A Comparative Study on the Anti-Corrosive Performance of Zinc Phosphate in Powder Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Coating Formulae
2.2. Preparation of Coating Powder and Coated Panels
2.3. Characterization and Evaluation Techniques
2.3.1. Electrochemical Measurements
2.3.2. Salt Spray Tests
2.3.3. General Coating Property Measurements
2.3.4. Imaging and Spectroscopy Analyses
3. Results and Discussion
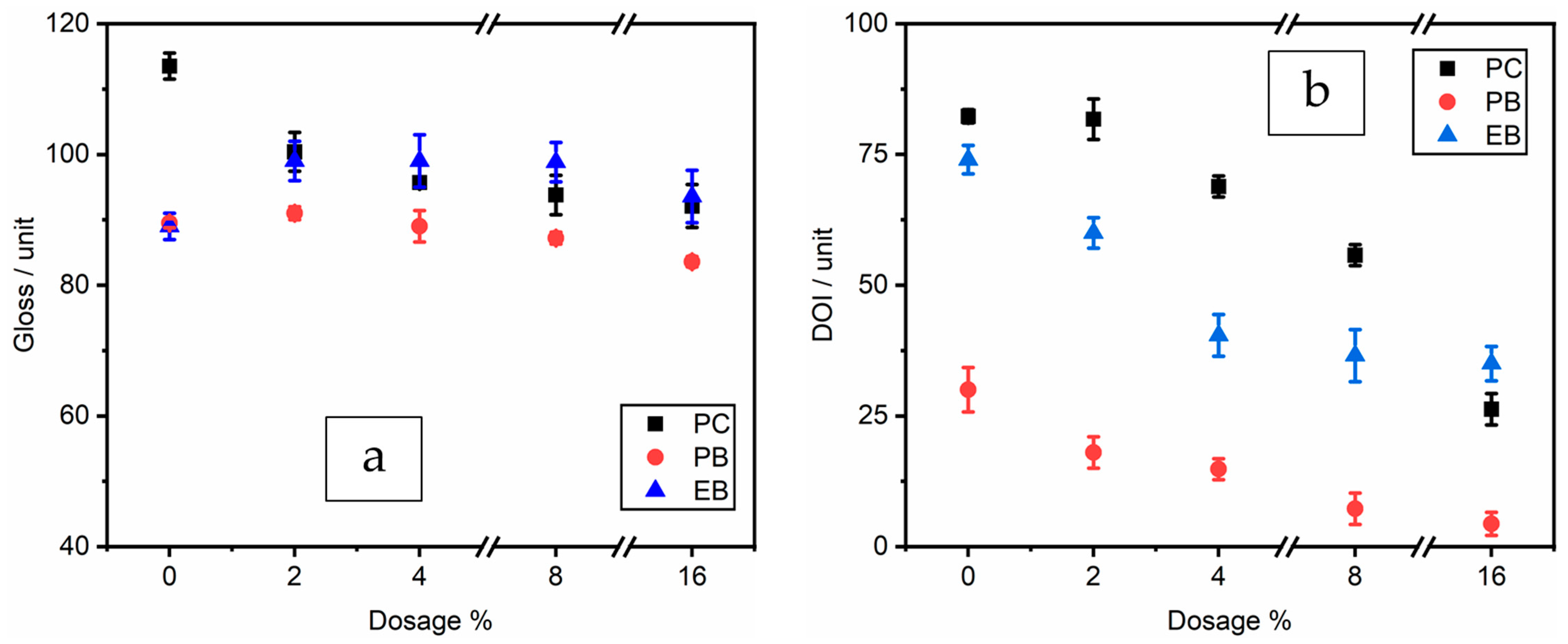
