Defective ZnO Nanoflowers Decorated by Ultra-Fine Pd Clusters for Low-Concentration CH4 Sensing: Controllable Preparation and Sensing Mechanism Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of ZnO NFs
2.2. Synthesis of ZnO1−x NFs
2.3. Synthesis of Pd Cs/ZnO1−x
2.4. Characterizations
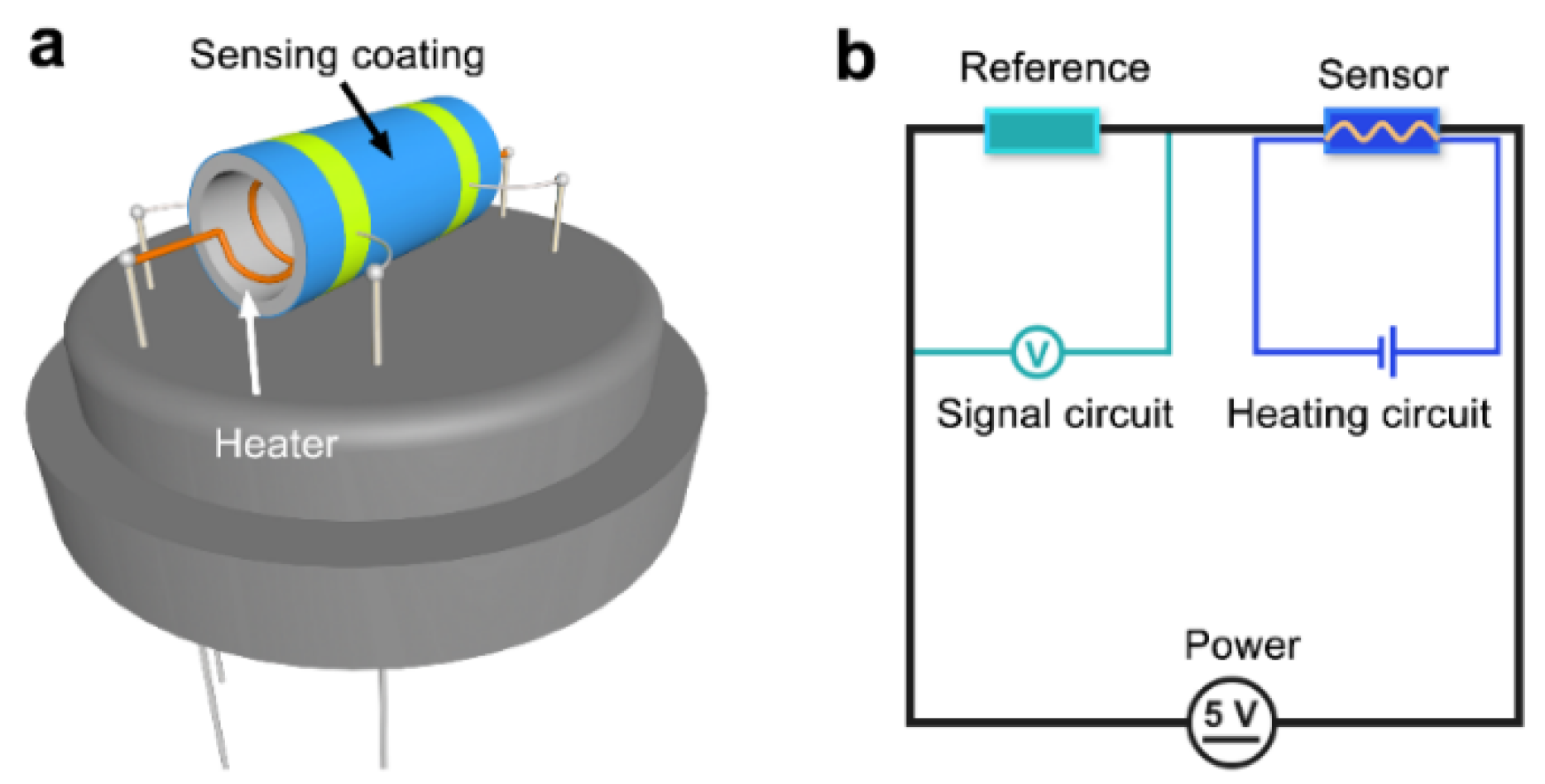
2.5. Fabrication and Performance Testing of Gas Sensors
3. Results
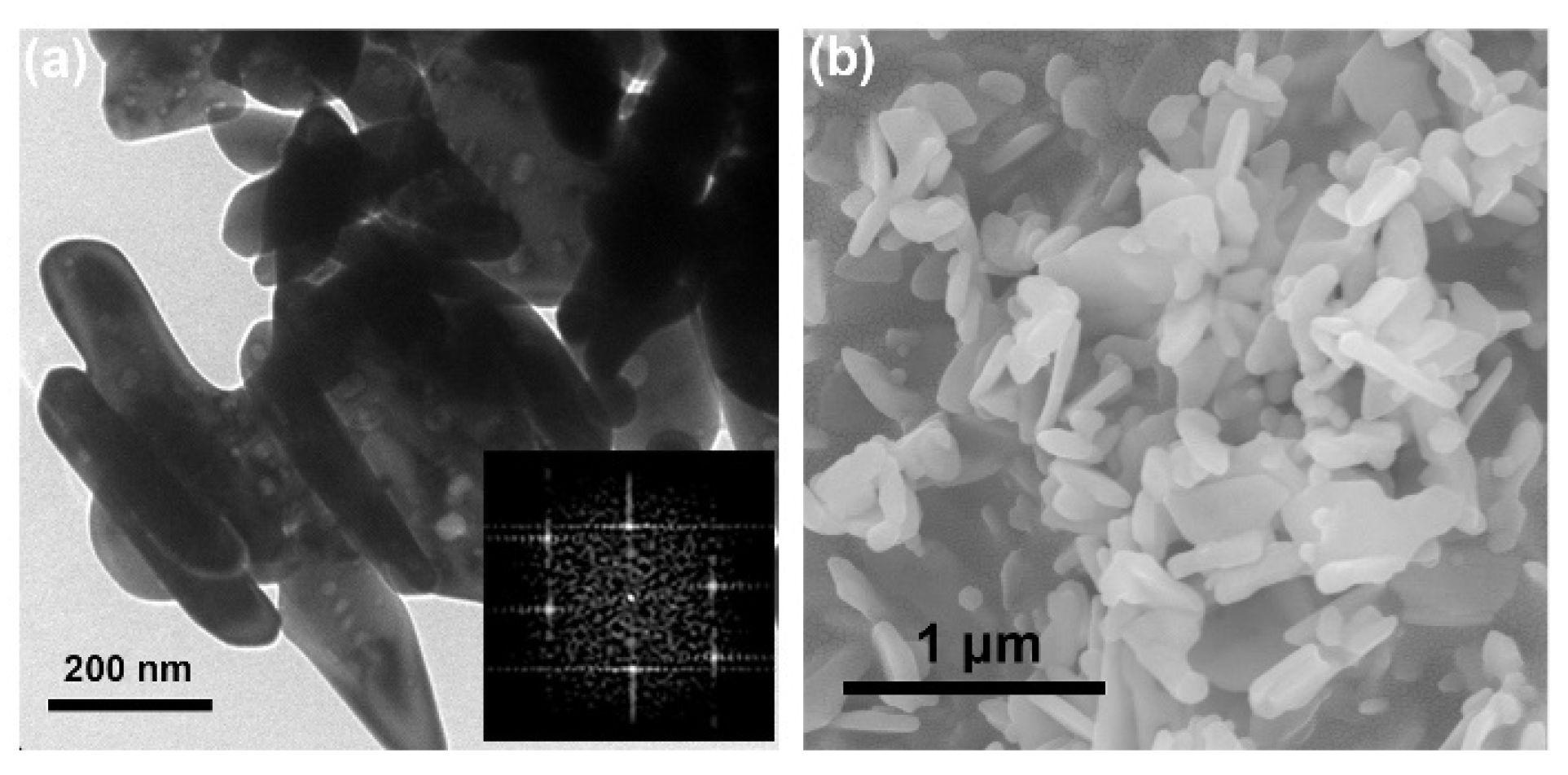
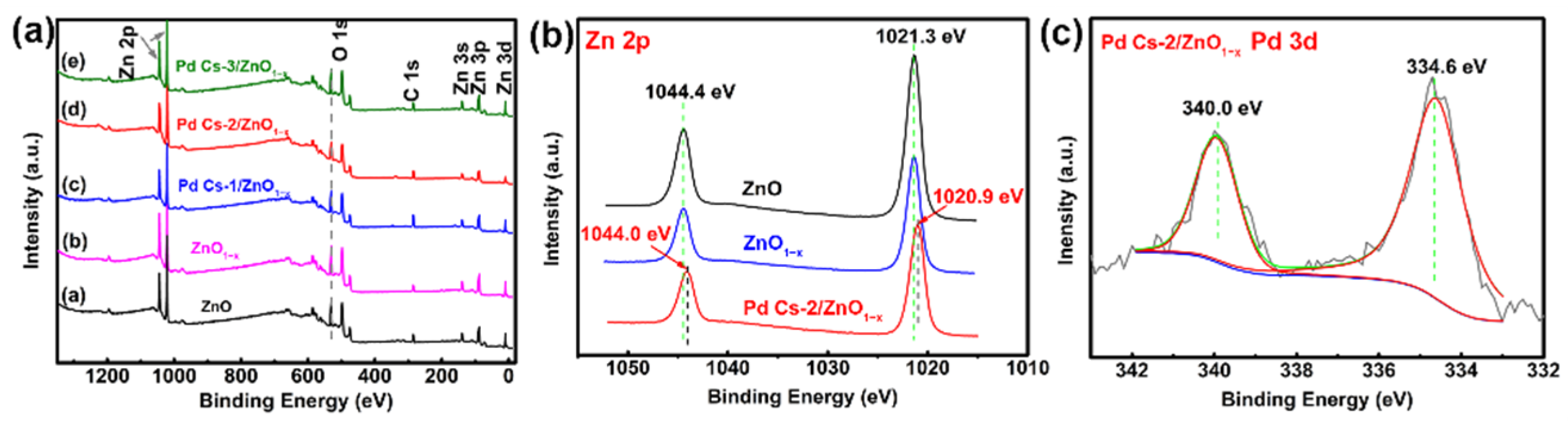
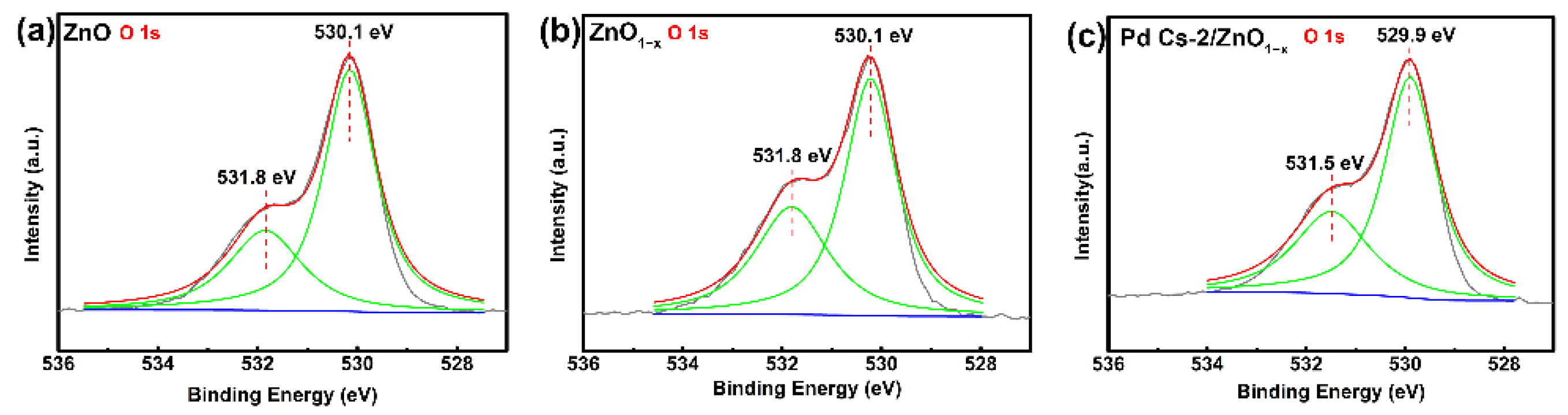
3.1. Characterizations and Analysis
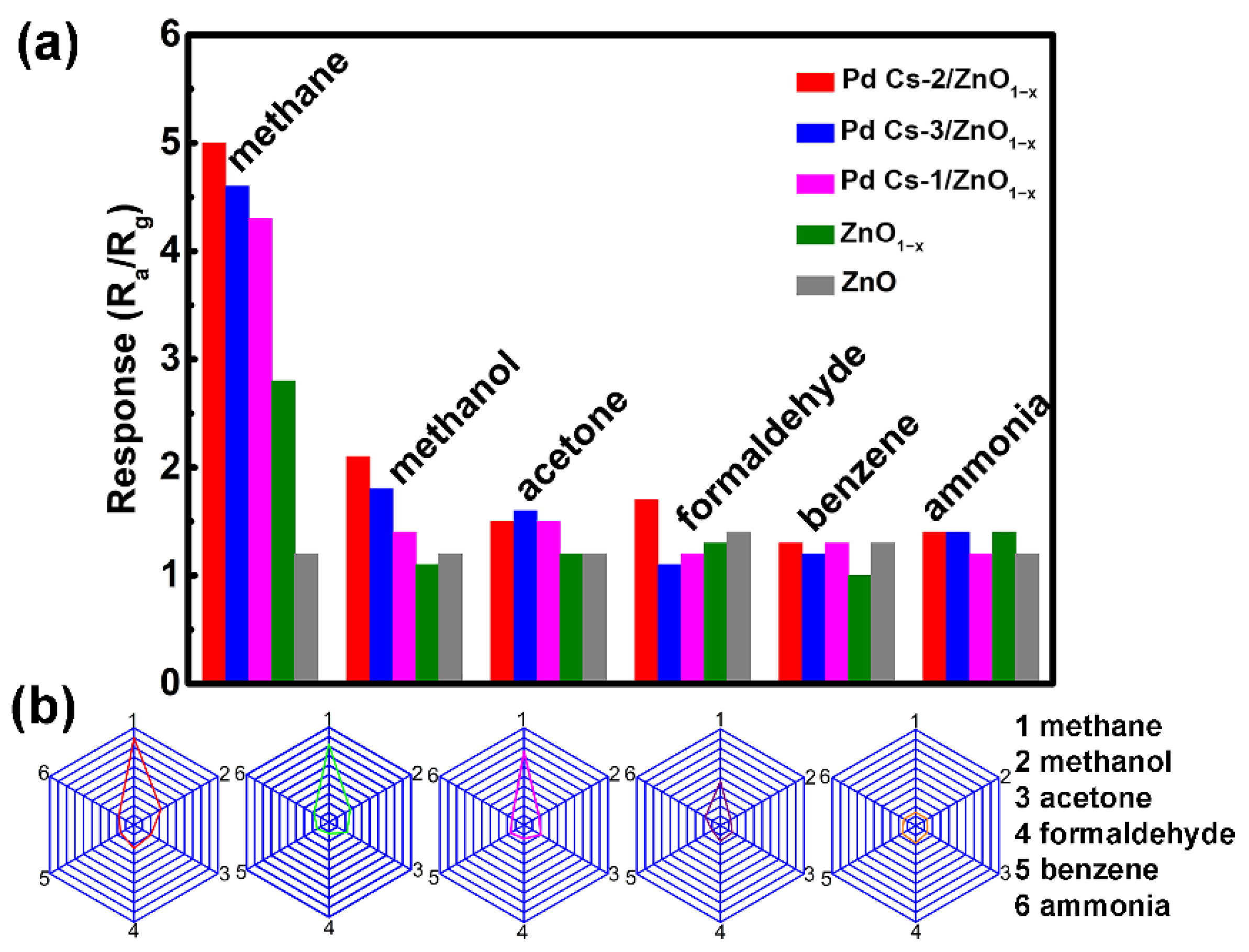
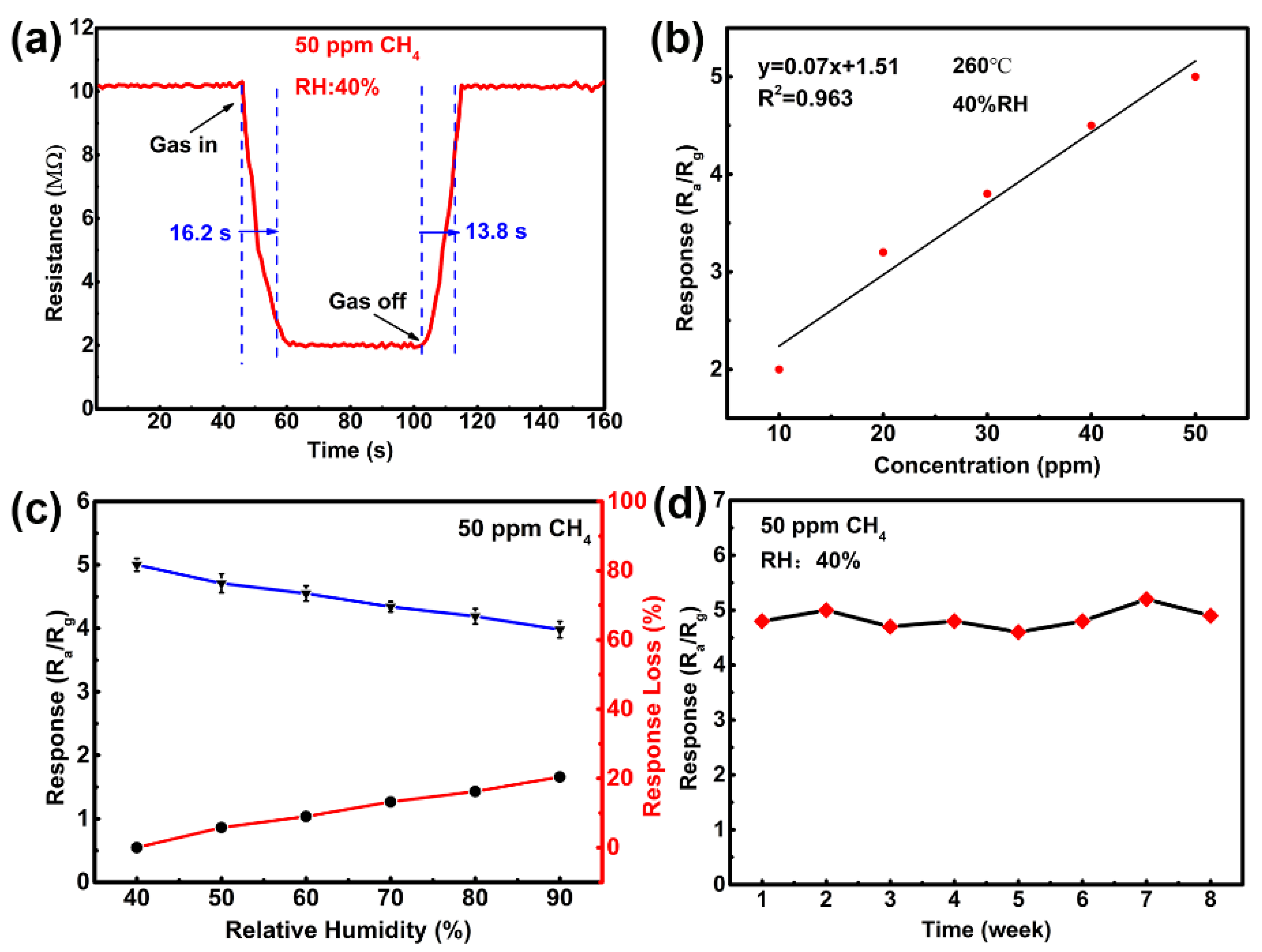
3.2. Gas-Sensing Properties
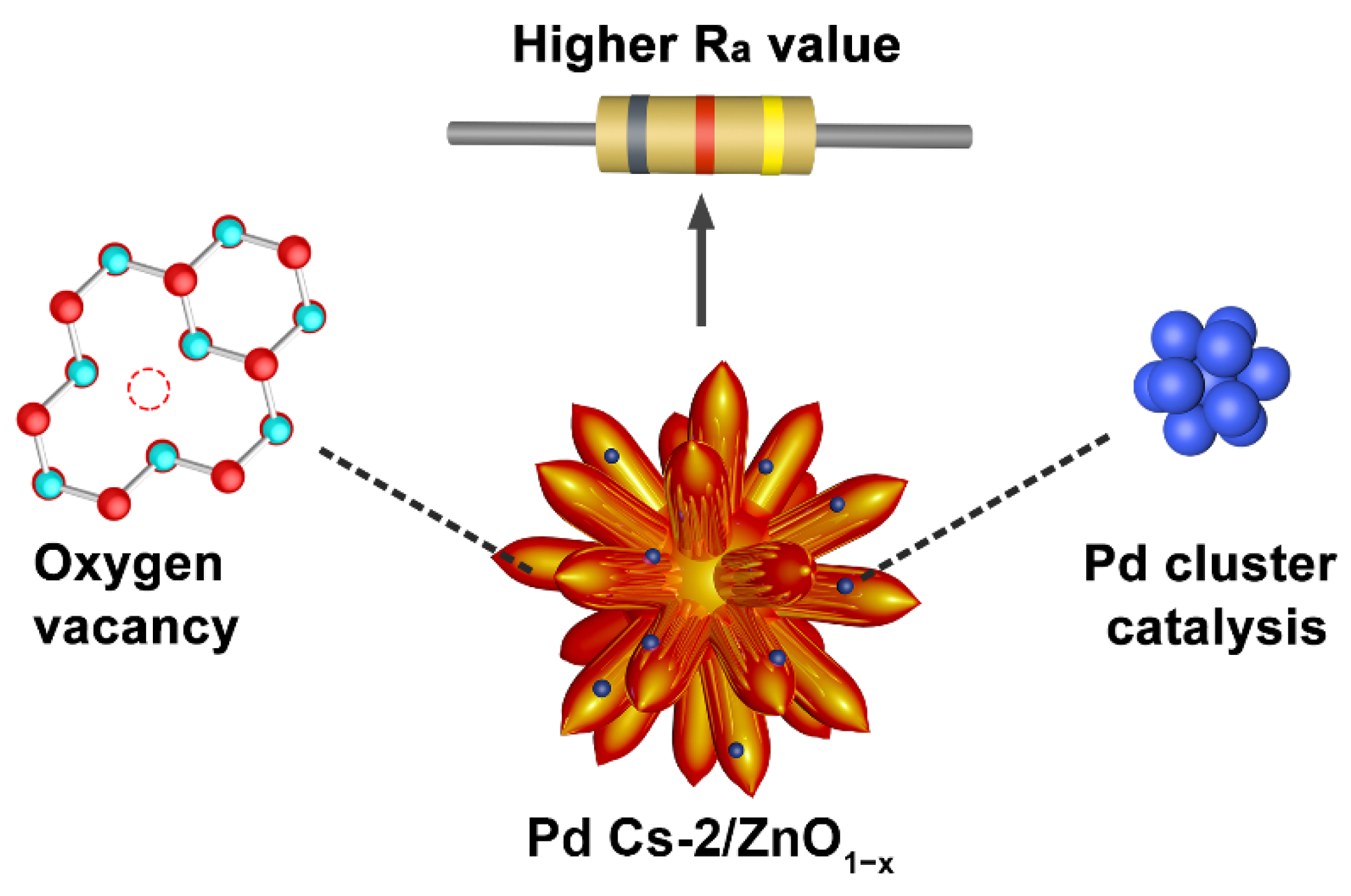
3.3. Mechanism of Gas-Sensing Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghosh, S.; Roy Chaudhuri, C.; Bhattacharya, R.; Saha, H.; Mukherjee, N. Palladium–Silver-Activated ZnO Surface: Highly Selective Methane Sensor at Reasonably Low Operating Temperature. ACS Appl. Mater. Interfaces 2014, 6, 3879–3887. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Li, C.; Sanchez, N.P.; Gluszek, A.K.; Tittel, F.K. Compact CH4 sensor system based on a continuous-wave, low power consumption, room temperature interband cascade laser. Appl. Phys. Lett. 2016, 108, 011106. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Yuan, T.; Wang, X.; Cheng, Z.; Xu, J. Coal mine gases sensors with dual selectivity at variable temperatures based on a W18O49 ultra-fine nanowires/Pd@Au bimetallic nanoparticles composite. Sens. Actuators B Chem. 2021, 354, 131004. [Google Scholar] [CrossRef]
