Colorimetric Measurement of Deltamethrin Pesticide Using a Paper Sensor Based on Aggregation of Gold Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemical Reagents
2.2. Instruments and Software
2.3. Nanoparticles Synthesis
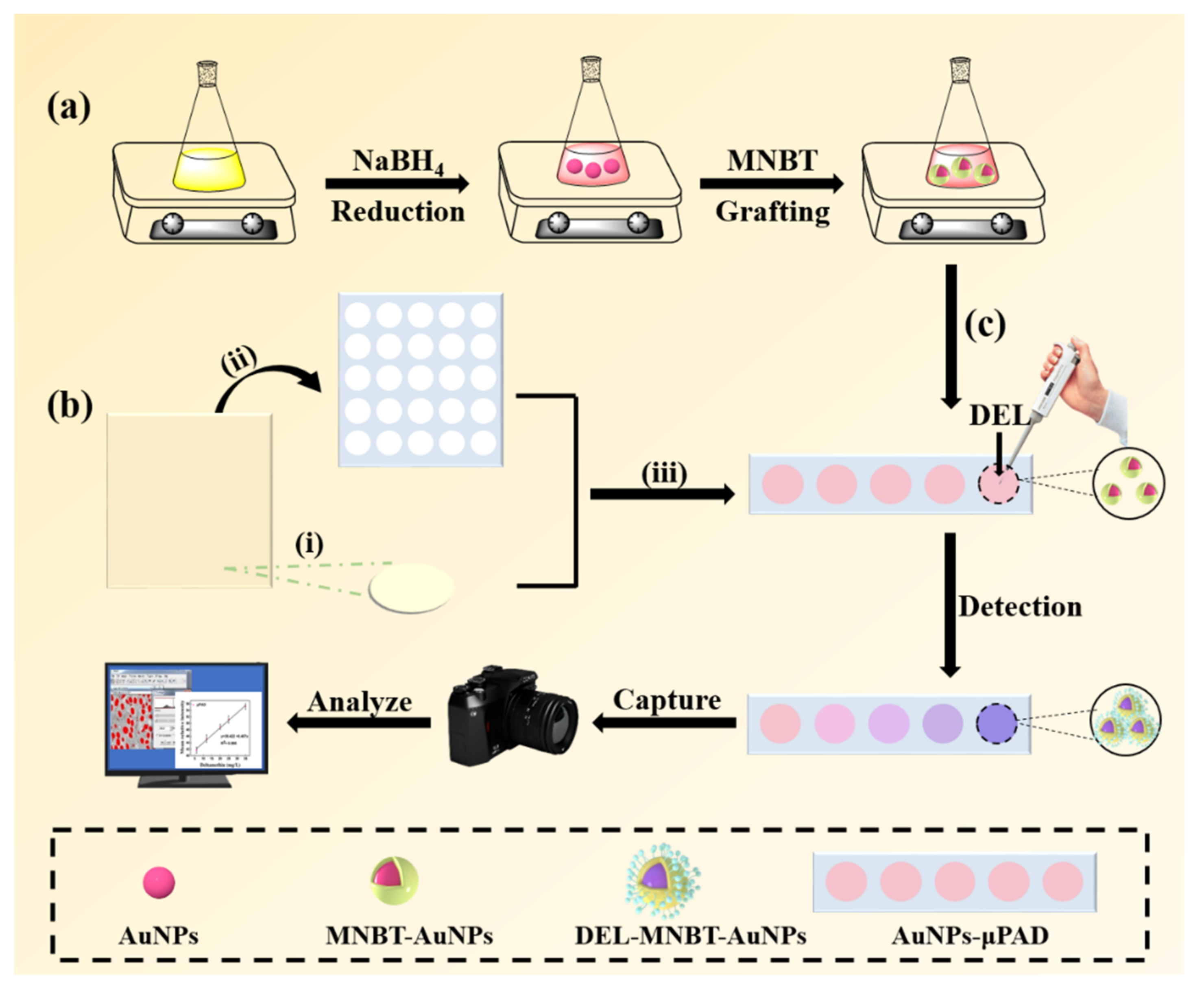
2.4. Fabrication of Microfluidic Paper Device (µPAD)
2.5. Sensing Procedure and Data Processing
2.6. Real Samples Pretreatment and Measurements
2.7. Statistical Analysis
3. Results
3.1. Mechanism of AuNPs Detecting DEL
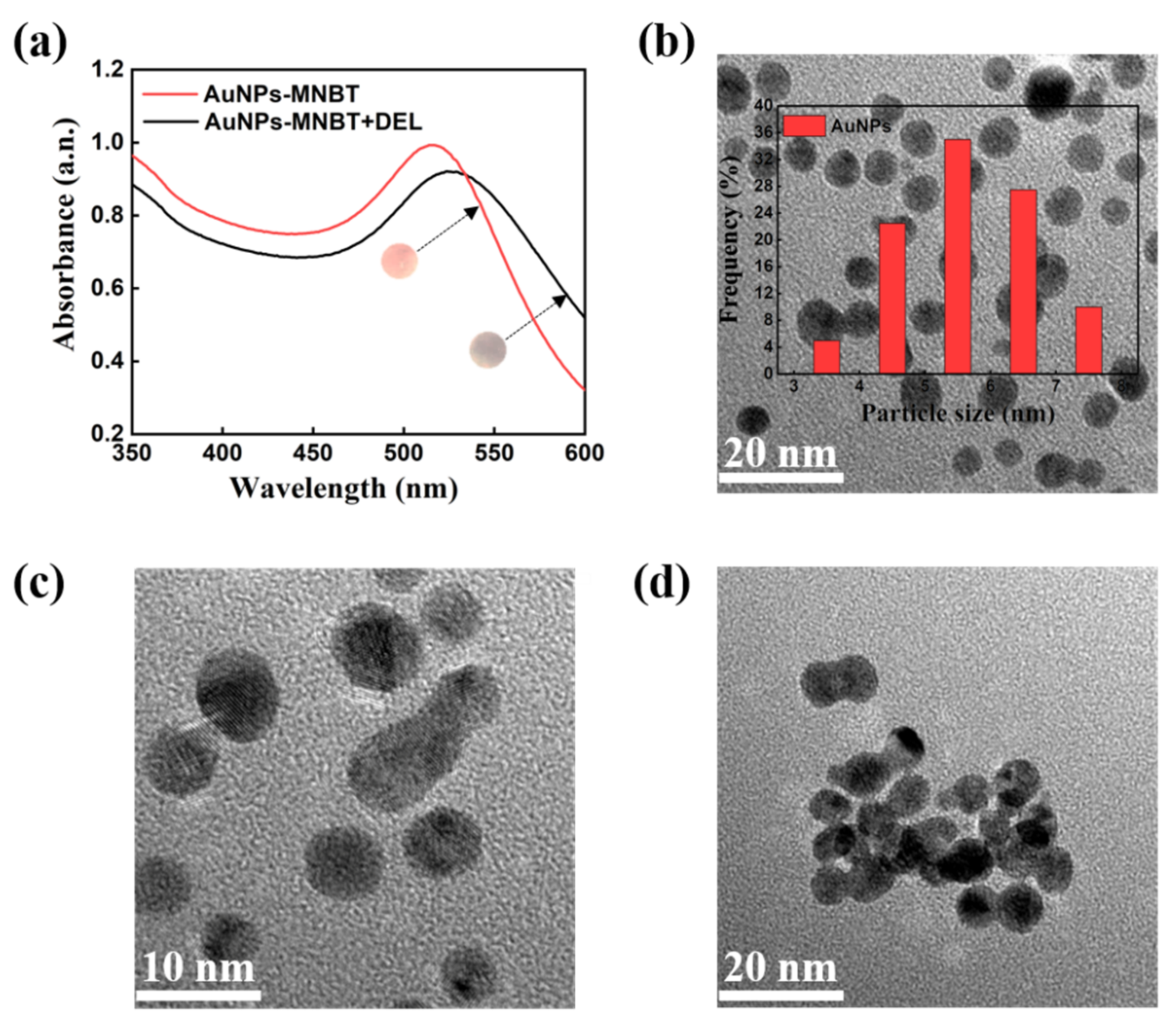
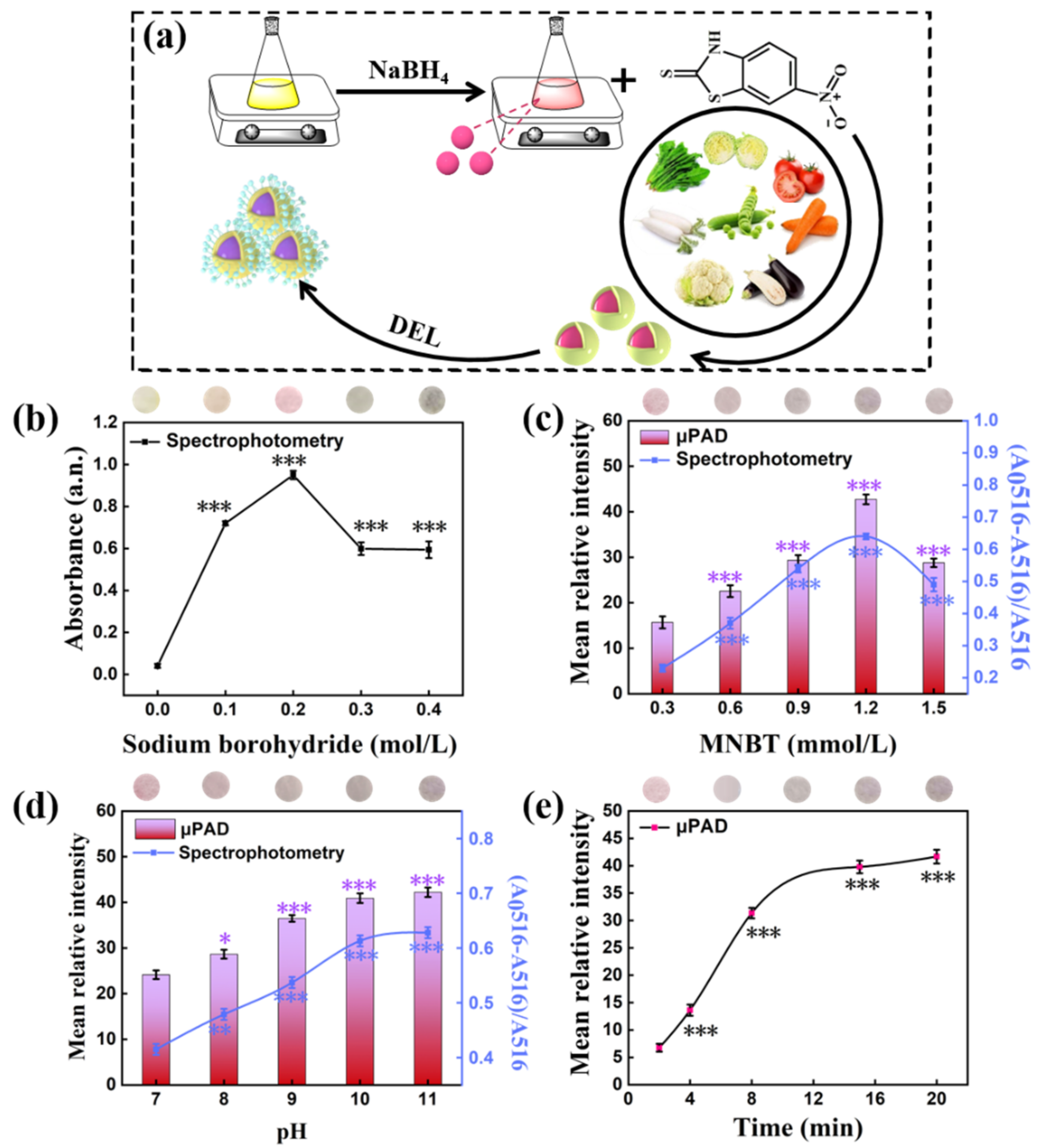
3.2. Effect of AuNPs Synthesis Condition
3.3. Optimization of Reaction Conditions
3.4. Sensitive Detection of DEL
3.5. Application of the μPAD to Detect DEL in Fruit and Vegetable
4. Discussion
4.1. Mechanism of AuNPs Detecting DEL
4.2. Effect of Synthesis Condition
4.3. Optimization of Reaction Conditions
4.4. Sensitive Detection of DEL
4.5. Application of the μPAD to Detect DEL in Fruit and Vegetable
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, T.; Wang, C.; Zhou, H.; Zhang, Y.; Ma, L. A multifunctional near-infrared fluorescent sensing material based on core-shell upconversion nanoparticles@magnetic nanoparticles and molecularly imprinted polymers for detection of deltamethrin. Microchim Acta. 2021, 188, 165. [Google Scholar] [CrossRef]
- Bhamore, J.R.; Jha, S.; Singhal, R.K.; Murthy, Z.V.P.; Kailasa, S.K. Amylase protected gold nanoclusters as chemo- and bio- sensor for nanomolar detection of deltamethrin and glutathione. Sens. Actuators B Chem. 2019, 281, 812–820. [Google Scholar] [CrossRef]
- Liu, X.; Li, L.; Liu, Y.Q.; Shi, X.B.; Li, W.J.; Yang, Y.; Mao, L.G. Ultrasensitive detection of deltamethrin by immune magnetic nanoparticles separation coupled with surface plasmon resonance sensor. Biosens. Bioelectron. 2014, 59, 328–334. [Google Scholar] [CrossRef]
