Effect of Si3N4/TaC Particles on the Structure and Properties of Microarc Oxidation Coatings on TC4 Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
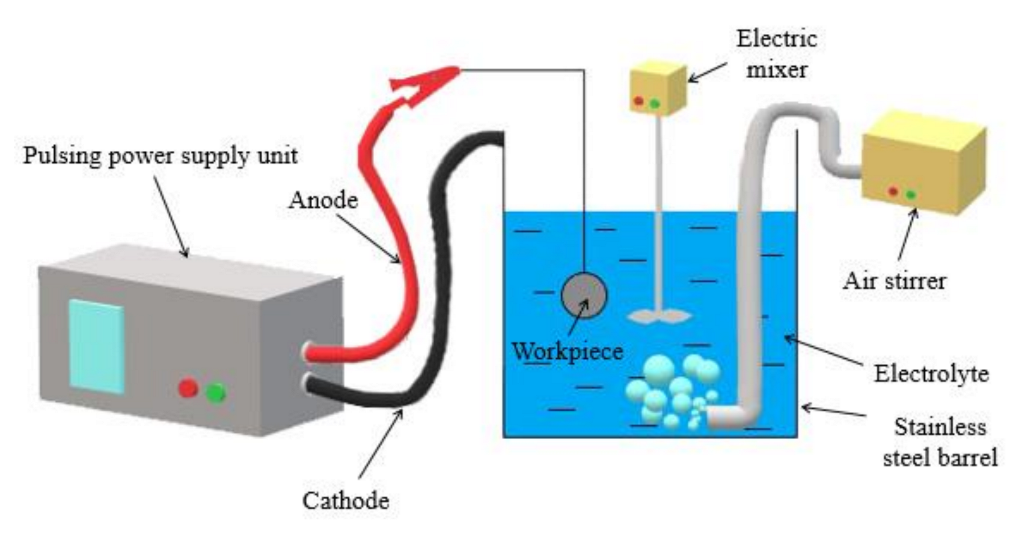
2.2. MAO Treatment
2.3. Surface Morphologies
2.4. Phase Composition
2.5. Electrochemical Research
2.6. Friction Tests
3. Results
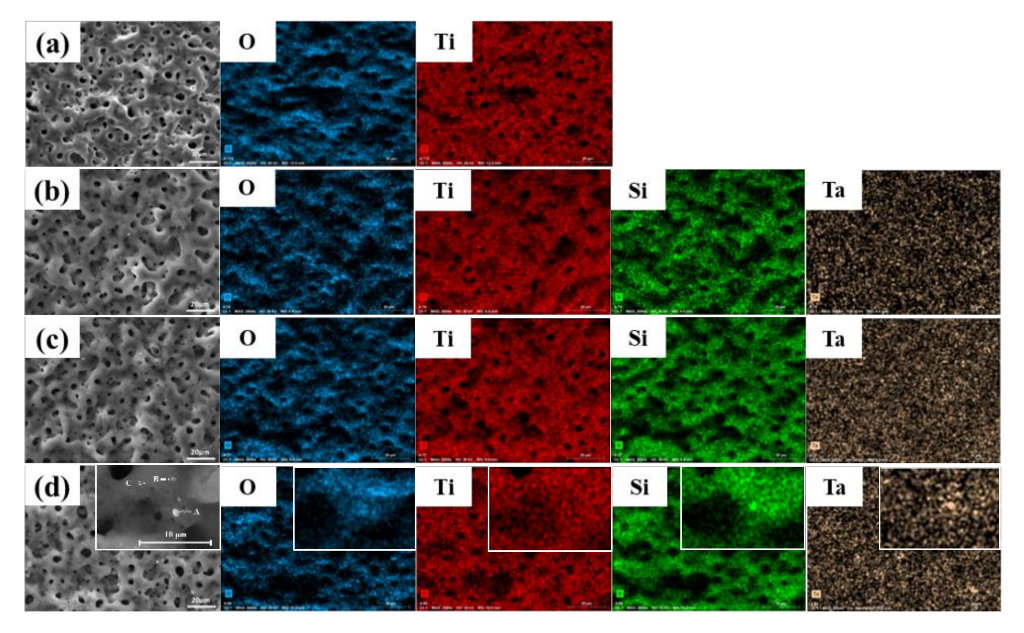
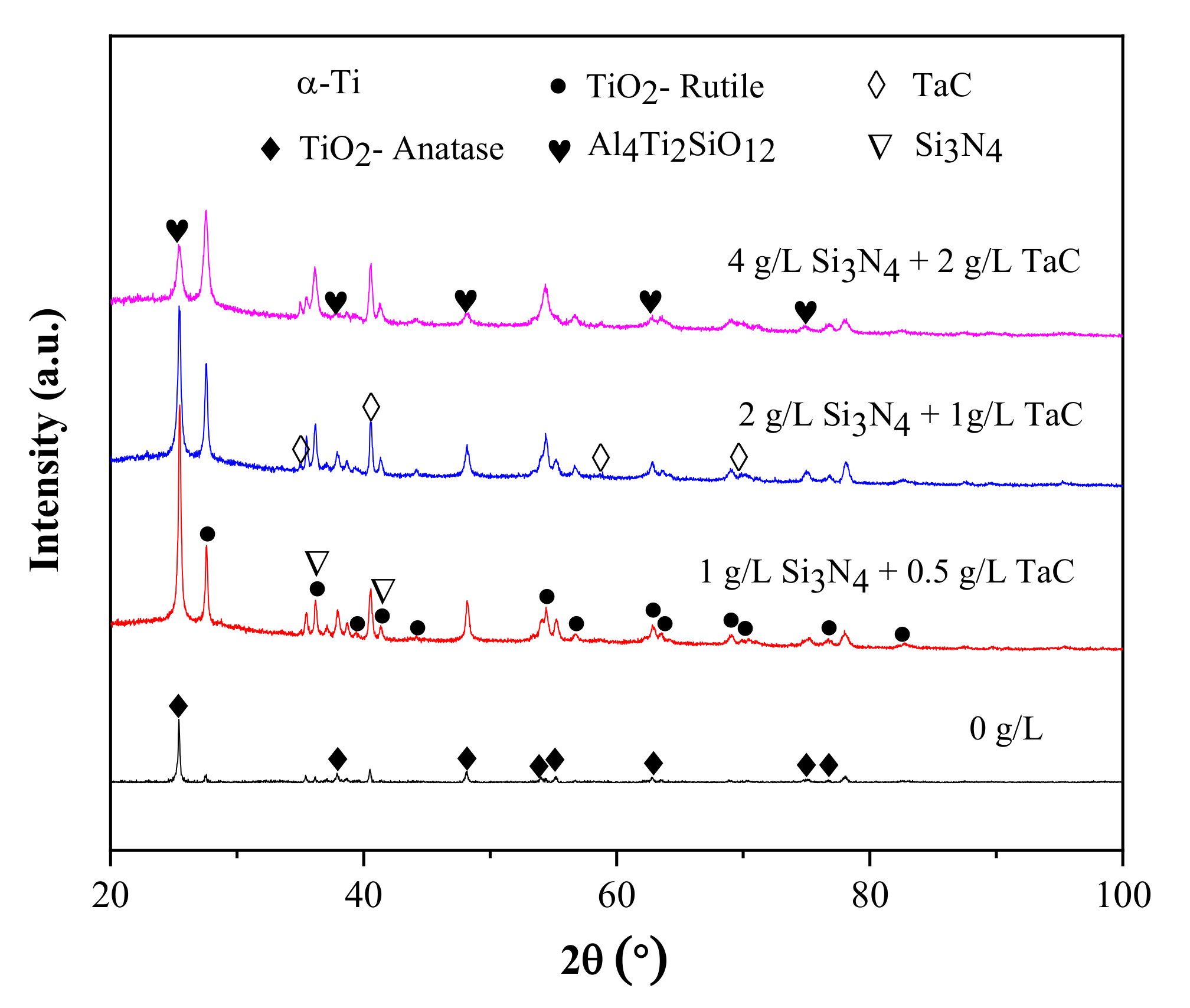
3.1. Composition and Microstructure
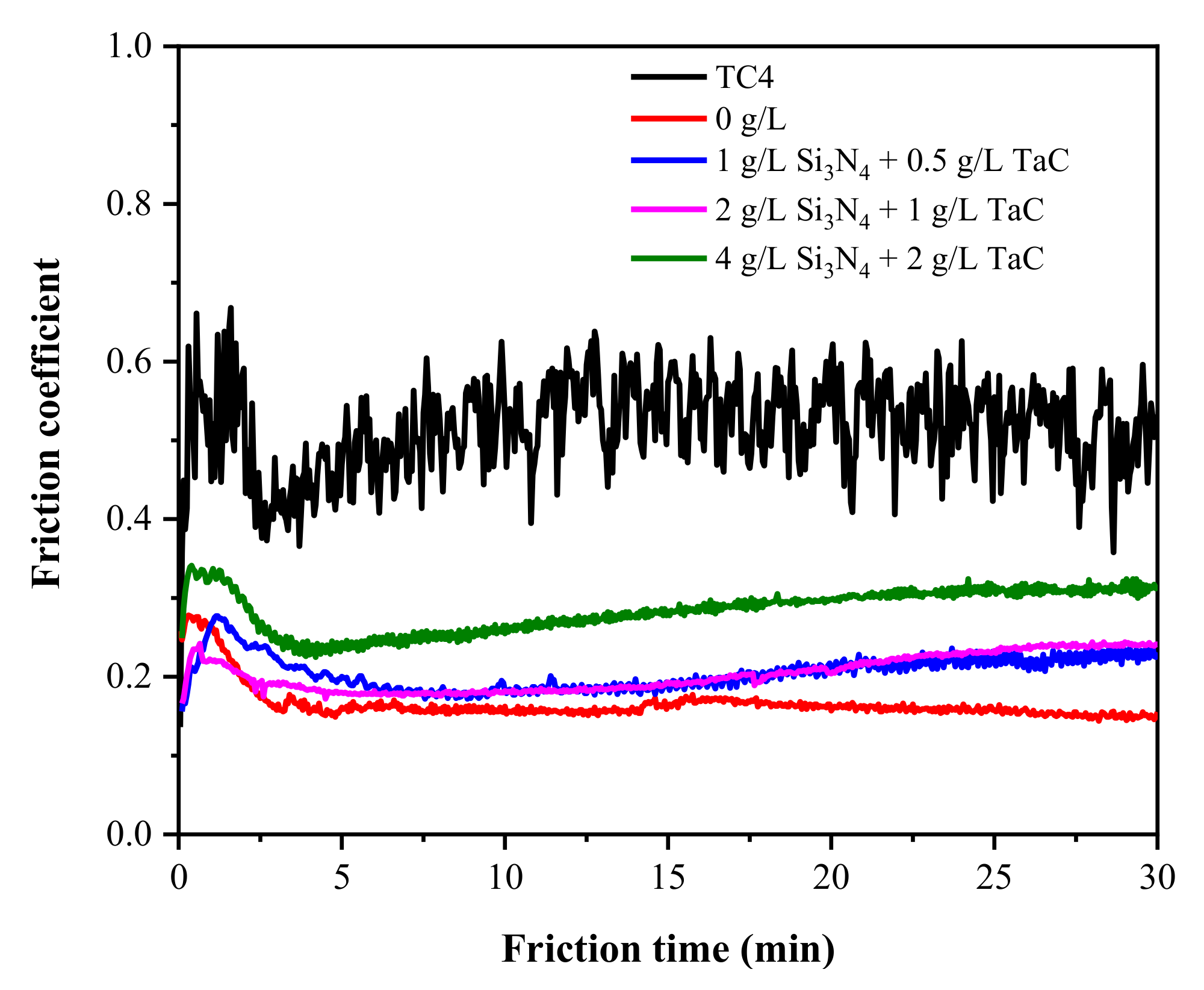
3.2. Tribological Properties
3.3. Corrosion Resistance
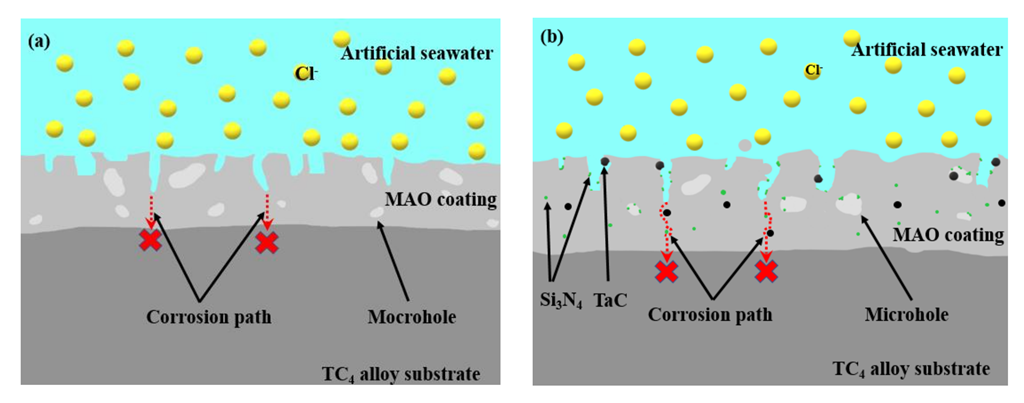
4. Discussions
5. Conclusions
- (1)
- The phase constituents of the composite MAO coatings with different contents of Si3N4/TaC particles were mainly composed of anatase TiO2, rutile TiO2, TaC, Si3N4, and Al4Ti2SiO12. By increasing the concentration of the Si3N4/TaC particles, the peak intensity of rutile TiO2 and TaC gradually increased, and the content of the metastable anatase-TiO2 decreased.
- (2)
- For the inert or partly-reactive incorporation of Si3N4/TaC particles during the MAO process, the thickness of the composite MAO coatings gradually increased with the increment of the concentration of Si3N4/TaC particles. The porosity of the MAO coatings first decreased and then increased with the increasing concentration of Si3N4/TaC particles.
- (3)
- The MAO coatings greatly improved the tribological properties of the TC4 alloy in the artificial seawater solution, regardless of the addition of the Si3N4/TaC particles. The composite MAO coatings with 1 g/L Si3N4 + 0.5 g/L TaC particles presented a relatively lower friction coefficient and the narrowest wear scar, thus showing the best tribological properties.
- (4)
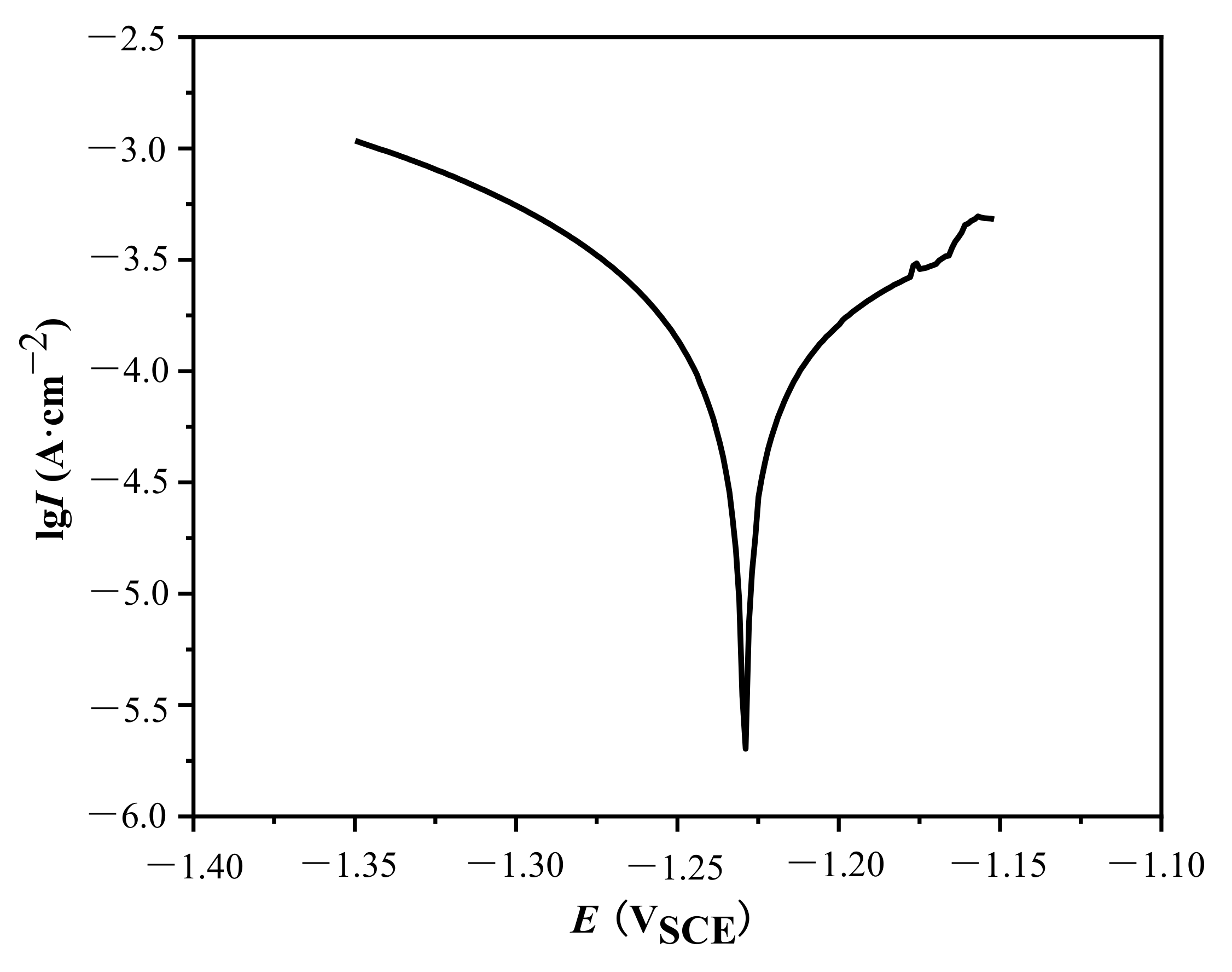
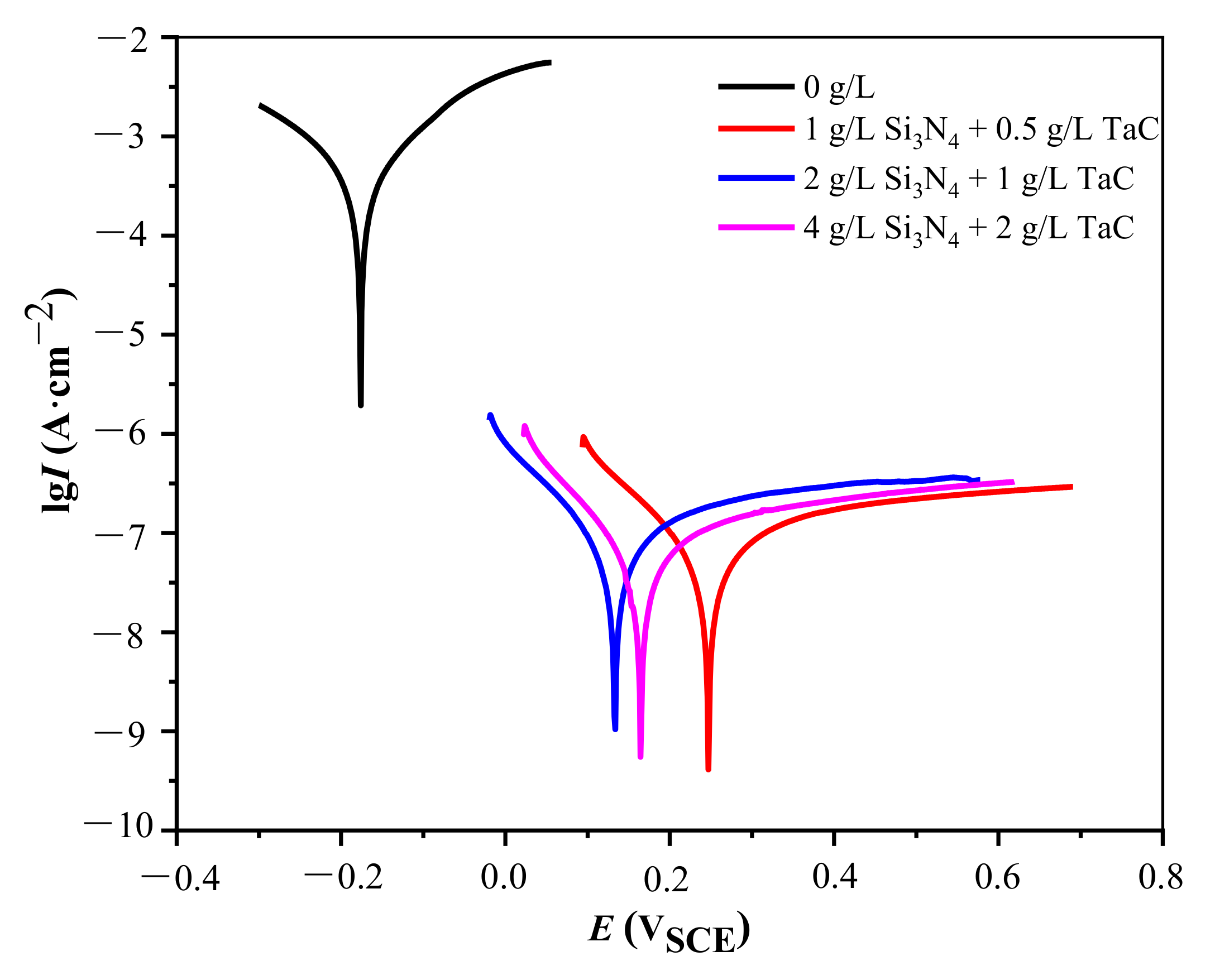
