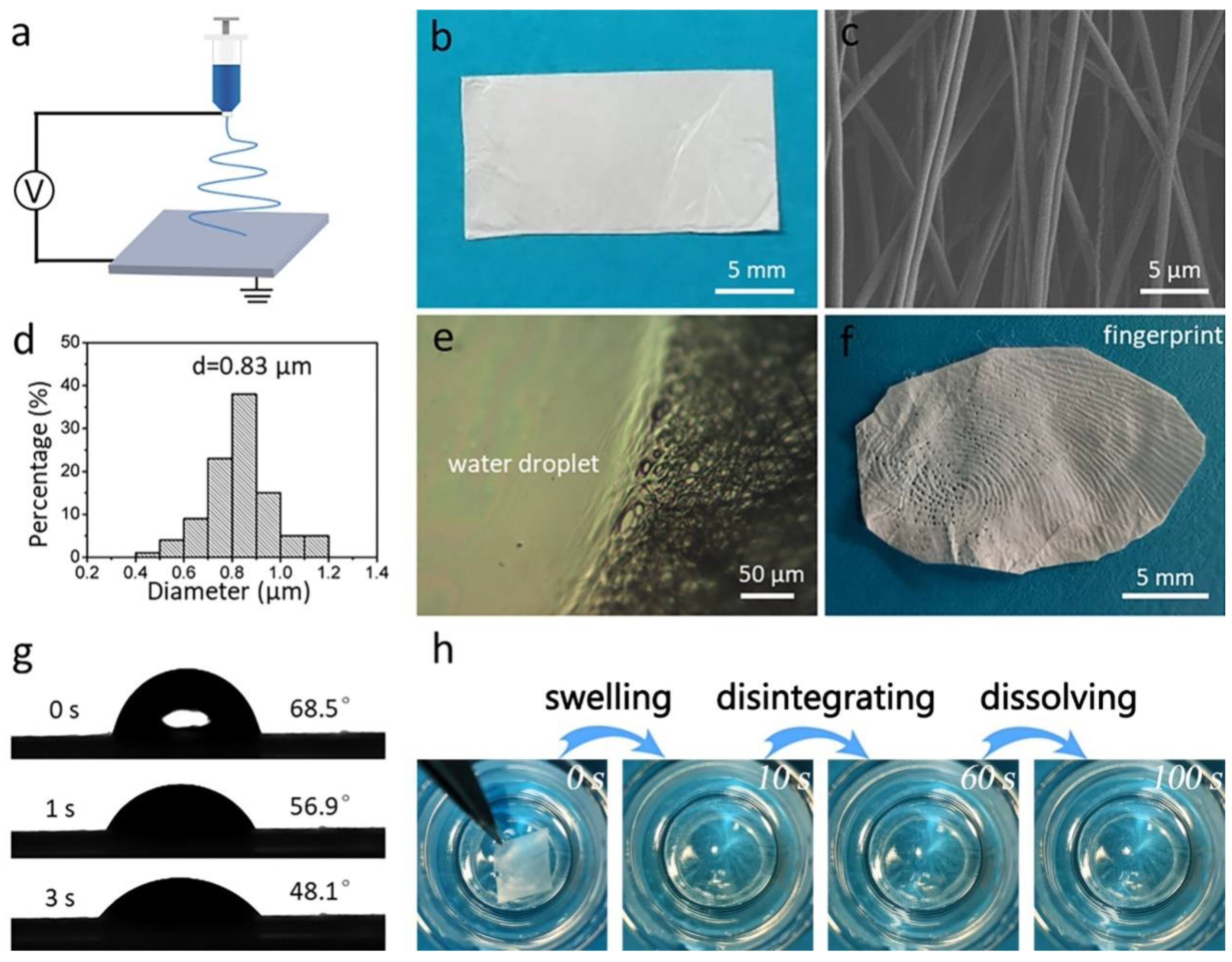
Figure 1a shows an illustration of the electrospinning fabrication process of the pristine gelatin membrane. The membrane has white appearance and a thickness of 46 μm (Figure 1b). The SEM image in Figure 1c shows that the membrane contains many bead-free nanofibers with an average diameter of 0.83 μm (Figure 1d). Further, to investigate the solubility of gelatin nanofibrous membrane, a drop of water was dropped onto the membrane. As shown in Figure 1e, where the membrane contacted the water droplet, it completely lost its fibrous morphology. We found that a clear fingerprint could be observed after pressing onto the gelatin membrane for 2 s, which was due to the sweat secreted from the fingertip (Figure 1f) [38]. The wettability of the membrane was measured as 48.1°, indicating the strong water affinity of the gelatin membrane (Figure 1g). Finally, we placed the membrane into water, and found that the membrane first changed its color from white to transparent after contacting the water, then disappeared (Figure 1h). The above results indicate the gelatin membrane’s fast-dissolving performance.
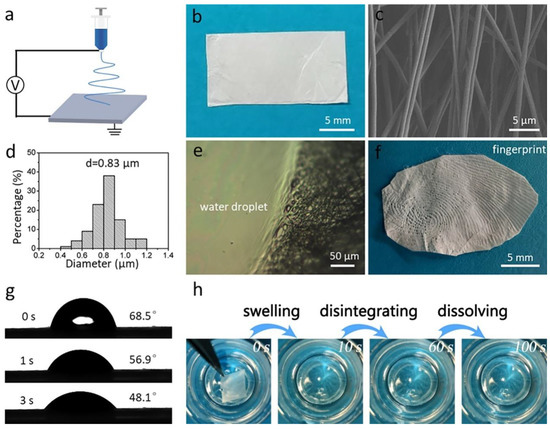
Figure 1.
