Environmental Fatigue Behavior of a Z3CN20.09M Stainless Steel in High Temperature Water
Abstract
1. Introduction
2. Materials and Experimental Procedures
3. Results and Discussion
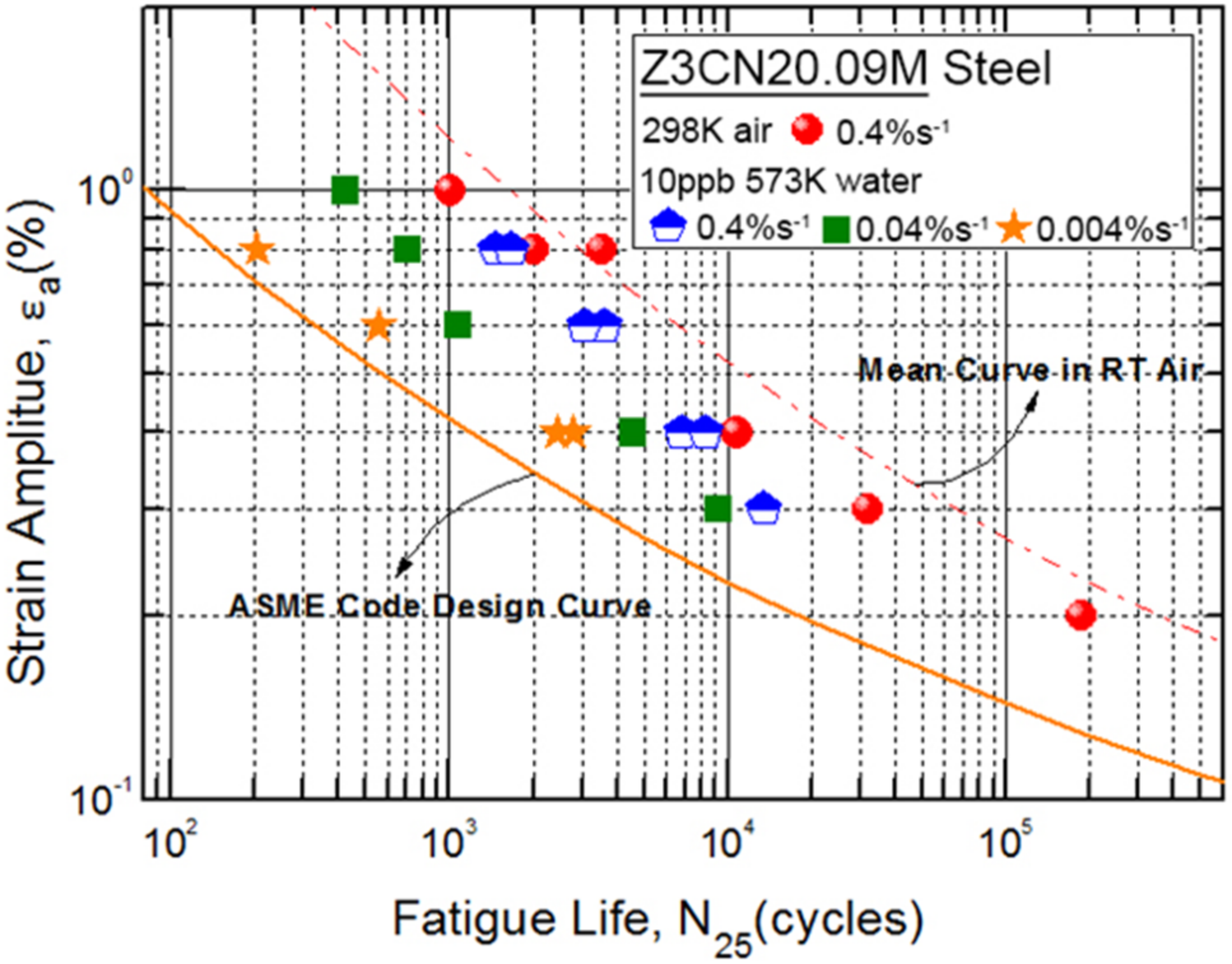
