Mechanical Properties Evolution and Damage Mechanism of Kevlar Fiber under Ozone Exposure in Near-Space Simulation
Abstract
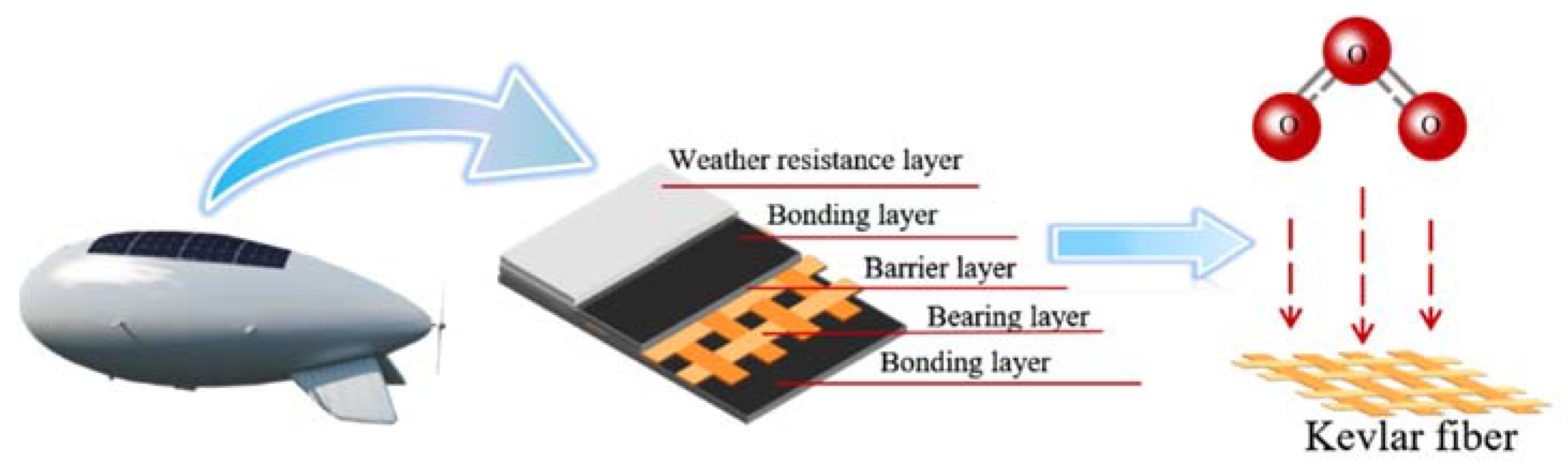
:1. Introduction
2. Materials and Methods
2.1. Materials
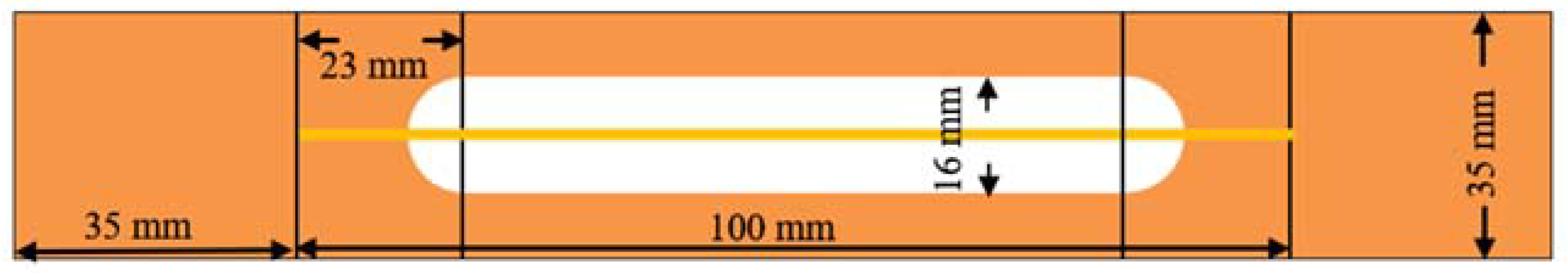
2.2. Testing and Characterization
2.3. Ozone Exposure
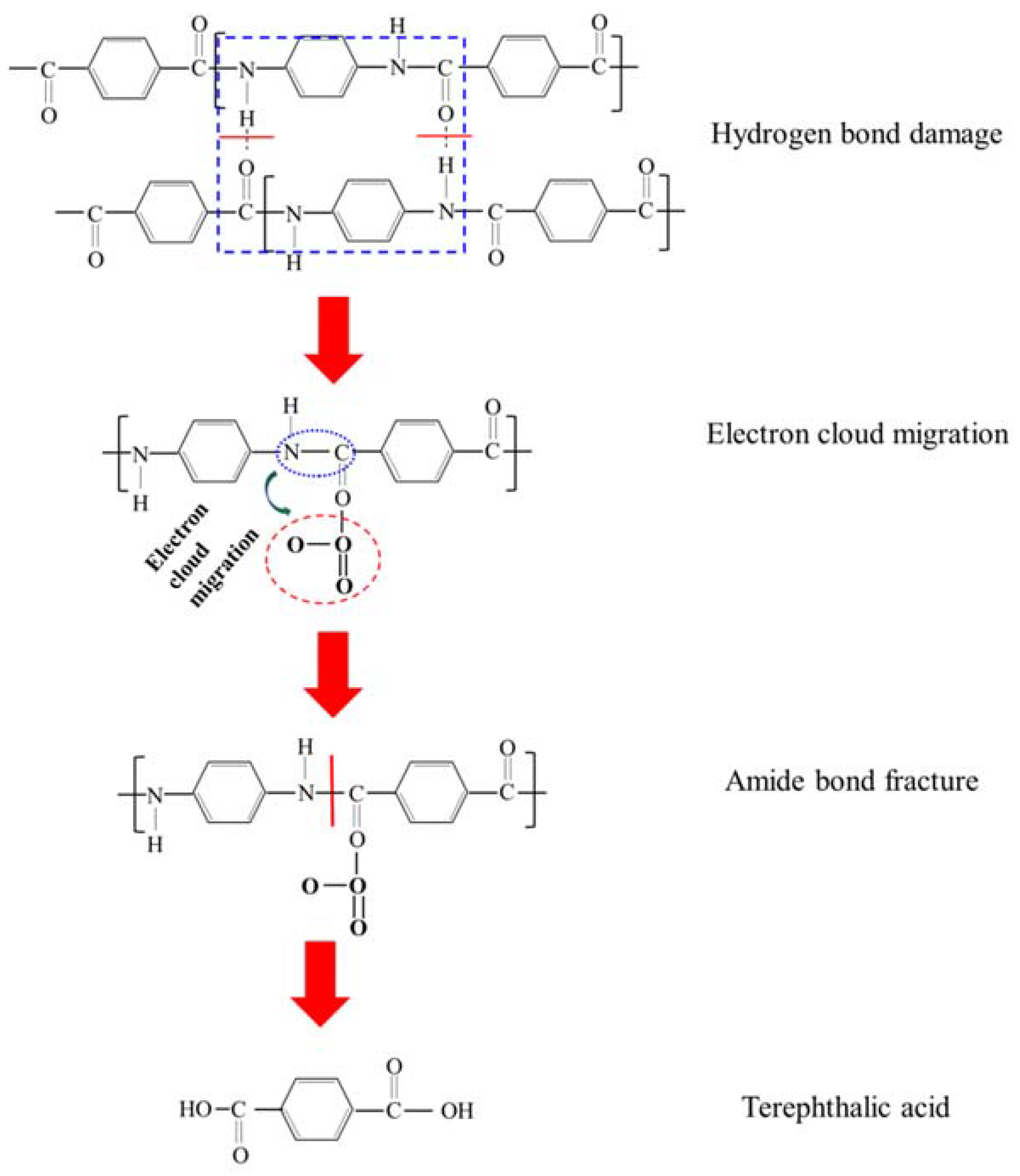
3. Results and Discussion
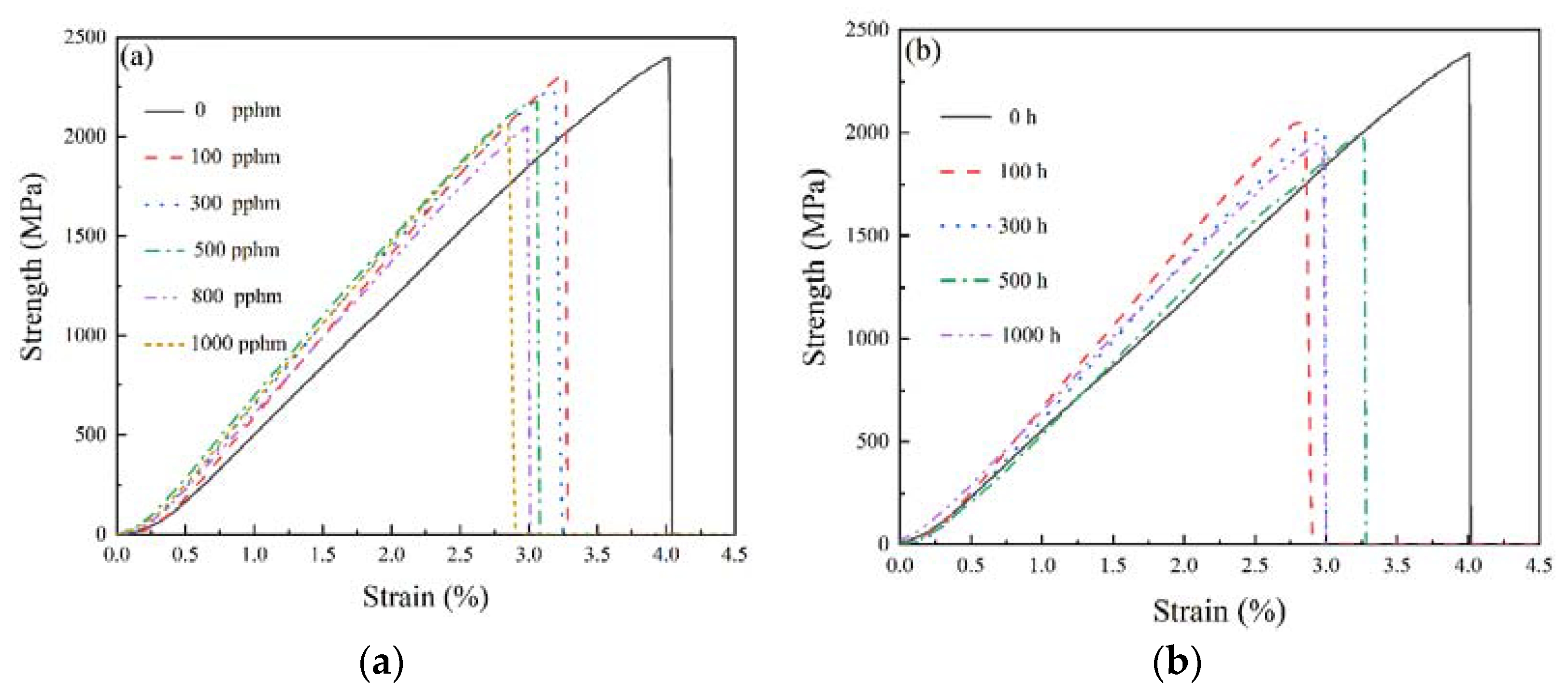
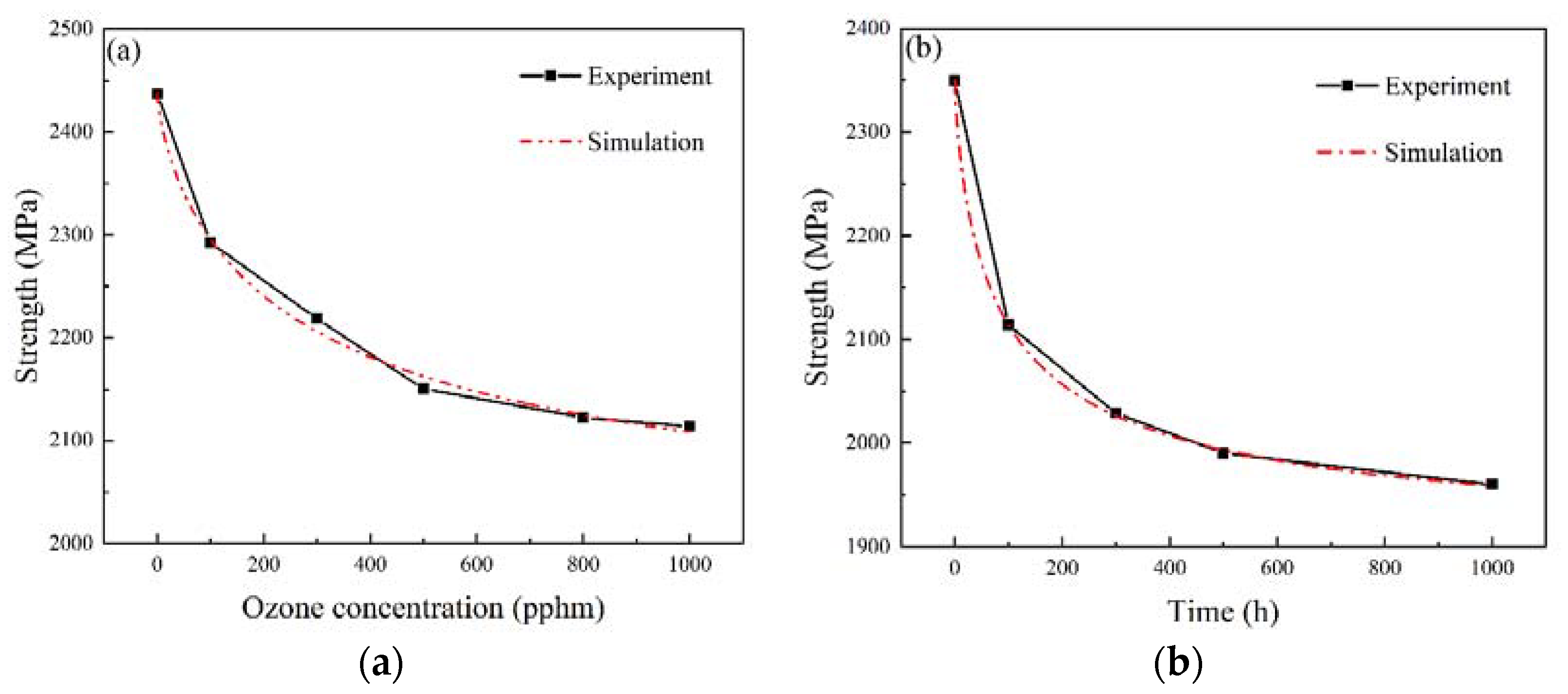
3.1. Mechanical Properties Characterization
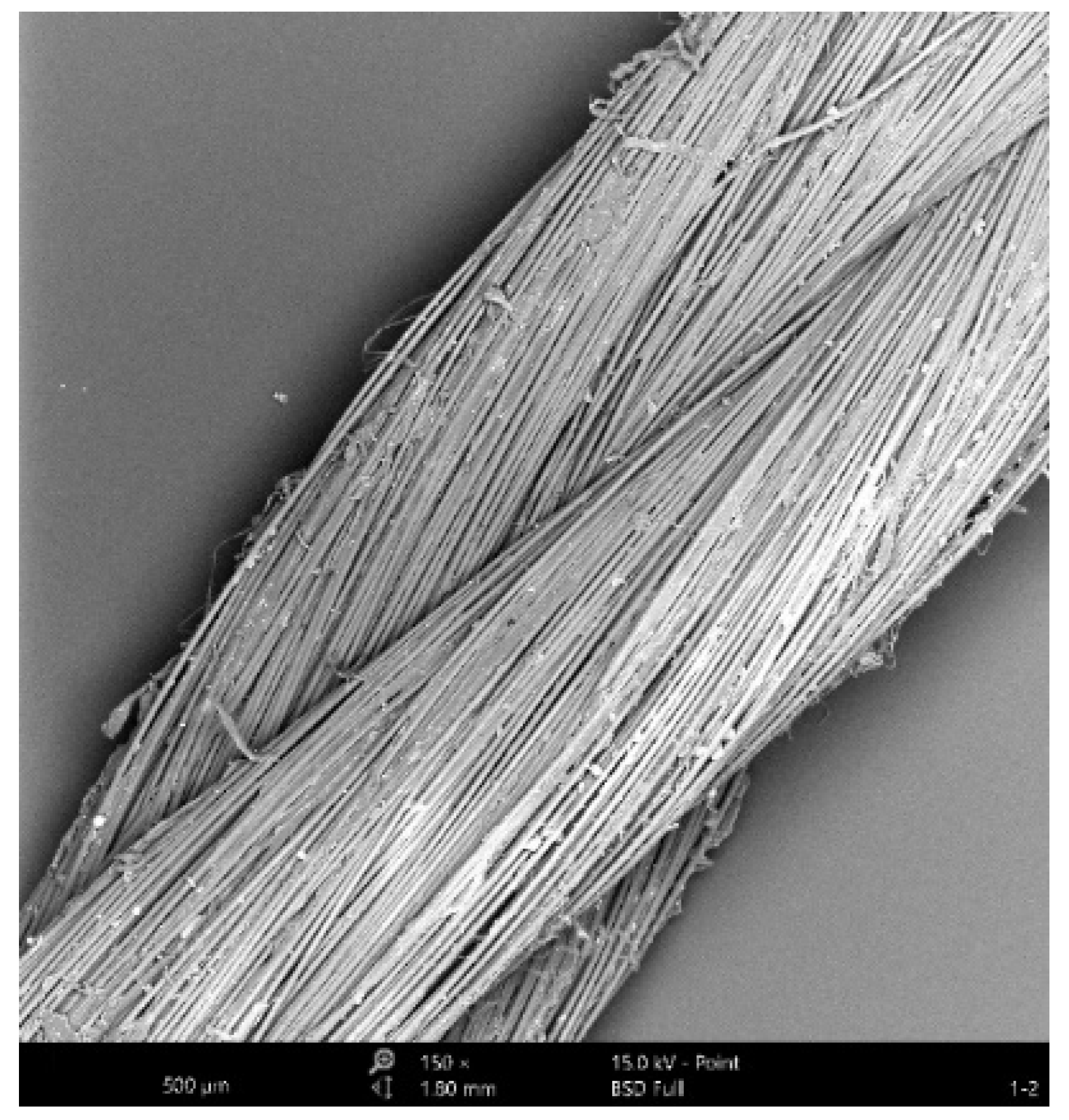
3.2. Morphology Analysis
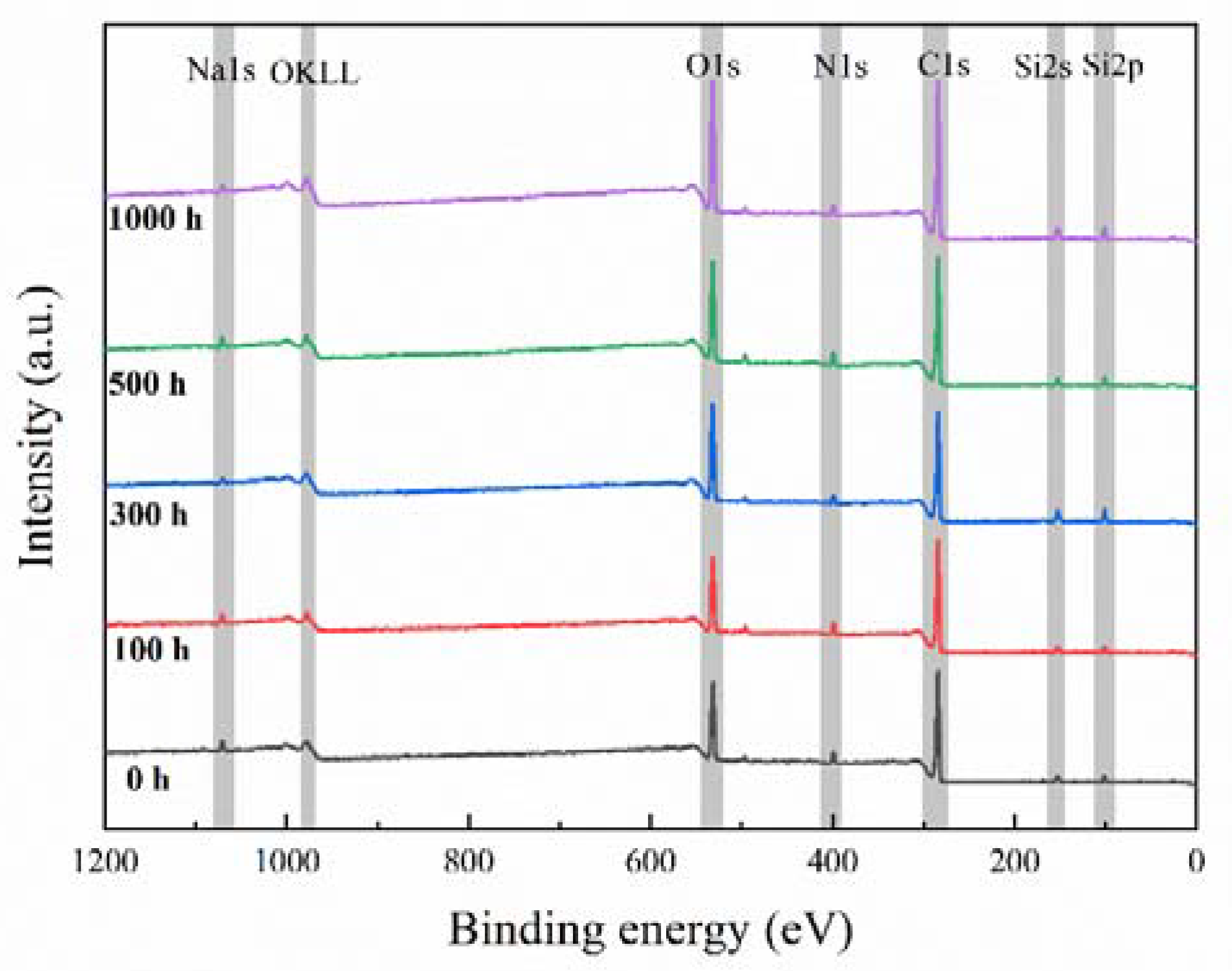
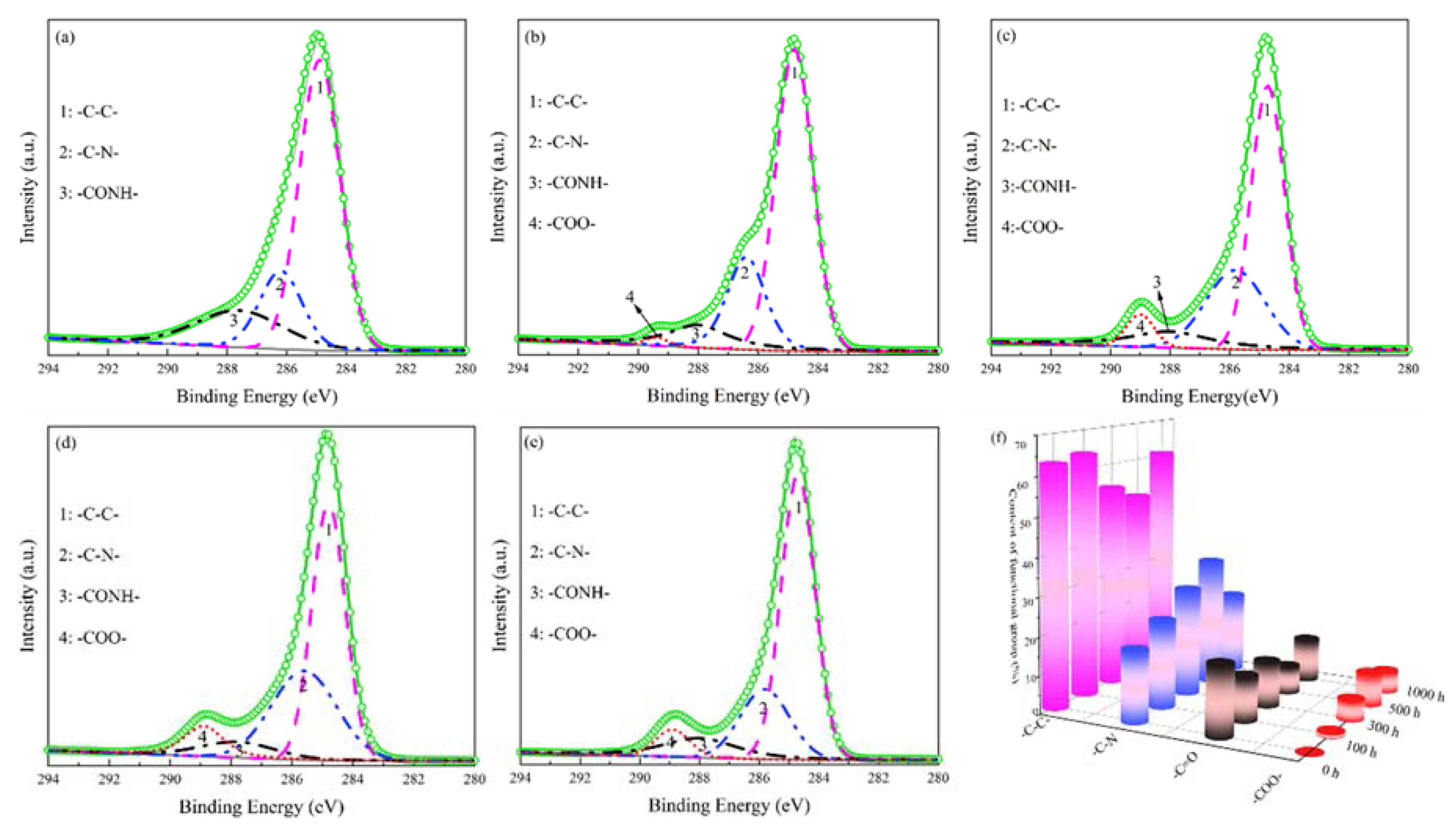
3.3. Surface Structure Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.Y.; Liu, Y.X.; Liu, S.Z.; Tan, H.F. Effect of Accelerated Xenon Lamp Aging on the Mechanical Properties and Structure of Thermoplastic Polyurethane for Stratospheric Airship Envelope. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2014, 29, 1270–1276. [Google Scholar] [CrossRef]
- Chen, F.G.; Chen, G.G.; Liu, K.H. Analysis of Near Space Environment and Its Effect. Equip. Environ. Eng. 2013, 10, 71–75. [Google Scholar] [CrossRef]
- Jamison, L.; Sommer, G.S.; Porche, I.R., III. High-Altitude Airships for the Future of The Force Army. Rand Corp. 2006, 75, 151–155. [Google Scholar]
