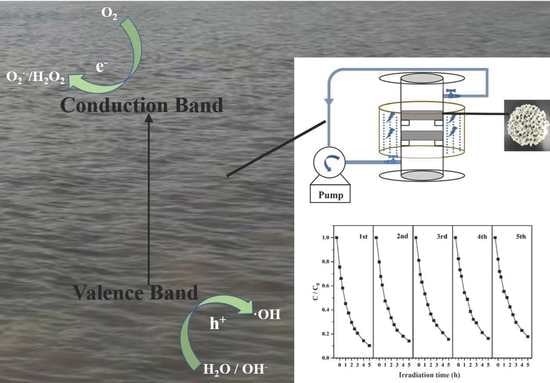
N-TiO2-Coated SiC Foam for the Treatment of Dyeing Wastewater under Blue Light LED Irradiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Synthesis of N-TiO2
2.3. Immobilization of N-TiO2/SiC Foams
2.4. Characterizations
2.5. Experimental Setups
3. Results
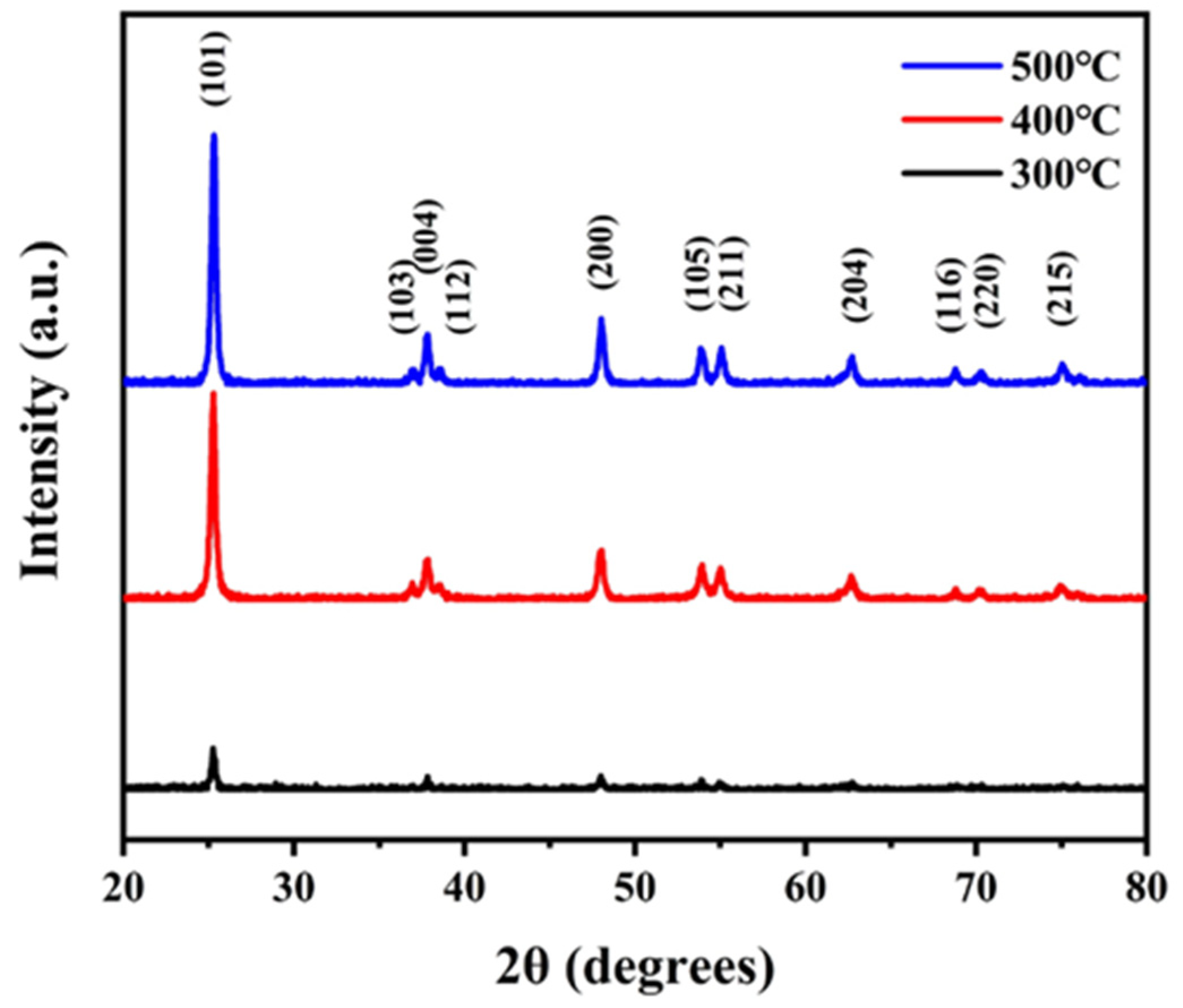
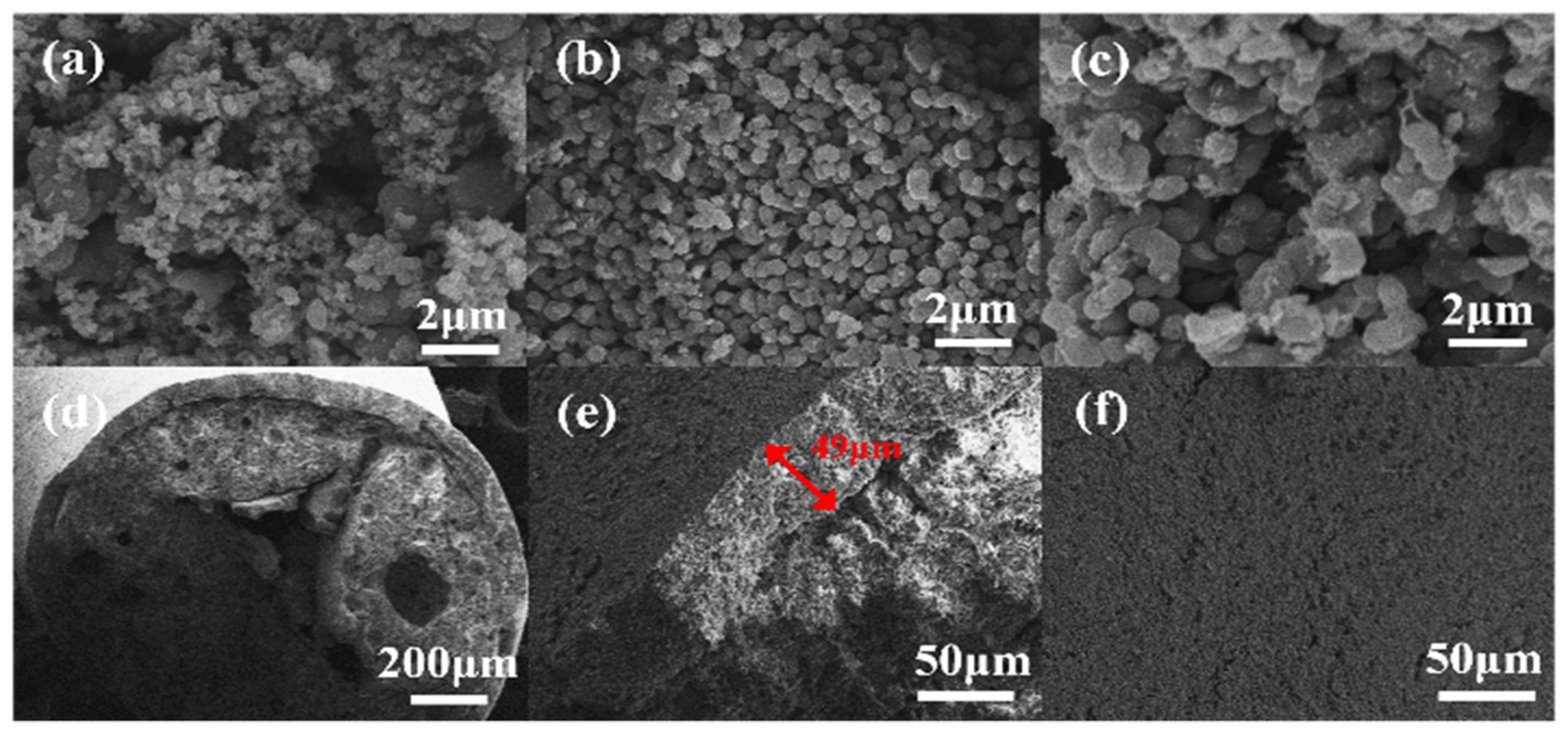
3.1. Catalyst Characterization
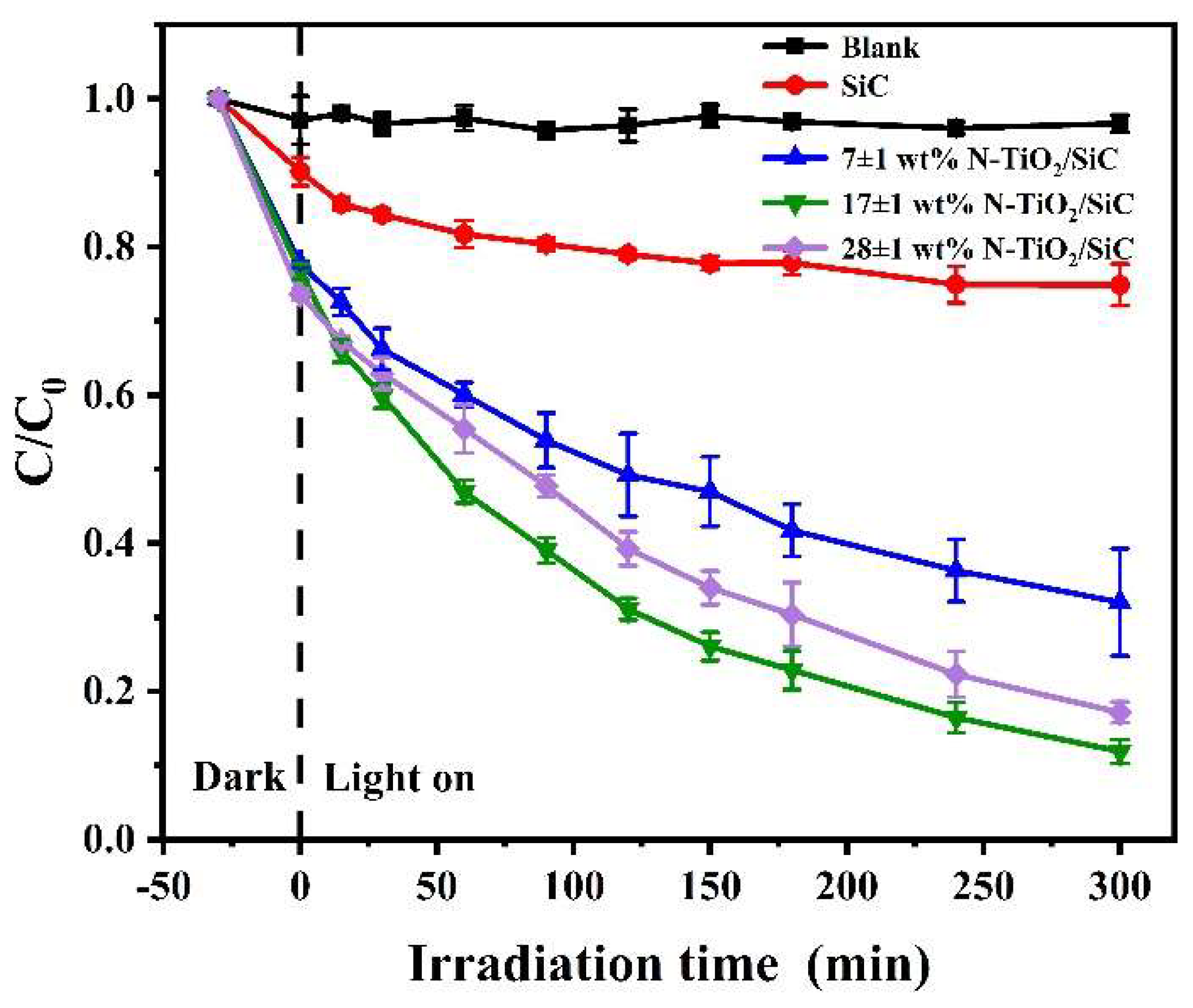
3.2. Photocatalytic Degradation of MB by N-TiO2
3.3. N-TiO2/SiC Foam Photocatalytic Activity
3.3.1. Effect of Initial MB Concentration
3.3.2. Effect of Initial pH
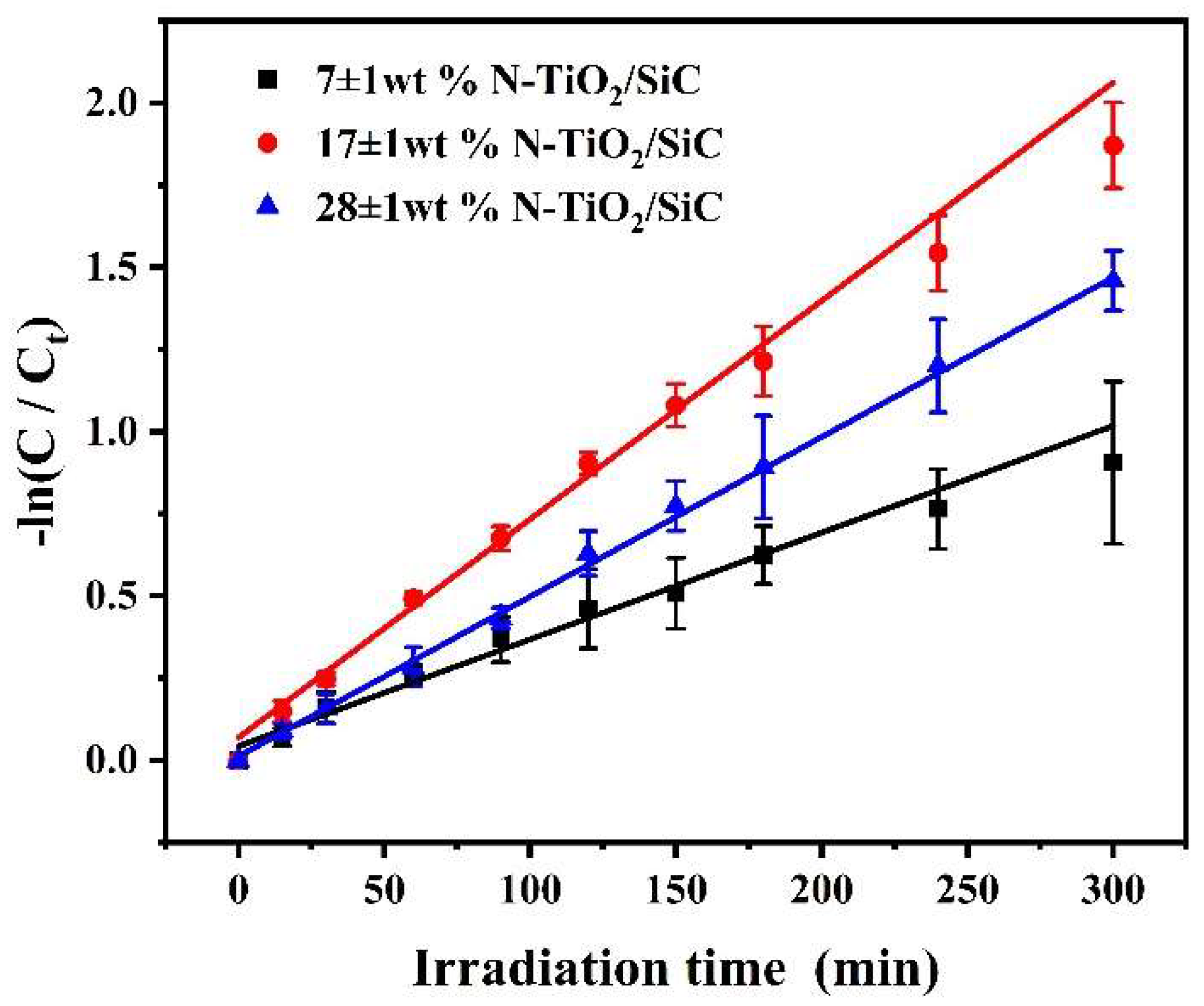
3.3.3. Effect of Catalyst Loading
3.4. Stability of N-TiO2/SiC Foam
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, L.; Chen, Z.; Shan, D.; Wu, Y.; Bai, L.; Wang, B. Treatment of industrial dye wastewater and pharmaceutical residue wastewater by advanced oxidation processes and its combination with nanocatalysts: A review. J. Water Process. Eng. 2021, 42, 102122. [Google Scholar] [CrossRef]
- Konicki, W.; Aleksandrzak, M.; Moszyński, D.; Mijowska, E. Adsorption of anionic azo-dyes from aqueous solutions onto graphene oxide: Equilibrium, kinetic and thermodynamic studies. E. J. Colloid Interface Sci. 2017, 496, 188–200. [Google Scholar] [CrossRef]
