Abstract
Micro-arc oxidation (MAO) treatment can effectively improve the wear resistance, corrosion resistance, and mechanical strength of aluminum alloy substrates. Improving the porous structure of MAO film and effectively sealing the pores is a significant research issue. In this study, the MAO treatment of 5052 aluminum alloy was carried out in silicate electrolytes. The MAO films were sealed with different concentrations of SiO2 nanoparticles. The effects of SiO2 nanoparticle content on the MAO films’ microstructure, mechanical properties, and corrosion performance were systematically investigated. When adding SiO2 nanoparticles to electrolytes, the particles were deposited at the micropores of the film, which could effectively seal the porous MAO film and significantly improve its corrosion and wear resistance. The corrosion resistance and wear resistance properties were optimal with 5.0 g/L SiO2 addition. Compared to the unsealed film, the corrosion current density and corrosion rate decreased from 1.24 × 10−9 A/cm2 and 1.47 × 10−5 mm/a to 7.78 × 10−10 A/cm2 and 9.15 × 10−6 mm/a, respectively. Moreover, the average friction coefficient of the sealed film was 0.606, which was ~19.3% lower than that of the substrate and 3.3% lower than for the unsealed film.
1. Introduction
Aluminum and its alloy materials are widely used in automotive, aerospace, and other industrial applications because of their high specific strength, strong corrosion resistance, superior machinability, good thermal conductivity, and recyclability [1,2]. However, aluminum has some disadvantages as a structural material, such as soft texture, poor wear resistance, low corrosion resistance, and poor heat resistance, which seriously affect the scaled-up applications [3,4].
To improve the performance of aluminum alloy, various surface engineering methods have been developed and applied. Common surface treatment methods include anodic oxidation [5,6,7], electroplating [8,9], plasma spraying, laser surface modification, and micro-arc oxidation [10,11]. Micro-arc oxidation (MAO) is a novel surface treatment technology that allows in situ deposition of autogenous ceramic films on magnesium, aluminum, and titanium [12,13,14]. MAO films are characterized by solid adhesion, compact structure, high strength, exceptional corrosion resistance [15,16], and excellent high-temperature impact resistance. However, many microcracks and discharge micropores inevitably appear on MAO films due to the special reaction process [17,18,19]. The corrosive medium can easily penetrate the membrane and interface layers, leading to the corrosion of substrate material [20].
In addition to optimizing the MAO process, it is necessary to seal the pores of the MAO film and enhance the shielding effect in the corrosive medium [21,22]. It has been found that the corrosion resistance of MAO films can be improved by applying different sealing materials and sealing processes [23,24]. The traditional sealing methods can be divided into hydrated, inorganic, and organic seals [25,26]. Inorganic seals have promising application prospects due to their simple process, low cost, and easy implementation [27,28,29]. Introducing highly stable oxide nanoparticles, such as TiO2, ZrO2, and SiO2, can effectively enhance corrosion protection [30,31]. The suspension of SiO2 nanoparticles can be used to prepare coatings with enhanced thermomechanical properties, reduced surface porosity, and improved wear resistance [32,33,34]. SiO2 nanoparticles can act as sintering aids to promote the formation of MAO films [35,36,37,38]. Meanwhile, SiO2 nanoparticles with high melting point and small size can reduce surface cracks and increase the density of ceramic layers, thus improving the corrosion and wear resistance of surface layers [39,40].
In the present study, the surface of 5052 Al alloy was treated by MAO process with different addition (0–10 g/L) of SiO2 nanoparticles (particle size of 20 nm) in silicate electrolytes. The nanoparticles were uniformly suspended in the electrolyte by high-speed stirring and ultrasonic shaking to seal the generated pores. The effects of SiO2 nanoparticle content on the microstructure, mechanical properties and corrosion resistance of MAO films were systematically investigated.
2. Experimental Method
2.1. Sample Preparation
The substrates used in this research were 5052 Al alloys with dimensions of 20 mm × 20 mm × 6 mm. The substrates were carefully pretreated before being subject to the MAO process. The samples were polished and ultrasonically cleaned in ethanol and deionized water for 5 min to remove the surface oil. The chemical composition of the 5052 Al alloy is detailed in Table 1.

Table 1.
Chemical Composition of 5052 Al Alloy.
The MAO treatment was conducted using micro-arc oxidation equipment (WHD-30, Harbin, China). The electrolyte composition and process parameters are shown in Table 2. The electrolyte was prepared with deionized water. All reagents were purchased from the Sigma company with analytical purity. We used the bidirectional pulse current. The positive and negative current density was 6 A/dm2. The positive and negative duty cycles were 30% & 40%.

Table 2.
Electrolyte Composition and MAO Process Parameters.
