Abstract
In this study, Ti/SnO2-Sb2Ox-TiO2 electrodes were produced using a sol-enhanced electrodeposition technique from methanesulfonate electrolytes. The surface microstructures of Ti/SnO2-Sb2Ox-TiO2 were observed, and their phase constituents were determined. The surface features were analyzed by X-ray photoelectron spectroscopy. Linear sweep voltammetry and degradation tests were also conducted to determine the degradation performance. The results show that the addition of TiO2 sol affects the microstructures of Ti/SnO2-Sb2Ox-TiO2 electrodes, while a uniform coating surface can be obtained at a proper sol concentration in electrolytes. Adding TiO2 sol also causes deep oxidation of Sb and generates more adsorbed oxygen on the electrode surface. The favorable surface features and the well-dispersed TiO2 in the coatings of 10 mL/L TiO2 modified Ti/SnO2-Sb2Ox-TiO2 electrodes award them the best electrocatalytic performance, and their uniform coating surface prolongs the electrode service life.
1. Introduction
The demand for effective effluents treatment technology has raised increasing research interests under growing public concern in environmental issues. The treatment of poorly biodegradable organics is essential in the effluents treatment industry. Advanced electrochemical oxidation technology (AEOT) is considered to be a promising method to deal with this issue, owing to its advantages in efficiency and flexibility [1,2,3]. Organic degradation reactions mostly occur on the anode surface, which plays a crucial role in the electrochemical treatment process. The primary anode materials applied in effluent treatment technology are precious metals (Pt, Au, etc.), carbon, boron-doped diamond (BDD), and metal oxide electrodes [4,5,6].
Titanium-supported antimony-tin oxide (Ti/SnO2-Sb2Ox) is a type of metal oxide electrode that can suppress the oxygen evolution reaction (OER) in order to allow for the effective degradation of organics by hydroxyl radicals. Originally, SnO2-Sb2Ox was utilized as a catalyst for organic conversion, whereas this mixed oxide was recently employed as an anode coating due to its adequate conductivity and chemical resistance. Moreover, SnO2-Sb2Ox can be efficiently and economically coated on a Ti substrate, making it into large areas for industrial applications. The inert Ti/SnO2-Sb2Ox electrodes, therefore, show unique advantages in effluent treatment technology due to their low preparation cost compared to precious metal and BDD, high OER potential, and solid electrocatalytic ability.
The conventional fabrication route for the Ti/SnO2-Sb2Ox electrode is thermal decomposition, in which, the precursor solution is transformed into SnO2-Sb2Ox after calcination [7]. However, the inferior stability and inadequate electrocatalytic ability limit the scale-up application of Ti/SnO2-Sb2Ox electrodes made from thermal decomposition. The electrodeposition fabrication route was recently proposed to manufacture Ti/SnO2-Sb2Ox-TiO2 electrodes. However, despite an enhanced stability, the electrodeposited Ti/SnO2-Sb2Ox-TiO2 electrode suffers from a relatively low electrocatalytic performance.
Previous literature has investigated various modification methods for electrode performance enhancement. Venkatesha et al. [8] and Wang et al. [9] constructed a porous SnO2-Sb2Ox coating based on anodized TiO2 nanotubes, boosting the electrode electrocatalytic performance and long-term stability. Chen et al. [10] synthesized the dense spherical Ti/Sb–SnO2 with a superior electrocatalytic performance using colloidal electrodeposition. Moreover, doping other elements and nanoparticles could effectively modify the electrodeposited Ti/SnO2-Sb2Ox [11]. Liu et al. [12] and Qiao et al. [13] fabricated the Ti/SnO2-Sb2Ox–Ce electrodes for efficient electrocatalytic oxidation. Wu et al. [14] prepared a high-performance duplex-structured Ti/SnO2-Sb2Ox-CNT composite anode using the electrodeposition method. In our recent studies, a sol-enhanced electrodeposition method has been proposed to efficiently dope inert nanoparticles and achieve their well-dispersion into the coating [15,16,17,18], which can serve as a promising method for Ti/SnO2-Sb2Ox modification.
This work explores the sol-enhanced electrodeposition route for the Ti/SnO2-Sb2Ox-TiO2 electrodes in methanesulfonate electrolytes. Compared to the traditional oxidizing sulfuric sources, the methanesulfonate electrolyte better reveals the advantages of a low toxicity, excellent chemical stability, and outstanding biodegradability [19,20,21]. The influence of TiO2 sol addition on the electrode’s properties and performance is systematically investigated. We propose a novel modification procedure for Ti/SnO2-Sb2Ox, which will benefit the scale-up application for Ti/SnO2-Sb2Ox in the effluent treatment industry. We also aim to thoroughly unveil the underlying mechanism and potential of its electrodeposition fabrication route.
