Preparation and Enhanced Antimicrobial Activity of Thymol Immobilized on Different Silica Nanoparticles with Application in Apple Juice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Silica Nanoparticles and Thymol Derivatives Preparation
2.3. Characterization
2.4. Antibacterial Activity Assays
2.4.1. Culture Conditions and Bacterial Strain
2.4.2. Antibacterial Activity Assays In Vitro
2.4.3. Antibacterial Activity Assays in a Real Food System
2.4.4. Determination of the Physicochemical Properties of the Apple Juice
2.5. Statistical Analysis
3. Results and Discussion
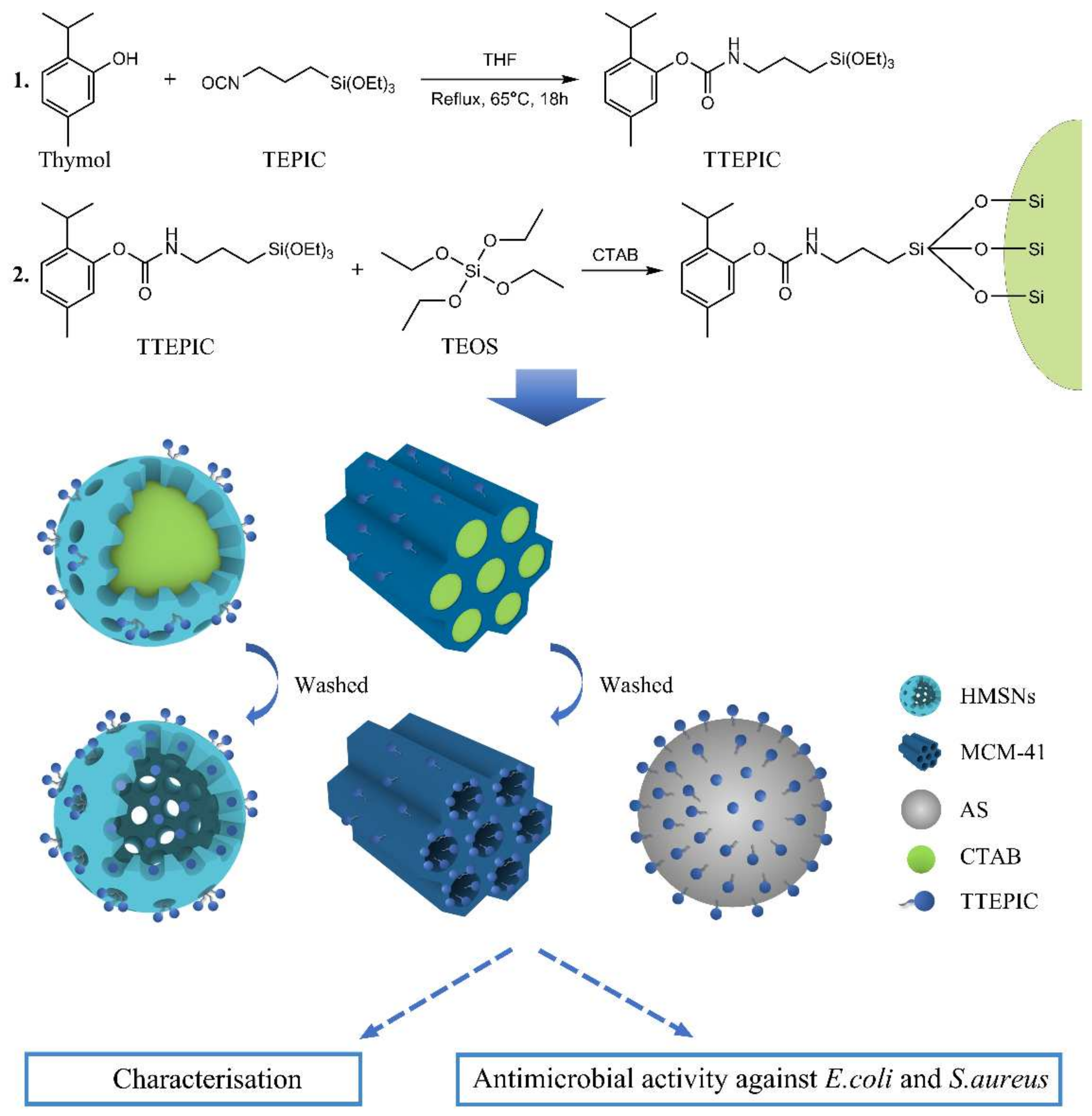
3.1. Design and Synthesis of the Antimicrobial Supports
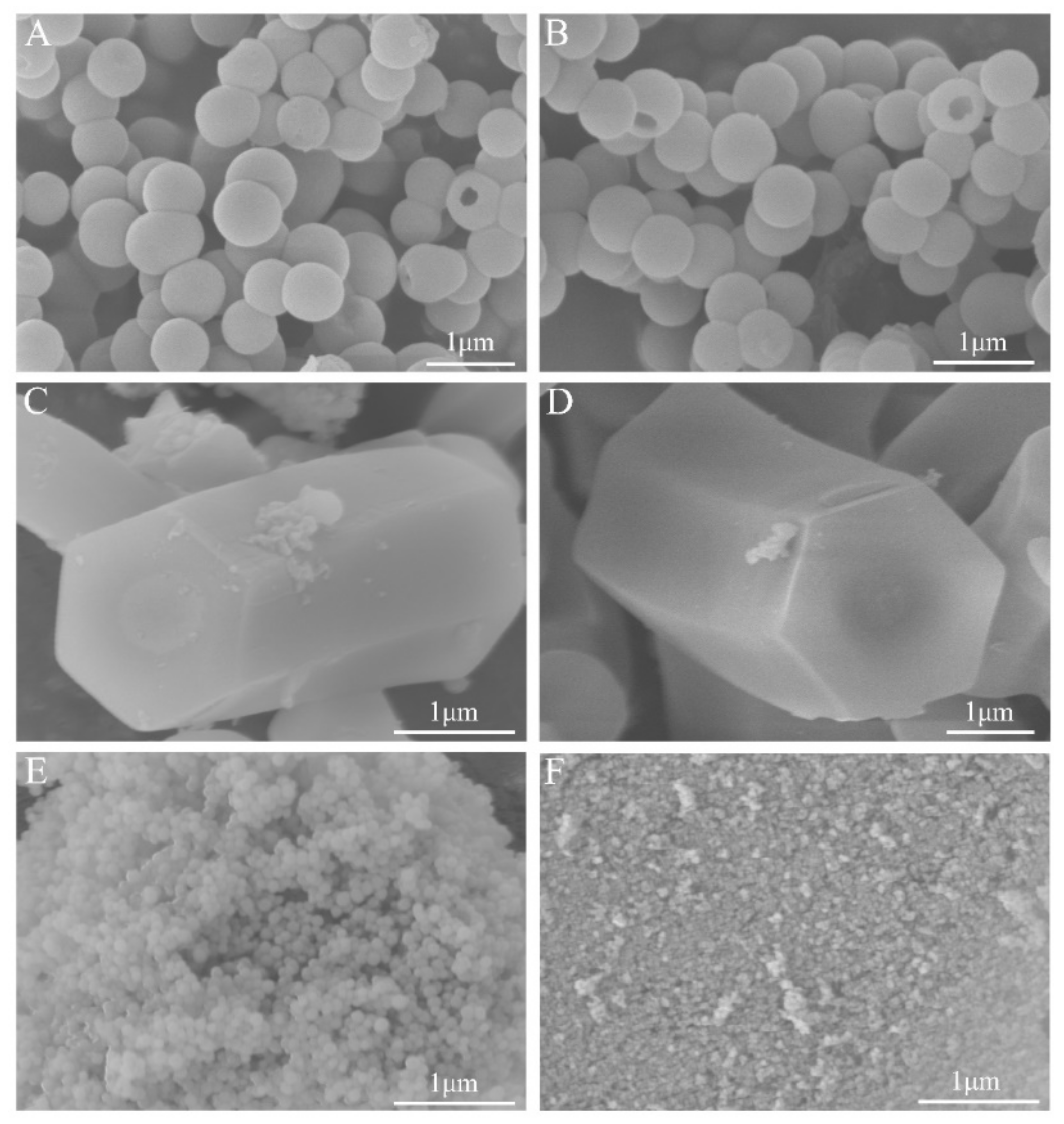
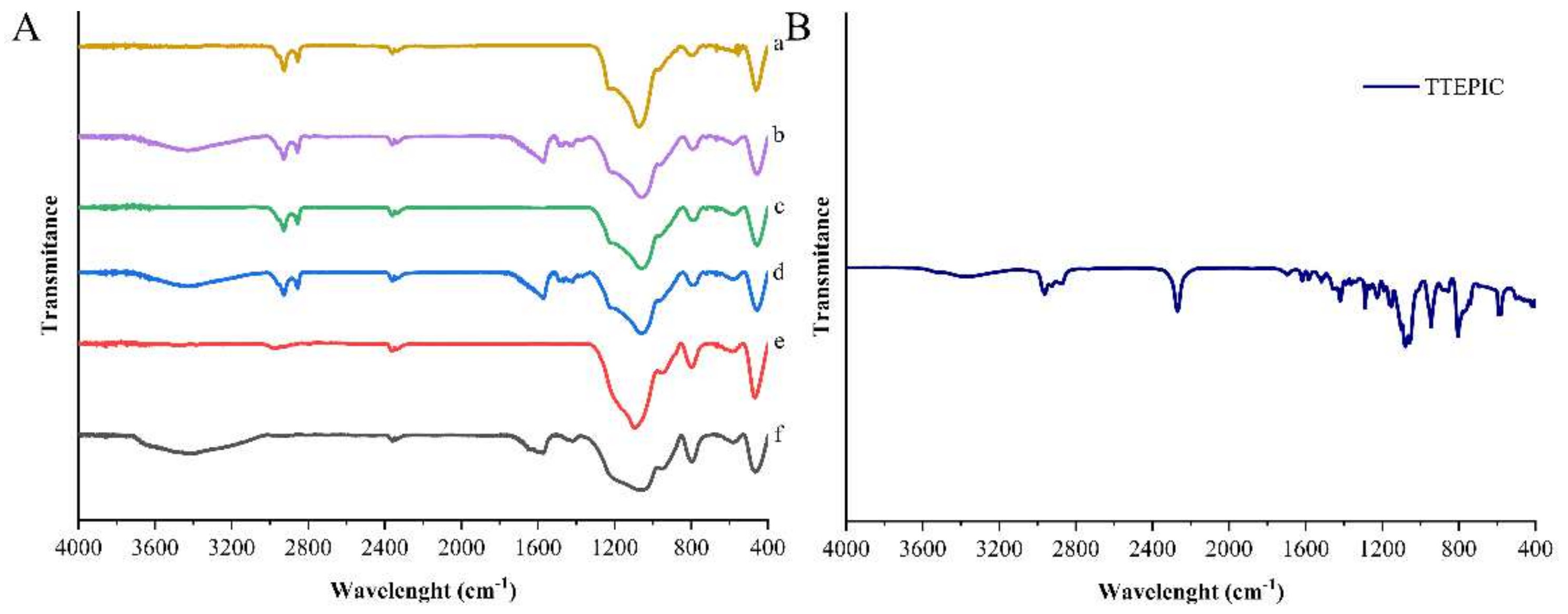
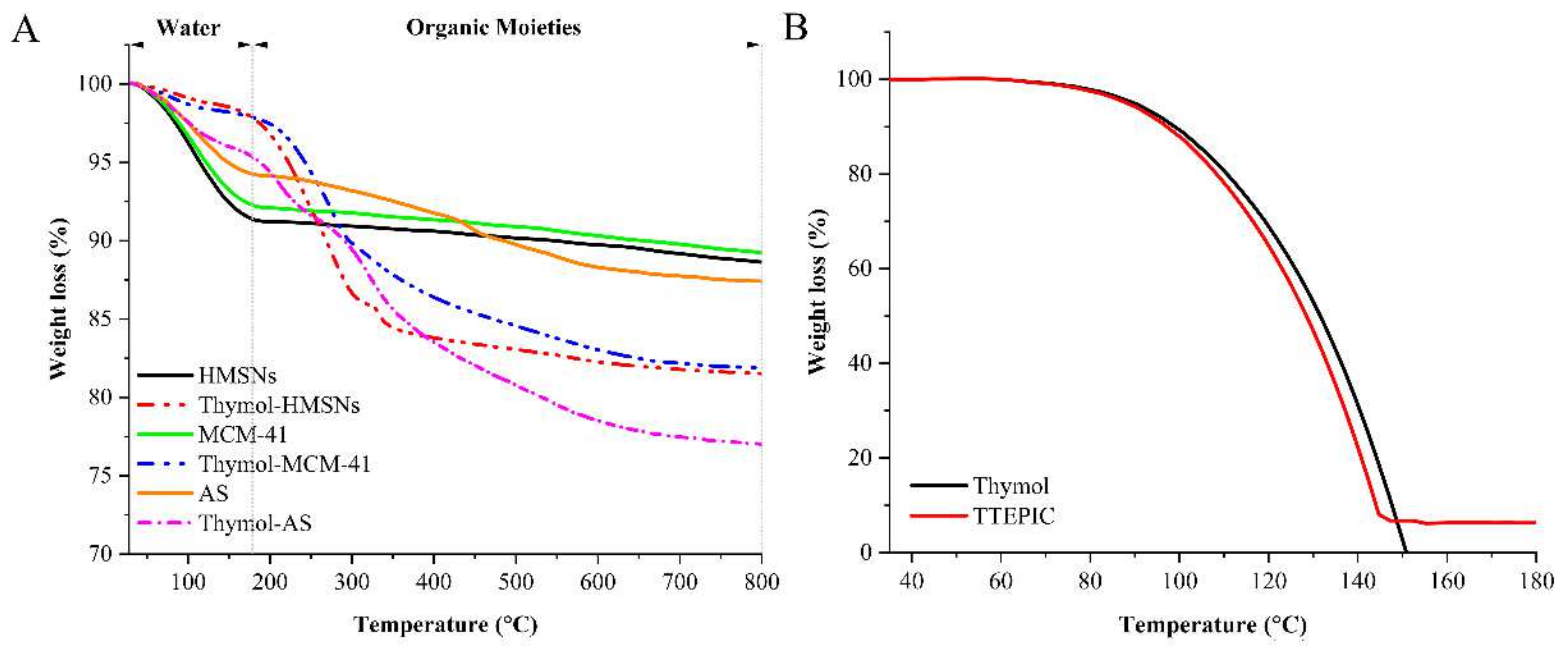
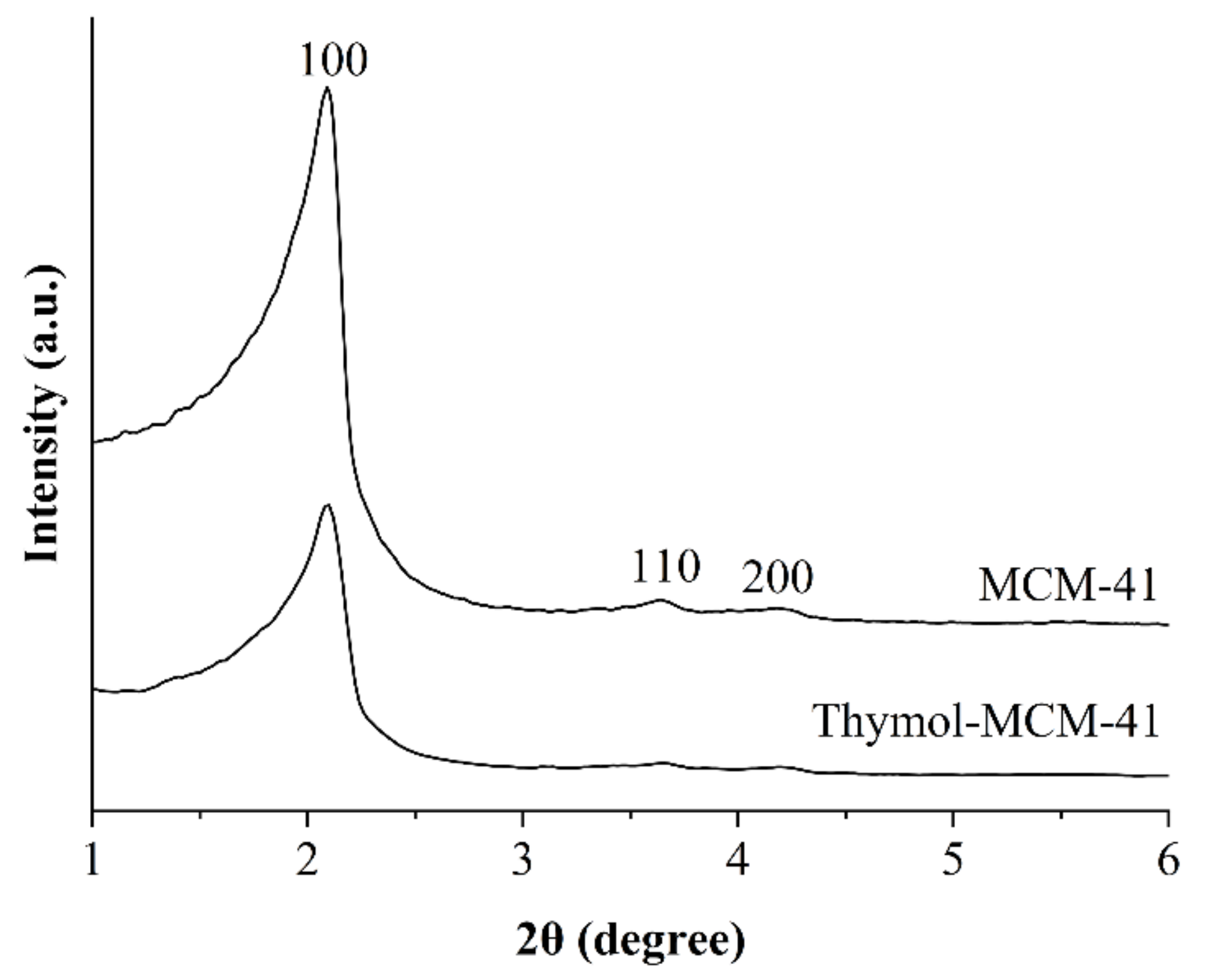
3.2. Characterization of Antimicrobial Supports
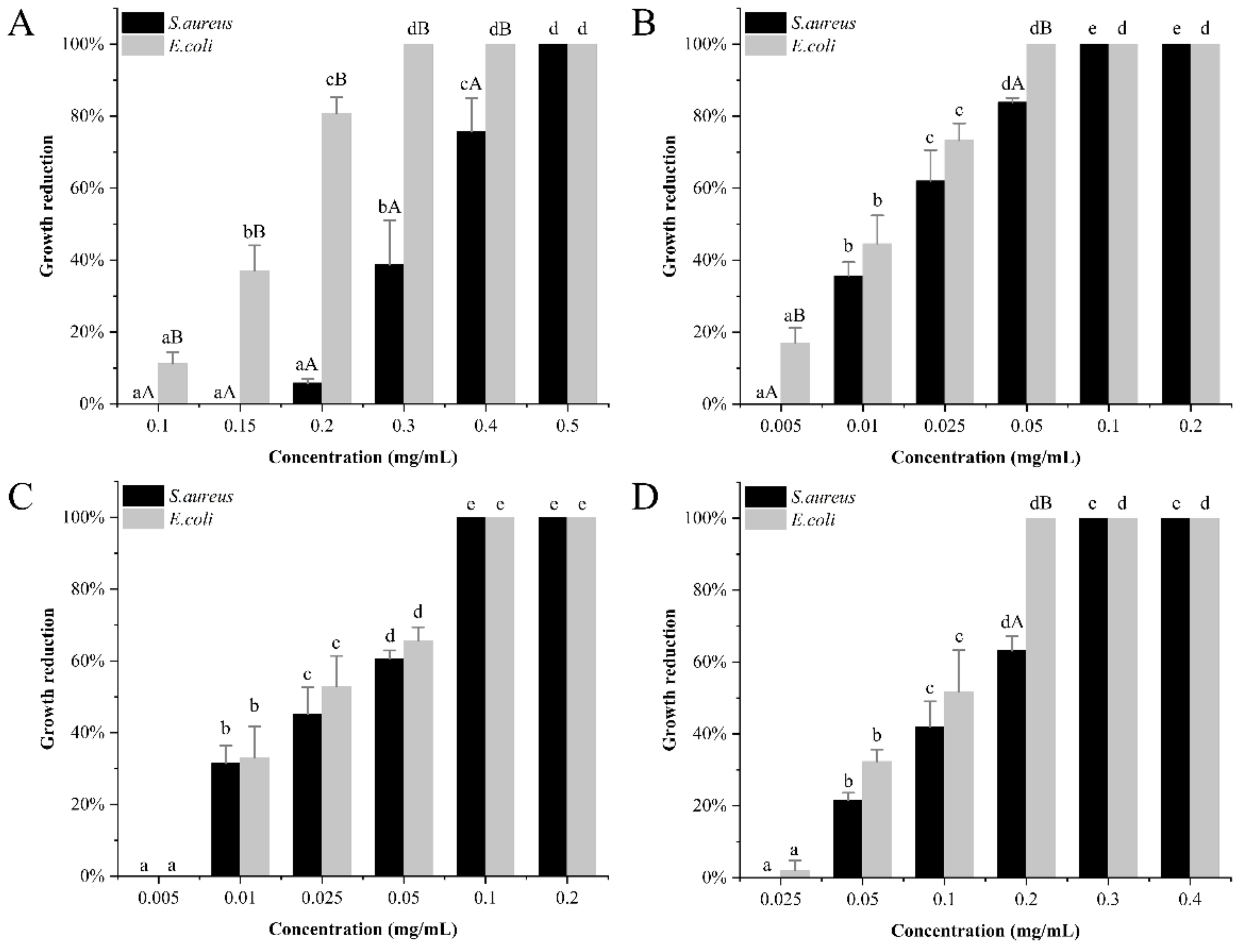
3.3. Antimicrobial Activity of Antimicrobial Supports In Vitro
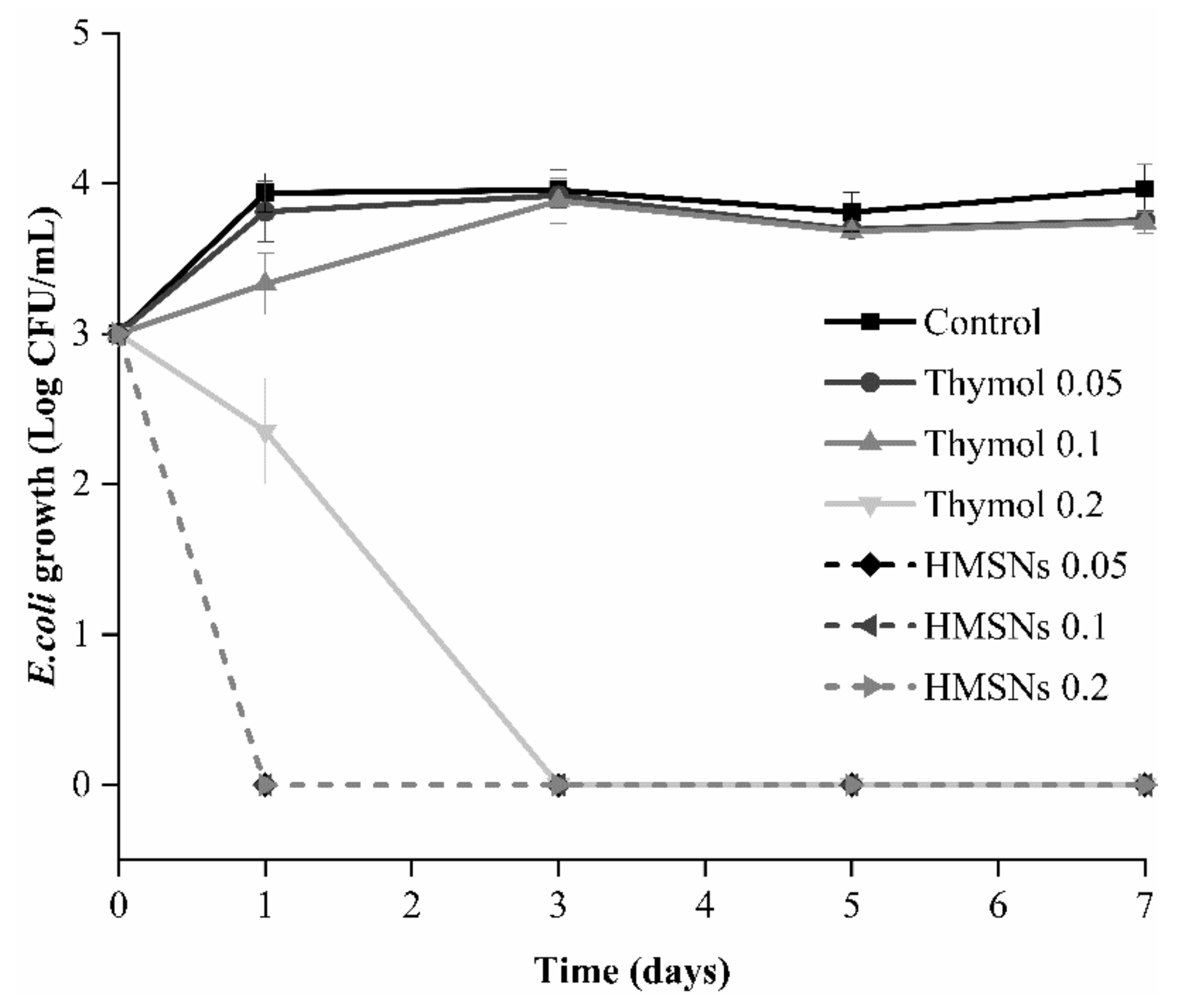
3.4. Antimicrobial Activity of Antimicrobial Supports in Apple Juice
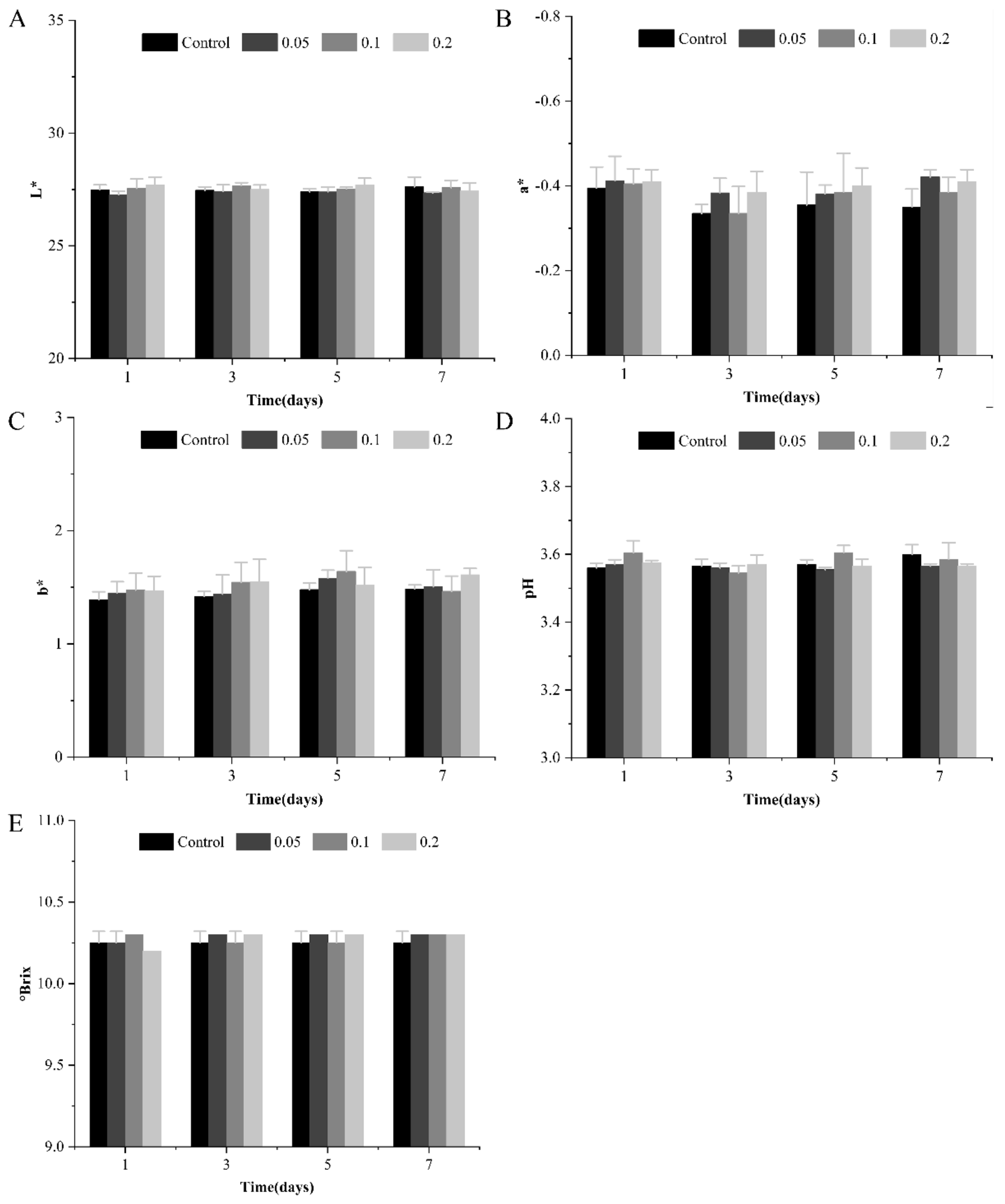
3.5. Physicochemical Properties of Apple Juice during Storage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Havelaar, A.H.; Kirk, M.D.; Torgerson, P.R.; Gibb, H.J.; Hald, T.; Lake, R.J.; Praet, N.; Bellinger, D.C.; de Silva, N.R.; Gargouri, N.; et al. World Health Organization Foodborne Disease Burden Epidemiology Reference, G. World Health Organization Global Estimates and Regional Comparisons of the Burden of Foodborne Disease in 2010. PLoS Med. 2015, 12, e1001923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, B.; Davidson, P.M.; Zhong, Q. Nanocapsular dispersion of thymol for enhanced dispersibility and increased antimicrobial effectiveness against Escherichia coli O157:H7 and Listeria monocytogenes in model food systems. Appl. Environ. Microbiol. 