- Haridas, D.; Gupta, V. Enhanced response characteristics of SnO2 thin film based sensors loaded with Pd clusters for methane detection. Sensors Actuators B: Chem. 2012, 166–167, 156–164. [Google Scholar] [CrossRef]
- Yuan, T.; Li, Z.; Zhang, W.; Xue, Z.; Wang, X.; Ma, Z.; Fan, Y.; Xu, J.; Yuen, W. Highly sensitive ethanol gas sensor based on ultrathin nanosheets as-sembled Bi2WO6 with composite phase. Sci. Bull. 2019, 64, 595–602. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Wang, X.; Yan, L.; Wang, Y.; Zhang, Z.; Xu, J. PdPt Bimetal-Functionalized SnO2 Nanosheets: Controllable Synthesis and its Dual Selectivity for Detection of Carbon Monoxide and Methane. ACS Appl. Mater. Interfaces 2019, 11, 26116–26126. [Google Scholar] [CrossRef]
- Rianjanu, A.; Fauzi, F.; Triyana, K.; Wasisto, H.S. Electrospun Nanofibers for Quartz Crystal Microbalance Gas Sensors: A Review. ACS Appl. Nanomater. 2021, 4, 9957–9975. [Google Scholar] [CrossRef]
- Rezki, M.; Septiani, N.L.W.; Iqbal, M.; Adhika, D.R.; Wenten, I.G.; Yuliarto, B. Review—Recent Advance in Multi-Metallic Metal Organic Frameworks (MM-MOFs) and Their Derivatives for Electrochemical Biosensor Application. J. Electrochem. Soc. 2022, 169, 017504. [Google Scholar] [CrossRef]
- Tian, W.; Liu, X.; Yu, W. Research Progress of Gas Sensor Based on Graphene and Its Derivatives: A Review. Appl. Sci. 2018, 8, 1118. [Google Scholar] [CrossRef] [Green Version]
- Pra, C.; Cra, B.; Is, A.; Jgp, A.; Hpan, A.; Mndsc, C. Molecularly imprinted polymer-based electrochemical sensors for en-vironmental analysis—ScienceDirect. Biosens. Bioelectron. 2010, 172, 112719–112727. [Google Scholar]