(a) Schematic illustration of the fabrication process of the electrospun nanofibrous gelatin membrane. (b) Optical image of the white gelatin membrane. (c) SEM image and (d) diameter distribution of the gelatin membrane. (e) Appearance of the membrane after contact with a water droplet. (f) A fingerprint that formed on the gelatin membrane. (g) WCA images of the gelatin membrane. (h) The fast dissolution process of the gelatin membrane.
In this study, two strategies were implemented to ensure the complete removal of TFEA. As was noted during the electrospinning process, nanofiber formation was accompanied by solvent evaporation. The nanofiber was ejected from the spinning nozzle to the collector at a very fast rate, and the fast movement of nanofiber favored the quick evaporation of TFEA. In addition, during the electrospinning process the nanofibers became thinner and thinner due to electrical repulsion. More solvent molecules could be diffused from the interior of the nanofiber to the surface of the nanofiber, benefiting solvent evaporation. Moreover, in order to ensure the complete removal of the TFEA solvent, we placed the nanofiber membrane into the oven at 37 °C for 24 h, which is a relatively long period. As the nanofiber membrane is highly porous and the nanofiber possesses a large specific surface area, it is possible to completely remove the residual solvent in the nanofiber.
3.2. Characterization and Fast-Dissolving Performance of the Single Drug-Loaded Gelatin Nanofibrous Membrane
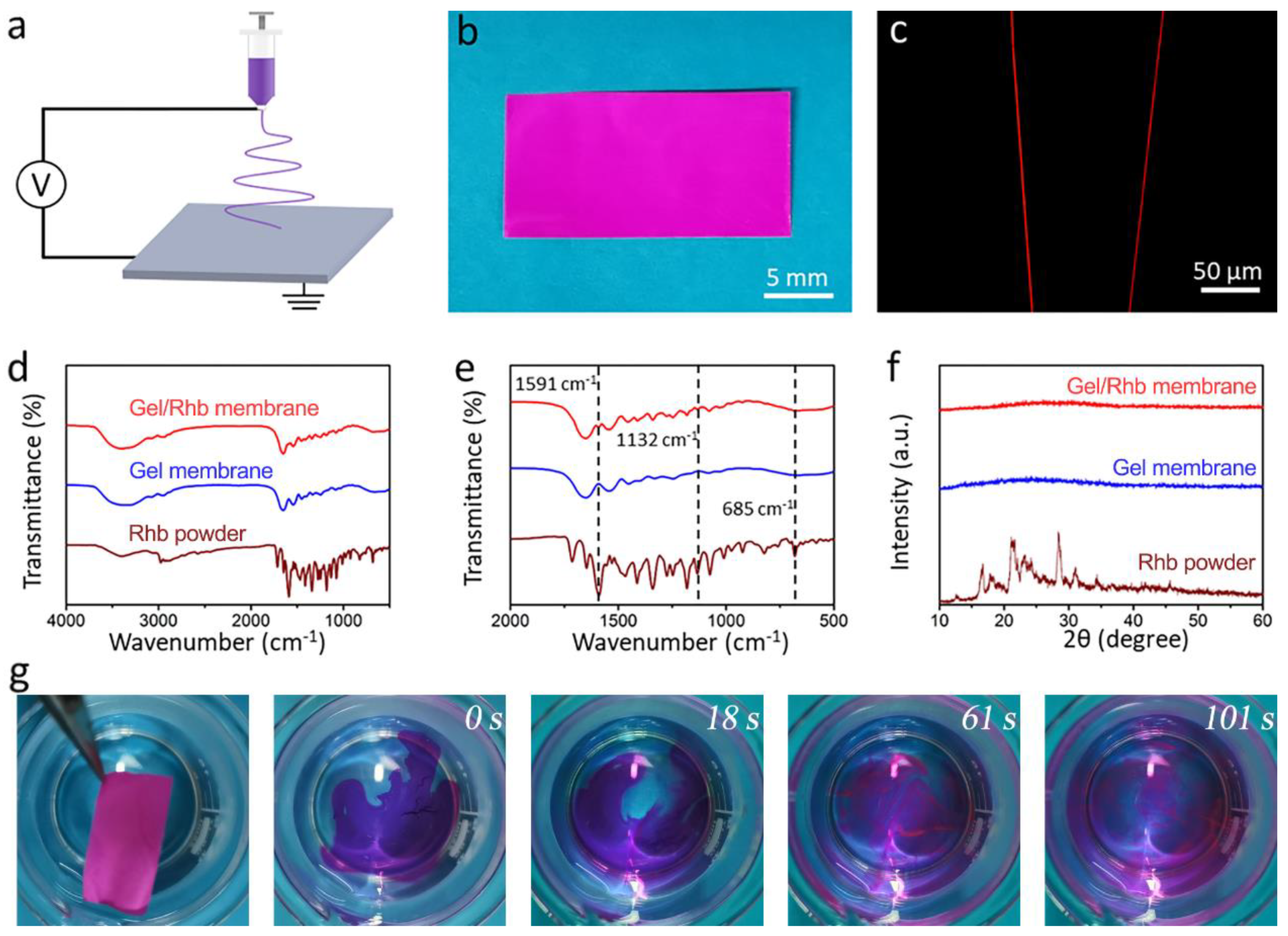
Rhb was used as a hydrophilic model drug, and the mixed Gel/Rhb solution was electrospun into nanofibrous structure by electrospinning (Figure 2a). The resultant nanofibrous membrane with a thickness of 44 μm showed a purplish-red color due to the incorporation of the red Rhb dye (Figure 2b). A fluorescence microscope was employed to test the distribution of the model drug within the nanofibers, and it could be seen that the red color was uniform within the whole nanofiber, indicating a uniform drug encapsulation of the hydrophilic model drug in the fiber (Figure 2c). We further detected the FTIR spectra of the three kinds of samples (Rhb powder, Gel membrane, and Gel/Rhb membrane) (Figure 2d,e). By comparing the FTIR spectra of different samples, characteristic peaks at 1591, 1132, and 685 cm−1 in the FTIR of the Gel/Rhb membrane were ascribed to Rhb, indicating that Rhb was successfully incorporated into the gelatin membrane. XRD studies were carried out to determine the physical state of Rhb in the gelatin nanofibers. From the results, it was found that the XRD pattern of the Rhb powder showed numerous distinct reflections, representing the crystalline areas of Rhb, while after electrospinning, the Rhb in the Gel membrane exhibited an amorphous state (Figure 2f). Compared with the crystalline state, the drug-loaded Rhb membrane in the amorphous state may be dissolved more quickly. In order to prove this, we further placed the red Gel/Rhb membrane into water, and found that the membrane could be completely dissolved and the model drug Rhb was fully released within 101 s (Figure 2g). We then measured the cumulative release profile of Rhb from the composite membrane; the results indicated the fast and complete release of the hydrophilic model drug Rhb (Figure S1).