2012, 78, 8448–8453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pisoschi, A.M.; Pop, A.; Georgescu, C.; Turcus, V.; Olah, N.K.; Mathe, E. An overview of natural antimicrobials role in food. Eur. J. Med. Chem. 2018, 143, 922–935. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Capeletti, L.B.; de Oliveira, L.F.; Goncalves, K.d.A.; Affonso de Oiveira, J.F.; Saito, A.; Kobarg, J.; Zimnoch dos Santos, J.H.; Cardoso, M.B. Tailored Silica-Antibiotic Nanoparticles: Overcoming Bacterial Resistance with Low Cytotoxicity. Langmuir 2014, 30, 7456–7464. [Google Scholar] [CrossRef]
- Ruiz-Rico, M.; Perez-Esteve, E.; Bernardos, A.; Sancenon, F.; Martinez-Manez, R.; Marcos, M.D.; Barat, J.M. Enhanced antimicrobial activity of essential oil components immobilized on silica particles. Food Chem. 2017, 233, 228–236. [Google Scholar] [CrossRef]
- Man, A.; Santacroce, L.; Jacob, R.; Mare, A.; Man, L. Antimicrobial Activity of Six Essential Oils Against a Group of Human Pathogens: A Comparative Study. Pathogens 2019, 8, 15. [Google Scholar] [CrossRef] [Green Version]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef]
- Prakash, B.; Kujur, A.; Yadav, A.; Kumar, A.; Singh, P.P.; Dubey, N.K. Nanoencapsulation: An efficient technology to boost the antimicrobial potential of plant essential oils in food system. Food Control 2018, 89, 1–11. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 25, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Rai, M.; Paralikar, P.; Jogee, P.; Agarkar, G.; Ingle, A.P.; Derita, M.; Zacchino, S. Synergistic antimicrobial potential of essential oils in combination with nanoparticles: Emerging trends and future perspectives. Int. J. Pharm. 