- Zhou, Q.; Zeng, W.; Chen, W.; Xu, L.; Kumar, R.; Umar, A. High sensitive and low-concentration sulfur dioxide (SO2) gas sensor application of heterostructure NiO-ZnO nanodisks. Sens. Actuators 2019, 298, 126870. [Google Scholar] [CrossRef]
- Xu, F.; Zhou, C.; Ho, H.P. A rule for operation temperature selection of a conductometric VOC gas sensor based on ZnO nan-otetrapods. J. Alloy. Compd. 2020, 858, 158294. [Google Scholar] [CrossRef]
- Suematsu, K.; Watanabe, K.; Tou, A.; Sun, Y.; Shimanoe, K. Ultraselective Toluene-Gas Sensor: Nanosized Gold Loaded on Zinc Oxide Nanoparticles. Anal. Chem. 2018, 90, 1959–1966. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, D.; Zhang, T.; Cui, Z. Ultrasensitive Silicon Nanowire Sensor Developed by a Special Ag Modification Process for Rapid NH3 Detection. ACS Appl. Mater. Interfaces 2017, 9, 28766–28773. [Google Scholar] [CrossRef]
- Kim, J.C.; Jun, H.K.; Huh, J.; Lee, D.D. Tin oxide-based methane gas sensor promoted by alumina-supported Pd catalyst. Sens. Actuators B Chem. 1997, 45, 271–277. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiang, Q.; Xu, J.; Xu, P.; Pan, Q.; Li, F. Self-assemblies of Pd nanoparticles on the surfaces of single crystal ZnO nan-owires for chemical sensors with enhanced performances. J. Mater. Chem. 2009, 19, 4701–4706. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, X.; Yao, M.; Sun, G.; Zhang, Z. Enhanced CH4 sensing properties of Pd modified ZnO nanosheets. Ceram. Int. 2019, 45, 13150–13157. [Google Scholar] [CrossRef]
