Abstract
Lacking osseointegration and peri-implantitis induced by bacterial infiltration are the pivotal issues for the long-term clinical success of implants. In order to improve the bioactivity and antibacterial properties of implant materials, volcano-shaped microporous TiO2 coatings doped with Zinc (Zn) were fabricated via a micro-arc oxidation (MAO) method on pure titanium (Ti). The microstructure, morphology, and chemical composition of the Zn-doped coatings were systematically studied. In cell culture tests, the formed coatings promoted the adhesion and proliferation of bone mesenchymal stem cells (BMSCs), exhibiting good biocompatibility. The antibacterial experiments revealed that Zn-TiO2 coatings possess excellent antibacterial properties against Staphylococcus aureus (S. aureus) and Porphyromonas gingivalis (P. gingivalis).
1. Introduction
Titanium (Ti) and its alloys are used in orthopedics, cardiovascular medicine, dentistry, and other applications because of their excellent mechanical properties and biocompatibility [1]. However, in recent years, several clinical issues, such as biomaterial-associated infections, peri-implantitis, and prosthetic joint infections, have been reported for Ti [2]. The biofilm formation due to bacterial adhesion was recognized as the major cause of these infections [3]. Once a biofilm is formed, pathogens are difficult to eliminate. The only way to solve the problem is to remove the contaminated implant from the patient. Prevention is the most effective approach to prevent implant-associated infections with antibacterial surface implants [4]. Therefore, antimicrobial surface coatings have attracted much attention due to the development of their surface properties to prolong the service life of implants.
There are many methods to modify the surface of a dental implant, such as HA coating, titanium plasma spray, sandblasted large-grit and acid-etching, micro-arc oxidation (MAO), etc. Among these methods, micro-arc oxidation (MAO) is one of the cost-effective and versatile ways to modify the surface of metallic implants with complex geometries [5]. MAO is a relatively simple technique with low cost compared to other surface modification methods. It can produce an oxide ceramic layer in situ on a metal substrate [6,7]. The TiO2 coating fabricated by MAO is generally on the micro/nanoscale, which can significantly reduce the elasticity modulus of Ti [8]. These porous structures can also reduce the elastic modulus of the implant surface, promote bone cell adhesion, and enhance implant–bone bonding [9]. In addition, a porous-structure coating prepared by MAO has been proven to favor cell adhesion and proliferation [10,11]. MAO results in a changed surface topography and a controllable thickness of the native oxide layer, which is suitable for osseointegration. The most striking characteristic of MAO is the formation of interconnecting pores, which can increase protein adsorption and stimulate osteoblast migration, resulting in faster osseointegration [12]. Some elements can be easily incorporated into the surface of titanium-based coatings via MAO, conferring dual functionalization and improving the compatibility of titanium alloys’ characteristics, such as their antibacterial properties and biocompatibility [13].
Antibacterial agents, such as antibiotics [14], organic cation compounds [15], and metal elements [16,17], have been incorporated on the implant surface to prevent the formation of biofilm. Among them, Zinc (Zn) is known as one of the most effective agents because of its excellent antibacterial effects. In addition, Zn is an important trace element for the human body, which can enhance cell proliferation, osteoblast marker gene expression, osteogenic function, and alkaline phosphatase (ALP) activity [18,19]. Although Zn possesses excellent antibacterial properties, it also exhibits a high cytotoxic threshold [20]. Excessive zinc doping will cause cytotoxic effects in osteoblasts, while moderate zinc doping can promote the osteogenic differentiation of cells [21]. Hence, the quantitative control of the incorporation of Zn will achieve a dual function on the Ti surface by MAO.
P. gingivalis is recognized as being most closely related to the development of peri-implantitis and even the ultimate loss of dental implants [22]. S. aureus is a frequent commensal of the nares and skin and is considered a transient oral resident, which is identified at higher numbers in biofilm obtained from implants with peri-implantitis than in healthy peri-implant conditions [23,24]. The biological rationale of S. aureus. in peri-implantitis is the capacity to attach onto titanium surfaces [25] and contribute to biofilm-associated and medical-device-related infections [26]. Therefore, an evaluation of the antibacterial effects of implants against P. gingivalis and S. aureus can determine its scope of application.
In this study, Zn-incorporated coatings (Zn-MAO) on the dental implant surface were prepared by a micro-arc oxidation technique, and the chemical stability was analyzed. Meanwhile, the cell biocompatibility and the antibacterial capacities of the coatings against P. gingivalis and S. aureus were evaluated in vitro.
