Application of a New Polymer Particle Adhesive for Lithium Battery Separators
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Polymer Particle Adhesive
2.3. Characterization of Physical and Chemical Properties of Materials
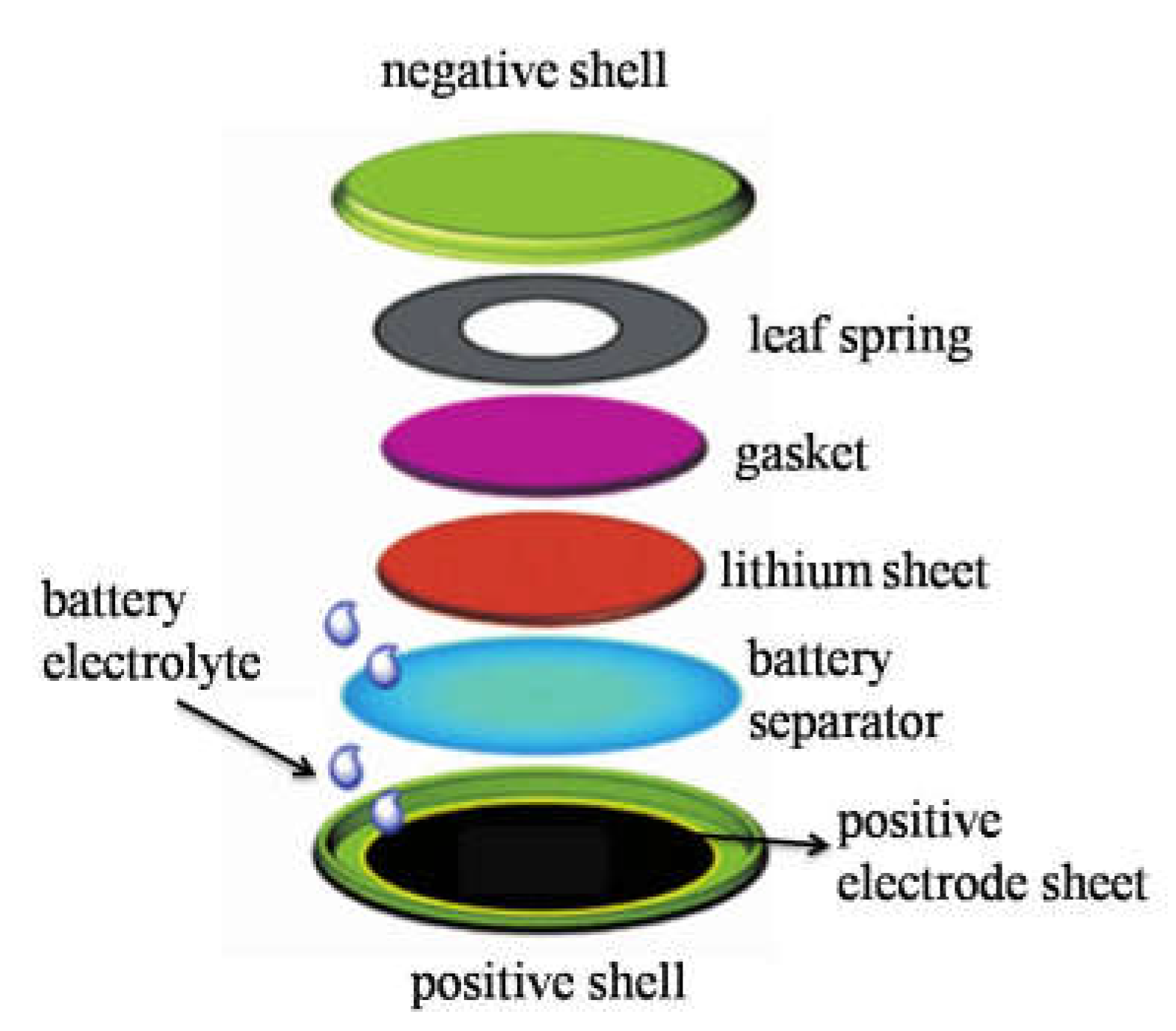
2.4. Button Battery Assembly
2.5. Characterization of Electrochemical Properties
- (a)
- Cyclic Voltammetry (CV)
- (b)
- Electrochemical Impedance Spectroscopy (EIS)
- (c)
- Charge and discharge performance test
3. Results and Discussion
3.1. The Particle Size of the Synthesized Polymer Particle Adhesive
3.2. The Glass Transition Temperature
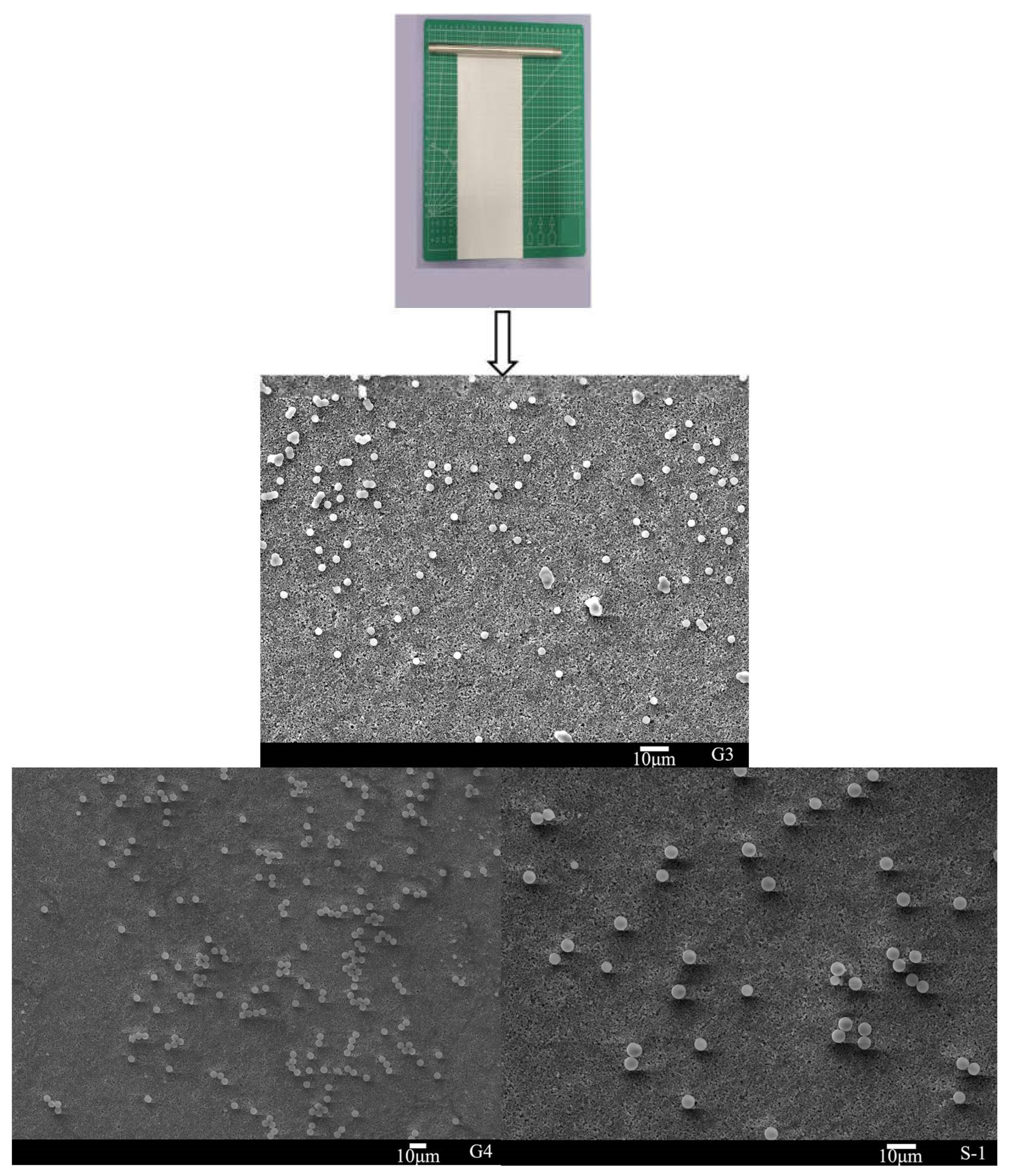
3.3. The Coating of Polymer Particle Adhesive
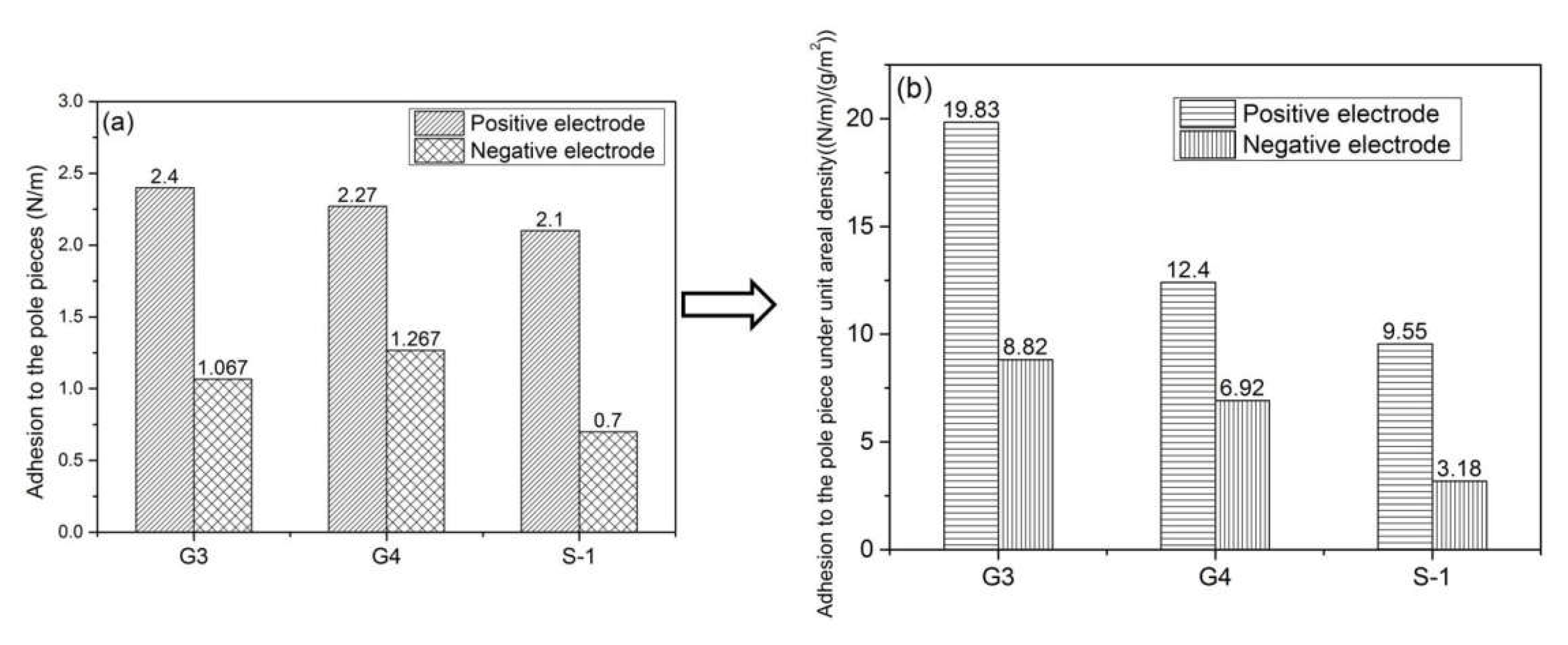
3.4. Adhesion Value between Battery Separator and Pole Piece
3.5. Electrochemical Performance of Assembled Lithium Battery
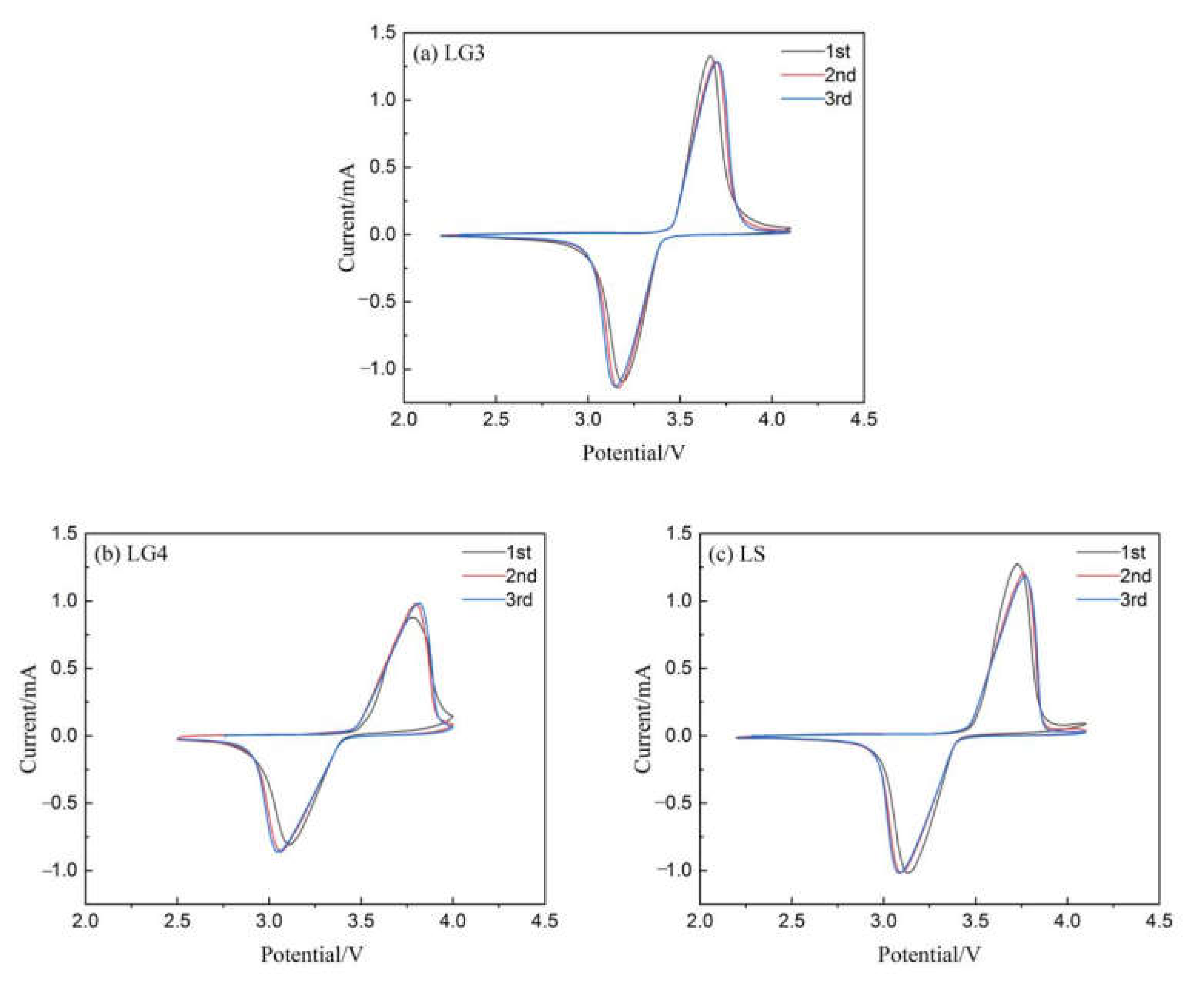
3.5.1. Cyclic Voltammetry Analysis
3.5.2. Electrochemical Impedance Spectroscopy
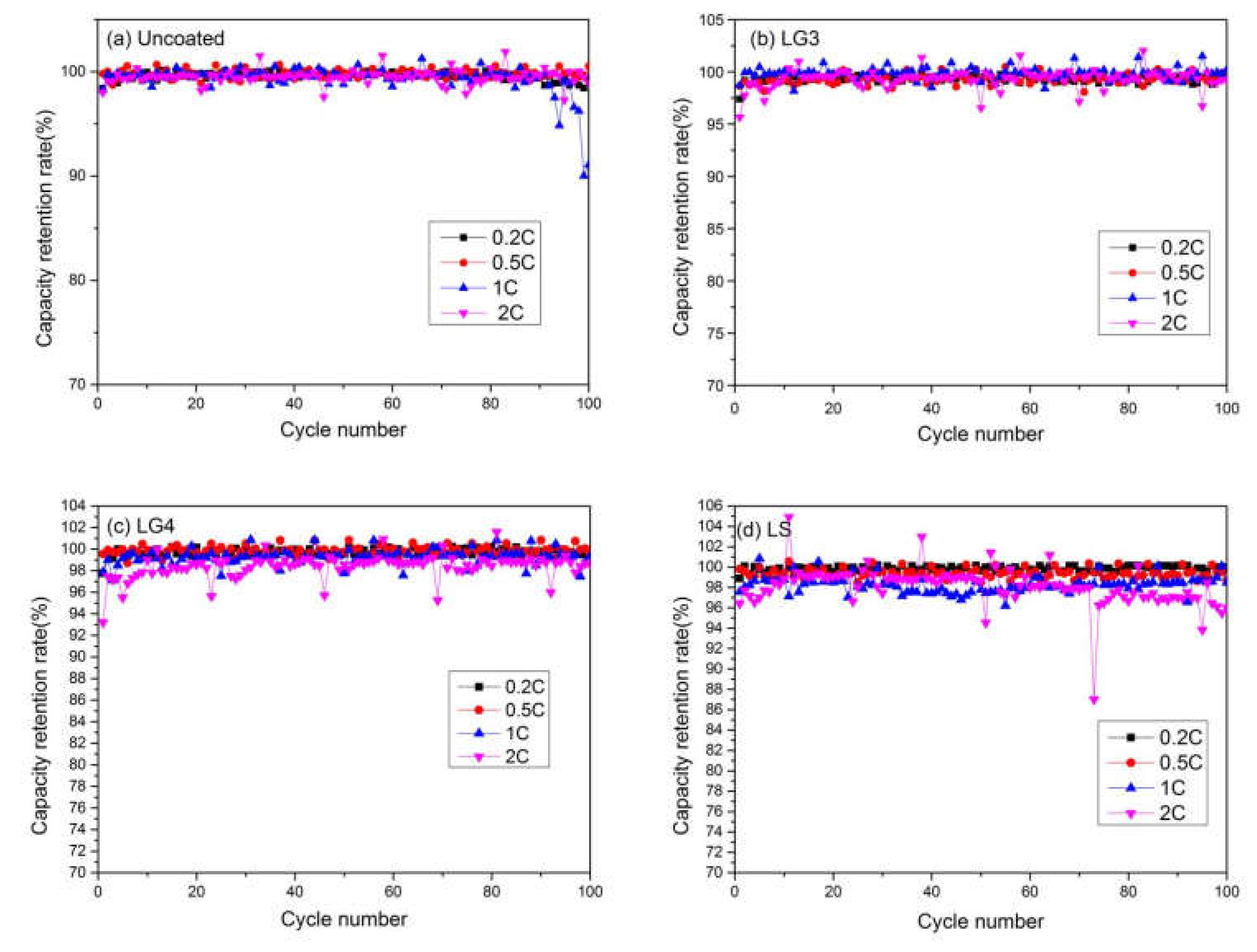
3.5.3. Charge and Discharge Performance Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Z.; Hao, H.; Cheng, X.; Zhao, F. Critical issues of energy efficient and new energy vehicles development in China. Energy Policy 2018, 115, 92–97. [Google Scholar] [CrossRef]