- Chen, J.; Hu, H.; Yang, J.; Xue, H.; Liu, Y. Removal behaviors and mechanisms for series of azo dye wastewater by novel nano constructed macro-architectures material. Bioresour. Technol. 2021, 322, 124556. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Ribeiro, J.P.; Nunes, M.I. Recent trends and developments in Fenton processes for industrial wastewater treatment—A critical review. Environ. Res. 2021, 197, 110957. [Google Scholar] [CrossRef] [PubMed]
- Giannakis, S.; Lin, K.Y.A.; Ghanbari, F. A review of the recent advances on the treatment of industrial wastewaters by Sulfate Radical-based Advanced Oxidation Processes (SR-AOPs). Chem. Eng. J. 2021, 406, 7012083. [Google Scholar] [CrossRef]
- Huang, D.; Zhang, G.; Yi, J.; Cheng, M.; Chen, S. Progress and challenges of metal-organic frameworks-based materials for SR-AOPs applications in water treatment. Chemosphere 2020, 263, 127672. [Google Scholar] [CrossRef]
- Velempini, T.; Prabakaran, E.; Pillay, K. Recent developments in the use of metal oxides for photocatalytic degradation of pharmaceutical pollutants in water—A review. Mater. Today Chem. 2021, 19, 100380. [Google Scholar] [CrossRef]
- Tsang, C.; Li, K.; Zeng, Y.; Zhao, W.; Zhang, T.; Zhan, Y.; Xie, R.; Leung, D.; Huang, H. Titanium oxide based photocatalytic materials development and their role of in the air pollutants degradation: Overview and forecast. Environ. Int. 2019, 125, 200–228. [Google Scholar] [CrossRef]