2017, 519, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Mousavi Khaneghah, A.; Gavahian, M.; Marszalek, K.; Es, I.; Munekata, P.E.S.; Ferreira, I.; Barba, F.J. Understanding the potential benefits of thyme and its derived products for food industry and consumer health: From extraction of value-added compounds to the evaluation of bioaccessibility, bioavailability, anti-inflammatory, and antimicrobial activities. Crit. Rev. Food Sci. Nutr. 2019, 59, 2879–2895. [Google Scholar] [CrossRef] [PubMed]
- Cho, T.J.; Park, S.M.; Yu, H.; Seo, G.H.; Kim, H.W.; Kim, S.A.; Rhee, M.S. Recent Advances in the Application of Antibacterial Complexes Using Essential Oils. Molecules 2020, 25, 1752. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils-Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [Green Version]
- Prakash, B.; Kiran, S. Essential oils: A traditionally realized natural resource for food preservation. Curr. Sci. 2016, 110, 1890–1892. [Google Scholar]
- Barros, C.H.N.; Casey, E. A Review of Nanomaterials and Technologies for Enhancing the Antibiofilm Activity of Natural Products and Phytochemicals. ACS Appl. Nano Mater. 2020, 3, 8537–8556. [Google Scholar] [CrossRef]
- Donsì, F.; Annunziata, M.; Sessa, M.; Ferrari, G. Nanoencapsulation of essential oils to enhance their antimicrobial activity in foods. LWT Food Sci. Technol. 2011, 44, 1908–1914. [Google Scholar] [CrossRef]
- Valencia-Sullca, C.; Jimenez, M.; Jimenez, A.; Atares, L.; Vargas, M.; Chiralt, A. Influence of liposome encapsulated essential oils on properties of chitosan films. Polym. Int. 2016, 65, 979–987. [Google Scholar] [CrossRef]