- Yuan, X.; Liu, X.; Zuo, J. The development of new energy vehicles for a sustainable future: A review. Renew. Sustain. Energy Rev. 2015, 42, 298–305. [Google Scholar] [CrossRef]
- Wang, H.; Fu, L.; Wang, M.; Yao, Z. Vehicle technologies, fuel-economy policies, and fuel-consumption rates of Chinese vehicle. Energy Policy 2012, 43, 30–36. [Google Scholar] [CrossRef]
- Wu, Y.A.; Ng, A.W.; Yu, Z.; Huang, J.; Meng, K.; Dong, Z.Y. A review of evolutionary policy incentives for sustainable development of electric vehicles in China: Strategic implications. Energy Policy 2021, 148, 111983. [Google Scholar] [CrossRef]
- Deimede, V.; Elmasides, C. Separators for Lithium-Ion Batteries: A Review on the Production Processes and Recent Developments. Energy Technol. 2015, 5, 453–468. [Google Scholar] [CrossRef]
- Shi, C.; Dai, J.; Li, C.; Shen, X.; Peng, L.; Zhang, P.; Wu, D.; Sun, D.; Zhao, J. A Modified Ceramic-Coating Separator with High-Temperature Stability for Lithium-Ion Battery. Polymers 2017, 9, 159. [Google Scholar] [CrossRef]
- Scrosati, B. Applications of Electroactive Polymer; Chapman & Hall: London, UK, 1993. [Google Scholar]
- Linden, D.; Reddy, T. Handbook of Batteries; McGraw-Hill: New York, NY, USA, 2002. [Google Scholar]