- Kang, W.; Suh, Y.; Woo, K.; Lee, I. Mechanical property characterization of film-fabric laminate for stratospheric airship envelope. Compos. Struct. 2006, 75, 151–155. [Google Scholar] [CrossRef]
- Fei, C.; Wang, Z.Q.; Zhou, X.Y.; Wang, F. Research Status of Near Space Airship Envelop Materials. Fiber Compos. 2016, 33, 24–26. [Google Scholar]
- Said, M.A.; Dingwall, B.; Gupta, A.; Seyam, A.M.; Mock, G.; Theyson, T. Investigation of ultra violet (UV) resistance for high strength fibers. Adv. Space Res. 2006, 37, 2052–2058. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Z.; Chen, W.; Gao, C. Initial configuration and nonlinear mechanical analysis of stratospheric nonrigid airship envelope. J. Aerosp. Eng. 2019, 32, 04018155. [Google Scholar] [CrossRef]
- Chen, L. Study on Comprehensive Performance of Several High-Performance Fibers. Ph.D. Thesis, Beijing University of Chemical Technology, Beijing, China, 2016. [Google Scholar]
- Dobb, M.G.; Johnson, D.J.; Saville, B.P. Supramolecular Structure of a High-modulus polyaromatic fiber (Kevlar 49). J. Polym. Sci. Polym. Phys. Ed. 2010, 15, 2201–2211. [Google Scholar] [CrossRef]
- Maekawa, S.; Nakadate, M.; Takegaki, A. On the Structures of the Low Altitude Stationary Flight Test Vehicle. J. Aircr. 2013, 44, 662–666. [Google Scholar] [CrossRef]
