Biopaper Based on Ultralong Hydroxyapatite Nanowires and Cellulose Fibers Promotes Skin Wound Healing by Inducing Angiogenesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Ultralong HAP Nanowires (HAPNWs)
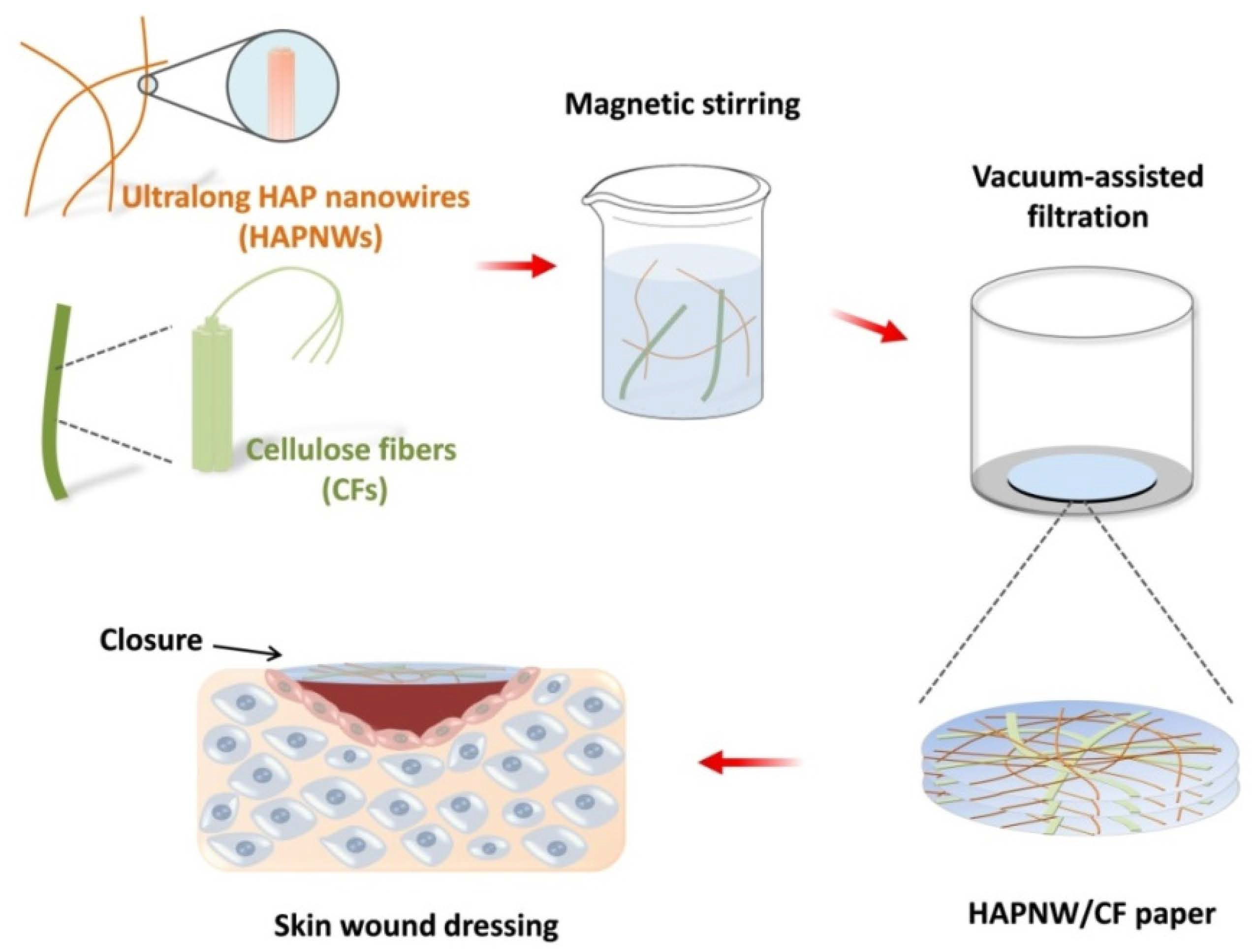
2.3. Fabrication of the HAPNW/CF Biopaper Consisting of HAPNWs and CFs
2.4. Materials Characterization
2.4.1. Structural Characterization
2.4.2. Degradation and Ca2+ Ions Release Test
2.4.3. Specific Surface Area Measurement
2.4.4. Density and Porosity Test
2.4.5. Water Absorption and Water Contact Angle Measurements
2.4.6. Mechanical Tests
2.5. In Vitro Cellular Studies
2.5.1. Cell Culture
2.5.2. Establishment of a Model of Cellular Ischemia and Hypoxia
2.5.3. Cell Viability
2.5.4. Western Blot Analysis
2.5.5. Cell Migration and Tube Formation
2.5.6. EdU Cell Proliferation Assay
2.6. In Vivo Experiments
2.6.1. Establishment of the Animal Model of Skin Wound
2.6.2. Histological Analysis
2.7. Statistical Analysis
3. Results
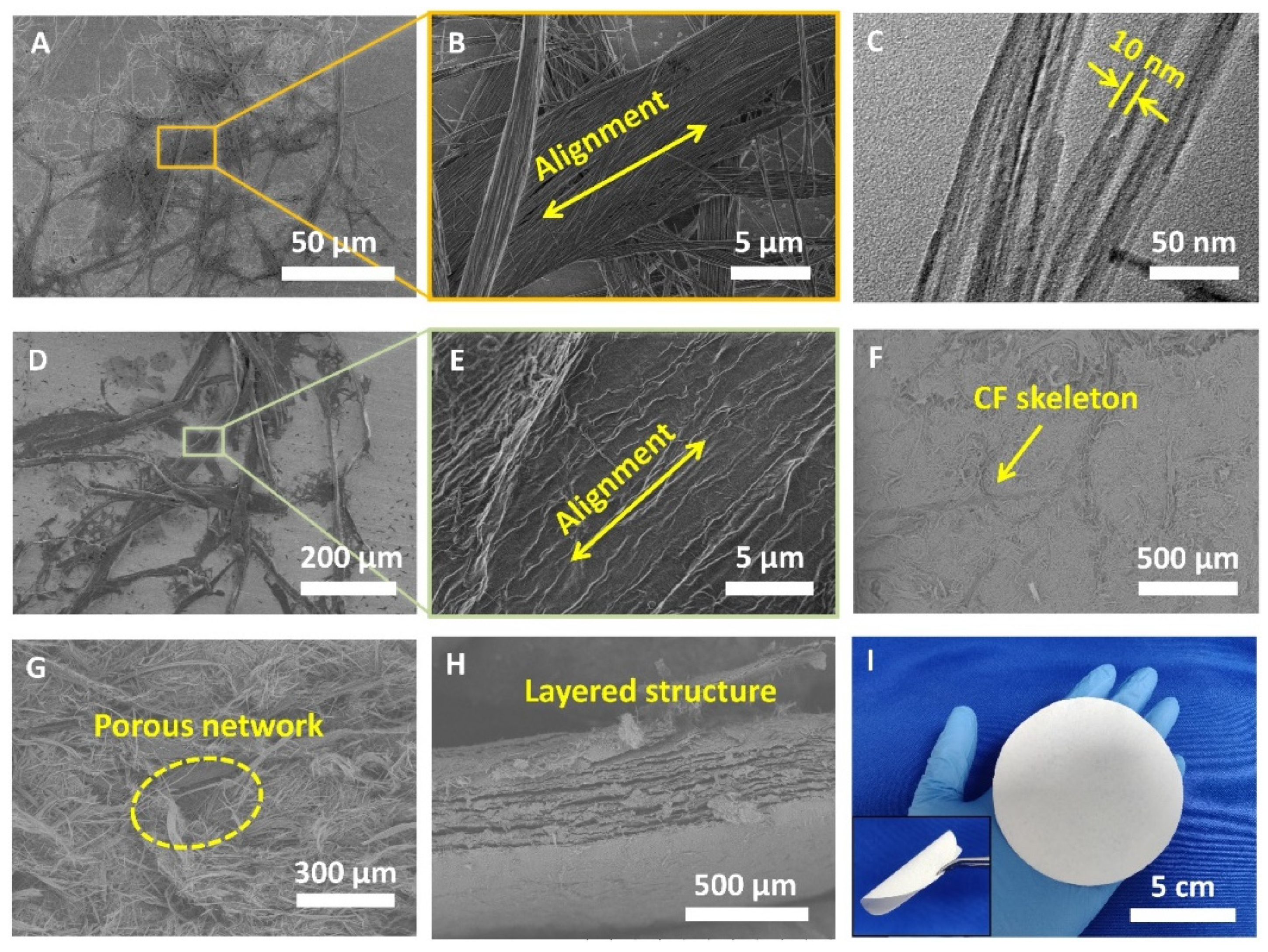
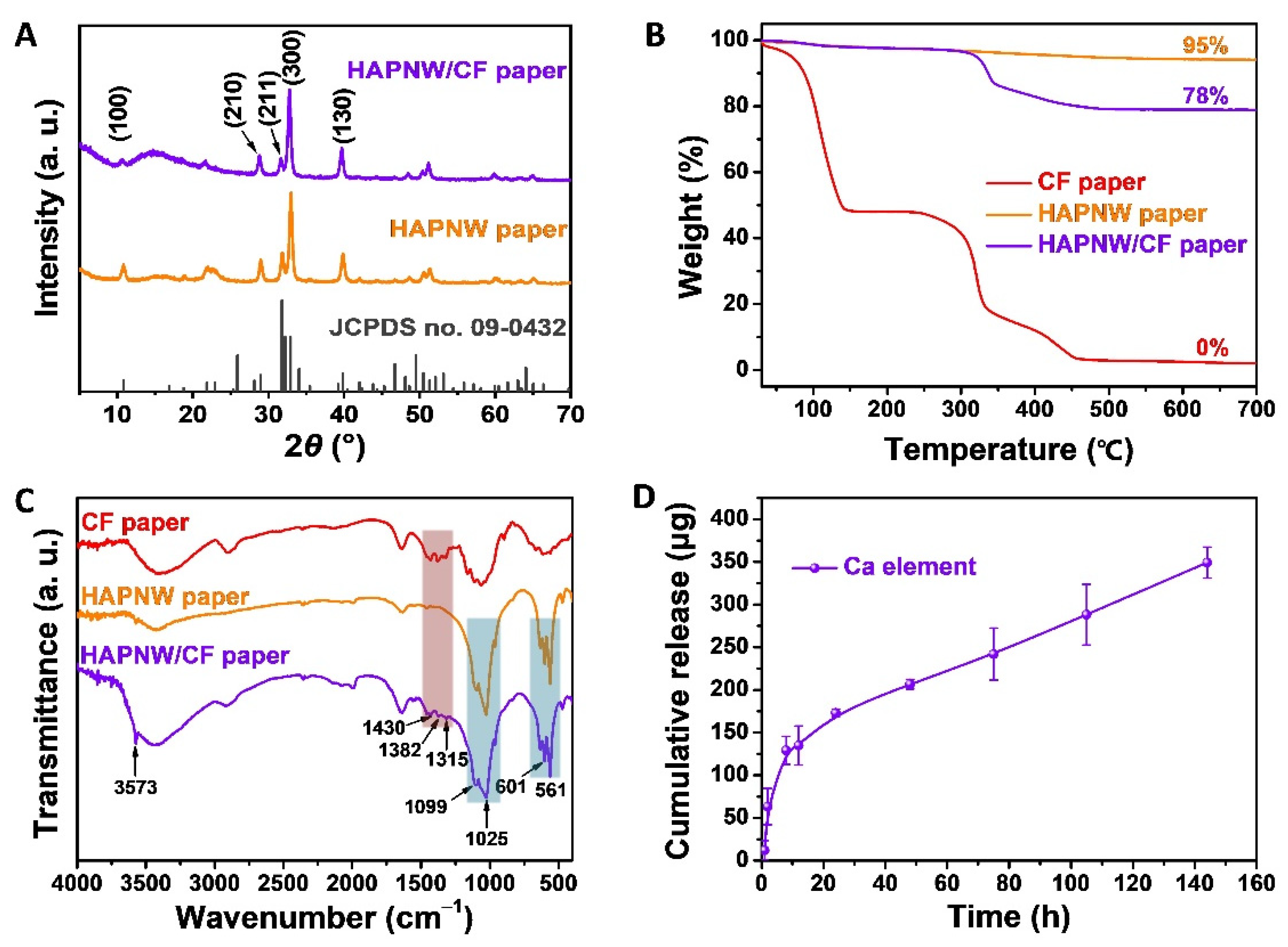
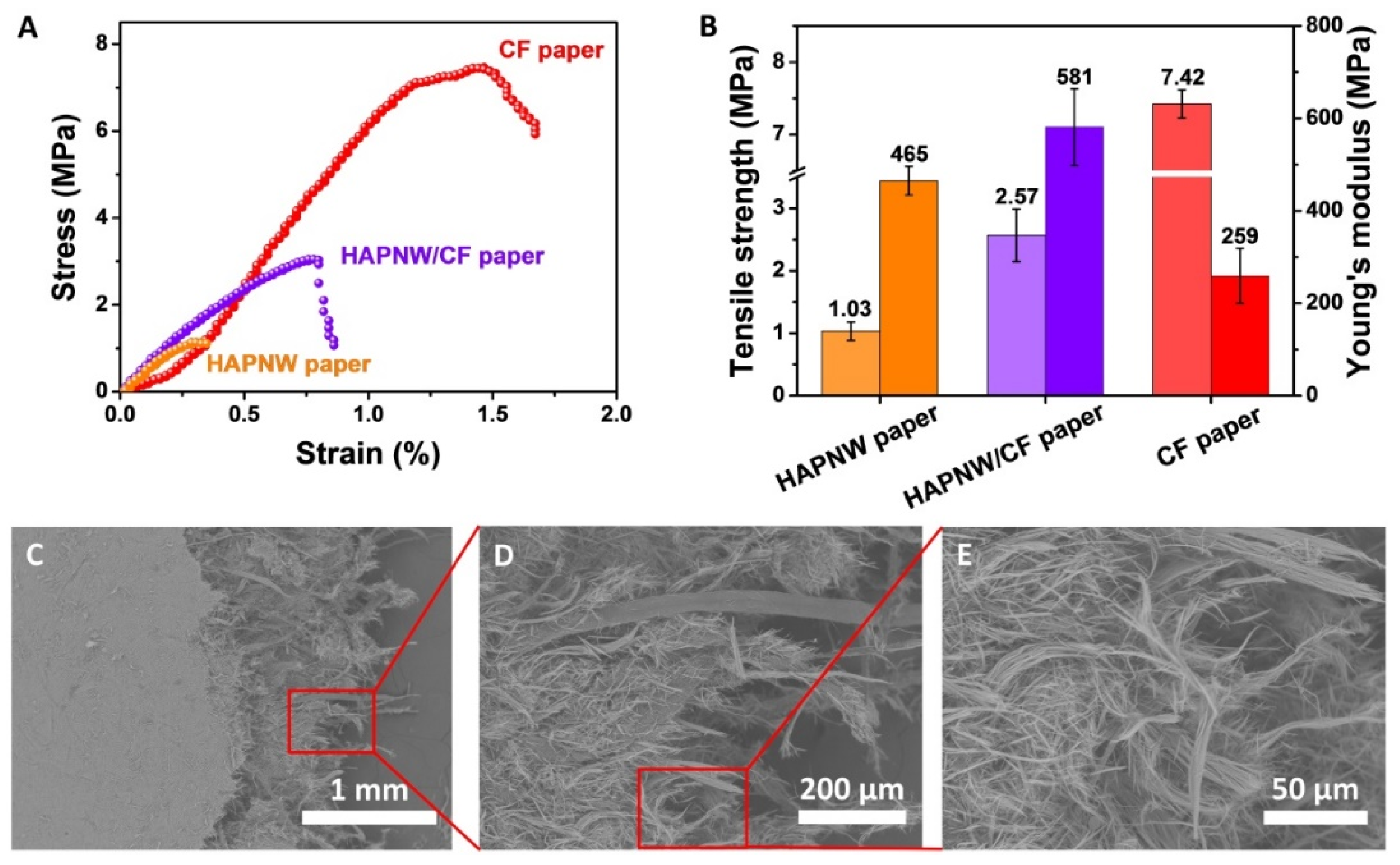
3.1. Characterization of the HAPNW/CF Biopaper and Control Samples
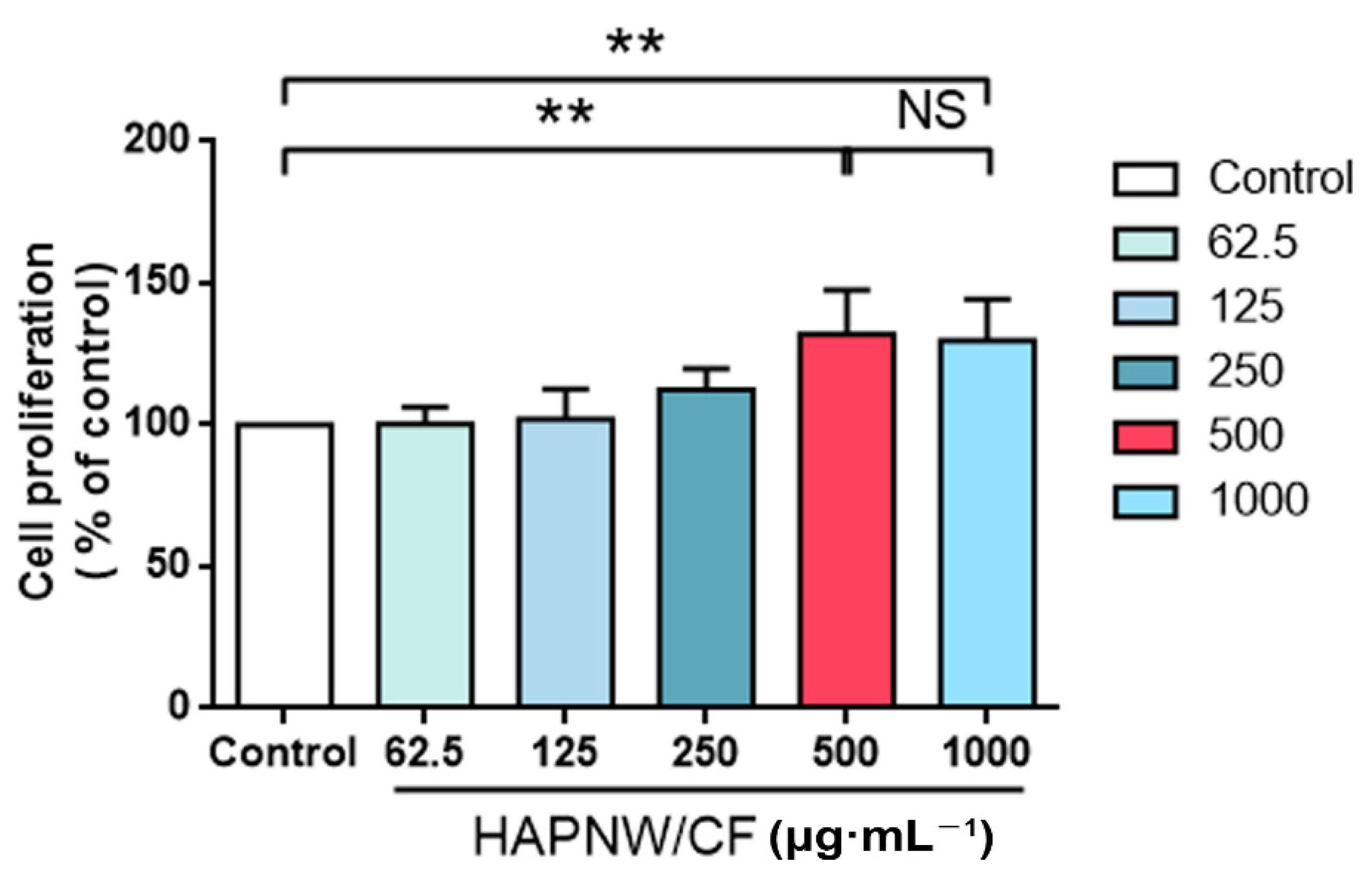
3.2. Safety Evaluation of the HAPNW/CF Biopaper
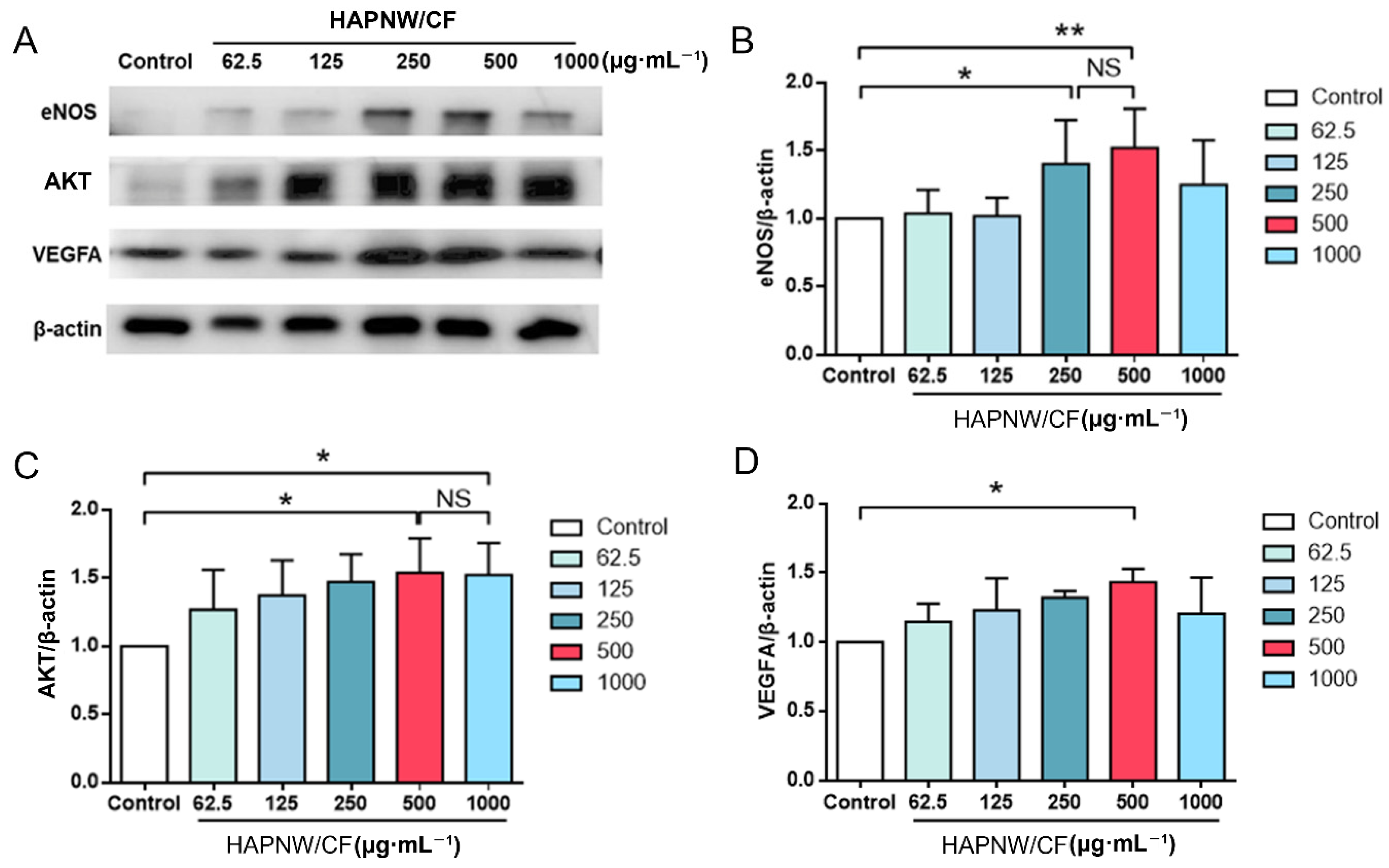
3.3. Evaluation of the Effect of the HAPNW/CF Biopaper on HUVECs
3.4. Effect of L-NAME on the Ability of the HAPNW/CF Biopaper to Promote Angiogenesis
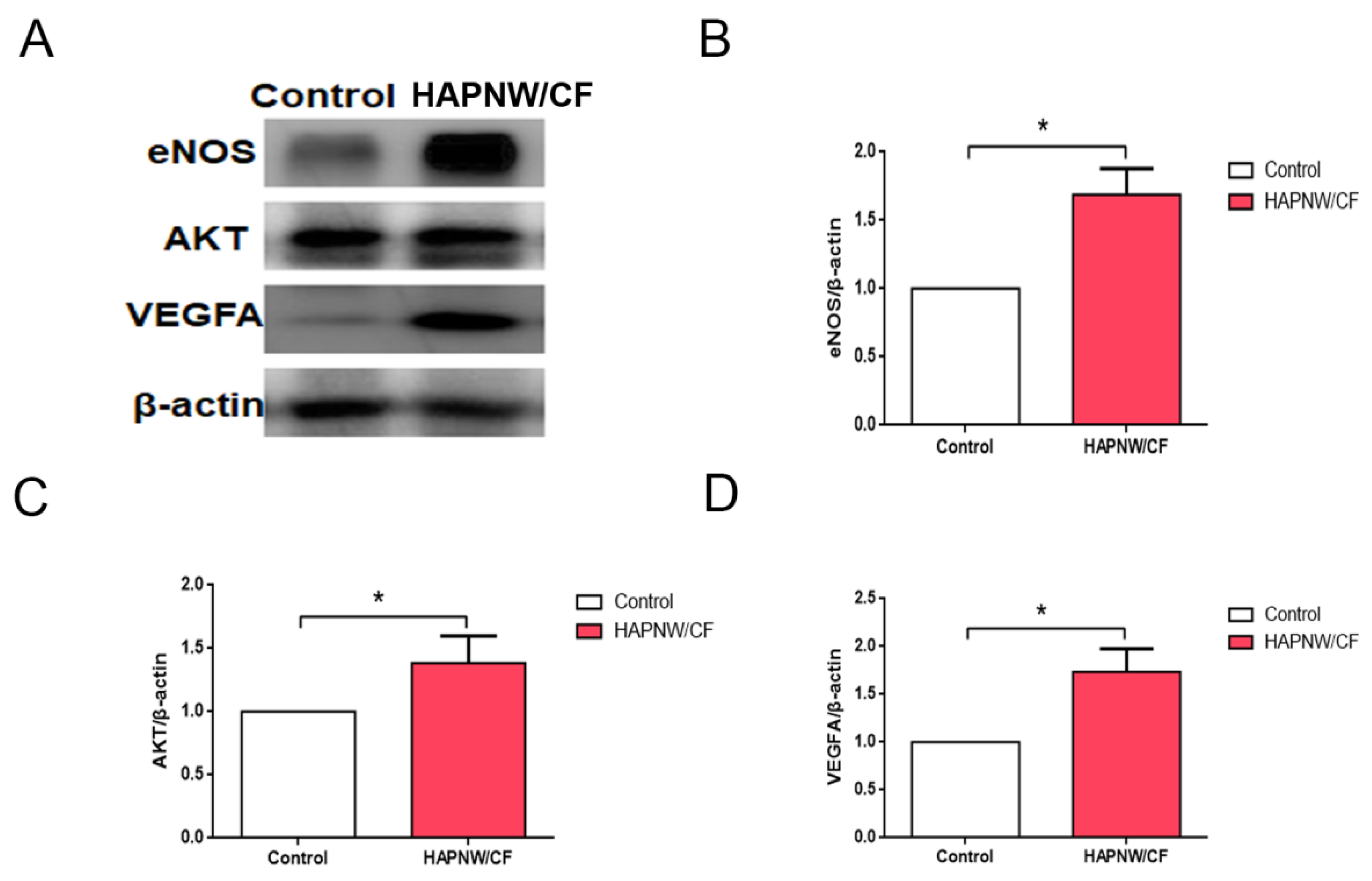
3.5. Effect of the HAPNW/CF Biopaper on the Expression of Pro-Angiogenic Protein in a Full-Thickness Skin Trauma Model
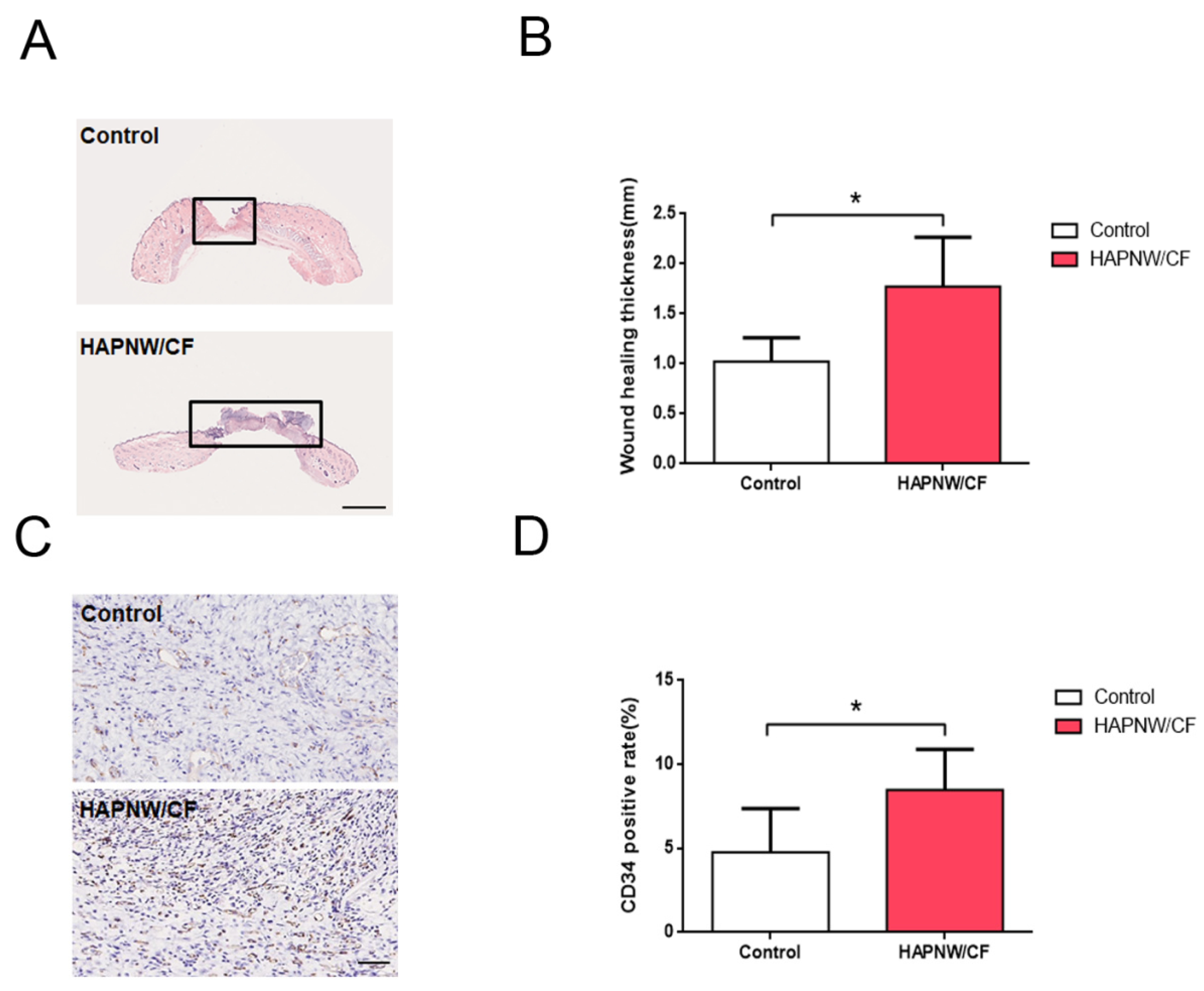
3.6. Histological Analysis of Angiogenesis in Full-Thickness Skin Trauma Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Subramaniam, T.; Fauzi, M.B.; Lokanathan, Y.; Law, J.X. The role of calcium in wound healing. Int. J. Mol. Sci. 2021, 22, 6486. [Google Scholar] [CrossRef] [PubMed]