- Bissacot, D.Z.; Vassilieff, I. Pyrethroid residues in milk and blood of dairy cows following single topical applications. Vet. Hum. Toxicol. 1997, 39, 6–8. [Google Scholar]
- Vinggaard, A.M.; Christiansen, S.; Laier, P.; Poulsen, M.E.; Breinholt, V.; Jarfelt, K.; Jacobsen, H.; Dalgaard, M.; Nellemann, C.; Hass, U. Perinatal exposure to the fungicide prochloraz feminizes the male rat offspring. Toxicol. Sci. 2005, 85, 886–897. [Google Scholar] [CrossRef] [Green Version]
- Poulsen, C.R.; Bokvist, K.; Olsen, H.L.; Hoy, M.; Capito, K.; Gilon, P.; Gromada, J. Multiple sites of purinergic control of insulin secretion in mouse pancreatic beta-cells. Diabetes 1999, 48, 2171–2181. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, Y.S.; Saad, T.T.; El-Bahr, S.M. Acute intoxication of deltamethrin in monosex Nile tilapia, Oreochromis niloticus with special reference to the clinical, biochemical and haematological effect. Environ. Toxicol. Pharmacol. 2007, 24, 212–217. [Google Scholar] [CrossRef]
- Brancato, A.; Brocca, D.; De Lentdecker, C.; Erdos, Z.; Ferreira, L.; Greco, L.; Jarrah, S.; Kardassi, D.; Leuschner, R.; Lythgo, C.; et al. Modification of the existing maximum residue level for deltamethrin in kale. EFSA J. 2018, 16, 5153–5179. [Google Scholar]
- Boonchiangma, S.; Ngeontae, W.; Srijaranai, S. Determination of six pyrethroid insecticides in fruit juice samples using dispersive liquid-liquid microextraction combined with high performance liquid chromatography. Talanta 2012, 88, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Fan, S.; Zou, J.H.; Miao, H.; Li, J.G.; Zhang, G.W.; Wu, Y.N. Application of Gas Chromatography-mass Spectrometry in Analyzing Pharmacokinetics and Distribution of Deltamethrin in Miniature Pig Tissues. Biomed. Environ. Sci. 2014, 27, 426–435. [Google Scholar]