- Liu, T.M.; Zheng, Y.S.; Hu, J. Surface modification of aramid fibers with novel chemical approach. Polym. Bull. 2011, 66, 259–275. [Google Scholar] [CrossRef]
- Xi, M.; Li, Y.L.; Shang, S.Y.; Li, D.H.; Dai, X.Y. Surface modification of aramid fiber by air DBD plasma at atmospheric pressure with continuous on-line processing. Surf. Coat. Technol. 2008, 202, 6029–6033. [Google Scholar] [CrossRef]
- Yue, C.Y.; Padmanabhan, K. Interfacial studies on surface modified Kevlar fiber/epoxy matrix composites. Compos. Part B Eng. 1999, 30, 205–217. [Google Scholar] [CrossRef]
- Yu, C.C.; Chen, Y.J.; Wu, C.Y. Effect of Relative Humidity on Adsorption Breakthrough of CO2 on Activated Carbon Fibers. Materials 2017, 10, 1296. [Google Scholar] [CrossRef] [Green Version]
- Kamedulski, P.; Lukaszewicz, J.P.; Witczak, U. The Importance of Structural Factors for the Electrochemical Performance of Graphene/Carbon Nanotube/Melamine Powders towards the Catalytic Activity of Oxygen Reduction Reaction. Materials 2021, 14, 2448. [Google Scholar] [CrossRef]
- Irshad, H.M.; Hakeem, A.S.; Raza, K.; Baroud, T.N.; Ehsan, M.A.; Ali, S.; Tahir, M.S. Design, Development and Evaluation of Thermal Properties of Polysulphone-CNT/GNP Nanocomposites. Nanomaterials 2021, 11, 2080. [Google Scholar] [CrossRef]