3.1. Fatigue Life
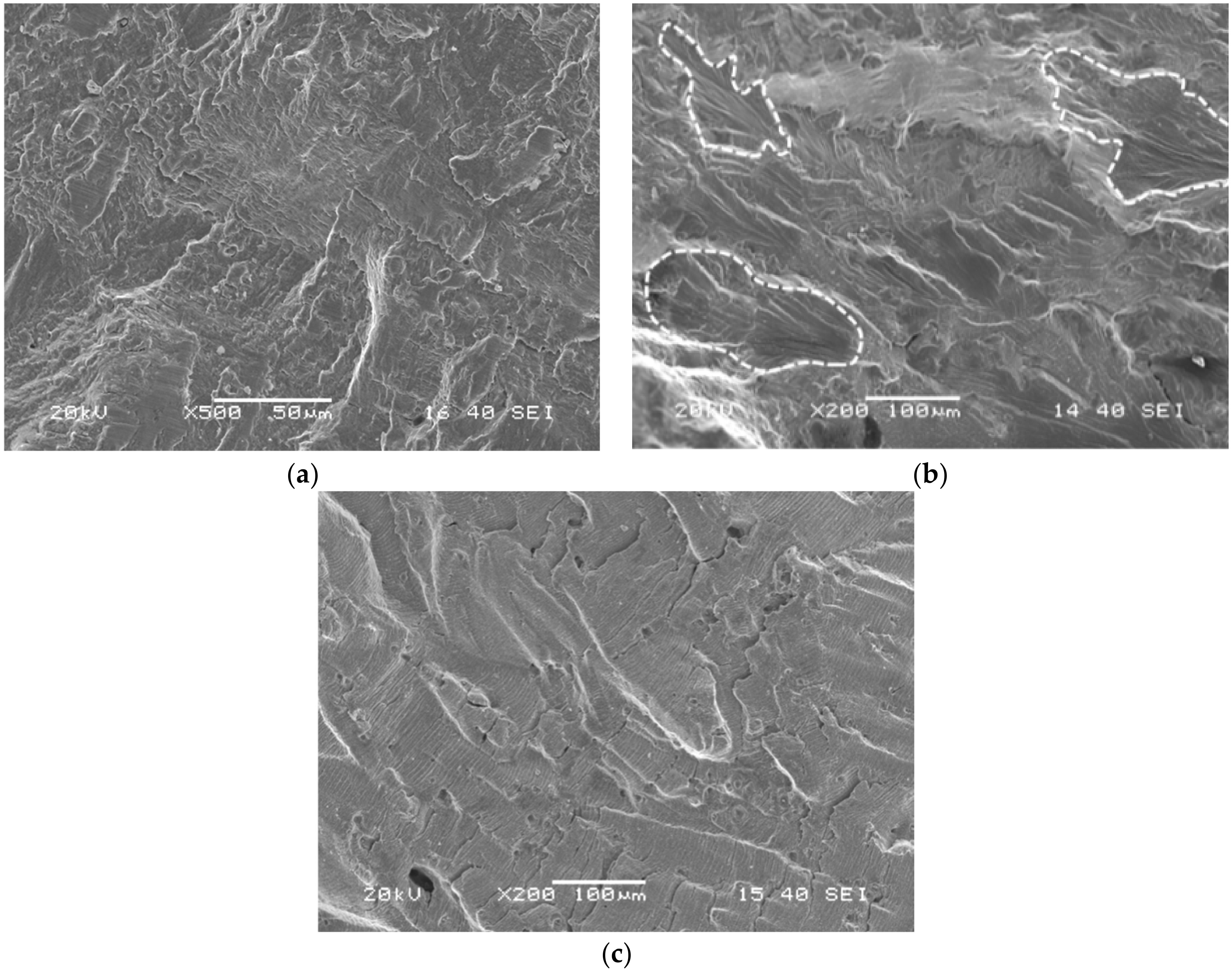
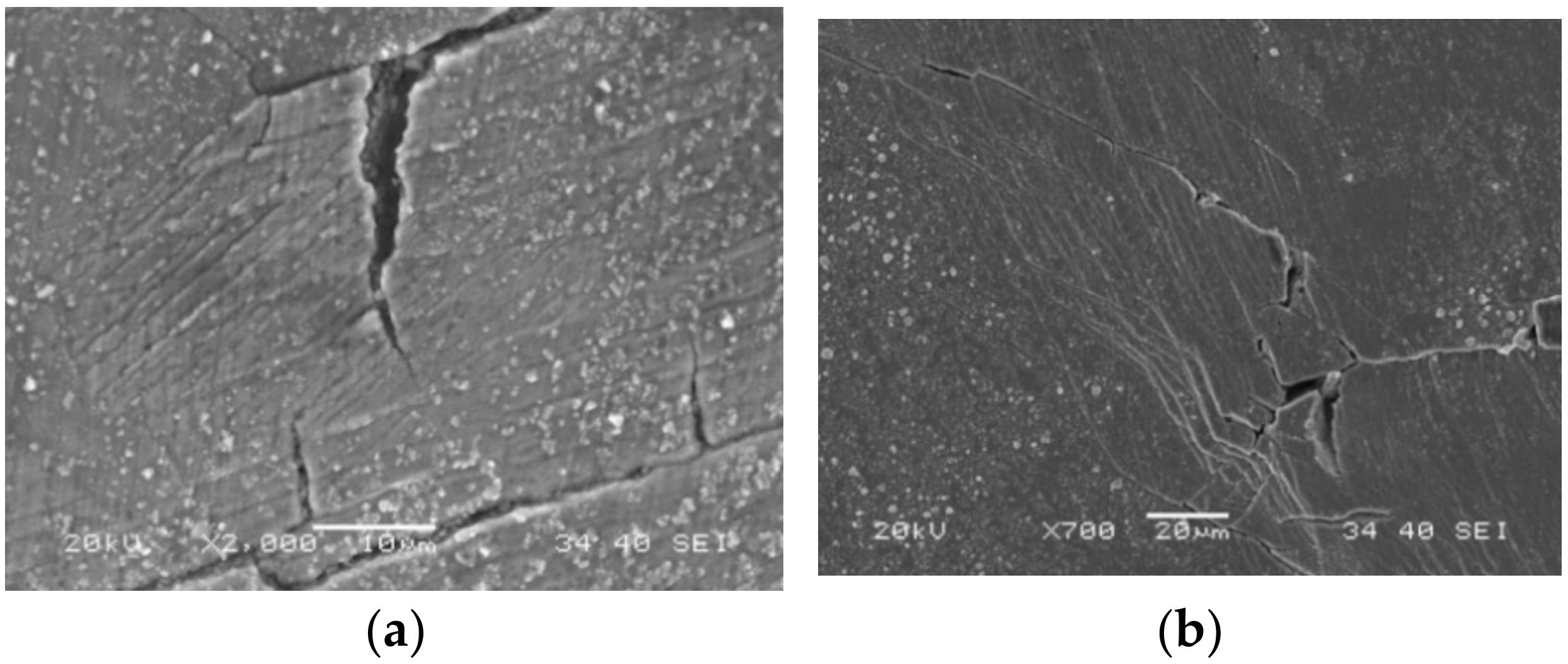
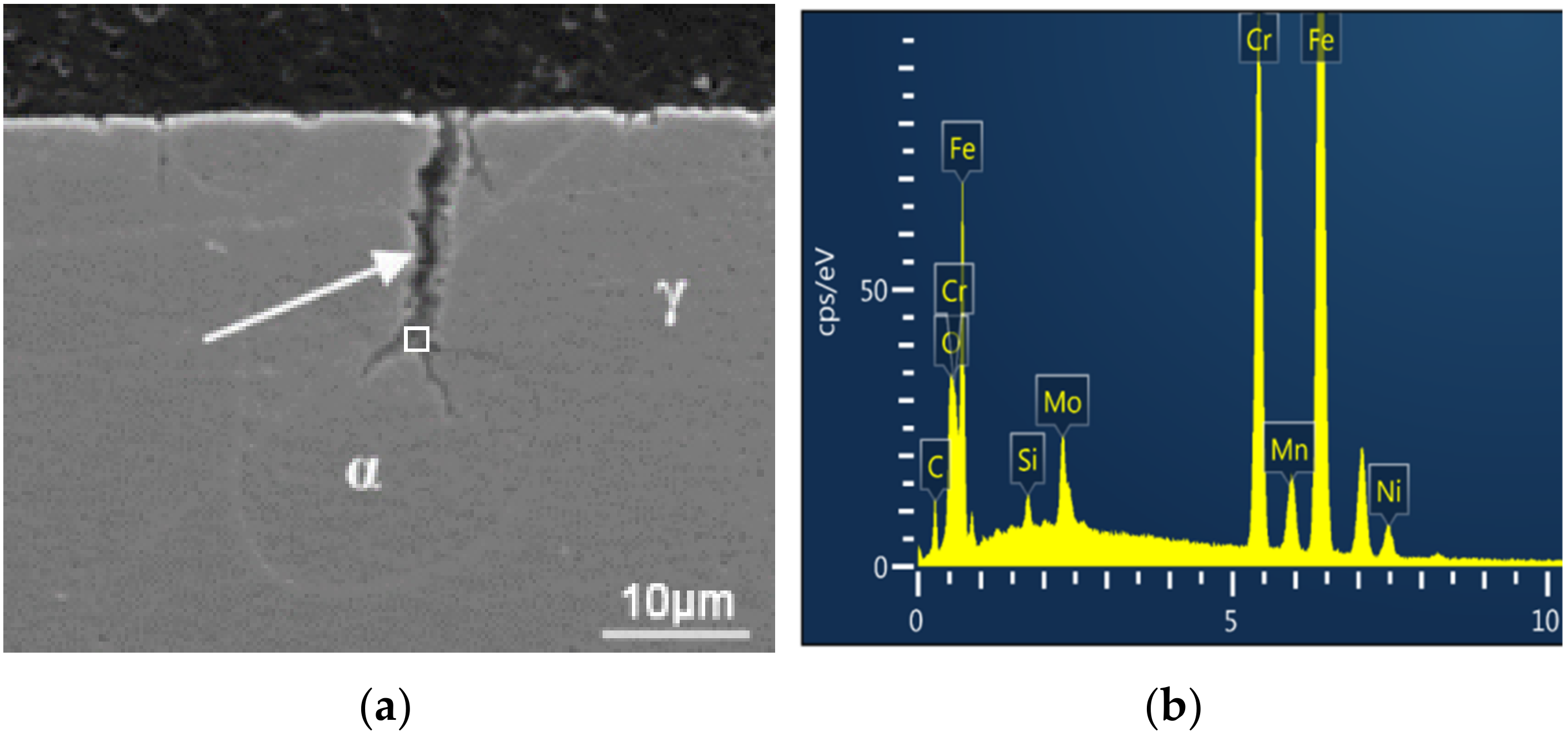
3.2. SEM Observation
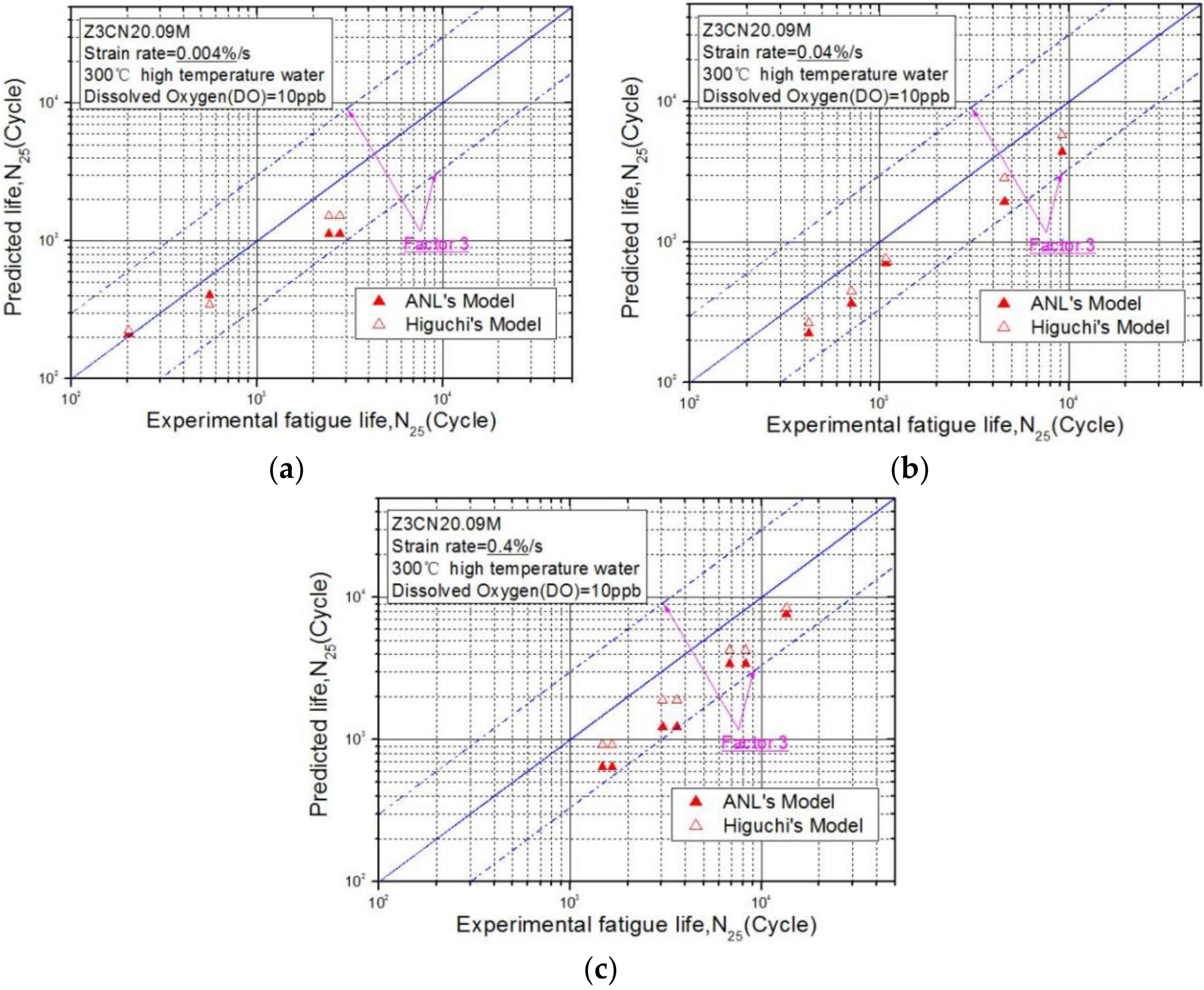
3.3. Difference between the Fatigue Life Data and the Prediction Models
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iida, K. A Review of fatigue failures in LWR plants in Japan. Nucl. Eng. Des. 1992, 138, 297–312. [Google Scholar] [CrossRef]
- Keisler, J.M.; Chopra, O.K.; Shack, W.J. Statistical models for estimating fatigue strain-life behavior of pressure boundary materials in light water reactor environments. Nucl. Eng. Des. 1996, 167, 129–154. [Google Scholar] [CrossRef]
- Chopra, O.K. Effects of LWR Coolant Environments on Fatigue Design Curves of Austenitic Stainless Steels; NUREG/CR-5704, ANL-98/31; U.S. Nuclear Regulatory Commission: Rockville, MD, USA, 1999.