- Almadiy, A.A.; Nenaah, G.E.; Al Assiuty, B.A.; Moussa, E.A.; Mira, N.M. Chemical composition and antibacterial activity of essential oils and major fractions of four Achillea species and their nanoemulsions against foodborne bacteria. LWT Food Sci. Technol. 2016, 69, 529–537. [Google Scholar] [CrossRef]
- Pinto, L.; Bonifacio, M.A.; De Giglio, E.; Santovito, E.; Cometa, S.; Bevilacqua, A.; Baruzzi, F. Biopolymer hybrid materials: Development, characterization, and food packaging applications. Food Packag. Shelf Life 2021, 28, 100676. [Google Scholar] [CrossRef]
- Ribes, S.; Ruiz-Rico, M.; Pérez-Esteve, É.; Fuentes, A.; Talens, P.; Martínez-Máñez, R.; Barat, J.M. Eugenol and thymol immobilised on mesoporous silica-based material as an innovative antifungal system: Application in strawberry jam. Food Control 2017, 81, 181–188. [Google Scholar] [CrossRef]
- Perez-Esteve, E.; Ruiz-Rico, M.; de la Torre, C.; Villaescusa, L.A.; Sancenon, F.; Marcos, M.D.; Amoros, P.; Martinez-Manez, R.; Barat, J.M. Encapsulation of folic acid in different silica porous supports: A comparative study. Food Chem. 2016, 196, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Bernardos, A.; Piacenza, E.; Sancenon, F.; Hamidi, M.; Maleki, A.; Turner, R.J.; Martinez-Manez, R. Mesoporous Silica-Based Materials with Bactericidal Properties. Small 2019, 15, e1900669. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Liu, X.; Bian, C.; Sheng, J.; Song, Y.; Zhu, Y. Fabrication linalool-functionalized hollow mesoporous silica spheres nanoparticles for efficiently enhance bactericidal activity. Chin. Chem. Lett. 2019, 31, 2137–2141. [Google Scholar] [CrossRef]
- Li, T.; Geng, T.; Md, A.; Banerjee, P.; Wang, B. Novel scheme for rapid synthesis of hollow mesoporous silica nanoparticles (HMSNs) and their application as an efficient delivery carrier for oral bioavailability improvement of poorly water-soluble BCS type II drugs. Colloid Surf. B Biointerfaces 2019, 176, 185–193. [Google Scholar] [CrossRef]