3.1. Evaluation Results of Coating Properties
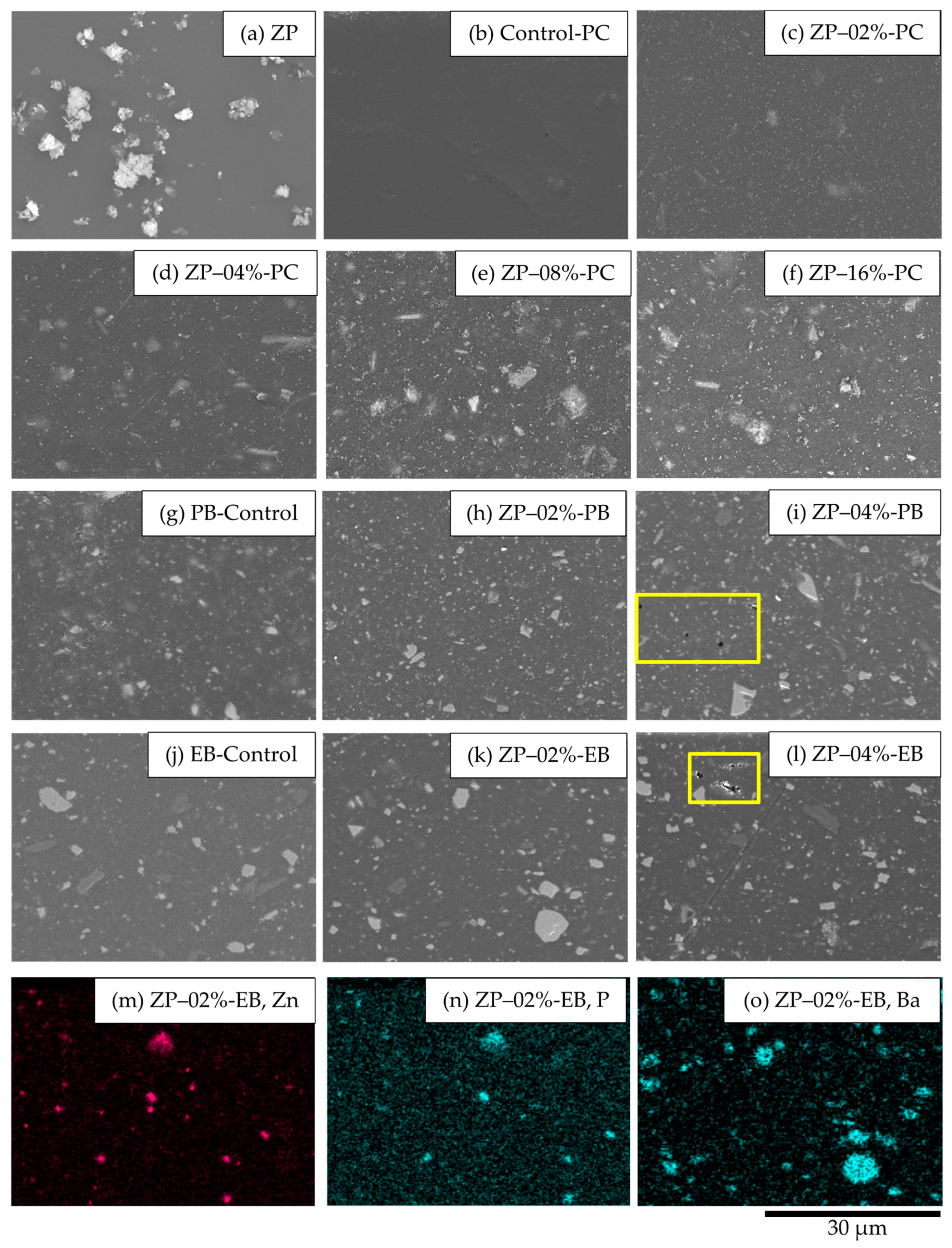
3.2. Coating Morphologies
3.3. Electrochemical Measurement Results