- Liu, L.; Xu, K.; Su, S.; He, L.; Qing, M.; Chi, H.; Liu, T.; Hu, S.; Wang, Y.; Xiang, J. Efficient Sm modified Mn/TiO2 catalysts for selective catalytic reduction of NO with NH3 at low temperature. Appl. Catal. A Gen. 2020, 592, 117413. [Google Scholar] [CrossRef]
- Chen, B.; Li, C.; Xu, H.; Gao, J.; Du, W.; Shangguan, L.; Zhang, X.; Lin, R.B.; Wu, H.; Zhou, W. Tunable titanium metal-organic frameworks with infinite 1D Ti-O rods for efficient visible-light-driven photocatalytic H2 evolution. J. Mater. Chem. A 2019, 7, 11928–11933. [Google Scholar]
- Huang, Y.U.; Zheng, X.; Yin, Z.; Tao, F.; Fang, B.; Hou, K. Preparation of Nitrogen-doped TiO2 Nanoparticle Catalyst and Its Catalytic Activity under Visible Light. Chin. J. Chem. Eng. 2007, 15, 802–807. [Google Scholar]
- Jin, X.; Zhou, X.; Sun, P.; Lin, S.; Cao, W. Photocatalytic degradation of norfloxacin using N-doped TiO2: Optimization, mechanism, identification of intermediates and toxicity evaluation. Chemosphere 2019, 237, 124433. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wang, Z.; Chen, X.; Chu, H.; Wang, C.C. Robust photocatalytic benzene degradation using mesoporous disk-like N-TiO2 derived from MIL-125(Ti). Chinese J. Catal. 2020, 41, 1186–1197. [Google Scholar] [CrossRef]
- Pestana, C.J.; Noronha, J.P.; Hui, J.; Edwards, C.; Gunaratne, H.Q.N.; Irvine, J.T.S.; Robertson, P.K.J.; Capelo-Nrto, J.; Lawton, L.A. Photocatalytic removal of the cyanobacterium Microcystis aeruginosa PCC7813 and four microcystins by TiO2 coated porous glass beads with UV-LED irradiation. Sci. Total Environ. 2020, 745, 141154. [Google Scholar] [CrossRef]