- He, H.; Xue, Y.-Q.; Wang, S.-Q.; Zhu, Q.-Q.; Chen, J.; Li, C.-P.; Du, M. A Double-Walled Bimetal–Organic Framework for Antibiotics Sensing and Size-Selective Catalysis. Inorg. Chem. 2018, 57, 15062–15068. [Google Scholar] [CrossRef]
- Su, P.-G.; Yu, J.-H. Enhanced NO2 gas-sensing properties of Au-Ag bimetal decorated MWCNTs/WO3 composite sensor under UV-LED irradiation. Sens. Actuators A Phys. 2019, 303, 111718. [Google Scholar] [CrossRef]
- Xing, L.; Ma, C.; Chen, Z.-H.; Chen, Y.-J.; Xue, X.-Y. High gas sensing performance of one-step-synthesized Pd–ZnO nanoflowers due to surface reactions and modifications. Nanotechnology 2011, 22, 215501. [Google Scholar] [CrossRef]
- Singh, B.V.; Sapana, R.; Saravanan, R.; Kumari, K.; Raliya, R.; Biswas, P.; Kumar, M. Improved Sensitivity with Low Limit of Detection of a Hydrogen Gas Sensor Based on rGO-Loaded Ni-Doped ZnO Nanostructures. ACS Appl. Mater. Interfaces 2018, 10, 11116–11124. [Google Scholar]
- Kim, H.; Jin, C.; Park, S.; Lee, C. Enhanced H2S gas sensing properties of multiple-networked Pd-doped SnO2-core/ZnO-shell nanorod sensors. Mater. Res. Bull. 2012, 47, 2708–2712. [Google Scholar] [CrossRef]
- Ning, Y.; Fielding, L.A.; Nutter, J.; Kulak, A.N.; Meldrum, F.C.; Armes, S.P. Spatially Controlled Occlusion of Polymer-Stabilized Gold Nanoparticles within ZnO. Angew. Chem. 2019, 131, 4346–4351. [Google Scholar] [CrossRef]
- Zabilskiy, M.; Sushkevich, V.L.; Newton, M.A.; Krumeich, F.; Nachtegaal, M.; Bokhoven, J.A.V. Mechanistic study of carbon dioxide hydrogenation over Pd/ZnO-based catalysts: The role of palladium-zinc alloy in selective methanol synthesis. Angew. Chem. 2021, 133, 17190–17196. [Google Scholar] [CrossRef]