2. Materials and Methods
2.1. Preparation of Ti/SnO2-Sb2Ox-TiO2
The analytical grade chemicals were bought from Aladdin Reagent, Shanghai, China. A pure titanium (99.9%) plate was used in our work. The titanium substrate was pretreated as described in the procedures below: (1) grinding and polishing; (2) soaking into 15% wt sodium hydroxide (NaOH) solution for 2 h; and (3) soaking in 12% wt oxalic acid solution for 3 h. The TiO2 sol was prepared as reported in the previous papers [22,23].
The proposed Ti/SnO2-Sb2Ox-TiO2 electrodes were fabricated from the electrodeposition method. The electrodeposition fabrication route consists of the electrodeposition process and the following heat-treatment process.
In the electrodeposition process, the electrolyte consisted of 60 mL/L tin methanesulfonate (C2H6O6S2Sn, 50% wt in H2O), 50 mL/L methane sulfonic acid (CH4O3S, 99%), antimony trichloride (SbCl3, 99%), 2 g/L gelatin, and 2 g/L hydroquinone. The electrodes were prepared in an electrolytic cell as follows: the tin plate (40 mm × 40 mm) was used as the anode and the pretreated titanium substrate (20 mm × 30 mm × 0.1 mm) was used as the cathode. TiO2 sol was prepared following the procedures in previous work [24,25,26]. A certain amount (5, 10, 15 mL/L) of as-prepared TiO2 sol was slowly dropped into the stirring electrolytes. The electrodeposition was carried out at a current density of 30 mA/cm2 for 20 min at 30 °C, and the electrolyte was stirred at 300 rpm. Then, in the heat treatment process, the samples were heat-treated in a muffle furnace at temperatures of 600 °C for 10 h to obtain the Ti/SnO2-Sb2Ox-TiO2 electrodes. The thickness of electrodeposited SnO2-Sb2Ox coating was ~26 μm, whereas TiO2 sol addition hardly modified the coating thickness.
The Ti/SnO2-Sb2Ox was also made by the traditional dip-coating method for comparison. Firstly, the pretreated titanium substrate was dipped into a mixture of 1.8 g of tin tetrachloride (SnCl4), 2 mL of antimony oxide (Sb2O3), and 8 mL of isopropyl alcohol for 15 s, followed by a drying treatment at 100 °C for 10 min and then a heating treatment in a muffle furnace for 10 min at 600 °C. The drying and heating treatment process was repeated six times, and, finally, the titanium substrate was placed in a muffle furnace for ten hours at 600 °C to acquire the Ti/SnO2-Sb2Ox.
2.2. Structural and Elemental Characterization
X-ray diffraction (XRD-6000X, Shimazu, Kyoto, Japan) identified the phase constituents at a step size of 0.1°/s. The surface morphology was observed by a scanning electron microscope (SEM, Phenom ProX, Eindhoven, The Netherlands) with an energy dispersive spectroscopy (EDS) detector, and the embedded simulation program completed the 3D surface imaging. The X-ray photoelectron spectroscopy (XPS, ESCALAB 250Xi, Thermo Fisher, Waltham, MA, USA) was utilized to analyze elemental details, during which, Al Kα radiation at 1486.6 eV was used.
2.3. Electrochemical and Degradation Tests
The electrochemical performance of the electrode was tested by linear sweep voltammetry (LSV) using an electrochemical workstation (CH660E, Chenhua Instrument, Shanghai, China). A three-electrode system was used in the tests. Ti/SnO2-Sb2Ox-TiO2 electrode (10 mm × 10 mm) was the working electrode. Platinum plate (20 mm × 20 mm) was the counter electrode, and saturated calomel electrode (SCE) was the reference electrode. A 0.25 mol/L Na2SO4 solution was used as the electrolyte solution. The scan range was from 0 to 4 V, at a scanning rate of 20 mV/s.
The benzoic acid degradation experiment was carried out in 100 mg/L benzoic acid solution at a volume of 200 mL. We used the Ti/SnO2-Sb2Ox-TiO2 electrode (20 mm ×30 mm) as the anode and the same-sized titanium sheet as the cathode. A direct current power was employed to control the current density at 40 mA/cm2, and the 300-rpm magnetic stirring was applied. The target solution sample was taken for detection after a certain degradation period. The degradation process was monitored using high-performance liquid chromatography (HPLC, Nexera, Shimadzu, Kyoto, Japan) equipped with a TC-C18 column. The mobile phase consisted of 90% water (0.1% trifluoroacetic acid) and 10% acetonitrile. The accelerated life was tested in 0.25 mol/L Na2SO4 at room temperature at 100 mA/cm2. The prepared sample was the anode, and a titanium sheet was the cathode. The accelerated lifetime was calculated when the potential steeply raised and exceeded 5.0 V.
3. Results and Discussion
3.1. Structural Characterizations
Figure 1 presents the XRD profiles of Ti/SnO2-Sb2Ox and Ti/SnO2-Sb2Ox-TiO2 electrodes. The electrode prepared by the conventional dip-coating method reveals broad SnO2 peaks, which indicates a large extent of the amorphous phase. In addition, the sharp Ti peaks imply the exposure of underneath titanium substrate. Such an observation is in good agreement with previous literature [7,27]. In contrast, distinct phase constituents are attained for these samples from the electrodeposition fabrication route. The crystallinity substantially increases, and several intense peaks of tetragonal rutile SnO2 (JCPDS 99-0024) are discovered. Similar phenomena have also been reported in some earlier literature [7].