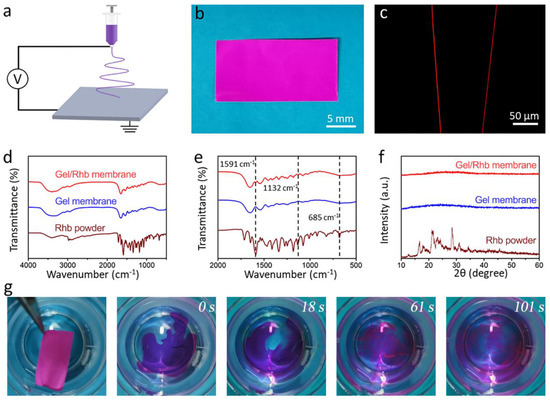
Figure 2.
(a) Schematic illustration of the process of fabricating of Gel/Rhb membrane by electrspinning. (b) Optical image exhibiting the red appearance of the Gel/Rhb membrane. (c) Image of the Gel/Rhb nanofibers from fluorescence microscopy. (d) FTIR spectra and (e) partial enlarged FTIR spectra. (f) XRD patterns of Rhb powder, pristine Gel membrane, and Gel/Rhb membrane. (g) Fast dissolution process of the Gel/Rhb membrane.
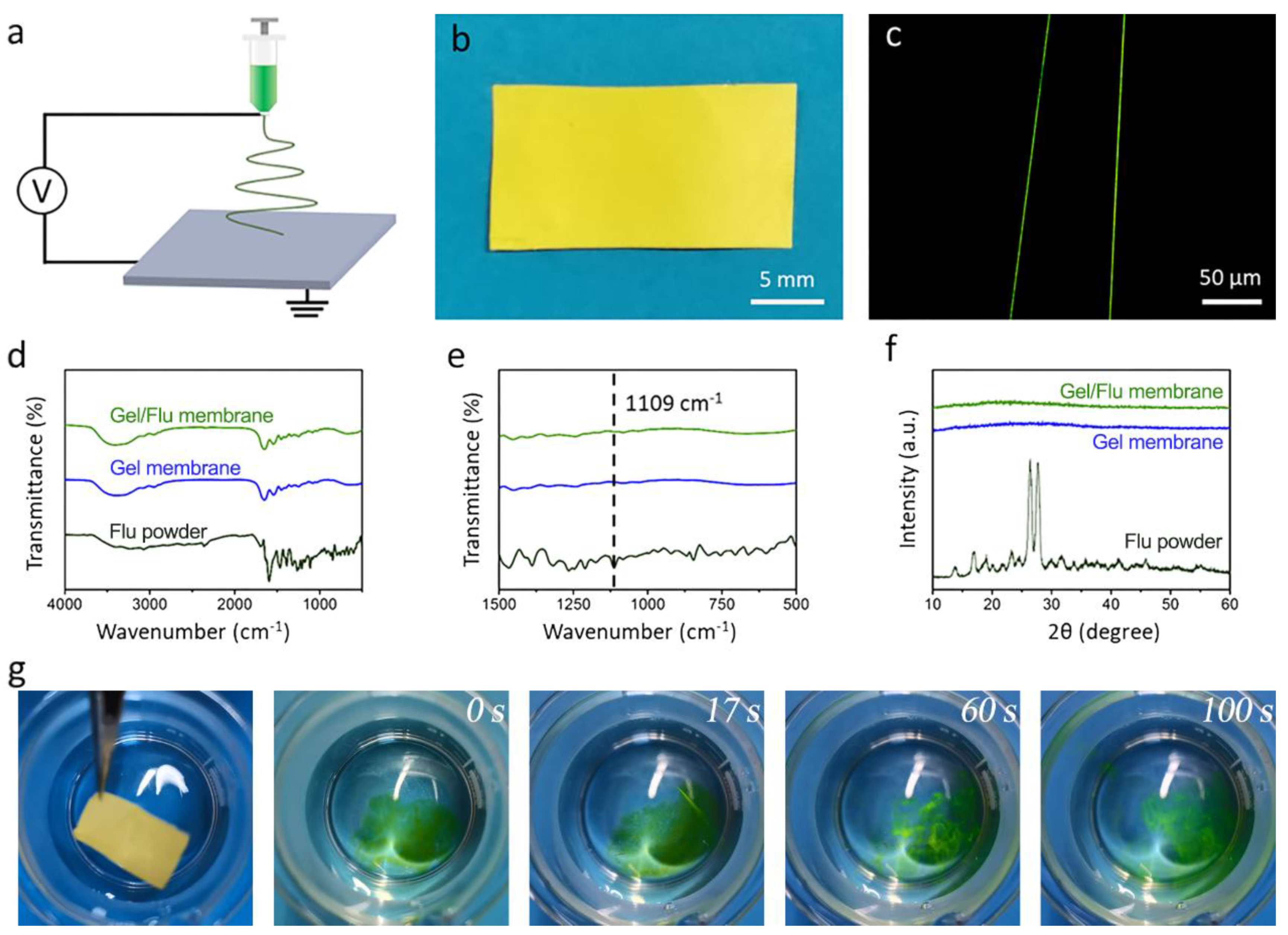
We then investigated the capacity of the gelatin nanofibrous membrane in terms of loading and fast release of a hydrophobic drug. Flu was chosen as a hydrophobic model drug, and the mixed Gel/Flu solution was manufactured into the nanofibrous membrane by one-step electrospinning (Figure 3a). As shown in Figure 3b, the membrane with a thickness of 45 μm showed a yellow appearance due to the incorporation of the dye Flu. Employing the fluorescent microscope, uniform green fibers could be observed, indicating the homogenous encapsulation of the model drug Flu (Figure 3c). The FTIR spectra of the Flu powder, Gel membrane, and Gel/Flu membrane (Figure 3d,e) were studied. In the Flu FTIR spectra, the C–H bending vibration of the benzene ring at 1109 cm−1 can be clearly observed. In contrast, in the FTIR spectra of the Gel/Flu nanofibrous membrane, the characteristic peaks at 1109 cm−1 imply the successful encapsulation of Flu in the gelatin membrane. The XRD patterns of the Flu powder, Gel membrane, and Gel/Flu membrane were further investigated (Figure 3f). It can be seen that Flu powder shows a typical crystalline state with many sharp peaks, while after incorporation into the gelatin membrane it transforms into an amorphous state. We finally investigated the fast-dissolving performance of the Gel/Flu nanofibrous membrane (Figure 3g); we found that after placing the membrane into water, the green dye Flu was immediately released and the membrane was completely dissolved in 100 s. The cumulative release profile of Flu from the composite membrane proved this (Figure S2). These results indicate that both hydrophilic and hydrophobic drugs can be loaded within the gelatin nanofibrous membrane, then the drugs can be quickly released from the membrane thanks to the fast-dissolving performance of the gelatin membrane along with the amorphous state of the drugs.