2.2. Sample Characterization
The microstructure and chemical distribution of the MAO films was characterized by a scanning electron microscope (SEM, Phenom Pro X, Eindhoven, The Netherlands) equipped with an energy dispersive spectrometer (EDS). The phase structure of the MAO films was studied using an X-ray diffractometer (XRD, Shimadzu XRD-6000, Kyoto, Japan). The electrochemical impedance spectroscopy and potentiodynamic polarization curve of the MAO films were tested using an electrochemical workstation (PGSTAT302 N, Metrohm Autolab B.V., Utrecht, The Netherlands). For the three-electrode corrosion cell, the counter electrode was made of platinum mesh, the reference electrode was a saturated calomel electrode, and the sample was the working electrode. The corrosion results were collected in 3.5 wt.% NaCl solution at room temperature. The microhardness of the MAO films was tested by a semi-automatic microhardness tester (HXS-1000 TAC, Shanghai, China). The load was 10 N and the loading time was set as 15 s. A reciprocating wear tester (HSR-2 M, Lanzhou, China) examined the friction properties of the MAO films. A Si3 N4 ceramic ball with a diameter of 4 mm was used for the test and the total sliding distance was set as 20 m for each sample. All the wear tests were conducted at room temperature for 10 mins under 5 N load.
3. Results and Discussion
3.1. Microstructures
The microstructures of the MAO films sealed using different concentrations of SiO2 nanoparticles were observed, as presented in Figure 1. Figure 1a shows the microstructure of unsealed MAO film prepared by the common electrolyte. It can be clearly seen that there are dense “honeycomb” holes on the film surface. These holes provide channels for the flow of corrosive media. During the MAO process, the surface film layer is constantly broken down by high voltage. The molten Al2 O3 was ejected and cooled rapidly in the external electrolyte, resulting in the formation of holes. When 2.5 g/L SiO2 nanoparticles were added into the electrolyte, SiO2 nanoparticles were deposited outside the discharge micropores and a small amount of aggregation occurred, as shown in Figure 1b. When the addition of SiO2 increased to 5 g/L, the micropores were more obviously covered by SiO2, as shown in Figure 1c, thereby blocking the corrosion channel. However, when excess SiO2 was added, as shown in Figure 1d–e, the nanoparticles gathered on the film surface under discharge, adsorbing a large number of free ions, and accentuating the micro-arc discharge, resulting in high surface energy and ablation, so that the molten oxide could not solidify well on the surface of the film. When the SiO2 concentration reached 10.0 g/L, film roughness was higher and surface oxide ablation was accentuated.
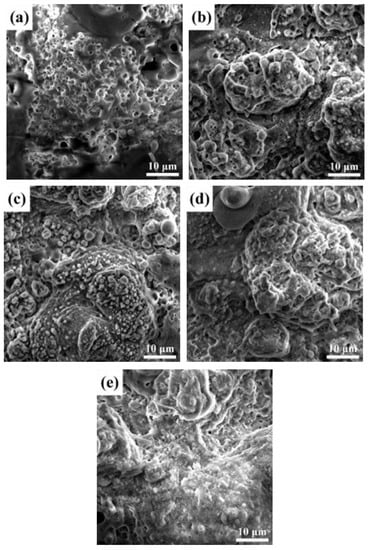
Figure 1.
Surface SEM images of (a) unsealed MAO film and (b) MAO films sealed by different concentrations of SiO2 nanoparticles: 2.5 g/L, (c) 5 g/L (d) 7.5 g/L and (e) 10 g/L.
The elemental content of MAO films sealed with different concentrations of SiO2 nanoparticles was determined by EDS analysis, as shown in Table 3. The main elements in the MAO films prepared by common electrolyte were O, Al and Si. O was mainly derived from SiO32− in the electrolyte and occurred as SiO2 and Al2 O3, while Al was derived from the matrix, and Si was derived from the residual SiO32− and SiO2 generated by the discharge of SiO32−. The atomic percentage of Si in the film increased continuously with addition of SiO2 nanoparticles and reached 27.22% when the concentration of SiO2 nanoparticles in the electrolyte was 10 g/L, confirming that the SiO2 nanoparticles had entered the film layer.

Table 3.
EDS Analysis (at%) of Film.
Figure 2 shows the cross-sectional morphology of MAO films prepared with different concentrations of SiO2 nanoparticles. The results showed that the thickness of the film layer increased with increase in SiO2 concentration. Figure 2a shows the MAO film layer without SiO2 nanoparticle addition, having a thickness of ~16 μm. The dense area near the substrate had fewer pores, while the outer area had some pores penetrating the film. Figure 2b shows the MAO film layer with 2.5 g/L SiO2 addition, having a thickness of ~20 μm. Many cracks and large inner pores appeared in the film. Figure 2c shows the MAO film layer prepared by adding 5.0 g/L SiO2 nanoparticles, having a thickness of ~33 μm. The internal dense area showed a certain degree of porosity with small pores, while cracks and holes were still evident. There were some particles deposited on the surface, blocking the micropores generated in the MAO process. Figure 2d shows the MAO film layer prepared by adding 7.5 g/L SiO2. The thickness of the film continued to increase to ~45 μm, yet the number of inner pores increased with larger pore diameter. Figure 2e shows that there was no significant increase in thickness when the nanoparticle concentration was further increased. The larger pore size holes inside them can seriously damage corrosion resistance.