- Sun, Y.; Li, G.; Gong, Y.; Sun, Z.; Yao, H.; He, J.; Dong, H.; Zhang, X.; Huang, H. Ag and TiO2 nanoparticles co-modified defective zeolite TS-1 for improved photocatalytic CO2 reduction. J. Hazard. Mater. 2021, 403, 124019. [Google Scholar] [CrossRef]
- Xuan, X.; Tu, S.; Yu, H.; Du, X.; Huang, H. Size-dependent selectivity and activity of CO2 photoreduction over black nano-titanias grown on dendritic porous silica particles. Appl. Catal. B 2019, 255, 117768. [Google Scholar] [CrossRef]
- Qiu, P.; Sun, X.; Lai, Y.; Gao, P.; Ge, L. N-doped TiO2@TiO2 visible light active film with stable and efficient photocathodic protection performance. J. Electroanal. Chem. 2019, 844, 91–98. [Google Scholar] [CrossRef]
- Marien, C.B.D.; Pivert, M.L.; Azas, A.; M’Bra, I.C.; Robert, D. Kinetics and mechanism of Paraquat’s degradation: UV-C photolysis vs UV-C photocatalysis with TiO2/SiC foams. J. Hazard. Mater. 2018, 370, 164–171. [Google Scholar] [CrossRef]
- Singha, J.; Kumar, S.; Rishikesh; Manna, A.K.; Soni, R.K. Fabrication of ZnO–TiO2 nanohybrids for rapid sunlight driven photodegradation of textile dyes and antibiotic residue molecules. Opt. Mater. 2020, 107, 110138. [Google Scholar] [CrossRef]