- The addition of Si3N4/TaC particles could enhance the corrosion resistance of the MAO coatings. The composite MAO coatings with 1 g/L Si3N4 + 0.5 g/L TaC particles exhibited the highest corrosion potential and the lower current density, i.e., the optimal corrosion resistance in the artificial seawater solution. This could be attributed to the fact that the coating had the lowest surface porosity.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jha, A.K.; Singh, S.K.; Kiranmayee, M.S.; Sreekumar, K.; Sinha, P.P. Failure analysis of titanium alloy (Ti6Al4V) fastener used in aerospace application. Eng. Fail. Anal. 2010, 17, 1457–1465. [Google Scholar] [CrossRef]
- Ding, R.; Guo, Z.X.; Wilson, A. Microstructural evolution of a Ti–6Al–4V alloy during thermomechanical processing. Mater. Sci. Eng. A 2002, 327, 233–245. [Google Scholar] [CrossRef]
- Rack, H.J.; Qazi, J.I. Titanium alloys for biomedical applications. Mater. Sci. Eng. C 2006, 26, 1269–1277. [Google Scholar] [CrossRef]
- Yang, W.; Gao, Y.; Guo, P.; Xu, D.P.; Wang, A.Y. Adhesion, biological corrosion resistance and biotribological properties of carbon films deposited on MAO coated Ti substrates. J. Mech. Behav. Biomed. 2020, 101, 103448. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Lin, N.; Peng, Z.; Zou, J.; Han, P.; Wang, Z.; Tang, B. Surface damage mitigation of TC4 alloy via micro arc oxidation for oil and gas exploitation application: Characterizations of microstructure and evaluations on surface performance. Appl. Surf. Sci. 2018, 436, 467–476. [Google Scholar] [CrossRef]
- Mousavi-Semnani, S.Z.; Yousefpour, M.; Zareidoost, A. Enhancing the biocompatibility of ZrO2 thin film on Zr–2.5Nb alloy by anodizing treatment using an electrolyte containing biofunctional groups. Thin Solid Films 2022, 753, 139279. [Google Scholar] [CrossRef]
- Deng, H.Y.; Xu, K.X.; Liu, S.G.; Zhang, C.F.; Zhu, X.W.; Zhou, H.R.; Xia, C.Q.; Shi, C.B. Impact of engineering surface treatment on surface properties of biomedical TC4 Alloys under an artificial human environment. Coatings 2022, 12, 157. [Google Scholar] [CrossRef]
- Huang, J.; Ma, J.; Wu, L.; Wei, J.; Li, H. Microstructure and wear resistance of WS2/W composite coating on TC4 titanium alloy surface. J. Phys. Conf. Ser. 2021, 1885, 032046. [Google Scholar] [CrossRef]