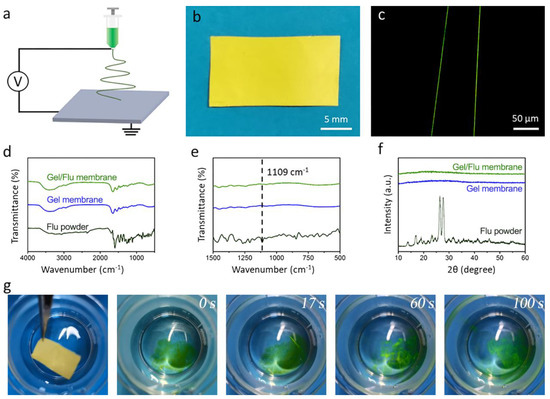
Figure 3.
(a) Schematic illustration of the electrospinning equipment. (b) Optical image exhibiting the prepared sample of the Gel/Flu membrane. (c) Image of the Gel/Flu nanofibers from the flurescent microscope. (d) FTIR spectra and (e) Partial enlarged FTIR spectra. (f) XRD images of the Flu powder, pristine Gel membrane, and Gel/Flu membrane. (g) Fast dissolution process of the Gel/Flu membrane.
3.3. Fabrication of Dual Drug-Loaded Bilayered Gelatin Nanofibrous Membrane and Its Fast-Dissolving Performance
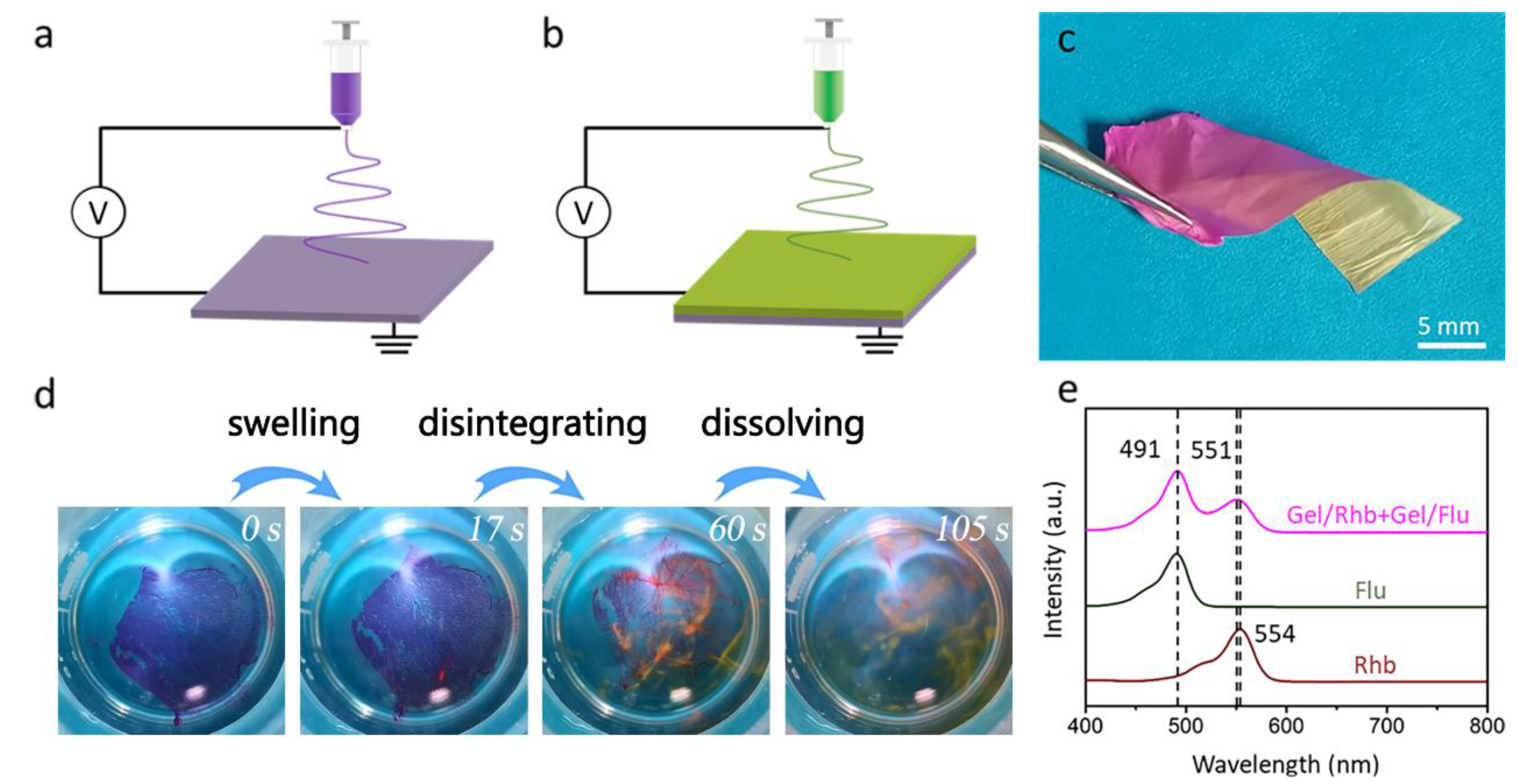
In order to load two different drugs in the nanofibrous membrane, we employed a novel sequential electrospinning technique to fabricate dual drug-loaded bilayered gelatin nanofibrous membranes. As shown in Figure 4a–c, the red Gel/Rhb nanofibrous membrane was first deposited on the collector by electrospinning. Then, the yellow Gel/Flu nanofibrous membrane was further stacked above the first layer to form a bilayered structure. The thickness of the bilayered membrane was measured as 88 μm. The bilayered membrane was incubated into water, and it was found that the membrane was first swollen, then disintegrated, and finally quickly dissolved in 105 s; meanwhile, the two model drugs were gradually released (Figure 4d). We employed a UV-Vis spectrophotometer to detect the two released drugs. As shown in Figure 4e, the Flu aqueous solution showed an adsorption peak at 491 nm, while the Rhb aqueous solution showed a peak at 554 nm. We then detected the spectra of the solution after dissolving the dual drug-loaded nanofibrous membrane, and two peaks at 491 and 551 nm were found, again attributed to Flu and Rhb, respectively. The above results indicate that the dual drug-loaded bilayered gelatin nanofibrous membrane was able to simultaneously load and release two different drugs.
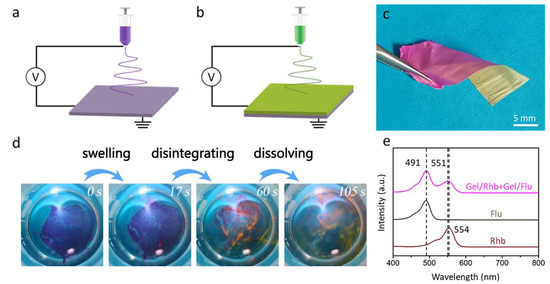
Figure 4.