- Kobayashi, H. Development of Residue Analysis for Pesticides by LC/MS and LC/MS/MS Methods. Bunseki Kagaku 2009, 58, 985–997. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Amaya, S.; Zhao, M.; Allebach, J.P.; Chiu, G.T.; Stanciu, L.A. Ionic Strength Influences on Biofunctional Au-Decorated Microparticles for Enhanced Performance in Multiplexed Colorimetric Sensors. ACS Appl. Mater. Inter. 2020, 12, 32397–32409. [Google Scholar] [CrossRef]
- Monisha; Shrivas, K.; Kant, T.; Patel, S.; Devi, R.; Dahariya, N.S.; Pervez, S.; Deb, M.K.; Rai, M.K.; Rai, J. Inkjet-printed paper-based colorimetric sensor coupled with smartphone for determination of mercury (Hg2+). J. Hazard. Mater. 2021, 414, 125440. [Google Scholar] [CrossRef]
- Lee, M.G.; Patil, V.; Na, Y.-C.; Lee, D.S.; Lim, S.H.; Yi, G.-R. Highly stable, rapid colorimetric detection of carbaryl pesticides by azo coupling reaction with chemical pre-treatment. Sens. Actuators B Chem. 2018, 261, 489–496. [Google Scholar] [CrossRef]
- Sun, Z.W.; Tian, L.Y.; Guo, M.; Xu, X.T.; Li, Q.; Weng, H.B. A double-film screening card for rapid detection of organophosphate and carbamate pesticide residues by one step in vegetables and fruits. Food Control. 2017, 81, 23–29. [Google Scholar] [CrossRef]
- Dong, L.; Hou, C.; Yang, M.; Fa, H.; Wu, H.; Shen, C.; Huo, D. Highly sensitive colorimetric and fluorescent sensor for cyanazine based on the inner filter effect of gold nanoparticles. J. Nanopart. Res. 2016, 18, 164. [Google Scholar] [CrossRef]
- Shariati, S.; Khayatian, G. Microfluidic paper-based analytical device using gold nanoparticles modified with N,N′-bis(2-hydroxyethyl)dithiooxamide for detection of Hg2+ in air, fish and water samples. New J. Chem. 2020, 44, 18662–18667. [Google Scholar] [CrossRef]