- Salman, S.H.; Shihab, A.A.; Kh.Elttayef, A.H. Design and construction of nanostructure TiO2 thin film gas sensor prepared by R.F magnetron sputtering technique. Energy Procedia 2019, 157, 283–289. [Google Scholar] [CrossRef]
- Wu, S.K.; Yang, W.; Gao, W.; Yao, Y.H.; Zhang, Y.; Chen, J. Characterization of MAO + Cu composite coatings on aluminum alloy. Coatings 2021, 11, 1172. [Google Scholar] [CrossRef]
- Da Silva Rodrigues, J.; Antonini, L.M.; da Cunha Bastos, A.A.; Zhou, J.; de Fraga Malfatti, C. Corrosion resistance and tribological behavior of ZK30 magnesium alloy coated by plasma electrolytic oxidation. Surf. Coat. Technol. 2021, 410, 126983. [Google Scholar] [CrossRef]
- Gao, J.; Zheng, K.; Yu, S.W.; Hei, H.J.; Wu, Y.X.; Gong, H.R.; Ma, Y. Characterization of quasi-static/dynamic contact mechanical properties of Mo surface-modified TC4. Coatings 2002, 12, 123. [Google Scholar] [CrossRef]
- Chen, C.A.; Jian, S.Y.; Lu, C.H.; Lee, C.Y.; Aktuğ, S.L.; Ger, M.D. Evaluation of microstructural effects on corrosion behavior of AZ31B magnesium alloy with a MAO coating and electroless Ni–P plating. J. Mater. Res. Technol. 2020, 9, 13902–13913. [Google Scholar] [CrossRef]
- Asl, V.Z.; Chini, S.F.; Zhao, J.; Palizdar, Y.; Shaker, M.; Sadeghi, A. Corrosion properties and surface free energy of the ZnAl LDH/rGO coating on MAO pretreated AZ31 magnesium alloy. Surf. Coat. Technol. 2021, 426, 127764. [Google Scholar]
- Song, Y.; Wang, H.; Liu, Q.; Li, G.; Wang, S.; Zhu, X. Sodium dodecyl sulfate (SDS) intercalated MgAl layered double hydroxides film to enhance the corrosion resistance of AZ31 magnesium alloy. Surf. Coat. Technol. 2021, 422, 127524. [Google Scholar] [CrossRef]
- Wang, Y.M.; Jiang, B.L.; Lei, T.Q.; Guo, L.X. Dependence of growth features of microarc oxidation coatings of titanium alloy on control modes of alternate pulse. Mater. Lett. 2004, 58, 1907–1911. [Google Scholar] [CrossRef]
- Wang, Y.M.; Jia, D.C.; Guo, L.X.; Lei, T.Q.; Liang, B.L. Effect of discharge pulsating on microarc oxidation coatings formed on Ti6Al4V alloy. Mater. Chem. Phys. 2005, 90, 128–133. [Google Scholar] [CrossRef]