- Järbrink, K.; Ni, G.; Sönnergren, H.; Schmidtchen, A.; Pang, C.; Bajpai, R.; Car, J. The humanistic and economic burden of chronic wounds: A protocol for a systematic review. Syst. Rev. 2017, 6, 15. [Google Scholar] [CrossRef] [Green Version]
- Keyes, B.E.; Liu, S.; Asare, A.; Naik, S.; Levorse, J.; Polak, L.; Lu, C.P.; Nikolova, M.; Pasolli, H.A.; Fuchs, E. Impaired epidermal to dendritic T cell signaling slows wound repair in aged skin. Cell 2016, 167, 1323–1338. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Sun, X.; Lee, J.H.; Kim, H.W.; Fu, X.; Leong, K.W. Advanced drug delivery systems and artificial skin grafts for skin wound healing. Adv. Drug Deliv. Rev. 2019, 146, 209–239. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P. Angiogenesis in health and disease. Nat. Med. 2003, 9, 653–660. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, G.; Wu, J.; Zhang, Y.; Liu, J.; Luo, H.; Shao, L. Insights into the angiogenic effects of nanomaterials: Mechanisms involved and potential applications. J. Nanobiotechnol. 2020, 18, 9. [Google Scholar] [CrossRef]
- Hendrickx, B.; Verdonck, K.; Van den Berge, S.; Dickens, S.; Eriksson, E.; Vranckx, J.J.; Luttun, A. Integration of blood outgrowth endothelial cells in dermal fibroblast sheets promotes full thickness wound healing. Stem Cells 2010, 28, 1165–1177. [Google Scholar] [CrossRef] [PubMed]
- Risau, W. Mechanisms of angiogenesis. Nature 1997, 386, 671–674. [Google Scholar] [CrossRef]
- Senger, D.R.; Davis, G.E. Angiogenesis. Cold Spring Harb. Perspect. Biol. 2011, 3, a005090. [Google Scholar] [CrossRef] [Green Version]