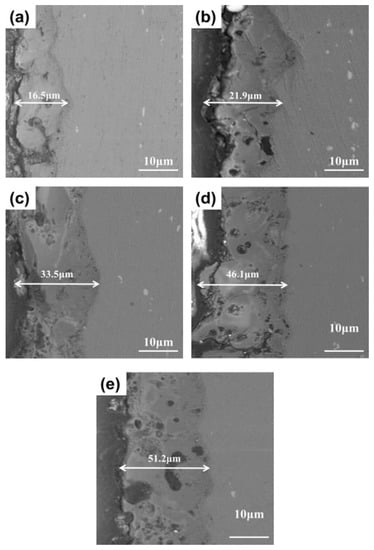
Figure 2.
Cross-sectional SEM images of (a) unsealed MAO film and, (b) MAO films sealed by different concentrations of SiO2 nanoparticles: 2.5 g/L, (c) 5 g/L (d) 7.5 g/L and (e) 10 g/L.
Figure 3 presents the cross-sectional elemental distribution of unsealed and 5.0 g/L SiO2 sealed films. The MAO film prepared with the addition of 5 g/L nano-SiO2 had a higher content and a more uniform distribution of Si compared to the unsealed layer. The Si concentrated in the outer sparse area, indicating that the nano-SiO2 particles were well-deposited on the surface. The distribution density of Si increased in the pores and cracks. It can be inferred that SiO2 nanoparticles were continuously deposited in the micropores, blocking the micropores and filling the cracks. Moreover, a certain amount of Si was also detected in the inner areas, implying that the SiO2 nanoparticles were successfully incorporated into the MAO film.
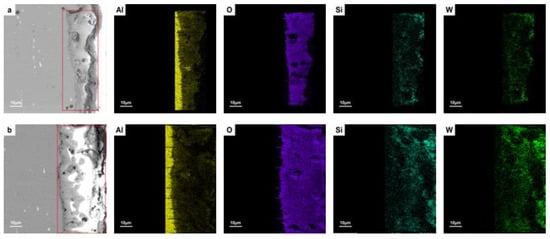
Figure 3.
Cross-sectional images and element distribution of (a) unsealed MAO film, and (b) MAO film sealed with 5 g/L concentration of SiO2 nanoparticles.
3.2. XRD Analysis
Figure 4 shows the XRD patterns of MAO thin films and MAO thin films sealed by SiO2 nanoparticles. It can be seen from the figure that the phase structure of MAO thin films was mainly composed of Al, α-Al2 O3, γ-Al2 O3 and mullite. The intensity of the diffraction peaks of Al was mainly from the matrix. During the MAO process, Al2 O3 with different crystal structures was formed in the MAO films as a result of the combined effect of chemical and electrochemical reactions. When aluminum is placed in alkaline electrolyte, a dense oxide film is rapidly formed on the substrate surface. The formation of different crystalline types of Al2 O3 was related to the cooling rate and cooling temperature. When the cooling rate was relatively fast, the molten Al2 O3 was converted mostly to γ-Al2 O3. When the cooling rate was relatively slow, the molten Al2 O3 was converted mostly to α-Al2 O3, and some γ-Al2 O3 was also converted to α-Al2 O3. At the same time, the presence of mullite peaks indicated the successful incorporation of nano-SiO2 into the film layer during the MAO process.
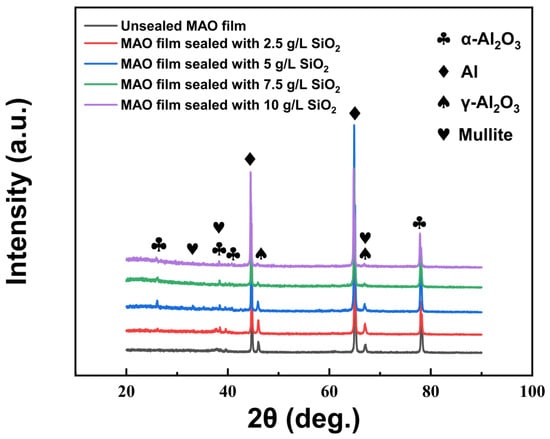
Figure 4.
XRD patterns of MAO film and MAO films sealed by SiO2 nanoparticles.
3.3. Mechanical Properties
Figure 5 shows the microhardness of unsealed MAO film and MAO film sealed by SiO2 nanoparticles. The microhardness of unsealed MAO film was ~HV 1241. The microhardness of the MAO films increased significantly after sealing. When the concentration of SiO2 nanoparticles in the electrolyte was 5 g/L, the microhardness of sealed MAO film reached a maximum of ~HV 1628, which was about 31.2% higher than that of the unsealed film. As the concentration of SiO2 nanoparticles increased, the hardness of the sealed MAO film decreased. However, the hardness of the MAO films after sealing was always higher than that of the film without sealing.