(a,b) Schematic illustration of the sequential electrospinning approach used to fabricate the dual drug-loaded bilayered gelatin nanofibrous membrane. (c) Digital image of the obtained dual drug-loaded nanofibrous membrane. (d) Fast-dissolving process of the dual drug-loaded bilayered gelatin nanofibrous membrane. (e) UV-Vis absorption spectra of Rhb solution, Flu solution, and the solution after dissolving the dual drug-loaded nanofibrous membrane.
Oral instant membranes are a unique kind of drug delivery system for patients with swallowing difficulties, with the benefits of high bioavailability, good patient compliance, and rapid drug release [39,40,41]. However, the reported oral instant membranes are only able to load and release one drug, which greatly hinders their application [42,43]. Previous studies have shown that combination therapy using dual-drug loaded nanofibrous membranes can enhance therapeutic efficiency [44]. In this study, we employed a two-step electrospinning technique to fabricate dual drug-loaded bilayered nanofibrous membranes. Unlike from the conventional one-step electrospinning technique, our sequential electrospinning technique can be used to construct bilayered or multiple-layered structures, each layer loaded with different drugs. In order to achieve fast-dissolving performance, we chose gelatin as the skeleton polymer to improve the dissolution velocity of the membrane. Gelatin has been proven to be biocompatible, and its amino acid degradation product is beneficial to the human body [45]. After being manufactured into the nanofibrous structure via electrospinning, the large surface area and big pore size of the membrane contribute to fast dissolution [46]. We observed that both a hydrophilic drug (Rhb) and a hydrophobic drug (Flu) were converted to an amorphous state after electrospinning, which might be due to the very fast drying process [47]. The two drugs in amorphous state in the nanofibrous membrane could be released faster than in their respective crystalline states [48]. Overall, owing to the unique sequential electrospinning technique, good hydrophilicity of gelatin, and the amorphous state of the two drugs, the dual drug-loaded nanofibrous membrane showed fast oral dissolving performance.