- Greco Song, H.H.; Rumma, R.T.; Ozaki, C.K.; Edelman, E.R.; Chen, C.S. Vascular tissue engineering: Progress, challenges, and clinical promise. Cell Stem Cell 2018, 22, 340–354. [Google Scholar] [CrossRef] [Green Version]
- Bae, H.; Puranik, A.S.; Gauvin, R.; Edalat, F.; Carrillo-Conde, B.; Peppas, N.A.; Khademhosseini, A. Building vascular networks. Sci. Transl. Med. 2012, 4, 160ps23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Huang, R.; Zheng, B.; Guo, W.; Li, C.; He, W.; Wei, Y.; Du, Y.; Wang, H.; Wu, D.; et al. Highly stretchable, adhesive, biocompatible, and antibacterial hydrogel dressings for wound healing. Adv. Sci. 2021, 8, 2003627. [Google Scholar] [CrossRef]
- Guo, M.; Wang, Y.; Gao, B.; He, B. Shark tooth-inspired microneedle dressing for intelligent wound management. ACS Nano 2021, 15, 15316–15327. [Google Scholar] [CrossRef]
- Kim, M.S.; Oh, G.W.; Jang, Y.M.; Ko, S.C.; Park, W.S.; Choi, I.W.; Kim, Y.M.; Jung, W.K. Antimicrobial hydrogels based on PVA and Diphlorethohydroxycarmalol (DPHC) derived from brown alga Ishige okamurae: An in vitro and in vivo study for wound dressing application. Mater. Sci. Eng. C 2020, 107, 110352. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Zhang, J.; Cao, L.; Jiao, Q.; Zhou, J.; Yang, L.; Zhang, H.; Wei, Y. Antifouling antioxidant zwitterionic dextran hydrogels as wound dressing materials with excellent healing activities. ACS Appl. Mater. Interfaces 2021, 13, 7060–7069. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, Z.; Zhao, Y.; Feng, Y.; Zvyagin, A.V.; Wang, J.; Yang, X.; Yang, B.; Lin, Q. Novel diabetic foot wound dressing based on multifunctional hydrogels with extensive temperature-tolerant, durable, adhesive, and intrinsic antibacterial properties. ACS Appl. Mater. Interfaces 2021, 13, 26770–26781. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liang, Y.P.; Huang, Y.; He, J.H.; Han, Y.; Guo, B.L. Physical double-network hydrogel adhesives with rapid shape adaptability, fast self-healing, antioxidant and NIR/pH stimulus-responsiveness for multidrug-resistant bacterial infection and removable wound dressing. Adv. Funct. Mater. 2020, 30, 1910748. [Google Scholar] [CrossRef]
- Deng, C.; Xu, C.; Zhou, Q.; Cheng, Y. Advances of nanotechnology in osteochondral regeneration. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1576. [Google Scholar] [CrossRef]
- Mofazzal Jahromi, M.A.; Sahandi Zangabad, P.; Moosavi Basri, S.M.; Sahandi Zangabad, K.; Ghamarypour, A.; Aref, A.R.; Karimi, M.; Hamblin, M.R. Nanomedicine and advanced technologies for burns: Preventing infection and facilitating wound healing. Adv. Drug Deliv. Rev. 2018, 123, 33–64. [Google Scholar] [CrossRef]
- Augustine, R.; Prasad, P.; Khalaf, I.M.N. Therapeutic angiogenesis: From conventional approaches to recent nanotechnology-based interventions. Mater. Sci. Eng. C 2019, 97, 994–1008. [Google Scholar] [CrossRef]
- Lu, B.Q.; Zhu, Y.J. One-dimensional hydroxyapatite materials: Preparation and applications. Can. J. Chem. 2017, 95, 1091–1102. [Google Scholar] [CrossRef]
- Zhu, Y.J.; Lu, B.Q. Deformable biomaterials based on ultralong hydroxyapatite nanowires. ACS Biomater. Sci. Eng. 2019, 5, 4951–4961. [Google Scholar] [CrossRef] [PubMed]
- Son, J.; Kim, J.; Lee, K.; Hwang, J.; Choi, Y.; Seo, Y.; Jeon, H.; Kang, H.C.; Woo, H.M.; Kang, B.J.; et al. DNA aptamer immobilized hydroxyapatite for enhancing angiogenesis and bone regeneration. Acta Biomater. 2019, 99, 469–478. [Google Scholar] [CrossRef]