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent biomedical applications of gold nanoparticles: A review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Guo, L.; Bao, Y.; Xie, J. A simple, label-free AuNPs-based colorimetric ultrasensitive detection of nerve agents and highly toxic organophosphate pesticide. Biosens. Bioelectron. 2011, 28, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Brasiunas, B.; Popov, A.; Ramanavicius, A.; Ramanaviciene, A. Gold nanoparticle based colorimetric sensing strategy for the determination of reducing sugars. Food Chem. 2021, 351, 129238. [Google Scholar] [CrossRef]
- Abnous, K.; Danesh, N.M.; Ramezani, M.; Alibolandi, M.; Emrani, A.S.; Lavaee, P.; Taghdisi, S.M. A colorimetric gold nanoparticle aggregation assay for malathion based on target-induced hairpin structure assembly of complementary strands of aptamer. Mikrochim. Acta. 2018, 185, 216. [Google Scholar] [CrossRef]
- Chang, C.C.; Chen, C.P.; Wu, T.H.; Yang, C.H.; Lin, C.W.; Chen, C.Y. Gold Nanoparticle-Based Colorimetric Strategies for Chemical and Biological Sensing Applications. Nanomaterials 2019, 9, 861. [Google Scholar] [CrossRef] [Green Version]
- Tai, H.; Duan, Z.; Wang, Y.; Wang, S.; Jiang, Y. Paper-Based Sensors for Gas, Humidity, and Strain Detections: A Review. Acs Appl. Mater. Inter. 2020, 12, 31037–31053. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, C.; Ji, D.; Wang, Y. Paper-Based Microfluidic Sensors for Onsite Environmental Detection: A Critical Review. Crit. Rev. Anal. Chem. 2021, 3, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Shrivas, K.; Kant, T.; Karbhal, I.; Kurrey, R.; Sahu, B.; Sinha, D.; Patra, G.K.; Deb, M.K.; Pervez, S. Smartphone coupled with paper-based chemical sensor for on-site determination of iron(III) in environmental and biological samples. Anal. Bioanal. Chem. 2020, 412, 1573–1583. [Google Scholar] [CrossRef] [PubMed]