2. Materials and Methods
2.1. Preparation of Zn-Doped TiO2 Coating (Zn-MAO)
We gradient polished the pure titanium discs with a diameter of 14 mm (thickness: 1.5 mm) to 1200 mesh. Then, we ultrasonically washed these titanium specimens with acetone and distilled water, and dried at 40 °C. We prepared the Zn-MAO coatings using a DC power supply in constant voltage mode. The MAO electrolyte solution consisted of 20 g/L trisodium phosphate dodecahydrate (Na3PO4·12H2O), 4 g/L potassium hydroxide (KOH), 10 g/L EDTA disodium, and zinc acetate. We set the concentration of zinc acetate ((CH₃COO)₂Zn) as 0 g/L, 0.05 g/L, 0.1 g/L, and 0.25 g/L, and recorded them as 0Zn-MAO, 0.05Zn-MAO, 0.1Zn-MAO, and 0.25Zn-MAO, respectively, according to Table 1. During MAO process, we used the stainless-steel plate as the cathode plate and the titanium sample as the anode. We maintained the temperature of electrolyte at 15 °C via circulating water treatment system. We conducted the fabrication under a constant voltage of 400 V for 4 min. After MAO, we washed the specimens with acetone and alcohol, then dried at room temperature.

Table 1.
Scheme for preparation coatings.
2.2. Surface Characterization
We observed the surface morphology of the coatings by scanning electron microscope (SEM, Quanta FEG 450, Hillsboro, OR, USA). We analyzed the surface and cross-sectional elemental compositions of the MAO coatings by energy-dispersive X-ray spectroscopy (EDS, Octane SDD, EDAX, Los Angeles, CA, USA). We performed the phase composition of all coatings by X-ray diffraction (XRD, D8 ADVANCE, Bruker, Munich, Germany). We subjected the coatings to X-ray microanalysis system (XPS, ESCALAB 250 Xi, Thermo Scientific, Cambridge, UK) to evaluate the changes in the chemical state of Zinc on the surface of Zn-doped coatings. We analyzed the hydrophilicity of the coatings (n = 4) by a contact angle meter (Dataphysics, OCA20, Stuttgart, Germany). We carried out the Zn2+ release experiments in saline at 36.5 °C for 0–4 weeks; then, we measured via inductively coupled plasma-optical emission spectrometry (ICP-OES, Optima 5300 DV, Perkin Elmer, Waltham, MA, USA). We measured three samples in each group.
2.3. Cytocompatibility for Zn-Doped Coating (Zn-MAO)
2.3.1. Preparation of Cell
In this study, we used four-week-old Sprague–Dawley (SD) rats to isolate and culture BMSCs. We isolated bone marrow from mice’s tibias and suspended it in 10% fetal bovine serum (FBS, Gibco, Carlsbad, CA, USA) and 1% penicillin–streptomycin–amino acids (PS, Gibco). We used an atmosphere of 5% CO2 and 37 °C to culture the primary cells for 48 h. Following that, we collected BMSCs from the bottom of the dishes. Every three days, we replaced the medium. In the following experiments, we used cells between passage 3 and 6.
2.3.2. Cell Viability Assays
We used a CCK-8 kit to detect the proliferation of BMSCs cultured on the surface of Zn-doped coatings. The seeding density was 7 × 104 cells/mL in 24-well plates. After 1, 3, and 5 days of culture, we measured cytotoxicity. Proliferation trials involved changing the medium every other day. After the prescribed incubation time, we added CCK-8 dye to each well; then, we cultured the plates for another 2 h at 37 °C. At last, we measured the optical density (OD, n = 5) at 450 nm by a plate reader. We employed five samples for each coating in the test.
After incubating the cells for 3 days, we washed with PBS, fixed with 4% paraformaldehyde for 10 min at room temperature, and then dehydrated in ethanol for 10 min per gradient, followed by vacuum drying. After gold sputtering, we observed the samples via SEM (Quanta FEG 450) for cell growth morphologies.
2.4. Antibacterial Properties of Zn-Doped Coating (Zn-MAO)
2.4.1. Preparation of Bacteria
We selected S. aureus (ATCC25923), the representative species of gram-positive bacteria, as the bacteria for the antibacterial experiment; furthermore, we selected P. gingivalis (ATCC 33277) as the representative species of gram-negative bacteria. We cultivated all species with brain heart infusion (BHI). We cultured P. gingivalis anaerobically with 80% N2, 10% H2, and 10% CO2, and cultured S. aureus aerobically at 37 °C. For each bacterium, we adjusted the concentration of inoculation to 106 colony-forming units and counted CFU/mL for antimicrobial assays.
2.4.2. Plate-Counting Method
We evaluated the antibacterial effects of Zn-doped coatings by the plate-counting method. We inoculated bacteria suspension (200 μL) on the surface of the Zn-MAO coating. We tightly covered the surface of the sample with a sterilized cover-slip to ensure adequate contact between bacteria and samples, and incubated at 37 °C and 5% CO2 for 24 h. We washed specimens with 1 mL of PBS to remove the bacteria in the cell culture dish.