- Tang, Y.B.; Shen, X.W.; Liu, Z.H.; Qiao, Y.X.; Yang, L.L.; Lu, D.H.; Zou, J.S.; Xu, J. Corrosion behaviors of selective laser melted inconel 718 alloy in NaOH solution. Acta Metall. Sin. 2022, 58, 324–333. [Google Scholar]
- Higuchi, M.; Tsutsumi, K.; Hirano, A. A proposal of fatigue life correction factor Fen for austenitic stainless steels in LWR water environments. J. Press. Vess. Tech. 2003, 125, 403–410. [Google Scholar] [CrossRef]
- Cho, H.; Kim, B.K.; Kim, I.S. Environmental fatigue testing of type 316 stainless steel in 310 °C water. In Proceedings of the ASME 2005 Pressure Vessels and Piping Conference, Denver, CO, USA, 17–21 July 2005. [Google Scholar]
- Tice, D.R.; McLennan, A.; Gill, P. Environmentally Assisted Fatigue (EAF) Knowledge Gap Analysis; EPRI: Palto Alto, CA, USA, 2018. [Google Scholar]
- ASME Boiler and Pressure Vessel Code III Division 1-Appendices; The American Society of Mechanical Engineers: New York, NY, USA, 1986.
- Tsutsumi, K.; Kanasaki, H.; Umakoshi, T. Fatigue life reduction in PWR water environment for stainless steels. In Proceedings of the 2000 ASME PVP Conference, Seattle, WA, USA, 23–27 July 2000. [Google Scholar]
- Higuchi, M.; Nakamura, T.; Sugie, Y. Development of an environmental fatigue evaluation method for nuclear power plants in JSME code. J. Environ. Eng. 2011, 6, 452–468. [Google Scholar] [CrossRef]
- Haan, F.H.E.; Wilde, M.H.C.; Blom, F.J. Overview of international implementation of environmental fatigue. In Proceedings of the 2013 ASME PVP Conference, Paris, France, 14–18 July 2013. [Google Scholar]
- Xue, F.; Shi, F.; Zhang, C.; Zheng, Q.; Yi, D.; Li, X.; Li, Y. The microstructure and mechanical and corrosion behaviors of thermally aged Z3CN20-09M cast stainless steel for primary coolant pipes of nuclear power plants. Coatings 2021, 11, 870. [Google Scholar] [CrossRef]
- Vincent, L.; Le Roux, J.C.; Taheri, S. On the high cycle fatigue behavior of a type 304L stainless steel at room temperature. Int. J. Fatigue 2012, 38, 84–91. [Google Scholar] [CrossRef]
- Chen, W.; Spätig, P.; Seifert, H.P. Fatigue behavior of 316L austenitic stainless steel in air and LWR environment with and without mean stress. MATEC Web Conf. 2018, 165, 03012. [Google Scholar] [CrossRef][Green Version]
- Zhang, W.Q.; Fang, K.W.; Wang, X.L. Investigation of stress corrosion cracking initiation in machined 304 austenitic stainless steel in magnesium chloride environment. J. Mater. Eng. Perform. 2020, 29, 191–204. [Google Scholar] [CrossRef]
- Wire, G.L.; Leax, T.R.; Kandra, J.T. Mean stress and environmental effects on fatigue in type 304 stainless steel. In Proceedings of the 1999 ASME PVP Conference, Boston, MA, USA, 1–5 August 1999. [Google Scholar]
- Solomon, H.D.; Amzallag, C.; Vallee, A.J.; De Lair, R.E. Influence of mean stress on the fatigue behavior of 304L SS in air and PWR water. In Proceedings of the 2005 ASME PVP Conference, Denver, CO, USA, 17–21 July 2005. [Google Scholar]