- Gao, F.; Zhou, H.; Shen, Z.; Qiu, H.; Hao, L.; Chen, H.; Zhou, X. Synergistic antimicrobial activities of tea tree oil loaded on mesoporous silica encapsulated by polyethyleneimine. J. Dispers. Sci. Technol. 2019, 41, 1859–1871. [Google Scholar] [CrossRef]
- Meena, J.; Gupta, A.; Ahuja, R.; Singh, M.; Bhaskar, S.; Panda, A.K. Inorganic nanoparticles for natural product delivery: A review. Environ. Chem. Lett. 2020, 18, 2107–2118. [Google Scholar] [CrossRef]
- Fruijtier-Pölloth, C. The safety of nanostructured synthetic amorphous silica (SAS) as a food additive (E 551). Arch. Toxicol. 2016, 90, 2885–2916. [Google Scholar] [CrossRef] [Green Version]
- Sokolik, C.G.; Lellouche, J.-P. Hybrid-silica nanoparticles as a delivery system of the natural biocide carvacrol. RSC Adv. 2018, 8, 36712–36721. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Jin, L.; Wang, C.; Sheng, J.; Song, Y. Thymol-functionalized hollow mesoporous silica spheres nanoparticles: Preparation, characterization and bactericidal activity. Bull. Mater. Sci. 2021, 44, 126. [Google Scholar] [CrossRef]
- Moghadam, H.D.; Sani, A.M.; Sangatash, M.M. Inhibitory Effect ofHelichrysum arenariumEssential Oil on the Growth of Food Contaminated Microorganisms. J. Essent. Oil Bear. Plants 2014, 17, 911–921. [Google Scholar] [CrossRef]
- Nguyen, T.N.T.; Le, N.T.T.; Nguyen, N.H.; Ly, B.T.K.; Nguyen, T.D.; Nguyen, D.H. Aminated hollow mesoporous silica nanoparticles as an enhanced loading and sustained releasing carrier for doxorubicin delivery. Microporous Mesoporous Mater. 2020, 309, 110543–110553. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Ramila, A.; del Real, R.P.; Perez-Pariente, J. A new property of MCM-41: Drug delivery system. Chem. Mater. 2001, 13, 308–311. [Google Scholar] [CrossRef]