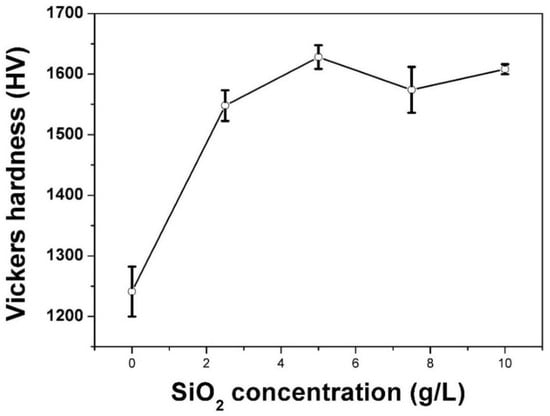
Figure 5.
Microhardness of unsealed MAO film and MAO films sealed by SiO2 nanoparticles.
The above results are related to the microstructure evolution of the MAO films. Appropriate addition of SiO2 nanoparticles can effectively adsorb free ions and promote a more uniform voltage breakdown on the film surface. Consequently, the hardness of the surface ceramic layer was significantly improved due to the better crystallization of Al2 O3 ejected from the homogeneously distributed holes.
3.4. Corrosion Resistance
Figure 6 shows the friction coefficient curves of different MAO films. The friction coefficient of the MAO film was more stable and fluctuated less than the matrix during the friction process. Table 4 presents the average friction coefficients of the substrate and the MAO films. The average friction coefficient of the MAO layer first decreased and then increased after sealing with SiO2 nanoparticles. When the concentration of SiO2 nanoparticles was 5 g/L, the average friction coefficient of the sealed film was 0.606, which was about 19.3% lower than the value of 0.773 for the matrix and 3.3% lower than the value of 0.627 for the unsealed film. This phenomenon was mainly attributed to the uniform surface structure and the reduced surface roughness after sealing. Further addition of SiO2 nanoparticles gradually increased the film’s average friction coefficient. Aggregation of nanoparticles aggravated the surface discharge of the film, resulting in the ablation of the film and the increase in surface roughness. When the concentration of SiO2 nanoparticles was 10 g/L, the average friction coefficient of the film increased to 0.91.
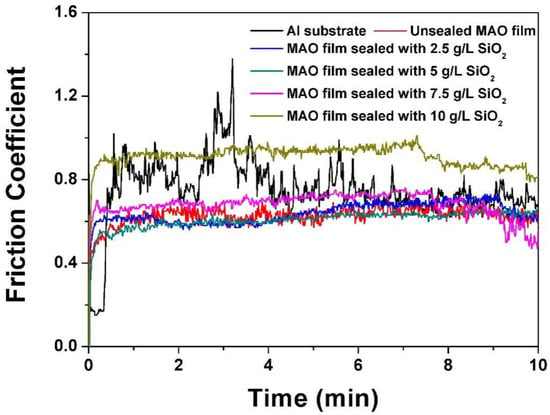
Figure 6.
Wear track images of unsealed MAO film and MAO films sealed by SiO2 nanoparticles.

Table 4.
Average friction coefficient of different specimens.
Figure 7 shows a comparison of wear track widths for different samples. Table 4 presents the average friction coefficient of different specimens. The wear track width of unsealed MAO film was ~595 μm. After sealing with SiO2 nanoparticles, the wear track width of the film was significantly reduced. Consistent with the variation trend of the average friction coefficient, the wear track width of the sealed film first decreased and then increased with increase in SiO2 nanoparticle concentration. When the addition of SiO2 nanoparticles was 5 g/L, the edge of the wear track was more uniform, and the wear track width decreased to ~425 μm. Due to the adsorption of nanoparticles on the film surface, further increase in the concentration of TiO2 nanoparticles led to film ablation, increasing surface roughness and deterioration in wear resistance. When the concentration of SiO2 nanoparticles was 10 g/L, the wear track width of the film rose to ~530 μm.
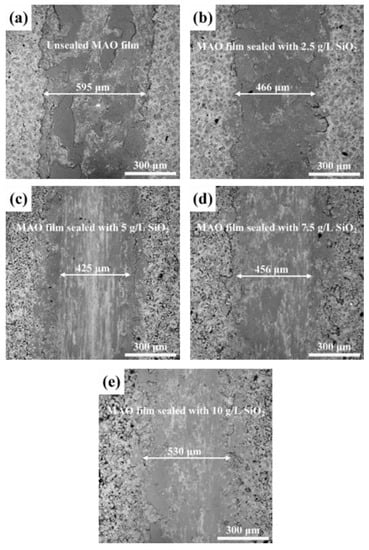
Figure 7.
Wear track images of unsealed MAO film (a) and MAO films sealed by different concentrations of SiO2 nanoparticles: (b) 2.5 g/L, (c) 5 g/L (d) 7.5 g/L and (e) 10 g/L.