As a new formation, the dual-loaded nanofibrous membrane was suitable for children and elderly patients with dysphagia. Such a membrane has many unique advantages. Drug combination therapy by dual-drug loaded oral instant membranes promise enhance efficacy compared to mono-therapy approaches. In addition, two drugs loaded into different layers could avoid interference during the preparation process, potentially maximizing the bioactivity retention of the two drugs. The doses of each drug could easily be tuned according to the needs of individual patients. In addition to the combination of hydrophilic and hydrophobic drugs, two hydrophilic drugs or two hydrophobic drugs could be encapsulated into the membrane as well, depending on the patient. Thus, this study provides a paradigm for the fabrication of dual drug-loaded oral instant membranes, which show great potential for patients with dysphagia.
4. Conclusions
In this paper, we describe an electrospinning approach for fabricating gelatin nanofibrous membranes. Thanks to the good hydrophilicity of gelatin along with its nanofibrous structure, pristine gelatin membranes could be quickly dissolved into water in 100 s. Two kinds of drugs with different water solubility were incorporated into the gelatin membrane to form a bilayered structure; and the results indicated that both the hydrophilic drug and hydrophobic drug were homogenously embedded within the gelatin nanofibers. In vitro dissolution results revealed that the bilayered membrane was quickly dissolved into water, and the two drugs could be completely released. This study provides an example of the design and fabrication of a new type of bilayered nanofibrous membrane as a fast-disintegrating dual drug delivery system; such dual drug-loaded oral instant membranes with high patient compliance may find tremendous applications in modern medicine.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/coatings13010023/s1. Figure S1: Cumulative release of Rhb from the composite membrane; Figure S2: Cumulative release of Flu from the composite membrane.
Author Contributions
S.M. (Shan Miao) and Z.C.: methodology. J.W., S.M. (Shan Miao) and Z.C.: investigation and writing (original draft preparation). X.S. and S.M. (Shan Miao): resources. S.M. (Shanbo Ma), L.L. and Y.C.: writing (review and editing). M.Z., F.W. and J.W.: visualization. X.G. and B.S.: conceptualization and writing (review and editing). B.S. and X.S.: conceptualization, supervision, writing (review and editing), and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Medical Promotion Program of Air Force Medical University (No.: 2020JSTS09).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting the findings of this manuscript are available from the corresponding authors upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lai, K.L.; Fang, Y.; Han, H.; Li, Q.; Zhang, S.; Li, H.Y.; Chow, S.F.; Lam, T.N.; Lee, W.Y.T. Orally-dissolving film for sublingual and buccal delivery of ropinirole. Colloids Surf. B Biointerfaces 2018, 163, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Senel, S.; Comoglu, T. Orally disintegrating tablets, fast-dissolving, buccal and sublingual formulations. Pharm. Dev. Technol. 2018, 23, 431. [Google Scholar] [CrossRef] [PubMed]
- Briatico-Vangosa, F.; Melocchi, A.; Uboldi, M.; Gazzaniga, A.; Zema, L.; Maroni, A. Effect of Polyethylene Glycol Content and Molar Mass on Injection Molding of Hydroxypropyl Methylcellulose Acetate Succinate-Based Gastroresistant Capsular Devices for Oral Drug Delivery. Polymers 2019, 11, 517. [Google Scholar] [CrossRef] [PubMed]
- Morath, B.; Sauer, S.; Zaradzki, M.; Wagner, A.H. Orodispersible films—Recent developments and new applications in drug delivery and therapy. Biochem. Pharmacol. 2022, 200, 115036. [Google Scholar] [CrossRef] [PubMed]
- Irfan, M.; Rabel, S.; Bukhtar, Q.; Qadir, M.I.; Jabeen, F.; Khan, A. Orally disintegrating films: A modern expansion in drug delivery system. Saudi Pharm. J. 2016, 24, 537–546. [Google Scholar] [CrossRef]
- Shah, K.A.; Gao, B.; Kamal, R.; Razzaq, A.; Qi, S.; Zhu, Q.N.; Lina, S.; Huang, L.; Cremin, G.; Iqbal, H.; et al. Development and Characterizations of Pullulan and Maltodextrin-Based Oral Fast-Dissolving Films Employing a Box-Behnken Experimental Design. Materials 2022, 15, 3591. [Google Scholar] [CrossRef]
- Musazzi, U.M.; Khalid, G.M.; Selmin, F.; Minghetti, P.; Cilurzo, F. Trends in the production methods of orodispersible films. Int. J. Pharm. 2020, 576, 118963. [Google Scholar] [CrossRef]
- Olechno, K.; Basa, A.; Winnicka, K. “Success Depends on Your Backbone”—About the Use of Polymers as Essential Materials Forming Orodispersible Films. Materials 2021, 14, 4872. [Google Scholar] [CrossRef]