- Hoffmann, F.; Cornelius, M.; Morell, J.; Froba, M. Silica-based mesoporous organic-inorganic hybrid materials. Angew. Chem. Int. Ed. Engl. 2006, 45, 3216–3251. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.M.P.; Griep, J.B.; Tavares, F.C.; de Oliveira, D.H.; Bianchini, D.; Jacob, R.G. Synthesis and characterization of imine-modified silicas obtained by the reaction of essential oil of Eucalyptus citriodora, 3-aminopropyltriethoxysilane and tetraethylorthosilicate. Vib. Spectrosc. 2013, 68, 272–278. [Google Scholar] [CrossRef]
- Fuentes, C.; Ruiz-Rico, M.; Fuentes, A.; Ruiz, M.J.; Barat, J.M. Degradation of silica particles functionalised with essential oil components under simulated physiological conditions. J. Hazard. Mater. 2020, 399, 123120. [Google Scholar] [CrossRef] [PubMed]
- Gholamzadeh, P.; Mohammadi Ziarani, G.; Zandi, F.; Abolhasani Soorki, A.; Badiei, A.; Yazdian, F. Modification of fumed silica surface with different sulfonamides via a postsynthesis method and their application as antibacterial agents. Comptes Rendus Chim. 2017, 20, 833–840. [Google Scholar] [CrossRef]
- Ruiz-Rico, M.; Moreno, Y.; Barat, J.M. In vitro antimicrobial activity of immobilised essential oil components against Helicobacter pylori. World J. Microbiol. Biotechnol. 2020, 36, 3. [Google Scholar] [CrossRef] [PubMed]
- Gamez, E.; Elizondo-Castillo, H.; Tascon, J.; Garcia-Salinas, S.; Navascues, N.; Mendoza, G.; Arruebo, M.; Irusta, S. Antibacterial Effect of Thymol Loaded SBA-15 Nanorods Incorporated in PCL Electrospun Fibers. Nanomaterials 2020, 10, 616. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, B.; Colilla, M.; Diez, J.; Pedraza, D.; Guembe, M.; Izquierdo-Barba, I.; Vallet-Regi, M. Mesoporous silica nanoparticles decorated with polycationic dendrimers for infection treatment. Acta Biomater. 2018, 68, 261–271. [Google Scholar] [CrossRef]