We evenly spread 50 μL of the remaining bacterial suspension on Columbia AGAR plates and further incubated for 24 h. After that, we counted each group’s colony-forming units (CFU). We carried out the experiments three times, and CFU was the average of three results.
We observed the bacterial growth morphology on Zn-MAO by SEM. We washed the bacterial cells with PBS after incubating on the surface for 24 h, fixed with 4% paraformaldehyde for 10 min at room temperature, and then dehydrated in ethanol for 10 min per gradient, followed by vacuum drying. After gold sputtering, we observed the samples via SEM (Quanta FEG 450) for bacterial growth morphologies.
2.5. Statistics
We repeated all assays at least three times. We collected and expressed data as the mean ± standard deviation. We considered statistical significance difference at * p < 0.05 and ** p < 0.01.
3. Results
3.1. Surface Characterization
Figure 1A shows the surface topography of the coatings deposited in different electrolytes. After the MAO process, volcano-shaped microporous TiO2 coatings were prepared on the surface of pure titanium. There was no obvious difference in the porous microstructure after the incorporation of Zn. The XRD spectra of Zn-doped coatings are presented in Figure 1B. They revealed the presence of only anatase and rutile phases in Zn-doped coatings without any Zn-related phase. Therefore, the Zn-existing states in Zn-doped coatings should be further analyzed using a high-resolution XPS spectrum.
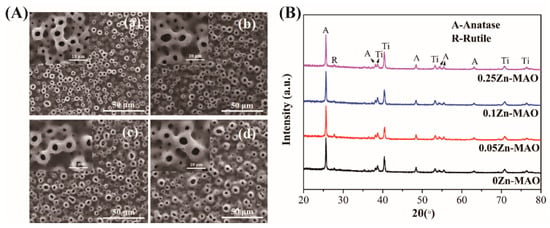
Figure 1.
Surface morphologies of different coatings (A): (a) 0Zn-MAO, (b) 0.05Zn-MAO, (c) 0.1Zn-MAO, (d) 0.25Zn-MAO. XRD patterns of different coatings (B).
The chemical compositions of the coatings were clearly revealed from the XPS full spectrum (Figure 2a). The Ti, O, and P were detected on all the surfaces. The high-resolution XPS spectra of Zn obtained from 0.05Zn-MAO, 0.1Zn-MAO, and 0.25Zn-MAO are shown in Figure 2b. Two peaks centered at 1044.4 and 1021.5 eV emerged in the high-resolution spectra for Zn2p, which revealed the presence of Zn2+ in the Zn-doped coating.
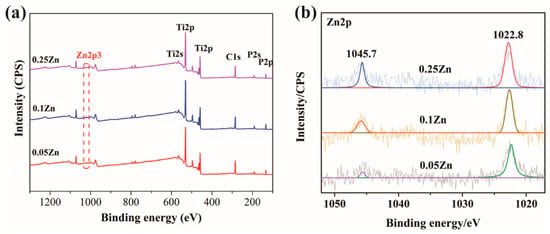
Figure 2.
XPS survey spectrum (a) and Zn2p high-resolution spectra of the 0.05Zn-MAO, 0.1Zn-MAO and 0.25Zn-MAO coatings (b).
Table 2 presents the concentration estimated by EDS. As concentrations of Zinc acetate added in the electrolyte increased, the amount of Zn increased from 0.45 wt.% (0.05Zn-MAO) to 3.45 wt.% (0.25Zn-MAO). This indicated that the Zn content in the coating can be adjusted by adjusting the concentration of the Zinc acetate in the electrolyte. Figure 3a–d show the cross-sectional SEM morphologies of the Zn-doped coatings. It can be seen that all the coatings have a cross-sectional thickness of 4–7 μm. The chemical composition of the 0.25Zn-MAO coating was examined by EDS. Figure 3e is the line-scan analysis along the yellow arrow in Figure 3d. This indicated that the Zn element is distributed throughout the thickness of the coating.

Table 2.
Element component (wt.%) on the surface of different coatings.
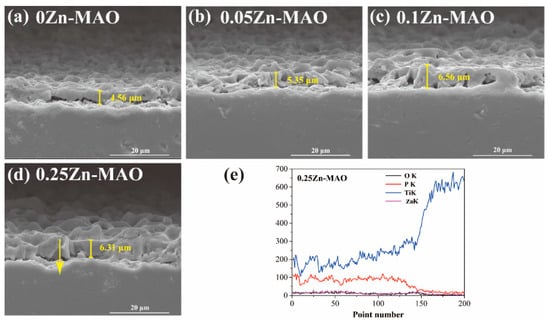
Figure 3.
Cross-sectional SEM morphologies of 0Zn-MAO (a), 0.05Zn -MAO (b), 0.1Zn-MAO (c), and 0.25ZN-MAO (d) coatings, and EDS line-scan analysis crossing the yellow arrow marked in Zn-doped coatings in (e).