To investigate the effect of the sealing process on MAO film corrosion resistance, potentiodynamic polarization tests and electrochemical impedance spectroscopy measurements were performed on the samples. The polarization curves are shown in Figure 8. The fitting results of the polarization curves are shown in Table 5. Ecorr, icorr, βa, βc were derived from the Tafel extrapolation method; Rp was calculated based on the Stern–Geary equation [41]. The corrosion potential of the films sealed with 5 g/L SiO2 nanoparticles increased to −1.05 V compared to the unsealed films, which showed a significant passivation trend in the anodic region. This was attributed to the formation of a barrier layer after sealing with SiO2 nanoparticles, which hindered the intrusion of the corrosive medium and thus prevented corrosion attack in the electrolyte. At the same time, the corrosion current density and corrosion rate decreased from 1.24 × 10−9 A/cm2 and 1.47 × 10−5 mm/a to 7.78 × 10–10 A/cm2 and 9.15 × 10−6 mm/a, respectively. The corrosion rate decreased by ~37%, indicating that the corrosion resistance of the sealed film was significantly improved.
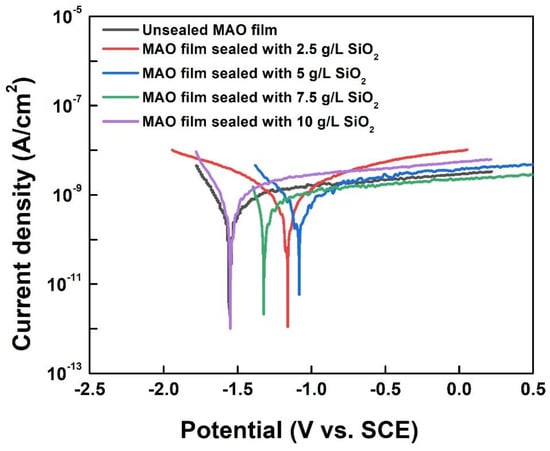
Figure 8.
Polarization curves of unsealed MAO film and MAO films sealed by SiO2 nanoparticles.

Table 5.
Fitting results of polarization curves.
Figure 9 shows the Nyquist plots of MAO film and MAO films sealed by SiO2 nanoparticles. With increase in SiO2 nanoparticle concentration in the electrolyte, the capacitive reactance arc radius of the film first increased and then decreased. When the concentration was 5 g/L, the impedance value reached a maximum, showing the best corrosion resistance. The increasing SiO2 adsorbed the free ions in the solution under the discharge, resulting in excessive high surface energy, intensified discharge process, and finally, ablation. The molten oxide could not solidify uniformly on the film surface, which increased the cracks and reduced the corrosion resistance. Accordingly, the impedance value decreased to a minimum when the concentration of SiO2 was 10 g/L.
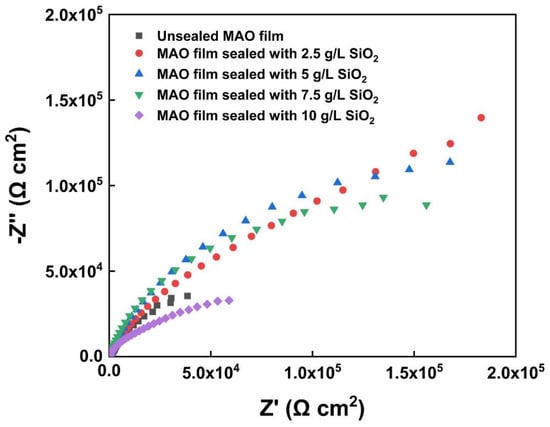
Figure 9.
Nyquist plots of unsealed MAO film and MAO films sealed by SiO2 nanoparticles.
Figure 10 shows the fitted equivalent circuit diagram of the electrochemical impedance spectrum, which consists mainly of two constant phase elements, since the MAO film usually consists of a dense inner layer and a sparse outer layer. R1 is the solution resistance depending on the corrosive medium, and CPE1 and CPE2 are the constant phase elements, corresponding to the outer sparse layer and the dense inner layer, respectively. Rct1 and Rct2 represent the outer and inner film layer charge transfer resistance values (Table 6 shows the fitted parameters of each component). As can be seen from Table 6, the addition of SiO2 nanoparticles increased the external Rct value by 1–2 orders of magnitude. When the concentration of SiO2 nanoparticles was 5 g/L, the value of Rct1 reached its maximum value, which was about 11 times higher than that of the unsealed membrane layer. The Bode diagram reflects the impedance to a certain extent.
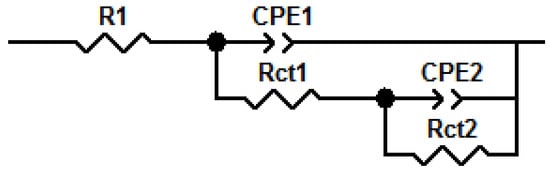
Figure 10.
The equivalent circuit diagram of EIS.

Table 6.
Equivalent circuit fitting parameters of EIS for different samples.