- Bing, Y.; Jian, Y.; Li, X.; Zhao, Y. The operating environment of near-space and its effects on the airship. Spacecr. Environ. Eng. 2008, 25, 555–557. [Google Scholar]
- Mabee, A.E. Ozone Effects on Construction Materials for The Stationary High-Altitude Relay Platform (SHARP) Aircraft. Ph.D. Thesis, University of Toronto (Canada), Toronto, ON, Canada, 1991. [Google Scholar]
- Zhang, Y.; Wang, Y.; Wang, C. Light weight optimization of stratospheric airship envelope based on reliability analysis. Chin. J. Aeronaut. 2020, 33, 2670–2678. [Google Scholar] [CrossRef]
- Mai, R.Q.; Wang, C.G.; Tan, H.F. Effect of Near Space Environment on Mechanical Properties of Airship Envelope Woven Materials. Equip. Environ. Eng. 2020, 17, 1–5. [Google Scholar]
- Yang, X.R.; Zhang, L.W.; Zhang, Y.Z. Natural Accelerated Environmental Test Technologies. Equip. Environ. Eng. 2004, 1, 7–11. [Google Scholar] [CrossRef]
- Ni, L.; Chemtob, A.; Barghorn, C.C.; Moreau, N.; Bouder, T.; Sebastien, C.; Nadine, P. Direct-to-metal UV-cured hybrid coating for the corrosion protection of aircraft aluminum alloy. Corros. Sci. 2014, 89, 242–249. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.C.; Li, H.S.; Zhang, P.S.; Zhou, Y.R. Highly Accelerated Ground Simulation Technology of Space Ultraviolet Radiation. Equip. Environ. Eng. 2021, 18, 57–61. [Google Scholar]