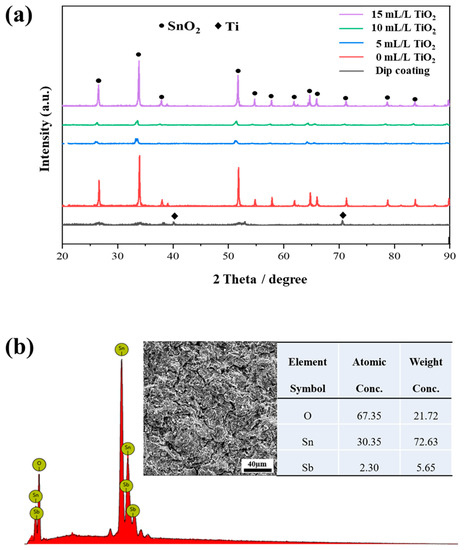
Figure 1.
(a) XRD profiles recorded on the Ti/SnO2-Sb2Ox-TiO2 with different concentrations of TiO2 sol, and (b) the EDS results recorded on the electrodeposited Ti/SnO2-Sb2Ox.
The incorporation of TiO2 addition affects the phase composition significantly. When the TiO2 addition was ≤10 mL/L, the SnO2 peak intensity sharply decreases, which indicates a refinement for the prepared SnO2 crystallites. As summarized in Table 1, the grain size of SnO2 is estimated according to the Scherrer equation through full width at half maximum (FWHM) [28]. The results show that adding 10 mL/L sol leads to a significantly decreased grain size, whereas larger grains form when further increasing the TiO2 sol concertation to 15 mL/L. During the electrodeposition process, the TiO2 nanoparticles are in situ generated and then co-deposited to generate SnO2-Sb2Ox-TiO2. These well-dispersed nanoparticles can serve as active sites for crystallite nucleation, thereby providing a driving force for nucleation and forming smaller SnO2 crystallites [29]. However, the sharp SnO2 peaks show an opposite phase variation at a 15 mL/L TiO2 concentration. Under this condition, the generated TiO2 nanoparticles tend to aggregate and decrease the active sites in the electrodeposition. It is noted that no TiO2 diffraction peak is detected in the composite coatings due to the limited amount of embedded TiO2 nanoparticles.

Table 1.
Estimated grain size of SnO2-Sb2Ox-TiO2 prepared at different TiO2 sol additions.
Figure 1b presents the elemental analysis recorded on the electrodeposited Ti/SnO2-Sb2Ox, proving the existence of antimony in the deposited coatings. The ratio of Sn/Sb is ~8 wt.% in SnO2-Sb2Ox, which is in good agreement with previous reports [7,30]. Such antimony content in the lattice provides good conductivity for the prepared electrode.
The surface microstructures of prepared Ti/SnO2-Sb2Ox-TiO2 are presented in Figure 2. A typical mud-like coating surface is obtained using the conventional dip-coating method, as shown in Figure 2a. The cracks and holes in such coating surfaces could damage the electrode stability due to the oxide generation in the electrolysis process [7]. In the meantime, the electrodeposited Ti/SnO2-Sb2Ox-TiO2 demonstrates a different surface morphology, showing a relatively compact coating surface with a much-improved uniformity. An increased addition of TiO2 sol (≤10 mL/L) gives rise to a more compact surface, whereas an addition of 15 mL/L TiO2 sol causes a relatively non-uniform surface morphology due to the nanoparticle’s aggregation.
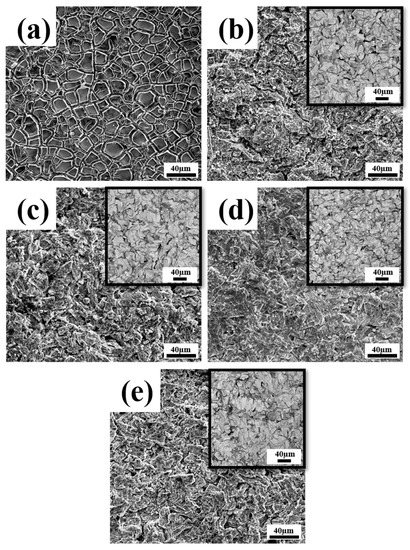
Figure 2.
The surface morphologies of (a) Ti/SnO2-Sb2Ox made by traditional dip-coating, and Ti/SnO2-Sb2Ox-TiO2 electrodeposited with different additions of TiO2 sol: (b) 0 mL/L (c) 5 mL/L, (d) 10 mL/L, and (e) 15 mL/L (insets are the corresponding Sn-Sb-TiO2 coatings before the heat treatment process).