Figure 11 presents the Bode diagrams of unsealed MAO film and MAO films sealed by SiO2 nanoparticles. The polarization resistance of the films after sealing was higher than that of the film without sealing as shown in Figure 11a. There were two time constants in the medium and high-frequency region of the unsealed film, as shown in Figure 11b. The unsealed film comprised a loose outer layer and a dense inner layer. When the unsealed film was in contact with the corrosive solution, the corrosive medium penetrated the porous outer layer and reacted with the compact inner layer. The time constant corresponding to the intermediate frequency region mainly derives from the contribution of the loose outer layer. In contrast, the time constant corresponding to the high frequency region primarily derives from the assistance of the dense inner layer. However, the phase angle diagram of the films after sealing has only one time constant in the high-frequency region. SiO2 nanoparticles filled the micropores of the films, and the corrosive medium could not quickly penetrate into the inner layer, thus improving the corrosion resistance of the film.
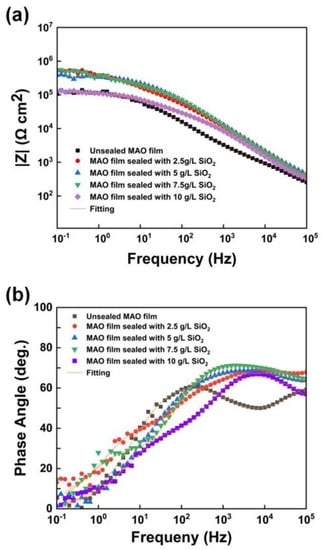
Figure 11.
Bode plots of unsealed MAO film and MAO films sealed by SiO2 nanoparticles: (a) relationship between frequency and Z; (b) relationship between frequency and phase angle.
4. Conclusions
The discharge micropores on the surface of MAO films can be uniformly filled and effectively sealed by SiO2 nanoparticles. When the concentration of SiO2 nanoparticles in the electrolyte was 5 g/L, the MAO films’ microhardness, wear resistance, and corrosion resistance were significantly improved and reached an optimal value. Compared with unsealed film, the microhardness of the film surface increased by ~31.2%, while the wear track width decreased from ~595 μm to ~425 μm. In addition, the corrosion rate reduced by ~37%. The current research provides valuable information on the MAO processing of Al alloy and solid grounds for future industrial application.
Author Contributions
Conceptualization, S.L. and Y.W.; methodology, S.L. and Y.W.; formal analysis, S.L. and J.C.; investigation, J.C. and D.Z.; resources: Y.W. and P.G.; writing—original draft preparation: S.L.; writing—review and editing: Y.W. and Z.H.; supervision, Y.W. and Z.H.; project administration, Y.W. and Z.H. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by the National Natural Science Foundation of China (51601073), the Research Project of Jiangsu Provincial Natural Science Fund (BK20211344), Jiangsu Provincial Six Talent Peaks (2018 XCL-028) and the Postgraduate Research and Practice Innovation Program of Jiangsu Province (KYCX21_3451).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to express their appreciation to Chris Goode (Cirrus Materials Science Ltd., NZ) for his generous assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fagiolari, L.; Bini, M.; Costantino, F.; Gatto, G.; Jeremy Kropf, A.; Marmottini, F.; Nocchetti, M.; Wegener, E.C.; Zaccaria, F.; Delferro, M.; et al. Iridium-Doped Nanosized Zn-Al Layered Double Hydroxides as Efficient Water Oxidation Catalysts. ACS Appl. Mater. Interfaces 2020, 12, 32736–32745. [Google Scholar] [CrossRef] [PubMed]
- Tacikowski, M.; Kamiński, J.; Rudnicki, J.; Borowski, T.; Trzaska, M.; Wierzchoń, T. The effect of the diffusive, composite chromium nitride layers produced by a hybrid surface treatment on the corrosion behavior of AZ91D magnesium alloy. Vacuum 2011, 85, 938–942. [Google Scholar] [CrossRef]
- Wang, Y.; Guan, L.; He, Z.; Zhang, S.; Singh, H.; Hayat, M.D.; Yao, C. Influence of pretreatments on physicochemical properties of Ni-P coatings electrodeposited on aluminum alloy. Mater. Des. 2021, 197, 109233. [Google Scholar] [CrossRef]
- Guo, P.Y.; Sun, H.; Shao, Y.; Ding, J.; Li, J.; Huang, M.; Mao, S.; Wang, Y.; Zhang, J.; Long, R.; et al. The evolution of microstructure and electrical performance in doped Mn-Co and Cu-Mn oxide layers with the extended oxidation time. Corros. Sci. 2020, 172, 108738. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, D.; Gao, W.; Qiao, Y.; Jin, Y.; Cheng, G.; Gao, W.; Zhi, Z. Microstructure and properties of sol-enhanced Co-P-TiO2 nano-composite coatings. J. Alloys Compd. 2019, 792, 617–625. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, D.; Qiao, Y.; He, Z.; Wang, Y.; Li, X.; Gao, W. Microstructure and Properties of Duplex Ni-P-TiO2/Ni-P Nanocomposite Coatings. Mater. Res. 2019, 22, 748. [Google Scholar] [CrossRef]