- Yimit, M.; Abla, S.; Zhang, S.; Sawut, A.; Di, S.X. Research on Experimental Methods of UV Artificially Accelerated Photoaging of Drip Irrigation Tape. China Plast. 2013, 27, 89–93. [Google Scholar]
- Wang, D.; Zhang, T.F.; Yang, X.H. Equivalent Relationship of Aviation Coatings Gloss Loss between Natural Exposure and Accelerated Aging Test. Equip. Environ. Eng. 2020, 17, 82–86. [Google Scholar]
- Li, B.T.; Xing, L.Y.; Zhou, Z.G.; Jiang, Z.G.; Chen, X.B. Study on Mechanical Properties of High-Performance Envelope Materials. J. Mater. Eng. 2010, 12, 1–13. [Google Scholar] [CrossRef]
- Yuan, H.; Wang, W.; Yang, D.; Zhou, X.; Zhao, Z.; Zhang, L.; Wang, S.; Feng, J. Hydrophilicity modification of aramid fiber using a linear shape plasma excited by nanosecond pulse. Surf. Coat. Technol. 2018, 344, 614–620. [Google Scholar] [CrossRef]
- American Society for Materials and Testing. Standard Test Method for Tensile Strength and Young’s Modulus of Fibers; ASTM: West Conshohocken, PA, USA, 2003. [Google Scholar]
- Liu, Y.X. Study on Photoaging Behavior and Protection of Vectran Fibers. Ph.D. Thesis, Harbin Institute of Technology, Harbin, China, 2014. [Google Scholar]