- Kim, J.; Lim, D. Surface-Modified Membrane as A Separator for Lithium-Ion Polymer Battery. Energies 2010, 3, 866–885. [Google Scholar] [CrossRef]
- Arora, P.; Zhang, Z. Battery separators. Chem. Rev. 2004, 104, 4419–4462. [Google Scholar] [CrossRef]
- Zhang, S. A review on the separators of liquid electrolyte Li-ion batteries. J. Power Sources 2007, 164, 351–364. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z.; Sun, Y.; Liang, X.; Xiang, H. Sb2O3 modified PVDF-CTFE electro spun fibrous membrane as a safe lithium-ion battery separator. J. Membr. Sci. 2019, 572, 512–519. [Google Scholar] [CrossRef]
- Yuan, X.; Wu, L.; He, X.; Zeinu, K.; Huang, L.; Zhu, X.; Hou, H.; Liu, B.; Hu, J.; Yang, J. Separator modified with N,S co-doped mesoporous carbon using egg shell as template for high performance lithium-sulfur batteries. Chem. Eng. J. 2017, 320, 178–188. [Google Scholar] [CrossRef]
- He, C.; Liu, J.; Li, J.; Zhu, F.; Zhao, H. Blending based polyacrylonitrile/poly(vinylalcohol) membrane for rechargeable lithium ion batteries. J. Membr. Sci. 2018, 560, 30–37. [Google Scholar] [CrossRef]
- Higuchi, H.; Matsushita, K.; Ezoe, M.; Shinomura, T. Porous Film, Process for Producing the Same and Use of the Same. U.S. Patent 5,385,777, 31 January 1995. [Google Scholar]