- Xue, W.B.; Wang, C.; Deng, Z.W.; Chen, R.Y.; Zhang, T.H. Influence of electrolytes on the microstructure of microarc oxidation coatings on Ti–6Al–4V alloy. J. Mater. Sci. Technol. 2002, 18, 37–39. [Google Scholar]
- Yang, W.; Xu, D.P.; Guo, Q.Q.; Chen, T.; Chen, J. Influence of electrolyte composition on microstructure and properties of coatings formed on pure Ti substrate by micro arc oxidation. Surf. Coat. Technol. 2018, 349, 522–528. [Google Scholar] [CrossRef]
- Yao, Z.P.; Xu, Y.J.; Jiang, Z.H.; Wang, F.P. Effects of cathode pulse at low frequency on the structure and composition of plasma electrolytic oxidation ceramic coatings. J. Alloys Compd. 2009, 488, 273–278. [Google Scholar] [CrossRef]
- Gao, W.; Liu, J.N.; Wei, J.P.; Yao, Y.H.; Ma, X.Q.; Yang, W. Enhanced properties of micro arc oxidation coating with Cu addition on TC4 alloy in marine environment. Coatings 2021, 11, 1168. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, W.; Yu, S.; Wang, L.Q.; Ma, X.Q.; Gao, W.; Lan, N.; Shao, W.T. Microstructure and properties in artificial seawater of copper-doped micro-arc coatings on TC4 alloy. Coatings 2022, 12, 883. [Google Scholar] [CrossRef]
- Rafieerad, A.R.; Ashra, M.R.; Mahmoodian, R.; Bushroa, A.R. Surface characterization and corrosion behavior of calcium phosphate-base composite layer on titanium and its alloys via plasma electrolytic oxidation: A review paper. Mater. Sci. Eng. C 2015, 57, 307–413. [Google Scholar] [CrossRef] [PubMed]
- Muhaffffel, F.; Kaba, M.; Cempura, G.; Derin, B.; Kruk, A.; Atar, E.; Cimenoglu, H. Influence of alumina and zirconia incorporations on the structure and wear resistance of titania-based MAO coatings. Surf. Coat. Technol. 2019, 377, 124900. [Google Scholar] [CrossRef]
- Lu, X.P.; Mohedano, M.; Blawert, C.; Matykina, E.; Arrabal, R.; Kainer, K.U.; Zheludkevich, M.L. Plasma electrolytic oxidation coatings with particle additions—A review. Surf. Coat. Technol. 2016, 307, 1165–1182. [Google Scholar] [CrossRef]