A similar morphologic variation can be seen for the Sn-Sb-TiO2 layers before the heat-treatment process, as shown in the inset images. The TiO2 addition refines the surface morphology of Sn-Sb-TiO2 at a TiO2 sol concentration ≤ 10 mL/L, whereas an excessive sol concentration caused relatively non-uniform morphology. The surface structures of Ti/SnO2-Sb2Ox-TiO2 electrodes are highly associated with the early electrodeposited Sn-Sb-TiO2 layers, with some features remaining after the oxidation reactions during heat treatment.
The surface features were further characterized by a 3D imaging technique, as shown in Figure 3. These images show the surface roughness and uniformity for the electrodeposited samples. The proper addition of TiO2 sol brings a more uniform and compact surface, depicted in Figure 3b,c. The observation corresponds with our earlier XRD and SEM findings, which proves that the 10 mL/L TiO2 sol addition refines the surface with a decreased crystallite size and leads to a relatively open surface morphology with an improved uniformity. Nevertheless, the 15 mL/L TiO2 addition causes a non-uniform coating surface, depicted in Figure 3d.
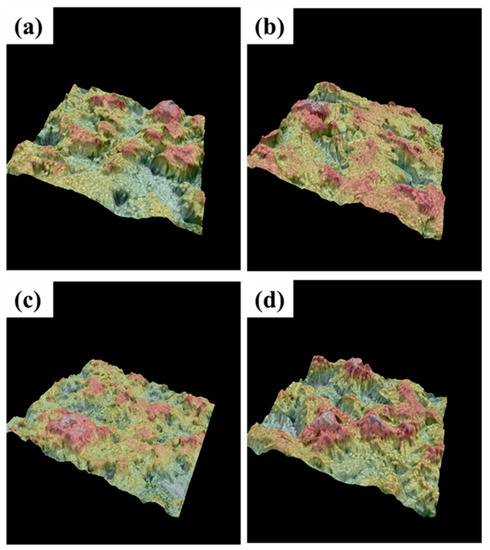
Figure 3.
The 3D-imaging surface (depicted with heat map) for the prepared Ti/SnO2-Sb2Ox-TiO2 samples electrodeposited with different additions of TiO2 sol: (a) 0 mL/L, (b) 5 mL/L, (c) 10 mL/L, (d) 15 mL/L.
3.2. Elemental and LSV Analysis
The elemental analysis characterizes the electrodeposited samples, as shown in Figure 4. Figure 4a shows the overall spectra of the electrodeposited Ti/SnO2-Sb2Ox and Ti/SnO2-Sb2Ox-TiO2 samples, where prominent peaks of Sb and Sn are detected. The presence of Ti peaks in Figure 4b proves the successful incorporation of TiO2 into the prepared SnO2-Sb2Ox-TiO2 coatings, whereas the SnO2-Sb2Ox sample shows no Ti peak. The weak Ti peaks correlate with the limited content of TiO2 existing on the electrode surface. During the sol-enhanced electrodeposition, the TiO2 nanoparticles are in situ generated in electrolytes. The attached organic chains avoid the agglomeration of nanoparticles, which are then co-deposited in the coating with excellent dispersion.
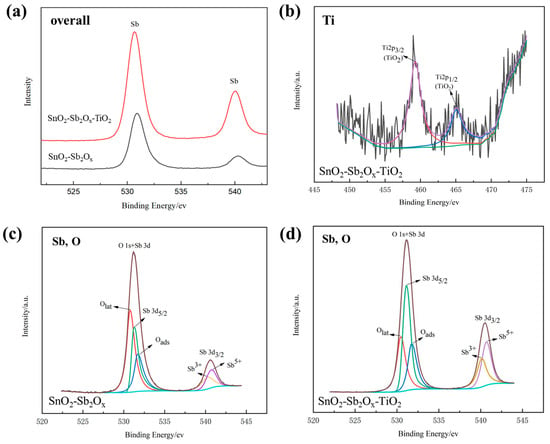
Figure 4.
XPS spectra of Ti/SnO2-Sb2Ox and Ti/SnO2-Sb2Ox-TiO2 (10 mL/L TiO2 sol addition): (a) the overall spectra, (b) Ti spectra for Ti/SnO2-Sb2Ox-TiO2, (c) Sb, O spectra for Ti/SnO2-Sb2Ox, and (d) Sb, O spectra for Ti/SnO2-Sb2Ox-TiO2.
Figure 4c,d compare the spectra of Sb and O on the electrodeposited Ti/SnO2-Sb2Ox and Ti/SnO2-Sb2Ox-TiO2. The results elaborate in detail on the state of antinomy oxide and absorbed oxygen with and without TiO2 sol addition. Both Sb3+ and Sb5+ exist in the prepared coatings, indicating that there are two oxidation states for antinomy after the heat treatment process. In addition, the detected oxygen can be categorized as Olat (i.e., lattice oxygen species) and Oads (hydroxyl oxygen species), which matches with previous studies [13,31].