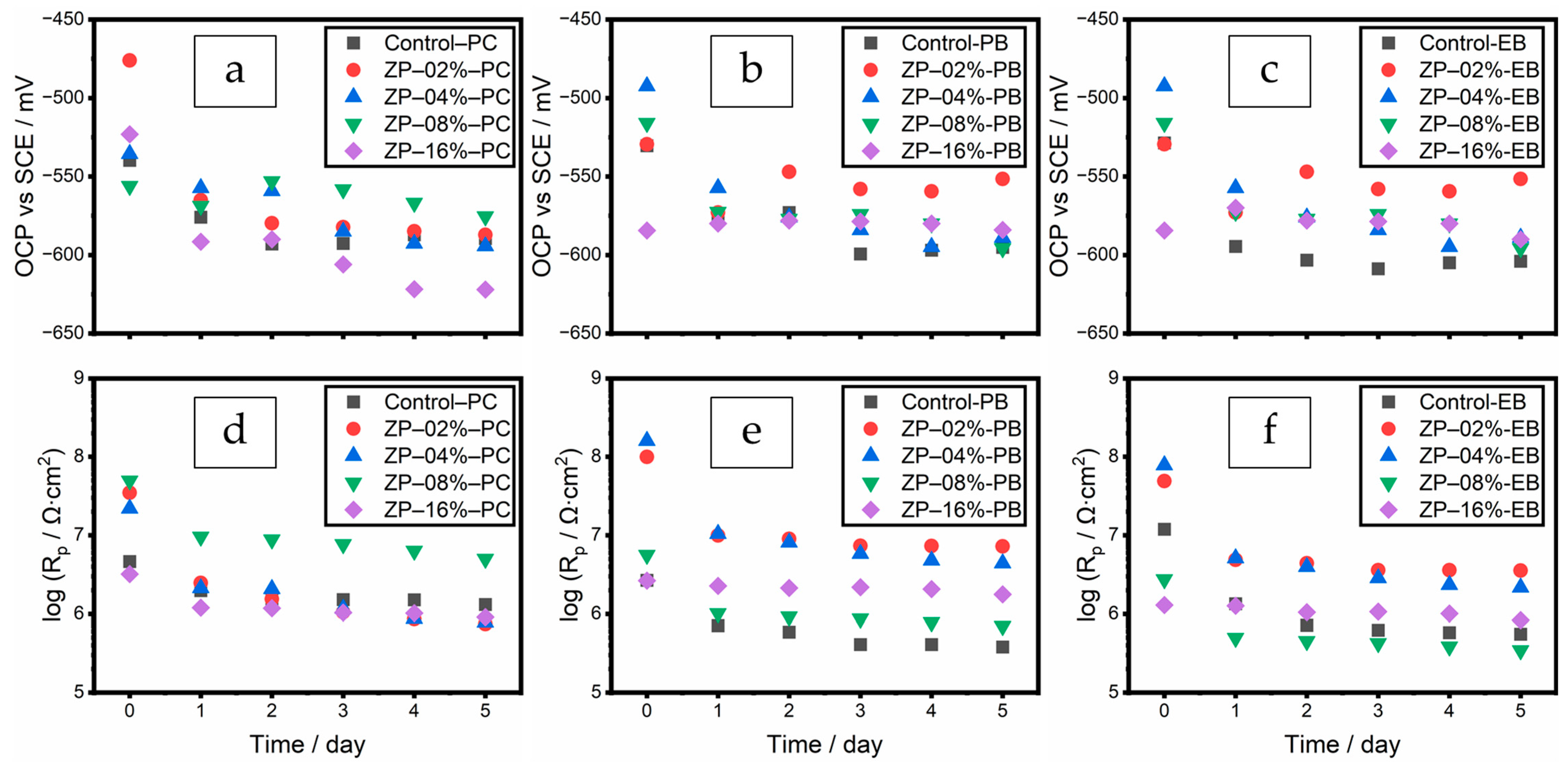
3.3.1. OCP and Rp Results
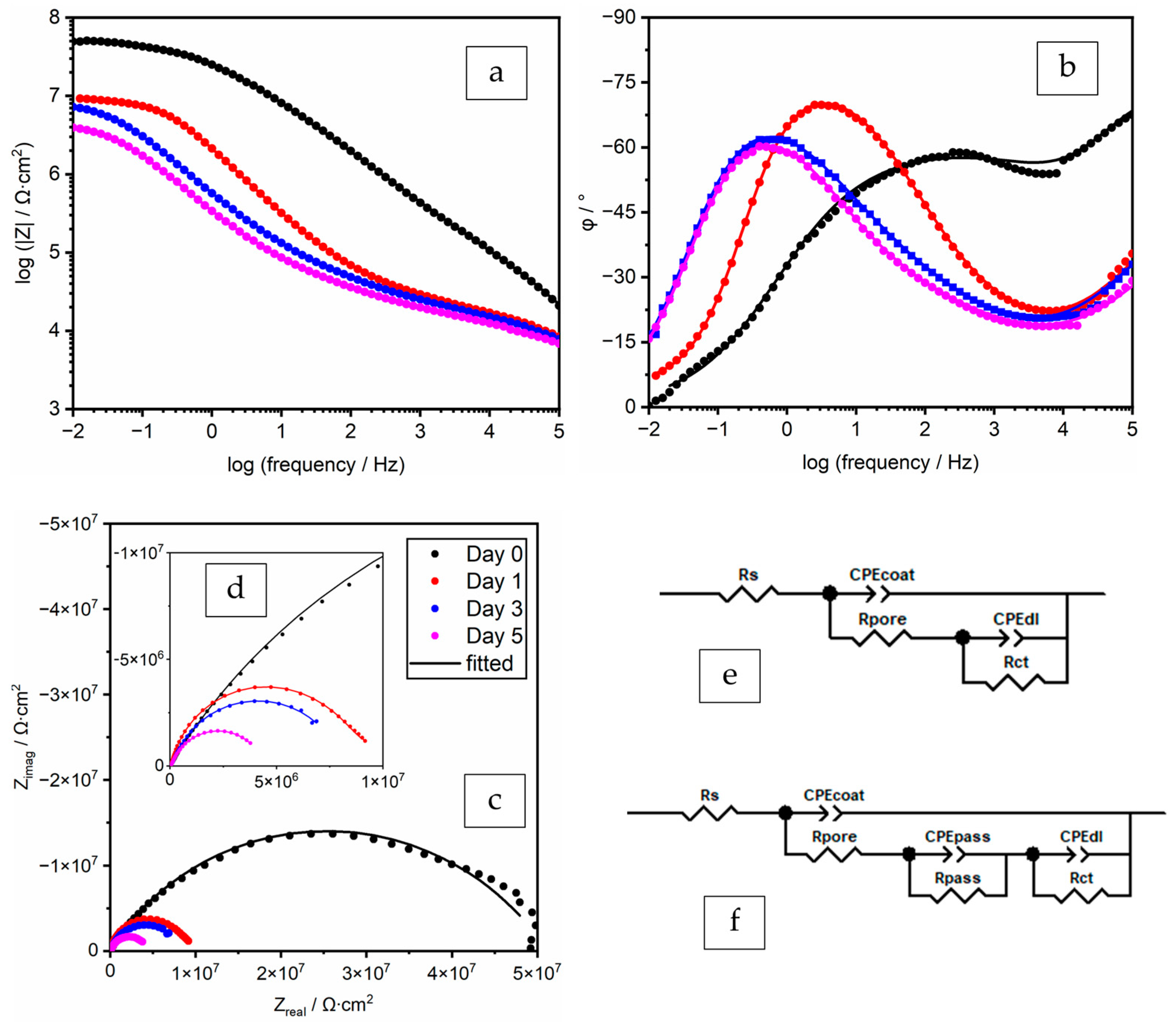
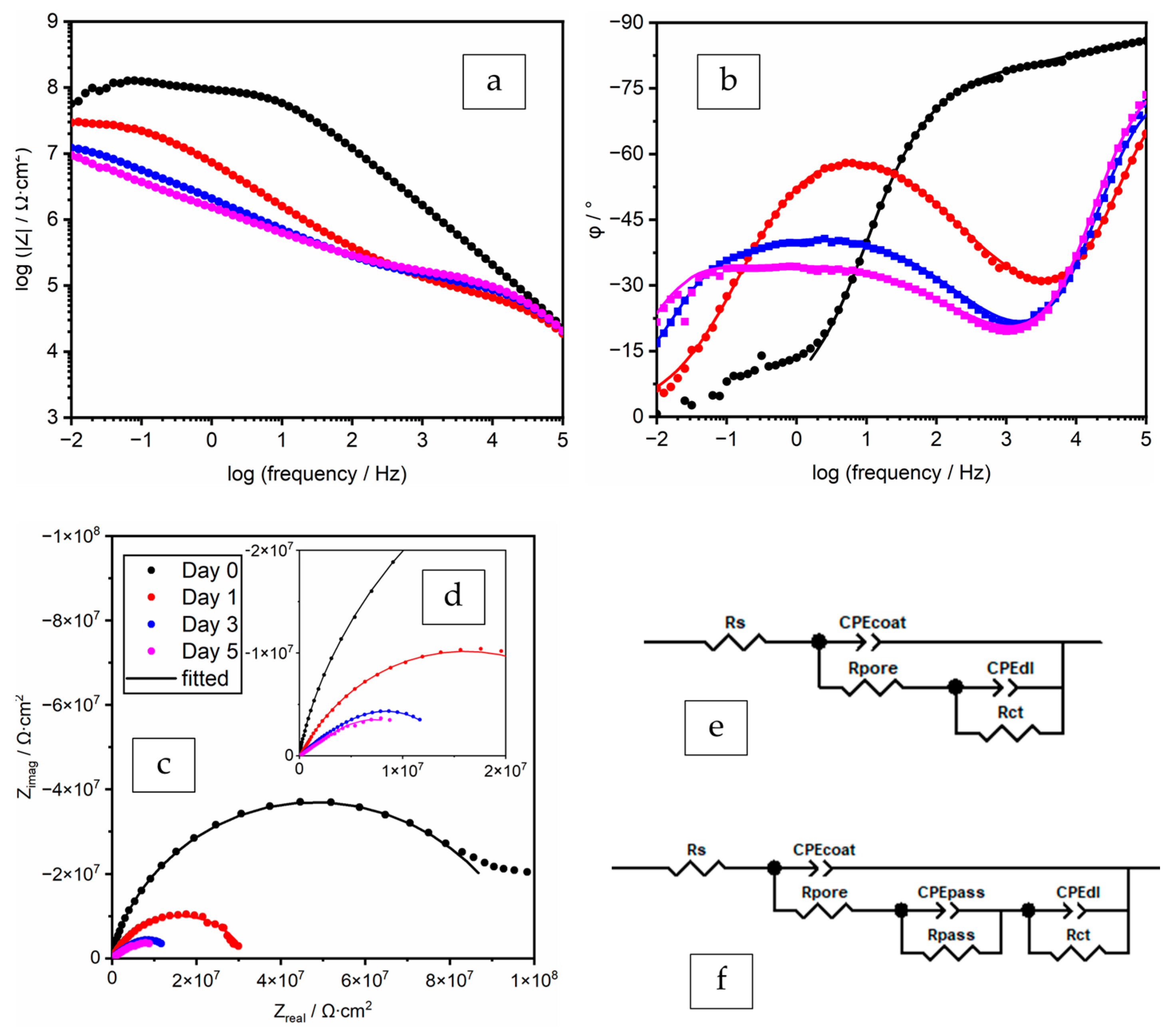