- Shrivas, K.; Nirmalkar, N.; Ghosale, A.; Thakur, S.S. Application of silver nanoparticles for a highly selective colorimetric assay of endrin in water and food samples based on stereoselective endo-recognition. RSC Adv. 2016, 6, 29855–29862. [Google Scholar] [CrossRef]
- Shrivas, K.; Monisha; Patel, S.; Thakur, S.S.; Shankar, R. Food safety monitoring of the pesticide phenthoate using a smartphone-assisted paper-based sensor with bimetallic Cu@Ag core-shell nanoparticles. Lab Chip 2020, 20, 3996–4006. [Google Scholar] [CrossRef]
- Wu, S.; Li, D.; Wang, J.; Zhao, Y.; Dong, S.; Wang, X. Gold nanoparticles dissolution based colorimetric method for highly sensitive detection of organophosphate pesticides. Sens. Actuators B Chem. 2017, 238, 427–433. [Google Scholar] [CrossRef]
- Patel, S.; Jamunkar, R.; Sinha, D.; Monisha; Patle, T.K.; Kant, T.; Dewangan, K.; Shrivas, K. Recent development in nanomaterials fabricated paper-based colorimetric and fluorescent sensors: A review. Trends Environ. Anal. 2021, 31, e00136. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, Y.; Wang, D.; Sun, L.; Dong, C.; Fang, L.; Zhang, Y.; Wu, A. A rapid colorimetric method for the detection of deltamethrin based on gold nanoparticles modified with 2-mercapto-6-nitrobenzothiazole. Anal. Methods 2018, 10, 1774–1780. [Google Scholar] [CrossRef]
- GB29705-2013; Food Safety National Standard—Determination of Organophosphorus Multi Pesticides Residue in Foods Gas Chromatography-Mass Spectrometry. The Standardization Administration of the People’s Republic of China: Beijing, China, 2016.