Table 2 summarizes the comparison between the Ti/SnO2-Sb2Ox and Ti/SnO2-Sb2Ox-TiO2 in XPS analysis. The listed atom ratios of Oads/Olat and Sb5+/Sb3+ provide a qualitative judgment for the element variation. The atom ratio of Oads/Olat is higher in Ti/SnO2-Sb2Ox-TiO2. It is noted that adsorbed oxygen is a potent oxidizing agent. In the meantime, the Sb 3d3/2 is split into two oxidation states, Sb5+ and Sb3+, respectively. The higher ratio of Sb5+/Sb3+ implies that deeper surface oxidation occurs in the heat treatment process. The higher valence of Sb generally offers more excessive electrons and functions as dominant donors for the SnO2-Sb semiconductor, improving the conductivity of the prepared electrode [32]. The natural donors of oxygen vacancies are inhibited simultaneously, and the lattice oxygen is reduced. To conclude, the results imply that the addition of TiO2 sol can help to generate favorable surface features of the chemical composition and oxygen state for the improved electrocatalytic performance.

Table 2.
Calculated atom ratios for Ti/SnO2-Sb2Ox and Ti/SnO2-Sb2Ox-TiO2.
Figure 5 presents the linear sweep voltammetry recorded on the Ti/SnO2-Sb2Ox and Ti/SnO2-Sb2Ox-TiO2. The oxygen evolution reaction (OER) potential decreases in the order below: 10 mL/L TiO2 modified sample (2.34 V) > 5 mL/L TiO2 modified sample (2.27 V) > 15 mL/L TiO2 modified sample (2.19 V) > 0 mL/L TiO2 modified sample (2.15 V). The picture indicates that adding a certain TiO2 sol increases the electrode’s OER potential. When the amount of TiO2 sol increases to 15 mL/L, the OER potential shows a downward tendency, resulting from the nanoparticle aggregation in the coating [33]. In general, the effective organic degradation process favors a high OER potential, which avoids the OER (i.e., the side-reaction) in the electrolysis process.
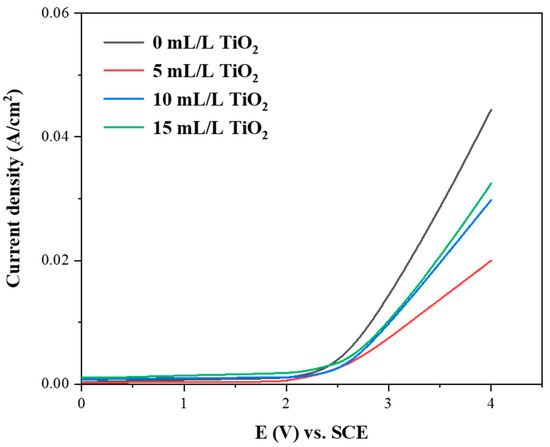
Figure 5.
LSV results of electrodeposited Ti/SnO2-Sb2Ox and Ti/SnO2-Sb2Ox-TiO2.
3.3. The Degradation Performance of Ti/SnO2-Sb2Ox-TiO2 Electrodes
The electrocatalytic performance of the prepared electrode was studied by degradation tests, as demonstrated in Figure 6. Benzoic acid (BA) is a typical organic pollutant used as a target in degradation tests. The results show that adding TiO2 sol influences the electrocatalytic ability. The degradation performance decreases following the order below: 10 mL/L TiO2 modified sample > 5 mL/L TiO2 modified sample > 15 mL/L TiO2 modified sample > 0 mL/L TiO2 modified sample. After the 10 h electrolysis, 98% BA is electrochemically combusted by the 10 mL/L TiO2 modified SnO2-Sb electrodes, whereas only 79% BA is degraded on the non-doped sample in the same duration.
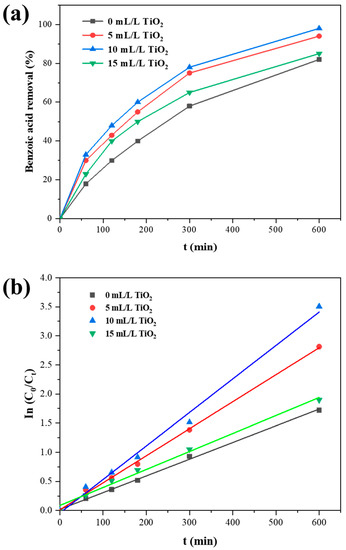
Figure 6.
(a) The benzoic concentration after a certain duration of degradation on different electrodes, (b) the fitted curve for the BA degradation process, where Ct is the BA concentration and C0 is the initial BA concentration.
Figure 6b shows the kinetic fitting curve of benzoic acid concentration with degradation time. The logarithm of benzoic acid concentration and degradation time showed a good linear relationship during the degradation process, which proved that the degradation process of benzoic acid follows the primary reaction kinetic model:
where C0 represents the initial concentration of benzoic acid, Ct represents the concentration of benzoic acid at a specific moment, and k is the reaction rate constant. Table 3 lists the calculated k value for the degradation process on different electrodes. The larger k value of the 10 mL/L TiO2 modified sample represents the electrode’s faster degradation rate of benzoic acid.

Table 3.
Degradation ability of Ti/SnO2-Sb2Ox-TiO2 electrodes with different concentrations of TiO2 sol in electrolytes, where k is kinetics coefficients.