- Song, Y.; Wu, H.; Gao, Y.; Li, J.; Lin, K.; Liu, B.; Lei, X.; Cheng, P.; Zhang, S.; Wang, Y.; et al. Zinc silicate/nano-hydroxyapatite/collagen scaffolds promote angiogenesis and bone regeneration via the p38 MAPK pathway in activated monocytes. ACS Appl. Mater. Interfaces 2020, 12, 16058–16075. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Yuan, X.; Ma, L.; Bi, B.; Zhu, H.; Lei, Z.; Liu, W.; Pu, H.; Jiang, J.; Jiang, X.; et al. Mesenchymal stem cell-loaded thermosensitive hydroxypropyl chitin hydrogel combined with a three-dimensional-printed poly(ε-caprolactone)/nano-hydroxyapatite scaffold to repair bone defects via osteogenesis, angiogenesis and immunomodulation. Theranostics 2020, 10, 725–740. [Google Scholar] [CrossRef]
- Dong, T.; Duan, C.; Wang, S.; Gao, X.; Yang, Q.; Yang, W.; Deng, Y. Multifunctional surface with enhanced angiogenesis for improving long-term osteogenic fixation of poly (ether ether ketone) implants. ACS Appl. Mater. Interfaces 2020, 12, 14971–14982. [Google Scholar] [CrossRef]
- Huang, G.J.; Yu, H.P.; Wang, X.L.; Ning, B.B.; Gao, J.; Shi, Y.Q.; Zhu, Y.J.; Duan, J.L. Highly porous and elastic aerogel based on ultralong hydroxyapatite nanowires for high-performance bone regeneration and neovascularization. J. Mater. Chem. B 2021, 9, 1277–1287. [Google Scholar] [CrossRef]
- Zhu, Y.J. Multifunctional fire-resistant paper based on ultralong hydroxyapatite nanowires. Chin. J. Chem. 2021, 39, 2296–2314. [Google Scholar] [CrossRef]
- Salama, A. Recent progress in preparation and applications of chitosan/calcium phosphate composite materials. Int. J. Biol. Macromol. 2021, 178, 240–252. [Google Scholar] [CrossRef]
- Kawai, K.; Larson, B.J.; Ishise, H.; Carre, A.L.; Nishimoto, S.; Longaker, M.; Lorenz, H.P. Calcium-based nanoparticles accelerate skin wound healing. PLoS ONE 2011, 6, e27106. [Google Scholar] [CrossRef] [Green Version]