- Wang, C.; Hao, J.M.; Xin, Y.Z.; Guo, C.F.; Chen, H. High temperature oxidation behavior of TiO2 + ZrO2 composite ceramic coatings prepared by microarc oxidation on Ti6Al4V alloy. Surf. Coat. Technol. 2015, 215, 201–207. [Google Scholar] [CrossRef]
- Wang, S.X.; Zhao, Q.; Liu, D.X.; Du, N. Microstructure and elevated temperature tribological behavior of TiO2/Al2O3 composite ceramic coating formed by microarc oxidation of Ti6Al4V alloy. Surf. Coat. Technol. 2015, 272, 343–349. [Google Scholar] [CrossRef]
- Mu, M.; Liang, J.; Zhou, X.; Xiao, Q. One-step preparation of TiO2/MoS2 composite coating on Ti6Al4V alloy by plasma electrolytic oxidation and its tribological properties. Surf. Coat. Technol. 2013, 214, 124–130. [Google Scholar] [CrossRef]
- Chen, X.W.; Li, M.L.; Zhang, D.F.; Cai, L.P.; Ren, P.; Hu, J.; Liao, D.D. Corrosion resistance of MoS2-modified titanium alloy micro-arc oxidation coating. Surf. Coat. Technol. 2022, 433, 128127. [Google Scholar] [CrossRef]
- Ao, N.; Liu, D.X.; Wang, S.X.; Zhao, Q.; Zhang, X.H.; Zhang, M.M. Microstructure and tribological behavior of a TiO2/hBN composite ceramic coating formed via micro-arc oxidation of Ti–6Al–4V alloy. J. Mater. Sci. Technol. 2016, 32, 1071–1076. [Google Scholar] [CrossRef]
- Lou, B.S.; Lin, Y.Y.; Tseng, C.M.; Lu, Y.C.; Duh, J.G.; Lee, J.W. Plasma electrolytic oxidation coatings on AZ31 magnesium alloys with Si3N4 nanoparticle additives. Surf. Coat. Technol. 2017, 332, 358–367. [Google Scholar] [CrossRef]
- Lu, X.P.; Blawert, C.; Scharnagl, N.; Kainer, K.N. Influence of incorporating Si3N4 particles into the oxide layer produced by plasma electrolytic oxidation on AM50 Mg alloy on coating morphology and corrosion properties. J. Magnes. Alloy 2013, 1, 267–274. [Google Scholar] [CrossRef] [Green Version]
- Aliofkhazraei, M.; Sabour Rouhaghdam, A.; Shahrabi, T. Abrasive wear behavior of Si3N4/TiO2 nanocomposite coatings fabricated by plasma electrolytic oxidation. Surf. Coat. Technol. 2010, 205, S41–S46. [Google Scholar] [CrossRef]