- Sogo, H. Separator for a Battery Using an Organic Electrolytic Solution and Method for Preparing the Same. U.S. Patent 5,641,565, 24 June 1997. [Google Scholar]
- Hashimoto, A.; Yagi, K.; Mantoku, H. Porous Film of High Molecular Weight Polyolefin and Process for Producing Same. U.S. Patent 6,048,607, 11 April 2000. [Google Scholar]
- Fisher, H.; Wensley, C. Polypropylene Microporous Membrane for Battery Separator. U.S. Patent 6,368,742, 29 April 2002. [Google Scholar]
- Lee, S.; Ahn, B.; Im, S.; Park, S.; Song, H.; Kyung, Y. High Crystalline Polypropylene Microporous Membrane, Multi-Component Microporous Membrane and Methods for Preparing the Same. U.S. Patent 6,830,849, 14 December 2004. [Google Scholar]
- Costa, C.M.; Silva, M.M.; Lanceros-Mendez, S. Battery separators based on vinylidene fluoride (VDF) polymers and copolymers for lithium ion battery applications. RSC Adv. 2013, 3, 11404–11417. [Google Scholar] [CrossRef]
- Wu, J.; Zeng, H.; Li, X.; Xiang, X.; Liao, Y.; Xue, Z.; Ye, Y.; Xie, X. Ultralight Layer-by-Layer Self-Assembled MoS2-Polymer Modified Separator for Simultaneously Trapping Polysulfides and Suppressing Lithium Dendrites. Adv. Energy Mater. 2018, 8, 1802430. [Google Scholar] [CrossRef]