- Kamaya, M. Influence of strain range on fatigue life reduction of stainless steel in PWR primary water. Fatigue Fract. Eng. Mater. Struct. 2017, 40, 2194–2203. [Google Scholar] [CrossRef]
- Spätig, P.; Heczko, M.; Kruml, T.; Seifert, H.P. Influence of mean stress and light water reactor environment on fatigue life and dislocation microstructures of 316L austenitic steel. J. Nucl. Mater. 2018, 509, 15–28. [Google Scholar] [CrossRef]
- Bradaï, S.; Gourdin, C. Equi-biaxial loading effect on austenitic stainless steel fatigue life. In Proceedings of the 2015 ASME PVP Conference, Boston, MA, USA, 19–23 July 2015. [Google Scholar]
- Dong, L.J.; Han, E.H.; Peng, Q.J.; Ke, W.; Wang, L. Environmentally assisted crack growth in 308L stainless steel weld metal in simulated primary water. Corros. Sci. 2017, 117, 1–10. [Google Scholar] [CrossRef]
- Seifert, H.P.; Ritter, S.; Leber, H.J. Corrosion fatigue crack growth behaviour of austenitic stainless steels under light water reactor conditions. Corros. Sci. 2012, 55, 61–75. [Google Scholar] [CrossRef]
- Seifert, H.P.; Ritter, S.; Leber, H.J. Corrosion fatigue initiation and short crack growth behaviour of austenitic stainless steels under light water reactor conditions. Corros. Sci. 2012, 59, 20–34. [Google Scholar] [CrossRef]
- Yuan, X.; Yu, W.; Fu, S.; Yu, D.; Chen, X. Effect of mean stress and ratcheting strain on the low cycle fatigue behavior of a wrought 316LN stainless steel. Mater. Sci. Eng. A 2016, 677, 193–202. [Google Scholar] [CrossRef]
- Miura, N.; Takahashi, Y. High-cycle fatigue behavior of type 316 stainless steel at 288 °C including mean stress effect. Int. J. Fatigue 2006, 28, 1618–1625. [Google Scholar] [CrossRef]
- Chen, W. Experimental Evaluation and Modelling of Fatigue of a 316L Austenitic Stainless Steels in High-Temperature Water and Air Environments. Ph.D. Thesis, EPFL, Lausanne, Switzerland, 2020. [Google Scholar]
- Zhang, Z.Y.; Tan, J.B.; Wu, X.Q.; Han, E.H.; Ke, W.; Rao, J.C. Effects of temperature on corrosion fatigue behavior of 316LN stainless steel in high-temperature pressurized water. Corros. Sci. 2019, 146, 80–89. [Google Scholar] [CrossRef]
- Paul, S.K. A critical review of experimental aspects in ratcheting fatigue: Microstructure to specimen to component. J. Mater. Res. Technol. 2019, 8, 4894–4914. [Google Scholar] [CrossRef]
- Ogawa, Y.; Birenis, D.; Matsunaga, H.; Thøgersen, A.; Prytz, Ø.; Takakuwa, O.; Yamabe, J. Multi-scale observation of hydrogen-induced, localized plastic deformation in fatigue-crack propagation in a pure iron. Scr. Mater. 2017, 140, 13–17. [Google Scholar] [CrossRef]
- Yi, X.N.; Ma, A.L.; Zhang, L.M.; Zheng, Y.G. Crystallographic anisotropy of corrosion rate and surface faceting of polycrystalline 90Cu-10Ni in acidic NaCl solution. Mater. Des. 2022, 215, 110429. [Google Scholar] [CrossRef]
- Ford, F.P.; Andresen, P.L. Corrosion Fatigue of Pressure Boundary Materials. In Proceedings of the 7th International Conference On Fracture, Houston, TX, USA, 20–24 March 1989. [Google Scholar]