- Singha, J.; Soni, R.K. Two-dimensional MoS2 nanosheet modified oxygen defect rich TiO2 nanoparticles for light emission and photocatalytic applications. New J. Chem. 2020, 35, 14936–14946. [Google Scholar] [CrossRef]
- Singha, J.; Soni, R.K. Enhanced sunlight driven photocatalytic activity of In2S3 nanosheets functionalized MoS2 nanoflowers heterostructures. Sci. Rep. 2021, 11, 15352. [Google Scholar] [CrossRef] [PubMed]
- Vaiano, V.; Sacco, O.; Sannino, D.; Ciambelli, P. Photocatalytic removal of spiramycin from wastewater under visible light with N-doped TiO2 photocatalysts. Chem. Eng. J. 2015, 261, 3–8. [Google Scholar] [CrossRef]
- Wang, C.; Ao, Y.; Wang, P.; Hou, J.; Jin, Q. Preparation of cerium and nitrogen co-doped titania hollow spheres with enhanced visible light photocatalytic performance. Powder Technol. 2011, 210, 203–207. [Google Scholar] [CrossRef]
- Parida, K.M.; Pany, S.; Naik, B. Green synthesis of fibrous hierarchical meso-macroporous N doped TiO2 nanophotocatalyst with enhanced photocatalytic H2 production. Int. J. Hydrogen Energy 2013, 38, 3545–3553. [Google Scholar] [CrossRef]
- Tichapondwa, J.; Newman, P.; Kubheka, O. Effect of TiO2 phase on the photocatalytic degradation of methylene blue dye. Phys. Chem. Earth. 2020, 118, 102900. [Google Scholar] [CrossRef]
- Huang, J.; Dou, L.; Li, J.; Zhong, J.; Wang, T. Excellent visible light responsive photocatalytic behavior of N-doped TiO2 toward decontamination of organic pollutants. J. Hazard. Mater. 2021, 403, 123857. [Google Scholar] [CrossRef]