- Gao, F.; Zhou, H.; Shen, Z.; Zhu, G.; Hao, L.; Chen, H.; Xu, H.; Zhou, X. Long-lasting anti-bacterial activity and bacteriostatic mechanism of tea tree oil adsorbed on the amino-functionalized mesoporous silica-coated by PAA. Colloids Surf. B Biointerfaces 2020, 188, 110784. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Rico, M.; Fuentes, C.; Pérez-Esteve, É.; Jiménez-Belenguer, A.I.; Quiles, A.; Marcos, M.D.; Martínez-Máñez, R.; Barat, J.M. Bactericidal activity of caprylic acid entrapped in mesoporous silica nanoparticles. Food Control 2015, 56, 77–85. [Google Scholar] [CrossRef]
- Hamoud, R.; Zimmermann, S.; Reichling, J.; Wink, M. Synergistic interactions in two-drug and three-drug combinations (thymol, EDTA and vancomycin) against multi drug resistant bacteria including E. coli. Phytomedicine 2014, 21, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Larraínzar, M.; Rúa, J.; Caro, I.; de Castro, C.; de Arriaga, D.; García-Armesto, M.R.; del Valle, P. Evaluation of antimicrobial and antioxidant activities of natural phenolic compounds against foodborne pathogens and spoilage bacteria. Food Control 2012, 26, 555–563. [Google Scholar] [CrossRef]
- Rua, J.; Fernandez-Alvarez, L.; de Castro, C.; del Valle, P.; de Arriaga, D.; Rosario Garcia-Armesto, M. Antibacterial Activity Against Foodborne Staphylococcus aureus and Antioxidant Capacity of Various Pure Phenolic Compounds. Foodborne Pathog. Dis. 2011, 8, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Peña-Gómez, N.; Ruiz-Rico, M.; Fernández-Segovia, I.; Barat, J.M. Study of apple juice preservation by filtration through silica microparticles functionalised with essential oil components. Food Control 2019, 106, 106749. [Google Scholar] [CrossRef]