- Kamaya, M. Development of disc bending fatigue test technique for equi-biaxial loading. Int. J. Fatigue 2016, 82, 561–571. [Google Scholar] [CrossRef]
- Nakamura, T.; Miyama, S. Environmental fatigue evaluation in PLM activities of PWR plant. E-J. Adv. Maint. 2010, 2, 82–100. [Google Scholar]
- Spätig, P.; Seifert, H.P. Mean stress effect on fatigue life of 316L austenitic steel in air and simulated boliing water reactor hydrogen water chemistry environment. In Proceedings of the 17th International Conference on Environmental Degradation of Materials in Nuclear Systems—Water Reactors, TMS, Ottawa, ON, Canada, 9–12 August 2015. [Google Scholar]
- Procopio, I.; Cicero, S.; Mottershead, K.; Bruchhausen, M.; Cuvilliez, S. Increasing safety in NPPs by covering gaps in environmental fatigue assessment. Pro. Str. Integrity 2018, 13, 97–103. [Google Scholar] [CrossRef]
- Mottershead, K.; Bruchhausen, M.; Métais, T.; Cicero, S.; Tice, D.; Platts, N. Increasing Safety in Nuclear Power Plants by Covering Gaps in Environmental Fatigue Assessment. In Proceedings of the 2016 ASME PVP Conference, Vancouver, BC, Canada, 17–21 July 2016. [Google Scholar]
- Kamaya, M.; Kawakubo, M. Mean stress effect on fatigue strength of stainless steel. Int. J. Fatigue 2015, 74, 20–29. [Google Scholar] [CrossRef]






| Properties | Temperature | Properties | Z3CN20.09M |
|---|---|---|---|
| Tensile Properties | 25 °C | Rp0.2 | 235 MPa |
| Rm | 655 MPa | ||
| A% (5d) | 56.8 % | ||
| 350 °C | Rtp0.2 | 155 MPa | |
| Rm | 395 MPa | ||
| KV Impact | 25 °C | Lowest Average Value | 265 J |
| Test conditions | Load ratio (R) | −1 |
| Control mode | Strain | |
| Wave form | Full reversed triangular | |
| Strain rate | 0.4, 0.04 and 0.004%/s | |
| Strain amplitude (εa) | 0.2–1.0% | |
| Water chemistry | Temperature | 300 °C |
| Pressure | 12 MPa | |
| Conductivity | <0.1 μS/cm | |
| Dissolved oxygen (DO) | 10 ± 1 ppb |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, K.; Luo, K.; Wang, L.; Li, C.; Wang, L.; Qiao, Y. Environmental Fatigue Behavior of a Z3CN20.09M Stainless Steel in High Temperature Water. Coatings 2022, 12, 317. https://doi.org/10.3390/coatings12030317
Fang K, Luo K, Wang L, Li C, Wang L, Qiao Y. Environmental Fatigue Behavior of a Z3CN20.09M Stainless Steel in High Temperature Water. Coatings. 2022; 12(3):317. https://doi.org/10.3390/coatings12030317
Chicago/Turabian StyleFang, Kewei, Kunjie Luo, Li Wang, Chengtao Li, Lei Wang, and Yanxin Qiao. 2022. "Environmental Fatigue Behavior of a Z3CN20.09M Stainless Steel in High Temperature Water" Coatings 12, no. 3: 317. https://doi.org/10.3390/coatings12030317
APA StyleFang, K., Luo, K., Wang, L., Li, C., Wang, L., & Qiao, Y. (2022). Environmental Fatigue Behavior of a Z3CN20.09M Stainless Steel in High Temperature Water. Coatings, 12(3), 317. https://doi.org/10.3390/coatings12030317