As shown in Figure 4a, the contact angle of 0Zn-MAO was 43°. With the increase of Zinc acetate in the electrolyte, the contact angle of the coating also increased linearly. However, the contact angles of the Zn-doped coatings were in the range of 43–49°, which indicated that adding Zn did not obviously alter their hydrophilicity. ICP-OES detected the release of Zn2+ at different time points. The amount of Zn released from the Zn-MAO coating as a function of immersion time in 0.9 wt.% NaCl solution at 36.5 °C was measured, and the result was shown in Figure 4b. The release curve shows that the release of Zn is slow and continuous in the prolonged immersion process.
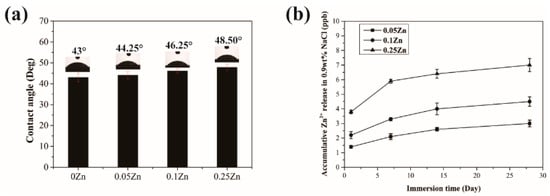
Figure 4.
Contact angles of Zn-MAO coatings (a). The release of Zn2+ from 0.05Zn-MAO, 0.1Zn, and 0.25Zn groups on 1, 7, 14, and 28 days (b).
3.2. The Cell Biocompatibility of Zn-MAO Coating
The cell viability was tested by CCK-8, the result of which showed the OD value was increased significantly in the 0Zn-MAO, 0.05Zn-MAO, 0.1Zn-MAO, and 0.25Zn-MAO groups at 5 days (p < 0.05) (Figure 5a). Compared with the Ti group, the Zn-doped groups showed much higher viability at 3 and 5 days. As shown in Figure 5b, the cell morphologies on different coatings after incubation for 3 days were observed by SEM. Compared with Ti, the cells attach and spread better on the surface of 0Zn-MAO and 0.25Zn-MAO. On Ti, the cells are spherical and spread less, whereas on 0Zn-MAO and 0.25Zn-MAO, the cells are polygonal and covered the pores. All the results present the good cell biocompatibility of Zn-MAO coatings in our study.
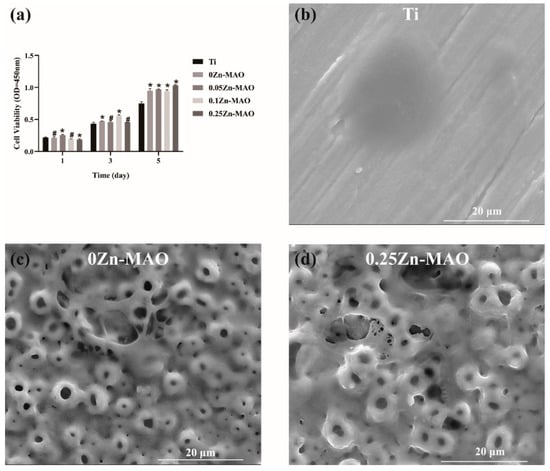
Figure 5.
The cell biocompatibility of Zn-MAO (a). Cytotoxicity was measured after 1, 3, and 5 days of culture. The BMSCs morphologies on Ti (b), 0Zn (c), and 0.25Zn (d) groups surface for 3 days under SEM. Data are presented as mean ± SD; (* p < 0.05, # p > 0.05, vs. Ti group).
3.3. Antibacterial Properties of Zn-MAO Coating
Figure 6a,b show images of S. aureus and P. gingivalis colonies cultured on the Zn-MAO coatings for 24 h. We found that the plates corresponding to the 0.25Zn coating had the lowest bacteria colony numbers compared to the other coatings. It revealed that the 0.25Zn-MAO coating exhibits powerful antibacterial activities. As showed in Figure 6c, the effects of those Zn-MAO coatings on bacterial morphology and cell-wall integrity were observed via SEM. The P. gingivalis and S. aureus grown on the surface of 0Zn-MAO coating are rounded with integrated membranes, while there were some bacteria collapsed, corrugated, and fractured on the surface of Zn-MAO coatings (marked with arrows in Figure 6c).
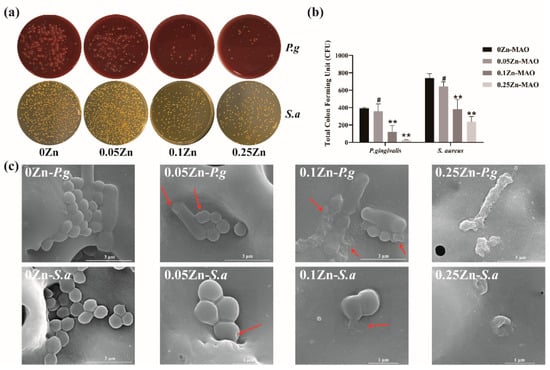
Figure 6.