- Zhu, X.; Jiang, X.; Ai, X.; Yang, H.; Cao, Y. A highly thermostable ceramic-grafted microporous polyethylene separator for safer lithium-ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 24119–24126. [Google Scholar] [CrossRef]
- Tseng, C.M.; Lu, Y.Y.; El-Aasser, M.S.; Vanderhoff, J.W. Uniform polymer particles by dispersion polymerization in alcohol. J. Polym. Sci. Pol. Chem. 1986, 24, 2995–3007. [Google Scholar] [CrossRef]
- Arshady, R. Suspension, emulsion, and dispersion polymerization: A methodological survey. Colloid Polym. Sci. 1992, 270, 717–732. [Google Scholar] [CrossRef]
- Airey, G.D.; Mohammed, M.H. Rheological properties of polyacrylates used as synthetic road binders. Rheol. Acta 2008, 47, 751–763. [Google Scholar] [CrossRef]
- Nguyen, M.H.T.; Oh, E.-S. Application of a new acrylonitrile/butylacrylate water-based binder fornegative electrodes of lithium-ion batteries. Electrochem. Commun. 2013, 35, 45–48. [Google Scholar] [CrossRef]
- Guan, Y.; Shen, J.; Wei, X.; Zhu, Q.; Zheng, X.; Zhou, S.; Xu, B. LiFePO4/activated carbon/graphene composite with capacitive-battery characteristics for superior high-rate lithium-ion storage. Electrochim. Acta 2018, 294, 148–155. [Google Scholar] [CrossRef]