- Gao, W.; Liu, J.N.; Ding, Z.S.; Zhang, Y.; Yao, Y.H.; Yang, W. Influence of Si3N4 nanoparticles on microstructure and properties of microarc oxidation coatings on TC4 alloy. Rare Metal Mater. Eng. 2022, 51, 1537–1542. [Google Scholar]
- Zhang, X.H.; Hilmas, G.E.; Fahrenholtz, W.G. Densification and mechanical properties of TaC-based ceramics. Mater. Sci. Eng. C 2009, 501, 37–43. [Google Scholar] [CrossRef]
- Ding, Z.S.; Gao, W.; Wei, J.P.; Jin, Y.H.; Zhao, C.; Yang, W. Effects of TaC microparticles on structure and properties of micro-arc oxidation coating on Ti–6Al–4V alloy. Acta Phys. Sin. 2022, 71, 028102. [Google Scholar] [CrossRef]
- Wang, F.; Wang, X.H.; Xie, W.E.; Wang, F.; Gan, Q.; Ping, S.; Wei, J.; Li, F.Q.; Wang, Z.M. Simultaneous incorporation of gallium oxide and tantalum microparticles into micro-arc oxidation coating of titanium possessing antibacterial effect and stimulating cellular response. Biomater. Adv. 2022, 135, 212736. [Google Scholar] [CrossRef]
- Zhang, S.L.; Zou, X.R.; Liu, N.; Wang, H.R.; Xia, C.Q.; Liang, C.Y. In situ preparation of a novel Ta2O5/MAO composite coating on magnesium for anti-corrosion protection. Surf. Coat. Technol. 2022, 430, 128003. [Google Scholar] [CrossRef]
- Du, N.; Wang, S.X.; Zhao, Q.; Zhu, W.H. Microstructure and tribological properties of microarc oxidation composite coating containing Cr2O3 particles on TC4 titanium alloy. Rare Metal Mater. Eng. 2013, 42, 621–624. [Google Scholar]
- Gleiter, H. Nanostructured materials: Basic concepts and microstructure. Acta Mater. 2000, 48, 1–29. [Google Scholar] [CrossRef]
- Qi, W.H.; Wang, M.P. Size and shape dependent melting temperature of metallic nanoparticles. Mater. Chem. Phys. 2004, 88, 280–284. [Google Scholar] [CrossRef]
- Xue, W.B.; Wang, C.; Chen, R.Y.; Deng, Z.W. Structure and properties characterization of ceramic coatings produced on Ti–6Al–4V alloy by microarc oxidation in aluminate solution. Mater. Lett. 2002, 52, 435–441. [Google Scholar] [CrossRef]