- Birer, M.; Acarturk, F. Electrospun orally disintegrating film formulation of telmisartan. Pharm. Dev. Technol. 2021, 26, 661–672. [Google Scholar] [CrossRef]
- Lau, E.T.L.; Steadman, K.J.; Cichero, J.A.Y.; Nissen, L.M. Dosage form modification and oral drug delivery in older people. Adv. Drug Deliv. Rev. 2018, 135, 75–84. [Google Scholar] [CrossRef]
- Cram, A.; Breitkreutz, J.; Desset-Brethes, S.; Nunn, T.; Tuleu, C.; European Paediatric Formulation Initiative. Challenges of developing palatable oral paediatric formulations. Int. J. Pharm. 2009, 365, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Mfoafo, K.A.; Omidian, M.; Bertol, C.D.; Omidi, Y.; Omidian, H. Neonatal and pediatric oral drug delivery: Hopes and hurdles. Int. J. Pharm. 2021, 597, 120296. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Zhu, L.; Yang, N.; Li, H.; Yang, Q. Recent advances of oral film as platform for drug delivery. Int. J. Pharm. 2021, 604, 120759. [Google Scholar] [CrossRef] [PubMed]
- Kadota, K.; Terada, H.; Fujimoto, A.; Nogami, S.; Uchiyama, H.; Tozuka, Y. Formulation and evaluation of bitter taste-masked orally disintegrating tablets of high memantine hydrochloride loaded granules coated with polymer via layering technique. Int. J. Pharm. 2021, 604, 120725. [Google Scholar] [CrossRef] [PubMed]
- Marquis, J.; Schneider, M.P.; Payot, V.; Cordonier, A.C.; Bugnon, O.; Hersberger, K.E.; Arnet, I. Swallowing difficulties with oral drugs among polypharmacy patients attending community pharmacies. Int. J. Clin. Pharm. 2013, 35, 1130–1136. [Google Scholar] [CrossRef]
- Howden, C.W. Management of acid-related disorders in patients with dysphagia. Am. J. Med. 2004, 117 (Suppl. S5A), 44S–48S. [Google Scholar] [CrossRef]
- Given, B.A.; Given, C.W.; Sikorskii, A.; Vachon, E.; Banik, A. Medication Burden of Treatment Using Oral Cancer Medications. Asia-Pac. J. Oncol. Nurs. 2017, 4, 275–282. [Google Scholar] [CrossRef]
- Perez-Jover, V.; Mira, J.J.; Carratala-Munuera, C.; Gil-Guillen, V.F.; Basora, J.; Lopez-Pineda, A.; Orozco-Beltran, D. Inappropriate Use of Medication by Elderly, Polymedicated, or Multipathological Patients with Chronic Diseases. Int. J. Environ. Res. Public Health 2018, 15, 310. [Google Scholar] [CrossRef]
- Owsiany, M.T.; Hawley, C.E.; Paik, J.M. Differential Diagnoses and Clinical Implications of Medication Nonadherence in Older Patients with Chronic Kidney Disease: A Review. Drugs Aging 2020, 37, 875–884. [Google Scholar] [CrossRef]
- Preis, M.; Pein, M.; Breitkreutz, J. Development of a taste-masked orodispersible film containing dimenhydrinate. Pharmaceutics 2012, 4, 551–562. [Google Scholar] [CrossRef]
- Visser, J.C.; Woerdenbag, H.J.; Crediet, S.; Gerrits, E.; Lesschen, M.A.; Hinrichs, W.L.J.; Breitkreutz, J.; Frijlink, H.W. Orodispersible films in individualized pharmacotherapy: The development of a formulation for pharmacy preparations. Int. J. Pharm. 2015, 478, 155–163. [Google Scholar] [CrossRef]
- Senta-Loys, Z.; Bourgeois, S.; Pailler-Mattei, C.; Agusti, G.; Briancon, S.; Fessi, H. Formulation of orodispersible films for paediatric therapy: Investigation of feasibility and stability for tetrabenazine as drug model. J. Pharm. Pharmacol. 2017, 69, 582–592. [Google Scholar] [CrossRef]
- Rashid, A.; Khan, I.U.; Khalid, S.H.; Asghar, S.; Munir, M.U. Development and evaluation of oral fast disintegrating film of ranitidine HCl by solvent casting method. Pak. J. Pharm. Sci. 2021, 34 (Suppl. S4), 1527–1534. [Google Scholar]
- Zheng, Y.; Pokorski, J.K. Hot melt extrusion: An emerging manufacturing method for slow and sustained protein delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1712. [Google Scholar] [CrossRef]
- Pimparade, M.B.; Vo, A.; Maurya, A.S.; Bae, J.; Morott, J.T.; Feng, X.; Kim, D.W.; Kulkarni, V.I.; Tiwari, R.; Vanaja, K.; et al. Development and evaluation of an oral fast disintegrating anti-allergic film using hot-melt extrusion technology. Eur. J. Pharm. Biopharm. 2017, 119, 81–90. [Google Scholar] [CrossRef]
- Mohammadreza, M.; Iraji, P.; Mahmoudi, Z.; Rahiman, N.; Akhgari, A. Design and physico-mechanical evaluation of fast-dissolving valsartan polymeric drug delivery system by electrospinning method. Iran. J. Basic Med. Sci. 2021, 24, 1683–1694. [Google Scholar]
- Sipos, E.; Szabo, Z.I.; Redai, E.; Szabo, P.; Sebe, I.; Zelko, R. Preparation and characterization of nanofibrous sheets for enhanced oral dissolution of nebivolol hydrochloride. J. Pharm. Biomed. Anal. 2016, 129, 224–228. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Electrospun formulation of acyclovir/cyclodextrin nanofibers for fast-dissolving antiviral drug delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111514. [Google Scholar] [CrossRef]