- Shi, P.; Ng, W.F.; Wong, M.H.; Cheng, F.T. Improvement of corrosion resistance of pure magnesium in Hanks’ solution by microarc oxidation with sol–gel TiO2 sealing. J. Alloys Compd. 2009, 469, 286–292. [Google Scholar] [CrossRef]
- Yang, S.; Cai, W.; Zeng, H.; Xu, X. Ultra-fine β-SiC quantum dots fabricated by laser ablation in reactive liquid at room temperature and their violet emission. J. Mater. Chem. 2009, 19, 7119. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Deng, Y.Z.; Shao, Y.W.; Wang, F.H. New sealing treatment of microarc oxidation coating. Surf. Eng. 2013, 30, 31–35. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, W.; He, Z.; Jin, Y.; Qiao, Y.; Cheng, G. Cu–Sn–Zn nanocomposite coatings prepared by TiO2 sol-enhanced electrodeposition. J. Appl. Electrochem. 2020, 50, 875–885. [Google Scholar] [CrossRef]
- Bahramian, A.; Raeissi, K.; Hakimizad, A. An investigation of the characteristics of Al2O3/TiO2 PEO nanocomposite coating. Appl. Surf. Sci. 2015, 351, 13–26. [Google Scholar] [CrossRef]
- Fatimah, S.; Kamil, M.P.; Kwon, J.H.; Kaseem, M.; Ko, Y.G. Dual incorporation of SiO2 and ZrO2 nanoparticles into the oxide layer on 6061 Al alloy via plasma electrolytic oxidation: Coating structure and corrosion properties. J. Alloys Compd. 2016, 707, 358–364. [Google Scholar] [CrossRef]
- Kaseem, M.; Ko, Y.G. Electrochemical Response of Al2O3-MoO2-TiO2 Oxide Films Formed on 6061 Al Alloy by Plasma Electrolytic Oxidation. J. Electrochem. Soc. 2016, 163, C587. [Google Scholar] [CrossRef]
- Arunnellaiappan, T.; Ashfaq, M.; Krishna, L.R.; Rameshbabu, N. Fabrication of corrosion-resistant Al2O3–CeO2 composite coating on AA7075 via plasma electrolytic oxidation coupled with electrophoretic deposition. Ceram. Int. 2016, 42, 5897–5905. [Google Scholar] [CrossRef]
- Raj, V.; Ali, M.M. Formation of ceramic alumina nanocomposite coatings on aluminium for enhanced corrosion resistance. J. Mater. Processing Tech. 2009, 209, 5341–5352. [Google Scholar] [CrossRef]
- Li, T.; Cao, M.; Liang, J.; Xie, X.; Du, G. Mechanism of Base-Catalyzed Resorcinol-Formaldehyde and Phenol-Resorcinol-Formaldehyde Condensation Reactions: A Theoretical Study. Polymers 2017, 9, 426. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, J.; Liang, J.; Chen, J. Microstructure and corrosion behavior of plasma electrolytic oxidation coated magnesium alloy pre-treated by laser surface melting. Surf. Coat. Technol. 2012, 206, 3109–3115. [Google Scholar] [CrossRef]
- Cui, X.-J.; Liu, C.-H.; Yang, R.-S.; Li, M.-T.; Lin, X.-Z. Self-sealing micro-arc oxidation coating on AZ91D Mg alloy and its formation mechanism. Surf. Coat. Technol. 2015, 269, 228–237. [Google Scholar] [CrossRef]
- Xiong, Y.; Hu, X.; Song, R. Characteristics of CeO2/ZrO2-HA composite coating on ZK60 magnesium alloy. J. Mater. Res. 2017, 32, 1073–1082. [Google Scholar] [CrossRef]
- Zahedi Asl, V.; Zhao, J.; Anjum, M.J.; Wei, S.; Wang, W.; Zhao, Z. The effect of cerium cation on the microstructure and anti-corrosion performance of LDH conversion coatings on AZ31 magnesium alloy. J. Alloys Compd. 2020, 821, 153248. [Google Scholar] [CrossRef]
- Zhou, M.; Pang, X.; Wei, L.; Gao, K. Insitu grown superhydrophobic Zn–Al layered double hydroxides films on magnesium alloy to improve corrosion properties. Appl. Surf. Sci. 2015, 337, 172–177. [Google Scholar] [CrossRef]
- Gong, C.; Zhou, Z.; Zhou, H.; Liu, R. Vacuum-assisted synthesis of tiny Au nanoparticles entrapped into mesoporous carbon matrix with superior catalytic activity for 4-nitrophenol reduction. Adv. Powder Technol. 2019, 30, 649–655. [Google Scholar] [CrossRef]
- Xi, J.J.; Zhao, J. Influence of Organic Sealed on the Corrosion Behaviors of Micro Arc Oxidation Coated ZM5 Magnesium Alloy. Adv. Mater. Res. 2011, 420, 844–847. [Google Scholar] [CrossRef]