The results prove that the proper addition of TiO2 sol enhances the electrocatalytic performance of Ti/SnO2-Sb2Ox-TiO2 electrodes. In general, the electrochemical combustion of organics is achieved by the hydroxyl radicals—a robust oxidizing agent that breaks the chemical bonds and finally turns the organic into H2O and CO2. The organic degradation pathway is proposed as follows in Equations (2) and (3) [13,34]:
where R stands for the organic and (OH)ads stands for the physic-adsorbed hydroxyl radicals. The higher content of Sb5+ increases the electrode conductivity and helps to generate surface adsorbed oxygen species. Besides, the uniform electrode surface with numerous peaks and valleys could provide many active sites for degradation reactions for the 5 and 10 mL/L TiO2 modified electrodes. The well-dispersed TiO2 nanoparticles also offer additional reaction sites, further improving the degradation ability for the TiO2 sol-enhanced electrodes. As for the 10 mL/L TiO2 modified Ti/SnO2-Sb2Ox-TiO2 electrode, both the superior surface feature and well-dispersed TiO2 award it the best electrocatalytic performance in the examined samples.
SnO2 + H2O → SnO2 (OH)ads + H+ + e
R + SnO2 (OH)ads → SnO2 + H+ + e + Oxidation products (such as CO2)
Accelerated life tests were also conducted to investigate the electrode performance, as shown in Figure 7, comprehensively. The dip-coated Ti/SnO2-Sb2Ox electrode was tested for comparison, which shows an inferior long-term stability due to its open surface structure being vulnerable to corrosion attack. During the degradation process, the corrosion attack results in the generation of titanium oxide, a non-conductive inert material that inhibits the degradation process and causes coating defoliation.
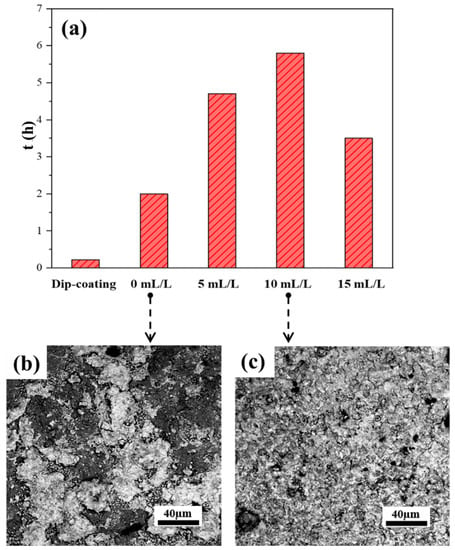
Figure 7.
The accelerated service life results, (a) the tested accelerated service life for samples preprepared by dip-coating and sol-enhanced electrodeposition with different TiO2 sol concentrations, (b) surface morphology of electrodeposited Ti/SnO2-Sb2Ox after 2 h test, and (c) surface morphology of Ti/SnO2-Sb2Ox-TiO2 (10 mL/L TiO2 sol doped) after 2 h test.
The electrodeposited Ti/SnO2-Sb2Ox-TiO2 shows a much-improved stability in the accelerated life tests. A relatively compact coating fully covers underneath the Ti substrate for these samples. An increasing TiO2 addition gradually prolongs the accelerated life at a concentration less than 10 mL/L. Figure 7b,c demonstrate the surface morphology of the undoped sample and 10 mL/L TiO2 sol modified samples after 2 h accelerated life tests. The undoped SnO2-Sb2Ox coating is almost peeled off from the substrate, whereas the electrolysis process only slightly changes the surface morphology of the Ti/SnO2-Sb2Ox-TiO2 electrode.
The electrode stability is highly correlated to the different morphologic features. The proper addition of TiO2 sol leads to a uniform surface with fewer cracks and holes, thereby improving the electrode stability. Moreover, well-dispersed nanoparticles in the coating can prevent corrosion penetration and enhance the corrosion resistance for electrodes. In contrast, excessive TiO2 addition harms the coating uniformity due to the nanoparticle aggregation, negatively affecting the electrode stability.
4. Conclusions
This study develops the Ti/SnO2-Sb2Ox-TiO2 electrodes by applying sol-enhanced electrodeposition from methanesulfonate electrolytes. The results show that adding TiO2 sol affects the surface morphologies of Ti/SnO2-Sb2Ox-TiO2 electrodes. A uniform coating surface can be obtained at a suitable sol addition. Furthermore, the addition of TiO2 sol leads to deep oxidation of Sb and helps to generate more adsorbed oxygen. The organic degradation performance decreases in the following order: 10 mL/L TiO2 sol modified sample > 5 mL/L TiO2 modified sample > 15 mL/L TiO2 modified sample > 0 mL/L TiO2 modified sample. The favored surface feature and well-dispersed TiO2 in the 10 mL/L TiO2 modified Ti/SnO2-Sb2Ox-TiO2 electrode results in the best electrocatalytic performance in the examined samples, and its uniform coating surface with fewer cracks and holes prolong the electrode service life.