- Wang, Y.M.; Jiang, B.L.; Lei, T.Q.; Guo, L.X. Microarc oxidation coatings formed on Ti6Al4V in Na2SiO3 system solution: Microstructure, mechanical and tribological properties. Surf. Coat. Technol. 2006, 201, 82–89. [Google Scholar] [CrossRef]
- Lan, N.; Yang, W.; Gao, W.; Guo, P.; Zhao, C.; Chen, J. Characteristic of ta–C film on micro arc oxidation coated titanium all in artificial seawater. Diam. Relat. Mater. 2021, 117, 108483. [Google Scholar] [CrossRef]
- Jin, F.Y.; Chu, P.K.; Tong, H.H.; Zhao, J. Improvement of surface porosity and properties of alumina films by incorporation of Fe micrograins in micro-arc oxidation. Appl. Surf. Sci. 2006, 253, 863–868. [Google Scholar] [CrossRef]
- Muhaffel, F.; Cimenoglu, H. Development of corrosion and wear resistant micro-arc oxidation coating on a magnesium alloy. Surf. Coat. Technol. 2019, 357, 822–832. [Google Scholar] [CrossRef]
- Lou, B.S.; Lee, J.W.; Tseng, C.M.; Lin, Y.Y.; Yen, C.A. Mechanical property and corrosion resistance evaluation of AZ31 magnesium alloys by plasma electrolytic oxidation treatment: Effect of MoS2 particle addition. Surf. Coat. Technol. 2018, 350, 813–822. [Google Scholar] [CrossRef]
- Laleh, M.; Rouhaghdam, A.S.; Shahrabi, T.; Shanghi, A. Effect of alumina sol addition to micro-arc oxidation electrolyte on the properties of MAO coatings formed on magnesium alloy AZ91D. J. Alloys Compd. 2010, 496, 548–552. [Google Scholar] [CrossRef]



| Chemical Reagents | Concentration, g/L |
|---|---|
| NaCl | 24.53 |
| MgCl2·6H2O | 11.11 |
| Na2SO4 | 4.09 |
| CaCl2 | 1.16 |
| KCl | 0.70 |
| NaHCO3 | 0.20 |
| KBr | 0.10 |
| Specimen | Surface Density of the Microholes, Number/mm2 | Average Diameter of the Microholes, μm | Surface Density of the Bright Particles, Number/mm2 |
|---|---|---|---|
| 0 g/L | 25,316 ± 1751 | 1.835 ± 1.275 | 0 |
| 1 g/L + 0.5 g/L | 16,652 ± 1335 | 1.740 ± 1.226 | 2005 ± 151 |
| 2 g/L + 1 g/L | 17,426 ± 1811 | 1.771 ± 0.861 | 3828 ± 202 |
| 4 g/L + 2 g/L | 17,543 ± 2002 | 1.794 ± 1.136 | 5845 ± 346 |
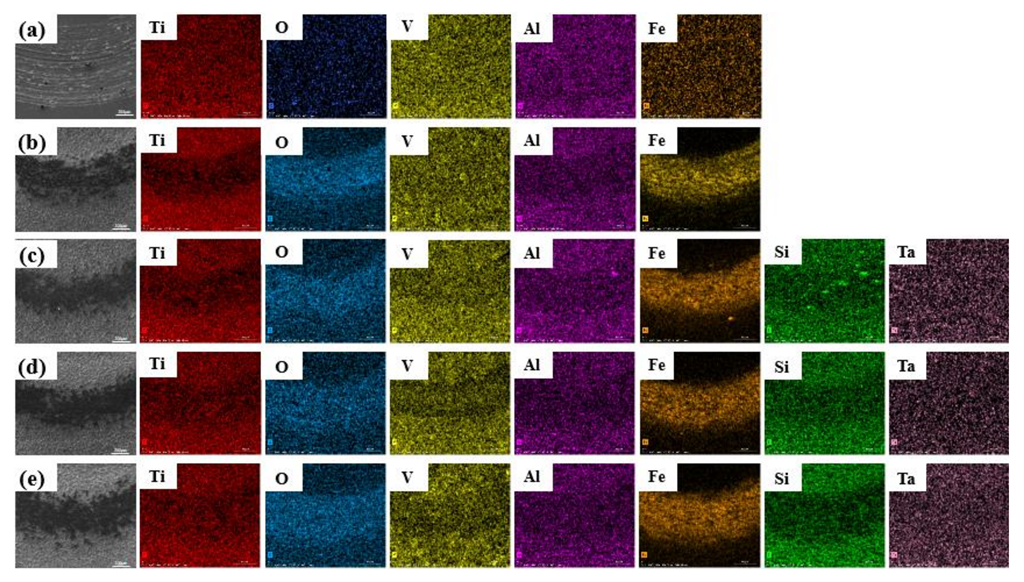