- Lu, B.Q.; Zhu, Y.J.; Chen, F. Highly flexible and nonflammable inorganic hydroxyapatite paper. Chem. Eur. J. 2014, 20, 1242–1246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.G.; Zhu, Y.J.; Chen, F.; Wu, J. Ultralong hydroxyapatite nanowires synthesized by solvothermal treatment using a series of phosphate sodium salts. Mater. Lett. 2015, 144, 135–137. [Google Scholar] [CrossRef]
- Jiang, Y.Y.; Zhu, Y.J.; Chen, F.; Wu, J. Solvothermal synthesis of submillimeter ultralong hydroxyapatite nanowires using a calcium oleate precursor in a series of monohydroxy alcohols. Ceram. Int. 2015, 41, 6098–6102. [Google Scholar] [CrossRef]
- Huang, J.J.; Shi, Y.Q.; Li, R.L.; Hu, A.; Lu, Z.Y.; Weng, L.; Han, Y.P.; Wang, S.Q.; Zhang, L.; Hao, C.N.; et al. Therapeutic ultrasound protects huvecs from Ischemia/Hypoxia-induced apoptosis via the PI3K-akt pathway. Am. J. Transl. Res. 2017, 9, 1990–1999. [Google Scholar]
- Reinke, J.M.; Sorg, H. Wound repair and regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Liu, S.; Xin, X.; Li, P.; Dou, J.; Han, X.; Kang, I.-K.; Yuan, J.; Chi, B.; Shen, J. S-nitrosated keratin composite mats with no release capacity for wound healing. Chem. Eng. J. 2020, 400, 125964. [Google Scholar] [CrossRef]
- Tonnesen, M.G.; Feng, X.; Clark, R.A. Angiogenesis in wound healing. J. Investig. Dermatol. Symp. Proc. 2000, 5, 40–46. [Google Scholar] [CrossRef] [Green Version]
- Dudzinski, D.M.; Igarashi, J.; Greif, D.; Michel, T. The regulation and pharmacology of endothelial nitric oxide synthase. Annu. Rev. Pharmacol. Toxicol. 2006, 46, 235–276. [Google Scholar] [CrossRef]
- Balligand, J.L.; Feron, O.; Dessy, C. eNOS Activation by physical forces: From short-term regulation of contraction to chronic remodeling of cardiovascular tissues. Physiol. Rev. 2009, 89, 481–534. [Google Scholar] [CrossRef]
- Wood, W. Wound healing: Calcium flashes illuminate early events. Curr. Biol. 2012, 22, R14–R16. [Google Scholar] [CrossRef] [Green Version]