- Shon, G.Y.S. Controlling Surface Ligand Density and Core Size of Alkanethiolate-Capped Pd Nanoparticles and Their Effects on Catalysis. Langmuir 2012, 28, 14502–14508. [Google Scholar]
- Zhou, H.; Yang, X.; Li, L.; Liu, X.; Huang, Y.; Pan, X.; Wang, A.; Li, J.; Zhang, T. PdZn Intermetallic Nanostructure with Pd–Zn–Pd Ensembles for Highly Active and Chemoselective Semi-Hydrogenation of Acetylene. ACS Catal. 2016, 6, 1054–1061. [Google Scholar] [CrossRef]
- Chang, Y.; Xu, J.; Zhang, Y.; Ma, S.; Xin, L.; Zhu, L.; Xu, C. Optical Properties and Photocatalytic Performances of Pd Modified ZnO Samples. J. Phys. Chem. C 2009, 113, 18761–18767. [Google Scholar] [CrossRef]
- Platonov, V.; Rumyantseva, M.; Khmelevsky, N.; Gaskov, A. Electrospun ZnO/Pd Nanofibers: CO Sensing and Humidity Effect. Sensors 2020, 20, 7333. [Google Scholar] [CrossRef]
- Zhu, Q.; Xie, C. Comparative study of ZnO nanorod array and nanoparticle film in photoelectric response and charge storage. J. Alloys Compd. 2014, 585, 267–276. [Google Scholar] [CrossRef]
- Geng, Z.; Kong, X.; Chen, W.; Su, H.; Liu, Y.; Cai, F.; Wang, G.; Zeng, J. Oxygen Vacancies in ZnO Nanosheets Enhance CO 2 Electrochemical Reduction to CO. Angew. Chem. 2018, 130, 6162–6167. [Google Scholar] [CrossRef]
- Xue, Z.; Cheng, Z.; Xu, J.; Xiang, Q.; Wang, X.; Xu, J. Controllable Evolution of Dual Defect Zni and VO Associate-Rich ZnO Nanodishes with (0001) Exposed Facet and Its Multiple Sensitization Effect for Ethanol Detection. ACS Appl. Mater. Interfaces 2017, 9, 41559–41567. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Fan, Y.; Yuan, T.; Lu, B.; Liu, Y.; Li, Z.; Li, G.; Cheng, Z.; Xu, J. Ultrafine Tungsten Oxide Nanowires: Synthesis and Highly Selective Acetone Sensing and Mechanism Analysis. ACS Appl. Mater. Interfaces 2019, 12, 3755–3763. [Google Scholar] [CrossRef] [PubMed]