- Kaseem, M.; Lee, Y.H.; Ko, Y.G. Incorporation of MoO2 and ZrO2 particles into the oxide film formed on 7075 Al alloy via micro-arc oxidation. Mater. Lett. 2016, 182, 260–263. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Zhang, J.-M.; Li, Y.; Bai, L.; Zhang, G. Enhanced corrosion resistance of micro-arc oxidation coated magnesium alloy by superhydrophobic Mg−Al layered double hydroxide coating. Trans. Nonferrous Met. Soc. China 2019, 29, 2066–2077. [Google Scholar] [CrossRef]
- Li, H.; Song, R.; Ji, Z. Effects of nano-additive TiO2 on performance of micro-arc oxidation coatings formed on 6063 aluminum alloy. Trans. Nonferrous Met. Soc. China 2013, 23, 406–411. [Google Scholar] [CrossRef]
- Guo, H.F.; An, M.Z.; Huo, H.B.; Xu, S.; Wu, L. Microstructure characteristic of ceramic coatings fabricated on magnesium alloys by micro-arc oxidation in alkaline silicate solutions. Appl. Surf. Sci. 2006, 252, 7911–7916. [Google Scholar] [CrossRef]
- Hung, J.C.; Ku, C.Y.; Fan, Z.W. Fabrication of an Electrode Insulated by Using Hot Dip Aluminizing and Micro-arc Oxidation Method for Electrochemical Microhole Machining. Procedia CIRP 2018, 68, 438–443. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, K.; Fu, Z.; Zhang, C.; Zhou, H.; Wang, L.; Zhu, S.; Guan, S.; Li, D.; Hu, J. Composite coating prepared by micro-arc oxidation followed by sol–gel process and in vitro degradation properties. Appl. Surf. Sci. 2012, 258, 2939–2943. [Google Scholar] [CrossRef]
- Sun, W.W.; Li, M.Q.; Gao, Y.; Liu, J. Double Sealing of Ultrasonic Micro-Arc Oxidation Coating on Pure Magnesium by Nano-SiO2 Particles and SiO2 Sol Sealing Agent. Adv. Mater. Res. 2014, 1030–1032, 48–51. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, L.; He, X.; Sun, F. Effect of cerium additive on aluminum-based chemical conversion coating on AZ91D magnesium alloy. Appl. Surf. Sci. 2013, 280, 467–473. [Google Scholar] [CrossRef]
- Tang, Y.; Shen, X.; Liu, Z.; Qiao, Y.; Yang, L.; Lu, D.; Zou, J.; Xu, J. Corrosion behaviors of laser melted inconel 718 alloy in NaOH solution. Acta Metallurgica Sinica 2022, 58, 324–333. [Google Scholar]
- Arun, S.; Arunnellaiappan, T.; Rameshbabu, N. Fabrication of the nanoparticle incorporated PEO coating on commercially pure zirconium and its corrosion resistance. Surf. Coat. Technol. 2016, 305, 264–273. [Google Scholar] [CrossRef]
- Erfanifar, E.; Aliofkhazraei, M.; Nabavi, H.F.; Sharifi, H.; Rouhaghdam, A.S. Growth kinetics and morphology of plasma electrolytic oxidation coating on aluminum. Mater. Chem. Phys. 2017, 185, 162–175. [Google Scholar] [CrossRef]
- Asgari, M.; Aliofkhazraei, M.; Darband, G.B.; Rouhaghdam, A.S. How nanoparticles and submicron particles adsorb inside coating during plasma electrolytic oxidation of magnesium? Surf. Coat. Technol. 2020, 383, 152252. [Google Scholar] [CrossRef]
- Matykina, E.; Arrabal, R.; Skeldon, P.; Thompson, G.E. Incorporation of zirconia nanoparticles into coatings formed on aluminium by AC plasma electrolytic oxidation. J. Appl. Electrochem. 2008, 38, 1375–1383. [Google Scholar] [CrossRef]
- Pezzato, L.; Rigon, M.; Martucci, A.; Brunelli, K.; Dabalà, M. Plasma Electrolytic Oxidation (PEO) as pre-treatment for sol-gel coating on aluminum and magnesium alloys. Surf. Coat. Technol. 2019, 366, 114–123. [Google Scholar] [CrossRef]
- Lu, X.; Mohedano, M.; Blawert, C.; Matykina, E.; Arrabal, R.; Kainer, K.U.; Zheludkevich, M.L. Plasma electrolytic oxidation coatings with particle additions—A review. Surf. Coat. Technol. 2016, 307, 1165–1182. [Google Scholar] [CrossRef]
- Pezzato, L.; Angelini, V.; Brunelli, K.; Martini, C.; Dabalà, M. Tribological and corrosion behavior of PEO coatings with graphite nanoparticles on AZ91 and AZ80 magnesium alloys. Trans. Nonferrous Met. Soc. China 2018, 28, 259–272. [Google Scholar] [CrossRef]
- Gnedenkov, S.V.; Sinebryukhov, S.L.; Egorkin, V.S.; Vyaliy, I.E. Wettability and electrochemical properties of the highly hydrophobic coatings on PEO-pretreated aluminum alloy. Surf. Coat. Technol. 2016, 307, 1241–1248. [Google Scholar] [CrossRef]
- Stern, M.; Geary, A.L. A Theoretical Analysis of the Shape of Polarization Curves. J. Electrochem. Soc. 1957, 104, 56–63. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).