- Qin, Z.Y.; Jia, X.W.; Liu, Q.; Kong, B.H.; Wang, H. Fast dissolving oral films for drug delivery prepared from chitosan/pullulan electrospinning nanofibers. Int. J. Biol. Macromol. 2019, 137, 224–231. [Google Scholar] [CrossRef]
- Li, X.; Kanjwal, M.A.; Lin, L.; Chronakis, I.S. Electrospun polyvinyl-alcohol nanofibers as oral fast-dissolving delivery system of caffeine and riboflavin. Colloids Surf. B Biointerfaces 2013, 103, 182–188. [Google Scholar] [CrossRef]
- Ponrasu, T.; Chen, B.H.; Chou, T.H.; Wu, J.J.; Cheng, Y.S. Fast Dissolving Electrospun Nanofibers Fabricated from Jelly Fig Polysaccharide/Pullulan for Drug Delivery Applications. Polymers 2021, 13, 241. [Google Scholar] [CrossRef]
- Balusamy, B.; Celebioglu, A.; Senthamizhan, A.; Uyar, T. Progress in the design and development of “fast-dissolving” electrospun nanofibers based drug delivery systems—A systematic review. J. Control. Release 2020, 326, 482–509. [Google Scholar] [CrossRef]
- Djagny, V.B.; Wang, Z.; Xu, S. Gelatin: A valuable protein for food and pharmaceutical industries: Review. Crit. Rev. Food Sci. Nutr. 2001, 41, 481–492. [Google Scholar] [CrossRef]
- Ahmady, A.; Abu Samah, N.H. A review: Gelatine as a bioadhesive material for medical and pharmaceutical applications. Int. J. Pharm. 2021, 608, 121037. [Google Scholar] [CrossRef]
- Al-Nimry, S.; Dayah, A.A.; Hasan, I.; Daghmash, R. Cosmetic, Biomedical and Pharmaceutical Applications of Fish Gelatin/Hydrolysates. Mar. Drugs 2021, 19, 145. [Google Scholar] [CrossRef]
- Shiao, W.C.; Wu, T.C.; Kuo, C.H.; Tsai, Y.H.; Tsai, M.L.; Hong, Y.H.; Huang, C.Y. Physicochemical and Antioxidant Properties of Gelatin and Gelatin Hydrolysates Obtained from Extrusion-Pretreated Fish (Oreochromis sp.) Scales. Mar. Drugs 2021, 19, 275. [Google Scholar] [CrossRef]
- Ayaz, F.; Demir, D.; Bölgen, N. Differential anti-inflammatory properties of chitosan-based cryogel scaffolds depending on chitosan/gelatin ratio. Artif. Cells Nanomed. Biotechnol. 2021, 49, 682–690. [Google Scholar] [CrossRef]
- Mansour, L.S.; Tian, Y.; Yeo, B.T.T.; Cropley, V.; Zalesky, A. High-resolution connectomic fingerprints: Mapping neural identity and behavior. Neuroimage 2021, 229, 117695. [Google Scholar] [CrossRef]
- Shimoda, H.; Taniguchi, K.; Nishimura, M.; Matsuura, K.; Tsukioka, T.; Yamashita, H.; Inagaki, N.; Hirano, K.; Yamamoto, M.; Kinosada, Y.; et al. Preparation of a fast dissolving oral thin film containing dexamethasone: A possible application to antiemesis during cancer chemotherapy. Eur. J. Pharm. Biopharm. 2009, 73, 361–365. [Google Scholar] [CrossRef]
- Fahmy, R.H.; Badr-Eldin, S.M. Novel delivery approach for ketotifen fumarate: Dissofilms formulation using 3(2) experimental design: In vitro/in vivo evaluation. Pharm. Dev. Technol. 2014, 19, 521–530. [Google Scholar] [CrossRef]
- Liu, C.; Chang, D.; Zhang, X.; Sui, H.; Kong, Y.; Zhu, R.; Wang, W. Oral fast-dissolving films containing lutein nanocrystals for improved bioavailability: Formulation development, in vitro and in vivo evaluation. AAPS PharmSciTech 2017, 18, 2957–2964. [Google Scholar] [CrossRef]
- Deckers, C.L.; Genton, P.; Sills, G.J.; Schmidt, D. Current limitations of antiepileptic drug therapy: A conference review. Epilepsy. Res. 2003, 53, 1–17. [Google Scholar] [CrossRef]
- Song, B.; Wu, C.; Chang, J. Ultrasound-triggered dual-drug release from poly(lactic-co-glycolic acid)/mesoporous silica nanoparticles electrospun composite fibers. Regen. Biomater. 2015, 2, 229–237. [Google Scholar] [CrossRef]
- Guo, J.; Wang, T.; Yan, Z.; Ji, D.; Li, J.; Pan, H. Preparation and evaluation of dual drug-loaded nanofiber membranes based on coaxial electrostatic spinning technology. Int. J. Pharm. 2022, 629, 122410. [Google Scholar] [CrossRef]
- Ebhodaghe, S.O. A short review on chitosan and gelatin-based hydrogel composite polymers for wound healing. J. Biomater. Sci. Polym. Ed. 2022, 33, 1595–1622. [Google Scholar] [CrossRef]
- Zhang, Q.; Lin, Z.; Zhang, W.; Huang, T.; Jiang, J.; Ren, Y.; Zhang, R.; Li, W.; Zhang, X.; Tu, Q. Fabrication of green poly(vinyl alcohol) nanofibers using natural deep eutectic solvent for fast-dissolving drug delivery. RSC Adv. 2020, 11, 1012–1021. [Google Scholar] [CrossRef]
- Song, B.; Wu, C.; Chang, J. Controllable delivery of hydrophilic and hydrophobic drugs from electrospun poly(lactic-co-glycolic acid)/mesoporous silica nanoparticles composite mats. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 2178–2186. [Google Scholar] [CrossRef]
- Topuz, F.; Kilic, M.E.; Durgun, E.; Szekely, G. Fast-dissolving antibacterial nanofibers of cyclodextrin/antibiotic inclusion complexes for oral drug delivery. J. Colloid Interface Sci. 2021, 585, 184–194. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).