- Rong, Q.; Xiao, B.; Zeng, J.; Yu, R.; Zi, B.; Zhang, G. Pt Single Atom-Induced Activation Energy and Adsorption En-hancement for an Ultrasensitive ppb-Level Methanol Gas Sensor. ACS Sens. 2021, 7, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Drobek, M.; Kim, J.-H.; Bechelany, M.; Vallicari, C.; Julbe, A.; Kim, S.S. MOF-Based Membrane Encapsulated ZnO Nanowires for Enhanced Gas Sensor Selectivity. ACS Appl. Mater. Interfaces 2016, 8, 8323–8328. [Google Scholar] [CrossRef]
- Kuang, Q.; Lao, C.; Wang, Z.L.; Xie, Z.; Zheng, L. High-Sensitivity Humidity Sensor Based on a Single SnO2 Nanowire. J. Am. Chem. Soc. 2007, 129, 6070–6071. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Jiang, Y.; Gao, X.; Wang, X.; Chen, W. Strongly Coupled Pd Nanotetrahedron/Tungsten Oxide Nanosheet Hybrids with Enhanced Catalytic Activity and Stability as Oxygen Reduction Electrocatalysts. J. Am. Chem. Soc. 2014, 136, 11687–11697. [Google Scholar] [CrossRef]
- Koo, W.-T.; Yu, S.; Choi, S.-J.; Jang, J.-S.; Cheong, J.Y.; Kim, I.-D. Nanoscale PdO Catalyst Functionalized Co3O4 Hollow Nanocages Using MOF Templates for Selective Detection of Acetone Molecules in Exhaled Breath. ACS Appl. Mater. Interfaces 2017, 9, 8201–8210. [Google Scholar] [CrossRef]
- Barsan, N.; Koziej, D.; Weimar, U. Metal oxide-based gas sensor research: How to? Sens. Actuators B Chem. 2007, 121, 18–35. [Google Scholar] [CrossRef]