Author Contributions
Investigation, S.Z.; methodology, C.Y.; writing—review and editing, J.T.; validation, Y.W.; writing—original draft preparation, Z.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Foundation of Jiangsu Province under Grant BK20201008, the Key Research Project of Zhenjiang under Grant GJ2020014.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Salazar-Banda, G.R.; Santos, G.d.O.S.; Duarte Gonzaga, I.M.; Dória, A.R.; Barrios Eguiluz, K.I. Developments in electrode materials for wastewater treatment. Curr. Opin. Electrochem. 2021, 26, 100663. [Google Scholar] [CrossRef]
- Tan, X.; Zhao, Y.; Sun, W.; Jin, C.; Chen, L.; Wei, H.; Sun, C. Three-dimensional hierarchically porous PbO2 electrode for electrochemical degradation of m-cresol. J. Electroanal. Chem. 2020, 856, 113726. [Google Scholar] [CrossRef]
- Syam Babu, D.; Nidheesh, P. A review on electrochemical treatment of arsenic from aqueous medium. Chem. Eng. Commun. 2020, 208, 1–22. [Google Scholar] [CrossRef]
- Wu, W.; Huang, Z.-H.; Lim, T.-T. Recent development of mixed metal oxide anodes for electrochemical oxidation of organic pollutants in water. Appl. Catal. A Gen. 2014, 480, 58–78. [Google Scholar] [CrossRef]
- Chaplin, B.P. Critical review of electrochemical advanced oxidation processes for water treatment applications. Environ. Sci. Process. Impacts 2014, 16, 1182–1203. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, L.; Liu, J.; Logan, B.E. Electrochemical technologies for wastewater treatment and resource reclamation. Environ. Sci. Water Res. Technol. 2016, 2, 800–831. [Google Scholar] [CrossRef]
- Ni, Q. Ti/SnO2-Sb2O5 as an Anode Material in Electro-Oxidation of Organic Compounds for Wastewater Treatment. Ph.D. Thesis, University of Toronto, Toronto, ON, Canada, 2016. [Google Scholar]
- Rao, A.N.S.; Venkatarangaiah, V.T. Preparation, characterization, and application of Ti/TiO2-NTs/Sb-SnO2 electrode in photo-electrochemical treatment of industrial effluents under mild conditions. Environ. Sci. Pollut. Res. 2018, 25, 11480–11492. [Google Scholar] [CrossRef]
- Yu, L.; Chen, Y.; Han, W.; Sun, X.; Li, J.; Wang, L. Preparation of porous TiO2-NTs/m-SnO2-Sb electrode for electrochemical degradation of benzoic acid. RSC Adv. 2016, 6, 19848–19856. [Google Scholar] [CrossRef]
- Duan, Y.; Chen, Y.; Wen, Q.; Duan, T. Fabrication of dense spherical and rhombic Ti/Sb-SnO2 electrodes with enhanced electrochemical activity by colloidal electrodeposition. J. Electroanal. Chem. 2016, 768, 81–88. [Google Scholar] [CrossRef]
- Yang, B.; Wang, J.; Jiang, C.; Li, J.; Yu, G.; Deng, S.; Lu, S.; Zhang, P.; Zhu, C.; Zhuo, Q. Electrochemical mineralization of perfluorooctane sulfonate by novel F and Sb co-doped Ti/SnO2 electrode containing Sn-Sb interlayer. Chem. Eng. J. 2017, 316, 296–304. [Google Scholar] [CrossRef]
- Yang, K.; Liu, Y.; Liu, J.; Qiao, J. Preparation optimization of multilayer-structured SnO2–Sb–Ce/Ti electrode for efficient electrocatalytic oxidation of tetracycline in water. Chin. J. Chem. Eng. 2018, 26, 2622–2627. [Google Scholar] [CrossRef]
- Yang, K.; Liu, Y.; Qiao, J. Electrodeposition preparation of Ce-doped Ti/SnO2-Sb electrodes by using selected addition agents for efficient electrocatalytic oxidation of methylene blue in water. Sep. Purif. Technol. 2017, 189, 459–466. [Google Scholar] [CrossRef]
- Wu, W.; Huang, Z.-H.; Hu, Z.-T.; He, C.; Lim, T.-T. High performance duplex-structured SnO2-Sb-CNT composite anode for bisphenol A removal. Sep. Purif. Technol. 2017, 179, 25–35. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, W.; He, Z.; Jin, Y.; Qiao, Y.; Cheng, G.; Gao, W.; Zhi, Z. Cu–Sn–Zn nanocomposite coatings prepared by TiO2 sol-enhanced electrodeposition. J. Appl. Electrochem. 2020, 50, 875–885. [Google Scholar] [CrossRef]
- He, Z.; Cao, D.; Wang, Y.; Yin, L.; Hayat, M.D.; Singh, H. Preparation of Co–P–TiO2 nanocomposite coatings via a pulsed electrodeposition process. Surf. Eng. 2020, 36, 975–981. [Google Scholar] [CrossRef]