Antibacterial activities of Zn-doped coatings against S. aureus and P. gingivalis (a); CFU results after S. aureus and P. gingivalis were cocultured with the Zn-MAO coating for 24 h (b). The SEM images (c) of S. aureus and P. gingivalis cultured on different surface after 24 h of incubation. Data are presented as mean ± SD; (** p < 0.01, # p > 0.05, vs. 0Zn-MAO group).
4. Discussion
After an implant is implanted in the body, the surface of the materials undergo a series of biochemical reactions, and the structure of the material surface plays a crucial role in the implant’s response to the internal environment. The microstructure and chemical composition of the implant surface can modify the adsorption of proteins that regulate cell adhesion and ultimately determine its function [27].
In our study, MAO coatings were prepared on pure titanium, and pores of a microscale size were distributed, as shown in Figure 1A. The microscale pores can significantly promote cell adhesion and osteogenic differentiation [28]. In addition, the volcano-shaped microporous surface can provide the required spatial environment for the growth of different cells, thus promoting the proliferation and adhesion of most osteoblasts on the surface of the material [29,30]. During the MAO process, a large number of micro-arcs struck over the anode surface, and the microscale pores are believed to be the discharge channels of these arcs [31]. It can be seen from the XRD results (Figure 1B) that the Zn-doped coatings comprised mainly anatase. Some anatase converted to rutile due to the high temperatures accompanying grain growth [32]. Many studies reported that anatase has unique features and advantages in medical applications. It also can absorb more and , which is favorable for the deposition of bone-like apatite in body fluids [10,33,34]. MAO treatment can only incorporate this element into the resulting oxide coating when the element is dissolved in the electrolyte [35,36]. In addition, phosphorus (P) is the bio-functional ion, while Zn is the essential trace element for human beings. Bacteria can be inhibited from adhering and reproducing [37]. The data via EDS analysis demonstrated that O, P, and Zn in the electrolyte are ultimately deposited throughout the MAO coatings (Table 1 and Figure 2). During the MAO process, the oxygen from the electrolyte was transported to the titanium substrate through the discharge channel to form TiO2 [38]. At the same time, the Zn2+ from the zinc acetate combined with the EDTA to form negatively charged complexes. These zinc-containing complexes then moved toward the anode, mainly by electromigration. The Zn2+ binding with from trisodium phosphate dodecahydrate formed in the end [39]. The main reactions of Zn2+ deposition may be described as follows:
The contact angles of the Zn-doped MAO coatings were from 43° to 48.5° (data in brief, Figure 4a), which shows hydrophilicity. Hydrophilic surfaces are beneficial in the early phases of osseointegration [40], which will be suitable for cell adhesion on the implant surface. The cell viability of BMSCs on Zn-MAO coatings was much higher than in the Ti group (Figure 5a), which proved that Zn-MAO coatings were more appropriate and suitable for BMSCs proliferation. In conclusion, the Zn-MAO coating possesses good cell biocompatibility in our study.
The antimicrobial coating on the surface of the implant is a method for preventing peri-implantitis. Antibacterial elements, such as Ag, Cu, and Zn, are easily used to incorporate onto the Ti surface to generate an antibacterial surface by MAO. The verifications have illustrated that the incorporation of Zn possesses certain antibacterial capabilities, and has been extensively used to improve the antibacterial abilities of implants [41,42]. In our study, Zn was chosen to incorporate into the TiO2 layer by the application of different concentrations of zinc acetate via the MAO electrolyte.
S. aureus is a frequent commensal of the nares and skin and is considered a transient oral resident, which is found identified at higher numbers in biofilm obtained from implants with peri-implantitis than peri-implant health [23]. P. gingivalis is recognized as most closely related to the development of peri-implantitis and even the ultimate loss of implants [22]. Therefore, S. sanguinis and P. gingivalis were selected as representative strains for peri-implantitis-associated biofilm experiments in vitro. Figure 6a shows the 0.25Zn-MAO coating achieved greater inhibition against two types of single-species biofilms by the CFU. The antibacterial rates of 0.25Zn-MAO coating against S.aureus and P. gingivalis bacterial were 68% and 93.4%, respectively. It indicated that the 0.25Zn-MAO coating possessed a strong bactericidal effect on P. gingivalis. SEM results visualized the collapse of S.aureus and P. gingivalis bacterial structures and the death of bacteria on Zn-MAO coatings. All of these suggest that the 0.25Zn-MAO coatings showed an excellent antibacterial effect on the peri-implant inflammatory pathogens. The antibacterial mechanism for Zn-MAO coating could be explained as through a mechanism of damaging bacterial cell membranes by the generation of reactive oxygen species [43,44]. It can not only penetrate the membrane of bacteria and interact with proteins but also damage the DNA of bacteria and prevent the proliferation of bacteria [45].