- Luo, N.; Wang, C.; Zhang, D.; Guo, M.; Wang, X.; Cheng, Z.; Xu, J. Ultralow detection limit MEMS hydrogen sensor based on SnO2 with oxygen vacancies. Sens. Actuators B Chem. 2021, 354, 130982. [Google Scholar] [CrossRef]
- Xu, Y.; Zheng, L.; Yang, C.; Zheng, W.; Liu, X.; Zhang, J. Oxygen Vacancies Enabled Porous SnO2 Thin Films for Highly Sensitive Detection of Triethylamine at Room Temperature. ACS Appl. Mater. Interfaces 2020, 12, 20704–20713. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Selamneni, V.; Thakur, U.N.; Sahatiya, P.; Hazra, A. Detection and discrimination of volatile organic compounds by noble metal nanoparticle functionalized MoS2 coated biodegradable paper sensors. New J. Chem. 2020, 44, 16613–16625. [Google Scholar] [CrossRef]
- Baek, D.-H.; Kim, J. MoS2 gas sensor functionalized by Pd for the detection of hydrogen. Sens. Actuators B Chem. 2017, 250, 686–691. [Google Scholar] [CrossRef]
- Penza, M.; Rossi, R.; Alvisi, M.; Cassano, G.; Signore, M.; Serra, E.; Giorgi, R. Pt- and Pd-nanoclusters functionalized carbon nanotubes networked films for sub-ppm gas sensors. Sens. Actuators B Chem. 2008, 135, 289–297. [Google Scholar] [CrossRef]
- Ayesh, A.I. Linear hydrogen gas sensors based on bimetallic nanoclusters. J. Alloy. Compd. 2016, 689, 1–5. [Google Scholar] [CrossRef]
- Luo, N.; Zhang, B.; Zhang, D.; Xu, J. Enhanced CO sensing properties of Pd modified ZnO porous nanosheets. Chin. Chem. Lett. 2020, 31, 2033–2036. [Google Scholar] [CrossRef]









| 2θ | (hkl) | d (Å) | I (f) | Grain Size (nm) |
|---|---|---|---|---|
| 31.77 | (100) | 2.84 | 51.7 | 5.05 |
| 34.32 | (002) | 2.63 | 42.5 | 5.22 |