- GB5009.110-2003; Determination of Cypermethrin, Fenvalerate and Deltamethrin Residues in Vegetable Foods. The Standardization Administration of the People’s Republic of China: Beijing, China, 2003.
- Dasary, S.S.R.; Zones, Y.K.; Barnes, S.L.; Ray, P.C.; Singh, A.K. Alizarin Dye based ultrasensitive plasmonic SERS probe for trace level Cadmium detection in drinking water. Sens. Actuators. B Chem. 2016, 224, 65–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Wu, Y. Investigation of the NaBH4-induced aggregation of Au nanoparticles. Langmiur 2010, 26, 9214–9223. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Ni, T.J.; Liu, H.Q.; Wu, L.; Pan, Y.J.; Zhao, Y.; Zhu, Y.H. Functional poly(carboxybetaine methacrylate) coated paper sensor for high efficient and multiple detection of nutrients in fruit. Chin. Chem. Lett. 2020, 31, 1099–1103. [Google Scholar] [CrossRef]
- Chen, H.Y.; Hu, O.; Fan, Y.; Lu, X.; Zhang, L.; Lan, W.; Hu, Y.; Xie, X.R.; Ma, L.X.; She, Y.B.; et al. Fluorescence paper-based sensor for visual detection of carbamate pesticides in food based on CdTe quantum dot and nano ZnTPyP. Food Chem. 2020, 327, 127075. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Handa, S.K.; Sharma, K.K. A new spray reagent for the detection of synthetic pyrethroids containing a nitrile group on thin-layer plates. Talanta 1998, 45, 1111–1114. [Google Scholar] [CrossRef]


| Sample | Found | Added (mg/L) | DEL Concentration (mg/L) | Statistical Significance | RSD (%) (n = 3) | |||
|---|---|---|---|---|---|---|---|---|
| µPAD | Spectrophotometry | µPAD | Spectrophotometry | µPAD | Spectrophotometry | |||
| Apple | ND | 10 | 10.29 | 9.98 | *** | *** | 4.9 | 5.5 |
| ND | 20 | 19.83 | 20.31 | *** | *** | 2.2 | 2.7 | |
| mandarin orange | ND | 10 | 9.89 | 10.38 | *** | *** | 3.8 | 4.1 |
| ND | 20 | 19.21 | 21.11 | *** | *** | 3.7 | 3.2 | |
| spinach | ND | 10 | 10.04 | 9.53 | *** | *** | 4.2 | 3.5 |
| ND | 20 | 20.17 | 19.57 | *** | *** | 2.8 | 3.7 | |
| tomato | ND | 10 | 9.28 | 9.32 | *** | *** | 5.3 | 4.7 |
| ND | 20 | 20.85 | 18.88 | *** | *** | 3 | 4.3 | |
| cucumber | ND | 10 | 9.58 | 10.62 | *** | *** | 5.2 | 4.8 |
| ND | 20 | 20.02 | 20.24 | *** | *** | 6.5 | 6.2 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Yin, L.; Zhang, W.; Chen, M.; Feng, D.; Zhao, Y.; Zhu, Y. Colorimetric Measurement of Deltamethrin Pesticide Using a Paper Sensor Based on Aggregation of Gold Nanoparticles. Coatings 2022, 12, 38. https://doi.org/10.3390/coatings12010038
Zhu J, Yin L, Zhang W, Chen M, Feng D, Zhao Y, Zhu Y. Colorimetric Measurement of Deltamethrin Pesticide Using a Paper Sensor Based on Aggregation of Gold Nanoparticles. Coatings. 2022; 12(1):38. https://doi.org/10.3390/coatings12010038
Chicago/Turabian StyleZhu, Jingyang, Lifeng Yin, Weiyi Zhang, Meilian Chen, Dongsheng Feng, Yong Zhao, and Yongheng Zhu. 2022. "Colorimetric Measurement of Deltamethrin Pesticide Using a Paper Sensor Based on Aggregation of Gold Nanoparticles" Coatings 12, no. 1: 38. https://doi.org/10.3390/coatings12010038
APA StyleZhu, J., Yin, L., Zhang, W., Chen, M., Feng, D., Zhao, Y., & Zhu, Y. (2022). Colorimetric Measurement of Deltamethrin Pesticide Using a Paper Sensor Based on Aggregation of Gold Nanoparticles. Coatings, 12(1), 38. https://doi.org/10.3390/coatings12010038