- Levchenko, A.A.; Antipov, E.M.; Plate, N.A.; Stamm, M. Comparative analysis of structure and temperature behavior of two Copolyimides-Regular KEVLAR and statistical ARMOS. Macromol. Symp. 1999, 146, 145–151. [Google Scholar] [CrossRef]
- Leal, A.A.; Deitzel, J.M.; Gillespie, J.W. Assessment of compressive properties of high-performance organic fibers. Compos. Sci. Technol. 2007, 67, 2786–2794. [Google Scholar] [CrossRef]
- Jin, Z.; Luo, Z.; Yang, S.; Lu, S. Influence of complexing treatment and epoxy resin coating on the properties of aramid fiber reinforced natural rubber. J. Appl. Polym. Sci. 2015, 132, 42122. [Google Scholar] [CrossRef]
- Xiong, L.; Yu, W.D. Application to the study on FTIR microspectroscopy in high-performance fibers. J. Donghua Univ. 2004, 30, 92–97. [Google Scholar] [CrossRef]
- Li, G.J.; Li, M.Y. The performance study on the Kevlar twaron fabric. New Chem. Mater. 2011, 8, 118–121. [Google Scholar] [CrossRef]
- Ban, H.J. Structure Design of Integrated Rope-Coring Soft Bag and Ultraviolet Protection. Ph.D. Thesis, Harbin Institute of Technology, Harbin, China, 2015. [Google Scholar]
- Su, M.; Gu, A.J.; Liang, G.Z.; Yuan, L. The effect of oxygen-plasma treatment on Kevlar fibers and the properties of Kevlar fibers/bismaleimide composites. Appl. Surf. Sci. 2011, 257, 3158–3167. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Jiang, Z.X.; Huang, Y.D.; Li, Q. The modification of Kevlar fibers in coupling agents by γ-ray co-irradiation. J. Fibers Polym. 2011, 12, 1014–1020. [Google Scholar] [CrossRef]
- Li, C. Study of Complexation on Surface Modification of Kevlar Fiber. Ph.D. Thesis, Guizhou University, Guizhou, China, 2015. [Google Scholar]
- Zhang, Y.; Jin, X.D.; Sun, S.B.; Tian, Y.L. Research Progress on Surface Modification of Polymer Materials Based on Ultraviolet-Ozone. J. Mater. Sci. Technol. 2021, 23, 1–17. [Google Scholar] [CrossRef]


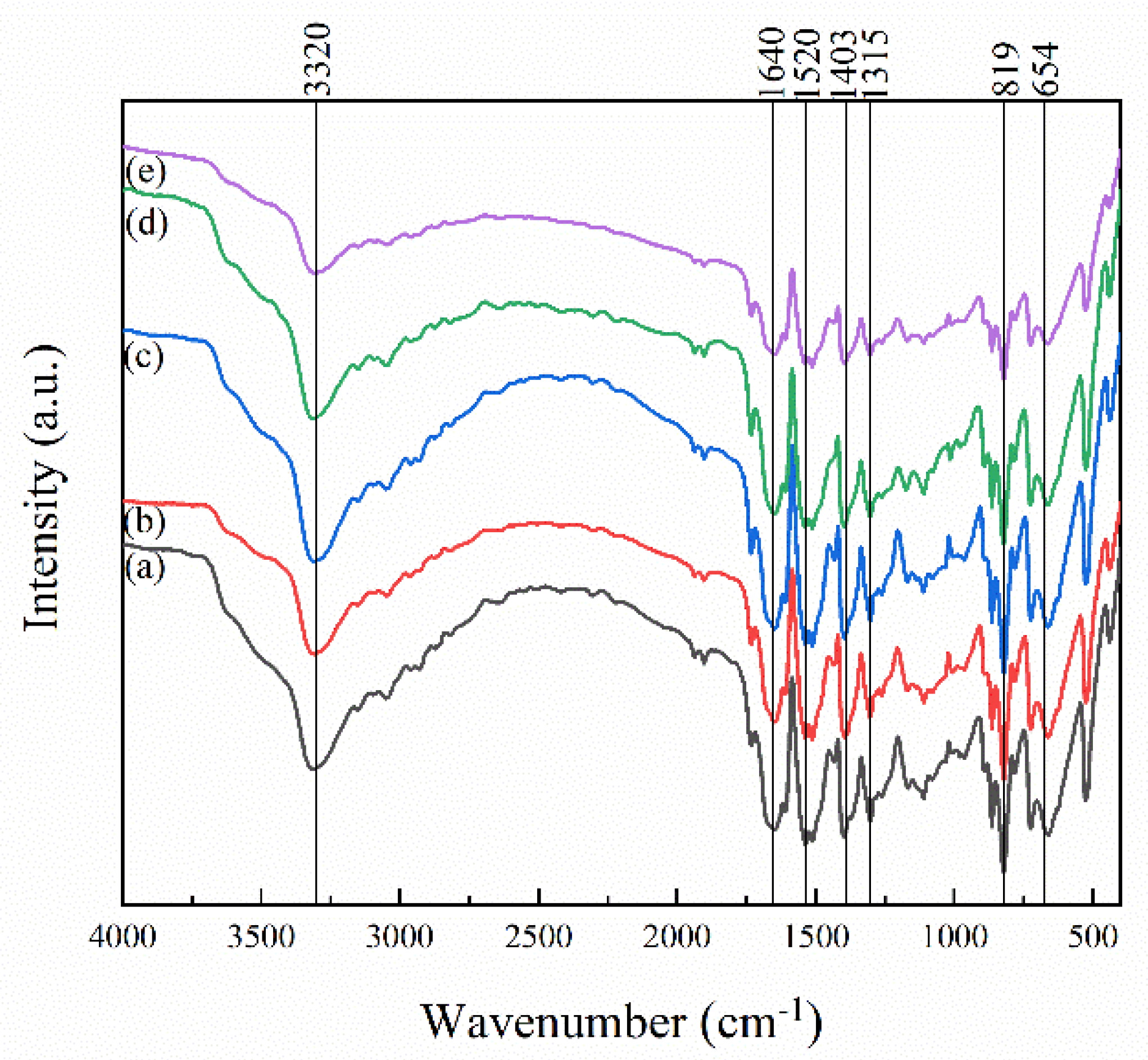
| Exposure Time (h) | Intensity Ratio of Absorption Band (%) | ||||
|---|---|---|---|---|---|
| Amide Group | Hydrogen Bond | ||||
| 1315 cm−1 | 1403 cm−1 | 1520 cm−1 | 1640 cm−1 | 3320 cm−1 | |
| 0 | 3.78 | 1.01 | 1.25 | 3.34 | 45.57 |
| 100 | 4.78 | 6.78 | 4.25 | 7.13 | 10.86 |
| 300 | 4.71 | 6.38 | 3.88 | 6.58 | 8.93 |
| 500 | 4.80 | 6.14 | 3.86 | 5.90 | 11.20 |
| 1000 | 4.70 | 6.17 | 3.55 | 5.96 | 10.10 |
| Exposure Time (h) | Surface Composition (at. %) | Atom Ratio | ||
|---|---|---|---|---|
| C | N | O | O/C | |
| 0 | 77.3 | 8.9 | 13.8 | 0.18 |
| 100 | 76.3 | 9.8 | 13.9 | 0.18 |
| 300 | 77.3 | 9.0 | 13.7 | 0.18 |
| 500 | 75.4 | 8.8 | 15.9 | 0.21 |
| 1000 | 75.9 | 8.1 | 16.0 | 0.21 |