- Budumuru, A.K.; Viji, M.; Jena, A.; Nanda, B.R.K.; Sudakar, C. Mn substitution controlled Li-diffusion in single crystallinenanotubular LiFePO4 high rate-capability cathodes: Experimental and theoretical studies. J. Power Sources 2018, 406, 50–62. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, X.; Wu, Q.; Shu, H.; Yang, X. Effects of Ni and Mn doping on physicochemical and electrochemical performances of LiFePO4/C. J. Alloys Compd. 2016, 675, 187–194. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; An, L.; Zhao, X.; Liang, G. Blended spherical lithium iron phosphate cathodes for high energy density lithium-ion batteries. Ionics 2019, 25, 61–69. [Google Scholar] [CrossRef]
- Bai, Y.; Qiu, P.; Wen, Z.; Han, S. Improvement of electrochemical performances of LiFePO4 cathode materials by coating of polythiophene. J. Alloys Compd. 2010, 508, 1–4. [Google Scholar] [CrossRef]
- Cai, Y.; Huang, D.; Ma, Z.; Wang, H.; Huang, Y.; Wu, X.; Li, Q. Construction of highly conductive network for improving electrochemical performance of lithium iron phosphate. Electrochim. Acta 2019, 305, 563–570. [Google Scholar] [CrossRef]
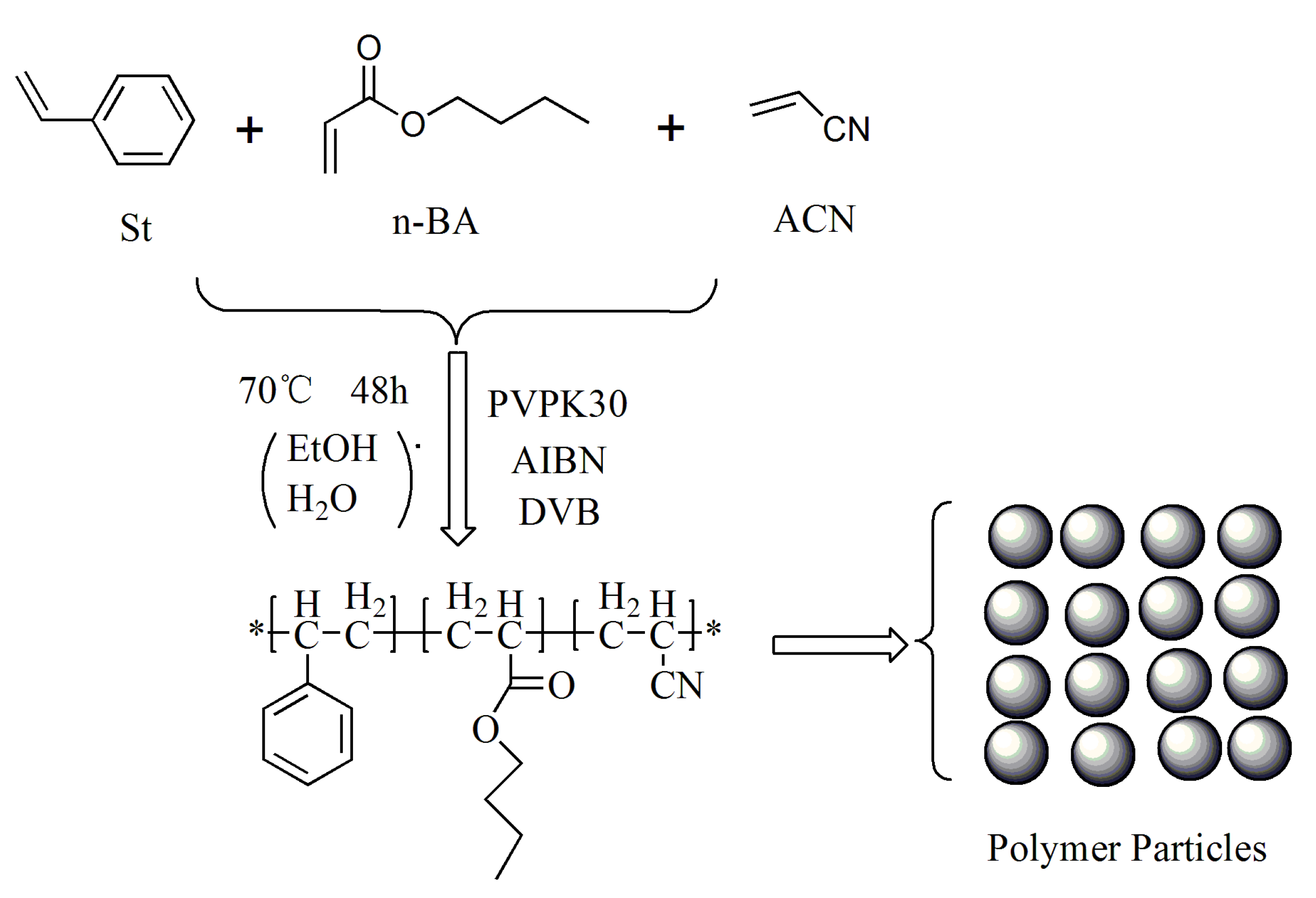

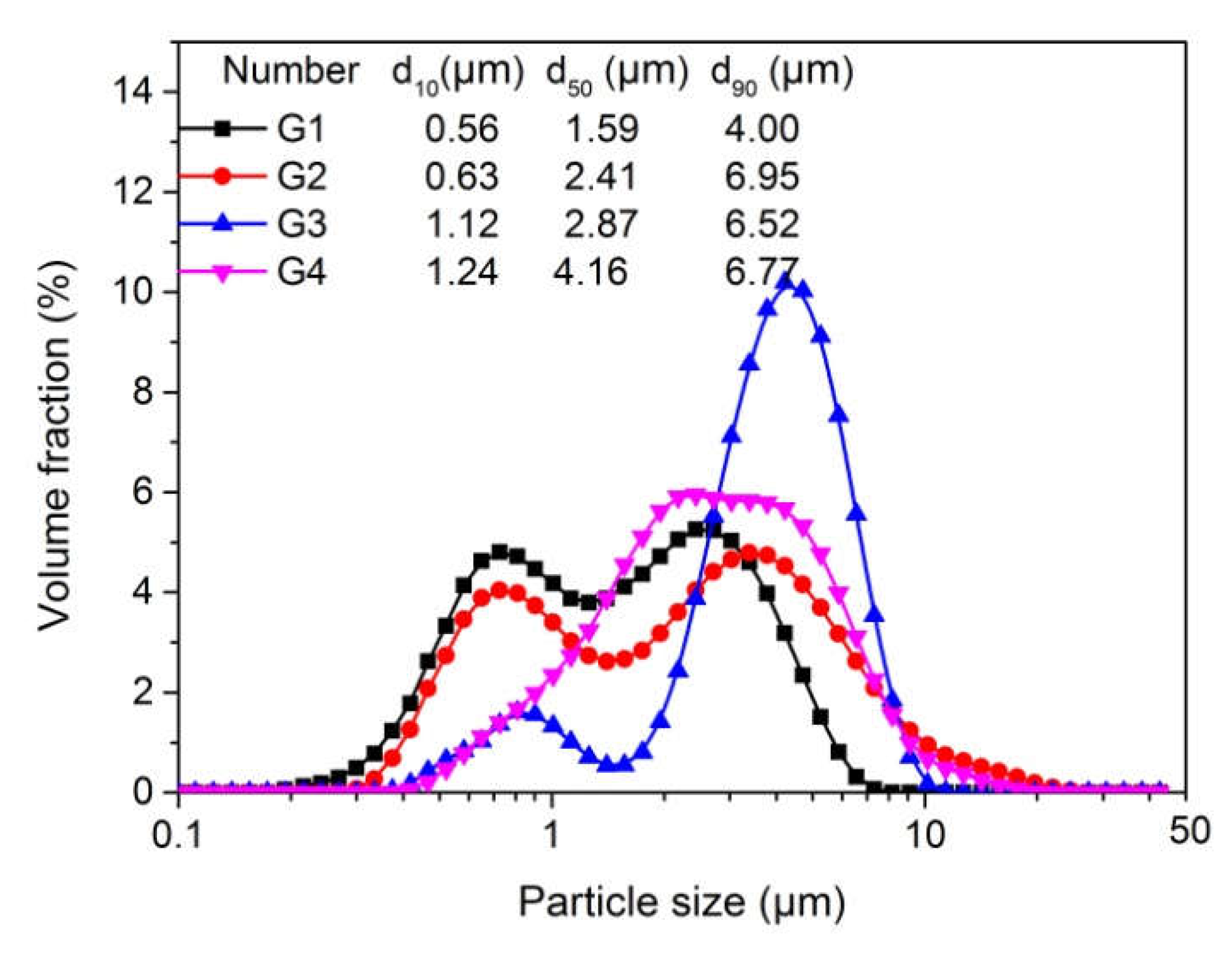




| Number | m (St:n-BA:ACN) | m (EtOH:H2O) | DVB | AIBN | PVPK30 | Temp | Time |
|---|---|---|---|---|---|---|---|
| G1 | 5:4.5:0.5 | 50:50 | 1.5% | 1% | 4.5% | 70 °C | 36 h |