| Specimen | O | Ti | Si | Ta |
|---|---|---|---|---|
| 0 g/L | 69.3 | 20.5 | - | - |
| 1 g/L + 0.5 g/L | 68.5 | 19.6 | 3.2 | 0.2 |
| 2 g/L + 1 g/L | 67.5 | 19.0 | 5.6 | 0.6 |
| 4 g/L + 2 g/L | 66.8 | 15.8 | 11.6 | 1.0 |
| Region | O | Ti | Si | Ta |
|---|---|---|---|---|
| A | 42.0 | 23.4 | 25.3 | 3.5 |
| B | 60.1 | 13.5 | 11.0 | 8.5 |
| C | 55.5 | 20.2 | 17.0 | 1.0 |
| Specimen | TC4 | 0 g/L | 1 g/L + 0.5 g/L | 2 g/L + 1 g/L | 4 g/L + 2 g/L |
|---|---|---|---|---|---|
| Ecorr, VSCE | −1.229 | −0.176 | 0.246 | 0.135 | 0.165 |
| Icorr, A·cm−2 | 8.47 × 10−5 | 2.67 × 10−4 | 4.23 × 10−8 | 6.43 × 10−8 | 4.15 × 10−8 |
| Specimen | O | Ti | V | Al | Si | Ta | Fe |
|---|---|---|---|---|---|---|---|
| TC4 | 19.4 | 71.7 | 3.1 | 6.3 | - | - | 0.1 |
| 0 g/L | 72.3 | 17.7 | 0.3 | 1.6 | - | - | 3.0 |
| 1 g/L + 0.5 g/L | 72.5 | 16.6 | 0.3 | 1.6 | 2.7 | 0.2 | 2.1 |
| 2 g/L + 1 g/L | 71.8 | 15.0 | 0.3 | 1.6 | 4.5 | 0.5 | 2.3 |
| 4 g/L + 2 g/L | 71.5 | 12.4 | 0.3 | 1.5 | 8.9 | 0.7 | 2.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, W.; Wang, L.; Jin, Y.; Yao, Y.; Ding, Z.; Yang, W.; Liu, J. Effect of Si3N4/TaC Particles on the Structure and Properties of Microarc Oxidation Coatings on TC4 Alloy. Coatings 2022, 12, 1247. https://doi.org/10.3390/coatings12091247
Gao W, Wang L, Jin Y, Yao Y, Ding Z, Yang W, Liu J. Effect of Si3N4/TaC Particles on the Structure and Properties of Microarc Oxidation Coatings on TC4 Alloy. Coatings. 2022; 12(9):1247. https://doi.org/10.3390/coatings12091247
Chicago/Turabian StyleGao, Wei, Liqun Wang, Yaohua Jin, Yuhong Yao, Zhisong Ding, Wei Yang, and Jiangnan Liu. 2022. "Effect of Si3N4/TaC Particles on the Structure and Properties of Microarc Oxidation Coatings on TC4 Alloy" Coatings 12, no. 9: 1247. https://doi.org/10.3390/coatings12091247
APA StyleGao, W., Wang, L., Jin, Y., Yao, Y., Ding, Z., Yang, W., & Liu, J. (2022). Effect of Si3N4/TaC Particles on the Structure and Properties of Microarc Oxidation Coatings on TC4 Alloy. Coatings, 12(9), 1247. https://doi.org/10.3390/coatings12091247