| Exposure Time (h) | Content of Functional Group (%) | |||
|---|---|---|---|---|
| -C-C- | -C-N- | -CONH- | -COO- | |
| 0 | 62.81 | 18.73 | 18.46 | 0.00 |
| 100 | 64.07 | 22.95 | 11.88 | 1.10 |
| 300 | 53.80 | 28.50 | 12.21 | 5.49 |
| 500 | 49.98 | 33.72 | 7.48 | 8.82 |
| 1000 | 60.85 | 21.58 | 11.76 | 5.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Wei, Q.; Fan, H.; Qi, Z.; Hu, N. Mechanical Properties Evolution and Damage Mechanism of Kevlar Fiber under Ozone Exposure in Near-Space Simulation. Coatings 2022, 12, 584. https://doi.org/10.3390/coatings12050584
Ma J, Wei Q, Fan H, Qi Z, Hu N. Mechanical Properties Evolution and Damage Mechanism of Kevlar Fiber under Ozone Exposure in Near-Space Simulation. Coatings. 2022; 12(5):584. https://doi.org/10.3390/coatings12050584
Chicago/Turabian StyleMa, Jie, Qiang Wei, Hongbo Fan, Zhengpan Qi, and Ning Hu. 2022. "Mechanical Properties Evolution and Damage Mechanism of Kevlar Fiber under Ozone Exposure in Near-Space Simulation" Coatings 12, no. 5: 584. https://doi.org/10.3390/coatings12050584
APA StyleMa, J., Wei, Q., Fan, H., Qi, Z., & Hu, N. (2022). Mechanical Properties Evolution and Damage Mechanism of Kevlar Fiber under Ozone Exposure in Near-Space Simulation. Coatings, 12(5), 584. https://doi.org/10.3390/coatings12050584