| G2 | 5:4.5:0.5 | 70:30 | 1.5% | 1% | 4.5% | 70 °C | 36 h |
| G3 | 5:4.5:0.5 | 85:15 | 1.5% | 1% | 4.5% | 70 °C | 36 h |
| G4 | 6.5:3:0.5 | 85:15 | 1.5% | 1% | 4.5% | 70 °C | 24 h |
| Type | Positive Electrode Sheet | Battery Electrolyte | Negative Electrode Sheet | Battery Separator |
|---|---|---|---|---|
| LG3 | LiFePO4 | LiPF6 | Lithium sheet | G3 coated |
| LG4 | LiFePO4 | LiPF6 | Lithium sheet | G4 coated |
| LS | LiFePO4 | LiPF6 | Lithium sheet | S-1 coated |
| Number | Tg (°C) | d50 (μm) | Coated Areal Density (g/m2) |
|---|---|---|---|
| G3 | 33 | 2.87 | 0.121 |
| G4 | 58 | 4.16 | 0.183 |
| S-1 | 61 | 5.1 | 0.22 |
| 1st | 2nd | 3rd | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Peak Voltage | Potential Difference | Peak Voltage | Potential Difference | Peak Voltage | Potential Difference | ||||
| LG3 | 3.666 | 3.181 | 0.485 | 3.690 | 3.159 | 0.531 | 3.696 | 3.148 | 0.548 |
| LG4 | 3.781 | 3.107 | 0.674 | 3.797 | 3.061 | 0.736 | 3.818 | 3.043 | 0.775 |
| LS | 3.726 | 3.129 | 0.597 | 3.759 | 3.089 | 0.67 | 3.772 | 3.082 | 0.69 |
| RCT (Ω) | DLi | ||
|---|---|---|---|
| LG3 | 39.63 | 125 | 1.40158 × 10−13 |
| LG4 | 50.88 | 323.2 | 1.00292 × 10−13 |
| LS | 46.85 | 183.6 | 8.50456 × 10−14 |