- Zhang, Y.I.; Gao, B.E.I.; Zhang, M.; Shi, J.; Xu, Y. Pulsed Electric Field Processing Effects on Physicochemical Properties, Flavor Compounds and Microorganisms of Longan Juice. J. Food Process. Pres. 2010, 34, 1121–1138. [Google Scholar] [CrossRef]
- Charles-Rodríguez, A.V.; Nevárez-Moorillón, G.V.; Zhang, Q.H.; Ortega-Rivas, E. Comparison of Thermal Processing and Pulsed Electric Fields Treatment in Pasteurization of Apple Juice. Food Bioprod. Process. 2007, 85, 93–97. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Li, X.; Sheng, J.; Lu, Y.; Sun, H.; Xu, Q.; Zhu, Y.; Song, Y. Preparation and Enhanced Antimicrobial Activity of Thymol Immobilized on Different Silica Nanoparticles with Application in Apple Juice. Coatings 2022, 12, 671. https://doi.org/10.3390/coatings12050671
Liu Y, Li X, Sheng J, Lu Y, Sun H, Xu Q, Zhu Y, Song Y. Preparation and Enhanced Antimicrobial Activity of Thymol Immobilized on Different Silica Nanoparticles with Application in Apple Juice. Coatings. 2022; 12(5):671. https://doi.org/10.3390/coatings12050671
Chicago/Turabian StyleLiu, Yuhao, Xutao Li, Jie Sheng, Yuyang Lu, Huimin Sun, Qixiang Xu, Yongheng Zhu, and Yishan Song. 2022. "Preparation and Enhanced Antimicrobial Activity of Thymol Immobilized on Different Silica Nanoparticles with Application in Apple Juice" Coatings 12, no. 5: 671. https://doi.org/10.3390/coatings12050671
APA StyleLiu, Y., Li, X., Sheng, J., Lu, Y., Sun, H., Xu, Q., Zhu, Y., & Song, Y. (2022). Preparation and Enhanced Antimicrobial Activity of Thymol Immobilized on Different Silica Nanoparticles with Application in Apple Juice. Coatings, 12(5), 671. https://doi.org/10.3390/coatings12050671