- Pastar, I.; Stojadinovic, O.; Yin, N.C.; Ramirez, H.; Nusbaum, A.G.; Sawaya, A.; Patel, S.B.; Khalid, L.; Isseroff, R.R.; Tomic-Canic, M. Epithelialization in wound healing: A comprehensive review. Adv. Wound Care 2014, 3, 445–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Ma, W.; Yang, Z.; Zhang, H.; Ma, J.; Li, T.; Niu, H.; Zhou, Y.; Yao, Q.; Chang, J.; et al. An ultralong hydroxyapatite nanowire aerogel for rapid hemostasis and wound healing. Chem. Eng. J. 2022, 430, 132912. [Google Scholar] [CrossRef]
- Dhand, C.; Venkatesh, M.; Barathi, V.A.; Harini, S.; Bairagi, S.; Goh Tze Leng, E.; Muruganandham, N.; Low, K.Z.W.; Fazil, M.; Loh, X.J.; et al. Bio-inspired crosslinking and matrix-drug interactions for advanced wound dressings with long-term antimicrobial activity. Biomaterials 2017, 138, 153–168. [Google Scholar] [CrossRef]
- Zhao, W.Y.; Fang, Q.Q.; Wang, X.F.; Wang, X.W.; Zhang, T.; Shi, B.H.; Zheng, B.; Zhang, D.D.; Hu, Y.Y.; Ma, L.; et al. Chitosan-calcium alginate dressing promotes wound healing: A preliminary study. Wound Repair Regen. 2020, 28, 326–337. [Google Scholar] [CrossRef]
- Sharma, D.; Ross, D.; Wang, G.; Jia, W.; Kirkpatrick, S.J.; Zhao, F. Upgrading prevascularization in tissue engineering: A review of strategies for promoting highly organized microvascular network formation. Acta Biomater. 2019, 95, 112–130. [Google Scholar] [CrossRef] [PubMed]
- Formentín, P.; Catalán, Ú.; Fernández-Castillejo, S.; Alba, M.; Baranowska, M.; Solà, R.; Pallarès, J.; Marsal, L.F. Human aortic endothelial cell morphology influenced by topography of porous silicon substrates. J. Biomater. Appl. 2015, 30, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Chen, X.; Cai, J.; Ye, D.; Wu, Y.; Fan, L.; Liu, P. Novel porous three-dimensional nanofibrous scaffolds for accelerating wound healing. Chem. Eng. J. 2019, 369, 253–262. [Google Scholar] [CrossRef]