| * Discharge Capacity/mAhg−1 | * Capacity Retention Rate/% | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.2 C | 0.5 C | 1 C | 2 C | 0.2 C | 0.5 C | 1 C | 2 C | ||
| Uncoated | 145.3 | 143.6 | 139 | 120.5 | 99.2 | 100.53 | 91.07 | 98.96 | |
| LG3 | 145.3 | 150.5 | 142.2 | 124.3 | 99.2 | 99.44 | 100.2 | 99.2 | |
| LG4 | 145.3 | 146.2 | 137.7 | 123.8 | 99.2 | 99.49 | 99.36 | 98.6 | |
| LS | 145.3 | 149 | 137.6 | 107.2 | 99.2 | 98.7 | 98.5 | 96 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, G.; Wu, H.; Cao, G.; Liu, Z.; Hu, H.; Guo, S. Application of a New Polymer Particle Adhesive for Lithium Battery Separators. Coatings 2023, 13, 21. https://doi.org/10.3390/coatings13010021
Huang G, Wu H, Cao G, Liu Z, Hu H, Guo S. Application of a New Polymer Particle Adhesive for Lithium Battery Separators. Coatings. 2023; 13(1):21. https://doi.org/10.3390/coatings13010021
Chicago/Turabian StyleHuang, Guanghua, Haohan Wu, Gongxun Cao, Zhijun Liu, Hanlin Hu, and Shifeng Guo. 2023. "Application of a New Polymer Particle Adhesive for Lithium Battery Separators" Coatings 13, no. 1: 21. https://doi.org/10.3390/coatings13010021
APA StyleHuang, G., Wu, H., Cao, G., Liu, Z., Hu, H., & Guo, S. (2023). Application of a New Polymer Particle Adhesive for Lithium Battery Separators. Coatings, 13(1), 21. https://doi.org/10.3390/coatings13010021