5. Conclusions
In this study, Zn-doped porous TiO2 coatings were prepared on the surface of titanium by MAO. The structures of Zn-doped coatings were systematically investigated. To evaluate the antibacterial effect of Zn-doped coatings, we cultured the gram-negative (P. gingivalis) and gram-positive bacteria (S. aureus) on the surface of Zn-doped coatings. The results showed that 0.25Zn-MAO coatings had the best antibacterial effect. Meanwhile, we found that 0.25Zn-MAO coatings had the best bioactivity, cell proliferation promotion, and adhesion effects relative to Ti and 0Zn-MAO through in vitro study. In conclusion, the Zn-doped TiO2 coatings had good biocompatibility and antimicrobial properties, which may be helpful in eradicating biomaterial-associated infections.
Author Contributions
Conceptualization, B.K. and S.Q.; methodology, B.K., L.L., D.L, R.D. and C.Y.; software, B.K. and D.L.; validation, B.K., L.L. and R.D.; formal analysis, S.Q.; investigation, B.K.; resources, P.L.; data curation, B.K., L.L. and R.D.; writing—original draft preparation, B.K.; writing—review and editing, S.Q. and B.K.; visualization, B.K.; supervision, S.Q., P.L., F.M. and X.C.; project administration, X.C.; funding acquisition, X.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The animal experiments were approved by the Animal Care and Use Committee of Department of the Laboratory Animal Science of Tongji University (ID: SHDSYY-2021-3063).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hanawa, T. Titanium-Tissue Interface Reaction and Its Control with Surface Treatment. Front. Bioeng. Biotechnol. 2019, 7, 170. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, M. Antibacterial Property and Biocompatibility of Silver, Copper, and Zinc in Titanium Dioxide Layers Incorporated by One-Step Micro-Arc Oxidation: A Review. Antibiotics 2020, 9, 716. [Google Scholar] [CrossRef] [PubMed]
- Paquette, D.W.; Brodala, N.; Williams, R.C. Risk factors for endosseous dental implant failure. Dent. Clin. N. Am. 2006, 50, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Smeets, R.; Henningsen, A.; Jung, O.; Heiland, M.; Hammacher, C.; Stein, J.M. Definition, etiology, prevention and treatment of peri-implantitis—A review. Head Face Med. 2014, 10, 34. [Google Scholar] [CrossRef]
- Durdu, S.J.C. Characterization, Bioactivity and Antibacterial Properties of Copper-Based TiO2 Bioceramic Coatings Fabricated on Titanium. Coatings 2018, 9, 1. [Google Scholar] [CrossRef]
- Khanna, R.; Kokubo, T.; Matsushita, T.; Nomura, Y.; Nose, N.; Oomori, Y.; Yoshida, T.; Wakita, K.; Takadama, H. Novel artificial hip joint: A layer of alumina on Ti-6Al-4V alloy formed by micro-arc oxidation. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 55, 393–400. [Google Scholar] [CrossRef]
- Li, L.H.; Kong, Y.M.; Kim, H.W.; Kim, Y.W.; Kim, H.E.; Heo, S.J.; Koak, J.Y. Improved biological performance of Ti implants due to surface modification by micro-arc oxidation. Biomaterials 2004, 25, 2867–2875. [Google Scholar] [CrossRef]
- Han, Y.; Chen, D.; Sun, J.; Zhang, Y.; Xu, K. UV-enhanced bioactivity and cell response of micro-arc oxidized titania coatings. Acta Biomater. 2008, 4, 1518–1529. [Google Scholar] [CrossRef]
- Xiu, P.; Jia, Z.; Lv, J.; Yin, C.; Cheng, Y.; Zhang, K.; Song, C.; Leng, H.; Zheng, Y.; Cai, H.; et al. Tailored Surface Treatment of 3D Printed Porous Ti6Al4V by Microarc Oxidation for Enhanced Osseointegration via Optimized Bone In-Growth Patterns and Interlocked Bone/Implant Interface. ACS Appl. Mater. Interfaces. 2016, 8, 17964–17975. [Google Scholar] [CrossRef]
- Akin, F.A.; Zreiqat, H.; Jordan, S.; Wijesundara, M.B.; Hanley, L. Preparation and analysis of macroporous TiO2 films on Ti surfaces for bone-tissue implants. J. Biomed. Mater. Res. 2001, 57, 588–596. [Google Scholar] [CrossRef]