- He, Z.; Cao, D.; Cao, F.; Zhang, S.; Wang, Y. Effects of heat treatment on the properties of Co–P–TiO2 nanocomposite coatings. Surf. Eng. 2020, 36, 720–726. [Google Scholar] [CrossRef]
- Wang, Y.X.; Cao, D.; Gao, W.D.; Qiao, Y.X.; Jin, Y.X.; Cheng, G.; Gao, W.; Zhi, Z. Microstructure and properties of sol-enhanced Co-P-TiO2 nano-composite coatings. J. Alloys Compd. 2019, 792, 617–625. [Google Scholar] [CrossRef]
- Liu, Z.; Luo, X.; Ji, D. Effect of phase composition of PbO2 on cycle stability of soluble lead flow batteries. J. Energy Storage 2021, 38, 102524. [Google Scholar] [CrossRef]
- He, Z.; Hayat, M.D.; Huang, S.; Wang, X.; Cao, P. PbO2 electrodes prepared by pulse reverse electrodeposition and their application in benzoic acid degradation. J. Electroanal. Chem. 2018, 812, 74–81. [Google Scholar] [CrossRef]
- He, Z.; Hayat, M.D.; Huang, S.; Wang, X.; Cao, P. Physicochemical characterization of PbO2 coatings electrosynthesized from a methanesulfonate electrolytic solution. J. Electrochem. Soc. 2018, 165, D670–D675. [Google Scholar] [CrossRef]
- Wang, Y.X.; Hu, B.; Tay, S.L.; Hou, F.Y.; Gao, W.; Xiong, C. The microstructure and properties of sol-enhanced Sn-TiO2 nanocomposite coatings. Int. J. Mod. Phys. B 2017, 31, 16–19. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, S.; Mao, Z.; Lin, Z.; Ren, X.; Yu, Z. High electrochemical activity of a Ti/SnO2-Sb electrode electrodeposited using deep eutectic solvent. Chemosphere 2020, 239, 124715. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhang, S.; Yin, L.; Hayat, M.D.; Cao, P. Cu–TiO2 nanocomposite coatings prepared from sol-enhanced electrodeposition. Int. J. Mod. Phys. B 2020, 34, 2040038. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, D.; Qiao, Y.; He, Z.; Wang, Y.; Li, X.; Gao, W. Microstructure and properties of duplex Ni-P-TiO2/Ni-P nanocomposite coatings. Mater. Res. 2019, 22. [Google Scholar] [CrossRef]
- Mao, A.; Xiang, H.-Z.; Zhang, Z.-G.; Kuramoto, K.; Yu, H.; Ran, S. Solution combustion synthesis and magnetic property of rock-salt (Co0.2Cu0.2Mg0.2Ni0.2Zn0.2) O high-entropy oxide nanocrystalline powder. J. Magn. Magn. Mater. 2019, 484, 245–252. [Google Scholar] [CrossRef]
- Ni, Q.; Kirk, D.W.; Thorpe, S.J. Characterization of the mixed oxide layer structure of the Ti/SnO2-Sb2O5 anode by photoelectron spectroscopy and impedance spectroscopy. J. Electrochem. Soc. 2014, 162, H40–H46. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer formula for X-ray particle size determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Allahkaram, S.R.; Nazari, M.H.; Mamaghani, S.; Zarebidaki, A. Characterization and corrosion behavior of electroless Ni–P/nano-SiC coating inside the CO2 containing media in the presence of acetic acid. Mater. Des. 2011, 32, 750–755. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, L.; He, J.; Zhang, J. Preparation of Ti/SnO2-Sb electrodes modified by carbon nanotube for anodic oxidation of dye wastewater and combination with nanofiltration. Electrochim. Acta 2014, 117, 192–201. [Google Scholar] [CrossRef]
- Wu, T.; Zhao, G.; Lei, Y.; Li, P. Distinctive tin dioxide anode fabricated by pulse electrodeposition: High oxygen evolution potential and efficient electrochemical degradation of fluorobenzene. J. Phys. Chem. C 2011, 115, 3888–3898. [Google Scholar] [CrossRef]
- Cui, Y.-H.; Feng, Y.-J.; Liu, Z.-Q. Influence of rare earths doping on the structure and electro-catalytic performance of Ti/Sb–SnO2 electrodes. Electrochim. Acta 2009, 54, 4903–4909. [Google Scholar] [CrossRef]
- Duan, Y.; Wen, Q.; Chen, Y.; Duan, T.; Zhou, Y. Preparation and characterization of TiN-doped Ti/SnO2-Sb electrode by dip coating for orange II decolorization. Appl. Surf. Sci. 2014, 320, 746–755. [Google Scholar] [CrossRef]
- Chen, Y.; Tu, Y.; Zhang, Y.H.; Lu, J.A.; Fang, B.B. Fabrication and enhanced electrocatalytic activity of TiO2 nanotubes based three-dimensionally macroporous SnO2 with mesoporous walls. Chem. Eng. J. 2017, 311, 100–110. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).