| 36.21 | (101) | 2.50 | 100 | 4.95 |
| 47.23 | (102) | 1.93 | 25.1 | 5.16 |
| 56.30 | (110) | 1.64 | 39.2 | 4.65 |
| 62.22 | (103) | 1.49 | 41.2 | 5.39 |
| 66.38 | (200) | 1.42 | 6.9 | 4.84 |
| 67.36 | (112) | 1.39 | 32 | 5.20 |
| 68.62 | (201) | 1.37 | 13.8 | 5.33 |
| Type of Oxygen | ZnO | ZnO1−x | Pd Cs-2/ZnO1−x |
|---|---|---|---|
| OI (lattice oxygen) | 68.40 | 61.18 | 62.15 |
| OII (oxygen defects) | 31.60 | 38.82 | 37.85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Zhang, W.; Luo, N.; Wang, W.; Xu, J. Defective ZnO Nanoflowers Decorated by Ultra-Fine Pd Clusters for Low-Concentration CH4 Sensing: Controllable Preparation and Sensing Mechanism Analysis. Coatings 2022, 12, 677. https://doi.org/10.3390/coatings12050677
Chen Y, Zhang W, Luo N, Wang W, Xu J. Defective ZnO Nanoflowers Decorated by Ultra-Fine Pd Clusters for Low-Concentration CH4 Sensing: Controllable Preparation and Sensing Mechanism Analysis. Coatings. 2022; 12(5):677. https://doi.org/10.3390/coatings12050677
Chicago/Turabian StyleChen, Yang, Wenshuang Zhang, Na Luo, Wei Wang, and Jiaqiang Xu. 2022. "Defective ZnO Nanoflowers Decorated by Ultra-Fine Pd Clusters for Low-Concentration CH4 Sensing: Controllable Preparation and Sensing Mechanism Analysis" Coatings 12, no. 5: 677. https://doi.org/10.3390/coatings12050677
APA StyleChen, Y., Zhang, W., Luo, N., Wang, W., & Xu, J. (2022). Defective ZnO Nanoflowers Decorated by Ultra-Fine Pd Clusters for Low-Concentration CH4 Sensing: Controllable Preparation and Sensing Mechanism Analysis. Coatings, 12(5), 677. https://doi.org/10.3390/coatings12050677