- Sanchez-Martinez, A.; Ceballos-Sanchez, O.; Koop-Santa, C.; López-Mena, E.R.; Orozco-Guareño, E.; García-Guaderrama, M. N-doped TiO2 nanoparticles obtained by a facile coprecipitation method at low temperature. Ceram. Int. 2018, 44, 5273–5283. [Google Scholar] [CrossRef]
- Echabbi, F.; Hamlich, M.; Harkati, S.; Jouali, A.; Safi, M. Photocatalytic degradation of methylene blue by the use of titanium-doped Calcined Mussel Shells CMS/TiO2. J. Environ. Chem. Eng. 2019, 7, 103293. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, W.; Li, X.; Zou, J.; Guo, H. N-TiO2-Coated SiC Foam for the Treatment of Dyeing Wastewater under Blue Light LED Irradiation. Coatings 2022, 12, 585. https://doi.org/10.3390/coatings12050585
Sun W, Li X, Zou J, Guo H. N-TiO2-Coated SiC Foam for the Treatment of Dyeing Wastewater under Blue Light LED Irradiation. Coatings. 2022; 12(5):585. https://doi.org/10.3390/coatings12050585
Chicago/Turabian StyleSun, Wen, Xuankun Li, Jingtao Zou, and Han Guo. 2022. "N-TiO2-Coated SiC Foam for the Treatment of Dyeing Wastewater under Blue Light LED Irradiation" Coatings 12, no. 5: 585. https://doi.org/10.3390/coatings12050585
APA StyleSun, W., Li, X., Zou, J., & Guo, H. (2022). N-TiO2-Coated SiC Foam for the Treatment of Dyeing Wastewater under Blue Light LED Irradiation. Coatings, 12(5), 585. https://doi.org/10.3390/coatings12050585