- Webster, T.J.; Ergun, C.; Doremus, R.H.; Siegel, R.W.; Bizios, R. Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials 2000, 21, 1803–1810. [Google Scholar] [CrossRef]
- Hu, H.; Liu, X.; Ding, C. Preparation and in vitro evaluation of nanostructured TiO2/TCP composite coating by plasma electrolytic oxidation. J. Alloy. Compd. 2010, 498, 172–178. [Google Scholar] [CrossRef]
- Zhang, B.; Li, B.; Gao, S.; Li, Y.; Cao, R.; Cheng, J.; Li, R.; Wang, E.; Guo, Y.; Zhang, K.J.M. Y-doped TiO2 coating with superior bioactivity and antibacterial property prepared via plasma electrolytic oxidation. Mater. Des. 2020, 192, 108758. [Google Scholar] [CrossRef]
- Yamamura, K.; Iwata, H.; Yotsuyanagi, T. Synthesis of antibiotic-loaded hydroxyapatite beads and in vitro drug release testing. J. Biomed. Mater. Res. 1992, 26, 1053–1064. [Google Scholar] [CrossRef]
- Shirai, T.; Shimizu, T.; Ohtani, K.; Zen, Y.; Takaya, M.; Tsuchiya, H. Antibacterial iodine-supported titanium implants. Acta Biomater. 2011, 7, 1928–1933. [Google Scholar] [CrossRef]
- Lishchynskyi, O.; Shymborska, Y.; Stetsyshyn, Y. Passive antifouling and active self-disinfecting antiviral surfaces. Chem. Eng. J. 2022, 446, 137048. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, J.; Yan, T.; Han, Y.J.A.S.S. Fibroblast responses and antibacterial activity of Cu and Zn co-doped TiO2 for percutaneous implants. Appl. Surf. Sci. 2018, 434, 633–642. [Google Scholar] [CrossRef]
- Zhu, H.; Jin, G.; Cao, H.; Qiao, Y.; Liu, X. Influence of implantation voltage on the biological properties of zinc-implanted titanium. Surf. Coat. Technol. 2017, 312, 75–80. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, W.; Qiao, Y.; Jiang, X.; Liu, X.; Ding, C. Antibacterial activity and increased bone marrow stem cell functions of Zn-incorporated TiO2 coatings on titanium. Acta Biomater. 2012, 8, 904–915. [Google Scholar] [CrossRef]
- Ye, J.; Li, B.; Li, M.; Zheng, Y.; Wu, S.; Han, Y. ROS induced bactericidal activity of amorphous Zn-doped titanium oxide coatings and enhanced osseointegration in bacteria-infected rat tibias. Acta Biomater. 2020, 107, 313–324. [Google Scholar] [CrossRef]
- Zhao, B.H.; Zhang, W.; Wang, D.N.; Feng, W.; Liu, Y.; Lin, Z.; Du, K.Q.; Deng, C.F. Effect of Zn content on cytoactivity and bacteriostasis of micro-arc oxidation coatings on pure titanium. Surf. Coat. Technol. 2013, 228, S428–S432. [Google Scholar] [CrossRef]
- Qi, H.; Li, B.; Wang, H.; Cai, Q.; Quan, X.; Cui, Y.; Meng, W. Effects of d-valine on periodontal or peri-implant pathogens: Porphyromonas gingivalis biofilm. J. Periodontol. 2018, 89, 303–314. [Google Scholar] [CrossRef]
- Zhuang, L.F.; Watt, R.M.; Mattheos, N.; Si, M.S.; Lai, H.C.; Lang, N.P. Periodontal and peri-implant microbiota in patients with healthy and inflamed periodontal and peri-implant tissues. Clin. Oral. Implant. Res. 2016, 27, 13–21. [Google Scholar] [CrossRef]
- Renvert, S.; Aghazadeh, A.; Hallström, H.; Persson, G. Factors related to peri-implantitis–a retrospective study. Clin. Oral. Implant. Res. 2014, 25, 522–529. [Google Scholar] [CrossRef]
- Harris, L.; Richards, R. Staphylococcus aureus adhesion to different treated titanium surfaces. J. Mater. Sci. Mater. Med. 2004, 15, 311–314. [Google Scholar] [CrossRef]
- Costerton, J.W.; Montanaro, L.; Arciola, C.R. Biofilm in implant infections: Its production and regulation. Int. J. Artif. Organs. 2005, 28, 1062–1068. [Google Scholar] [CrossRef]
- Wang, L.J.; Ni, X.H.; Zhang, F.; Peng, Z.; Yu, F.X.; Zhang, L.B.; Li, B.; Jiao, Y.; Li, Y.K.; Yang, B. Osteoblast Response to Copper-Doped Microporous Coatings on Titanium for Improved Bone Integration. Nanoscale Res. Lett. 2021, 16, 146. [Google Scholar] [CrossRef]