- Bibire, T.; Yilmaz, O.; Ghiciuc, C.M.; Bibire, N.; Dănilă, R. Biopolymers for surgical applications. Coatings 2022, 12, 211. [Google Scholar] [CrossRef]
- Hamza, R.Z.; Al-Motaani, S.E.; Al-Talhi, T. Therapeutic and ameliorative effects of active compounds of combretum molle in the treatment and relief from wounds in a diabetes mellitus experimental model. Coatings 2021, 11, 324. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Hao, L.-S.; Ning, B.-B.; Zhu, Y.-K.; Guan, J.-B.; Ren, H.-W.; Yu, H.-P.; Zhu, Y.-J.; Duan, J.-L. Biopaper Based on Ultralong Hydroxyapatite Nanowires and Cellulose Fibers Promotes Skin Wound Healing by Inducing Angiogenesis. Coatings 2022, 12, 479. https://doi.org/10.3390/coatings12040479
Gao J, Hao L-S, Ning B-B, Zhu Y-K, Guan J-B, Ren H-W, Yu H-P, Zhu Y-J, Duan J-L. Biopaper Based on Ultralong Hydroxyapatite Nanowires and Cellulose Fibers Promotes Skin Wound Healing by Inducing Angiogenesis. Coatings. 2022; 12(4):479. https://doi.org/10.3390/coatings12040479
Chicago/Turabian StyleGao, Jing, Liang-Shi Hao, Bing-Bing Ning, Yuan-Kang Zhu, Ju-Bo Guan, Hui-Wen Ren, Han-Ping Yu, Ying-Jie Zhu, and Jun-Li Duan. 2022. "Biopaper Based on Ultralong Hydroxyapatite Nanowires and Cellulose Fibers Promotes Skin Wound Healing by Inducing Angiogenesis" Coatings 12, no. 4: 479. https://doi.org/10.3390/coatings12040479
APA StyleGao, J., Hao, L.-S., Ning, B.-B., Zhu, Y.-K., Guan, J.-B., Ren, H.-W., Yu, H.-P., Zhu, Y.-J., & Duan, J.-L. (2022). Biopaper Based on Ultralong Hydroxyapatite Nanowires and Cellulose Fibers Promotes Skin Wound Healing by Inducing Angiogenesis. Coatings, 12(4), 479. https://doi.org/10.3390/coatings12040479