- Zhou, W.; Huang, O.; Gan, Y.; Li, Q.; Zhou, T.; Xi, W. Effect of titanium implants with coatings of different pore sizes on adhesion and osteogenic differentiation of BMSCs. Artif. Cells Nanomed. Biotechnol. 2019, 47, 290–299. [Google Scholar] [CrossRef]
- Gittens, R.A.; McLachlan, T.; Olivares-Navarrete, R.; Cai, Y.; Berner, S.; Tannenbaum, R.; Schwartz, Z.; Sandhage, K.H.; Boyan, B.D. The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation. Biomaterials 2011, 32, 3395–3403. [Google Scholar] [CrossRef]
- Li, X.; Wang, M.; Zhang, W.; Bai, Y.; Liu, Y.; Meng, J.; Zhang, L. A Magnesium-Incorporated Nanoporous Titanium Coating for Rapid Osseointegration. Int. J. Nanomed. 2020, 15, 6593–6603. [Google Scholar] [CrossRef]
- Mi, T.; Jiang, B.; Liu, Z.; Fan, L. Plasma formation mechanism of microarc oxidation. Electrochim. Acta. 2014, 123, 369–377. [Google Scholar] [CrossRef]
- Tang, G.-X.; Zhang, R.-J.; Yan, Y.-N.; Zhu, Z.-X. Preparation of porous anatase titania film. Mater. Lett. 2004, 58, 1857–1860. [Google Scholar] [CrossRef]
- Nie, X.; Leyland, A.; Matthews, A.; Jiang, J.C.; Meletis, E.I. Effects of solution pH and electrical parameters on hydroxyapatite coatings deposited by a plasma-assisted electrophoresis technique. J. Biomed. Mater. Res. 2001, 57, 612–618. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Nie, X.; Leyland, A.; Matthews, A. Characterisation of oxide films produced by plasma electrolytic oxidation of a Ti–6Al–4V alloy. Surf. Coat. Technol. 2000, 130, 195–206. [Google Scholar] [CrossRef]
- Ha, J.Y.; Tsutsumi, Y.; Doi, H.; Nomura, N.; Kim, K.H.; Hanawa, T. Enhancement of calcium phosphate formation on zirconium by micro-arc oxidation and chemical treatments. Surf. Coat. Technol. 2011, 205, 4948–4955. [Google Scholar] [CrossRef]
- Shimabukuro, M.; Tsutsumi, H.; Tsutsumi, Y.; Manaka, T.; Chen, P.; Ashida, M.; Ishikawa, K.; Katayama, H.; Hanawa, T. Enhancement of antibacterial property of titanium by two-step micro arc oxidation treatment. Dent. Mater. J. 2021, 40, 592–598. [Google Scholar] [CrossRef]
- Mirastschijski, U.; Martin, A.; Jorgensen, L.N.; Sampson, B.; Agren, M.S. Zinc, copper, and selenium tissue levels and their relation to subcutaneous abscess, minor surgery, and wound healing in humans. Biol. Trace. Elem. Res. 2013, 153, 76–83. [Google Scholar] [CrossRef]
- Curran, J.A.; Clyne, T.W. Porosity in plasma electrolytic oxide coatings. Acta Mater. 2006, 54, 1985–1993. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, R.; Xu, T.; Xu, Y.; Ge, F.; Xi, Y.; Zhu, J.; He, H. Co-adsorption of phosphate and zinc(II) on the surface of ferrihydrite. Chemosphere 2016, 144, 1148–1155. [Google Scholar] [CrossRef]
- Buser, D.; Broggini, N.; Wieland, M.; Schenk, R.; Denzer, A.; Cochran, D.; Hoffmann, B.; Lussi, A.; Steinemann, S. Enhanced bone apposition to a chemically modified SLA titanium surface. J. Dent. Res. 2004, 83, 529–533. [Google Scholar] [CrossRef]
- Reddy, L.S.; Nisha, M.M.; Joice, M.; Shilpa, P.N. Antimicrobial activity of zinc oxide (ZnO) nanoparticle against Klebsiella pneumoniae. Pharm. Biol. 2014, 52, 1388–1397. [Google Scholar] [CrossRef]
- Xie, Y.; He, Y.; Irwin, P.L.; Jin, T.; Shi, X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl. Environ. Microbiol. 2011, 77, 2325–2331. [Google Scholar] [CrossRef]
- San Miguel, S.M.; Opperman, L.A.; Allen, E.P.; Zielinski, J.E.; Svoboda, K.K. Antioxidant combinations protect oral fibroblasts against metal-induced toxicity. Arch. Oral. Biol. 2013, 58, 299–310. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A. A Mini Review of Antibacterial Properties of ZnO Nanoparticles. Front. Phys. 2021, 9, 641481. [Google Scholar] [CrossRef]
- Tsutsumi, H.; Tsutsumi, Y.; Shimabukuro, M. Investigation of the long-term antibacterial properties of titanium by two-step micro-arc oxidation treatment. Coating 2021, 11, 798. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).