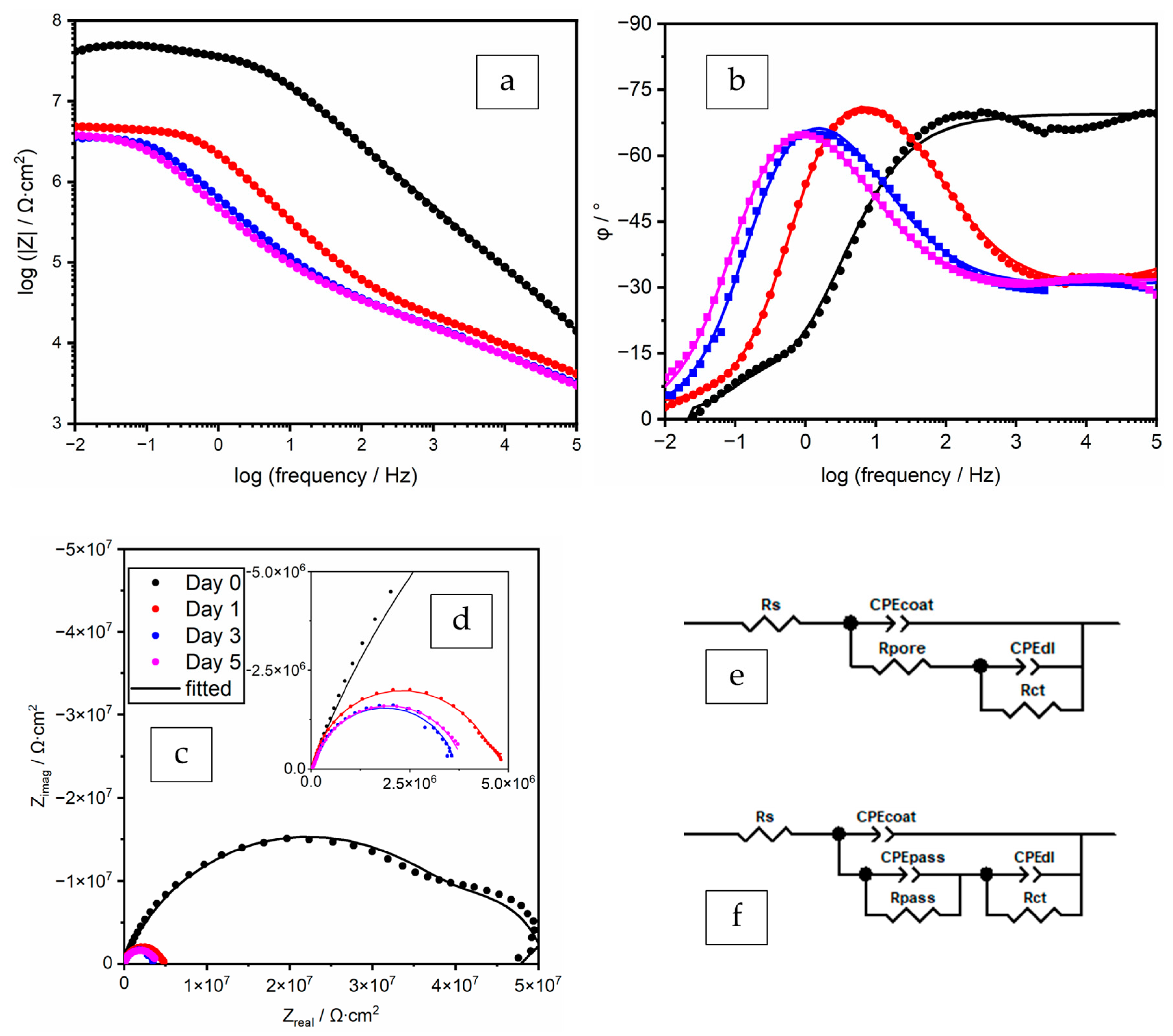
3.3.2. EIS Spectra Analyses and Data Fitting Results
ZP 8% in PC (Polyester/TGIC Clearcoat)
ZP 2% with Filler BaSO4 in PB (Polyester/TGIC) and EB (Epoxy/DICY) Coatings
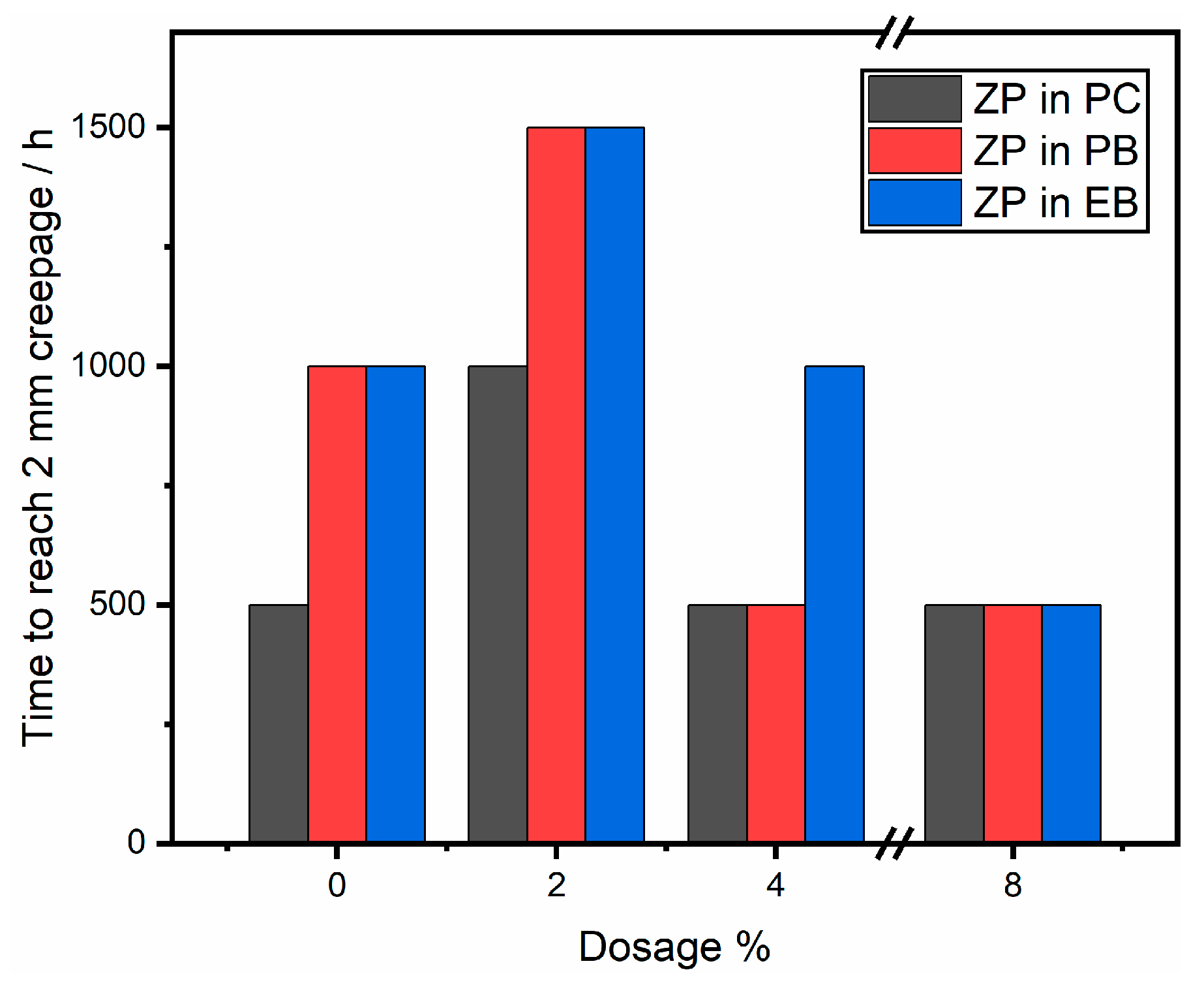
3.4. Neutral Salt Spray Test Results
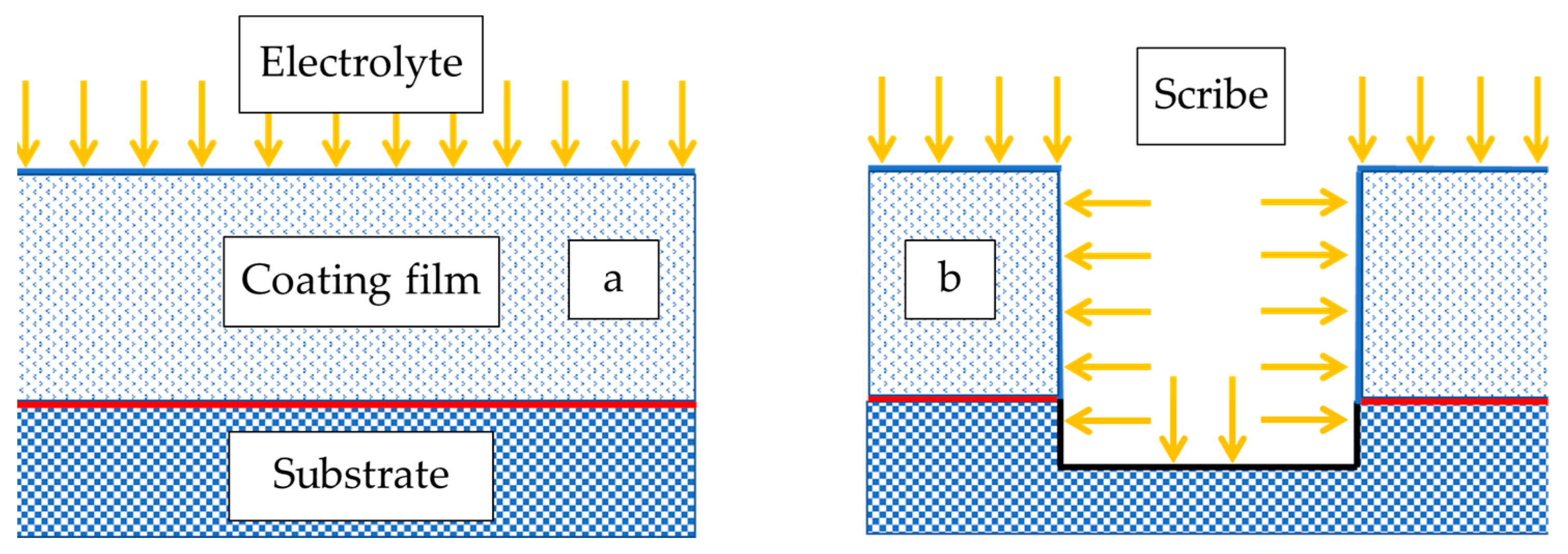
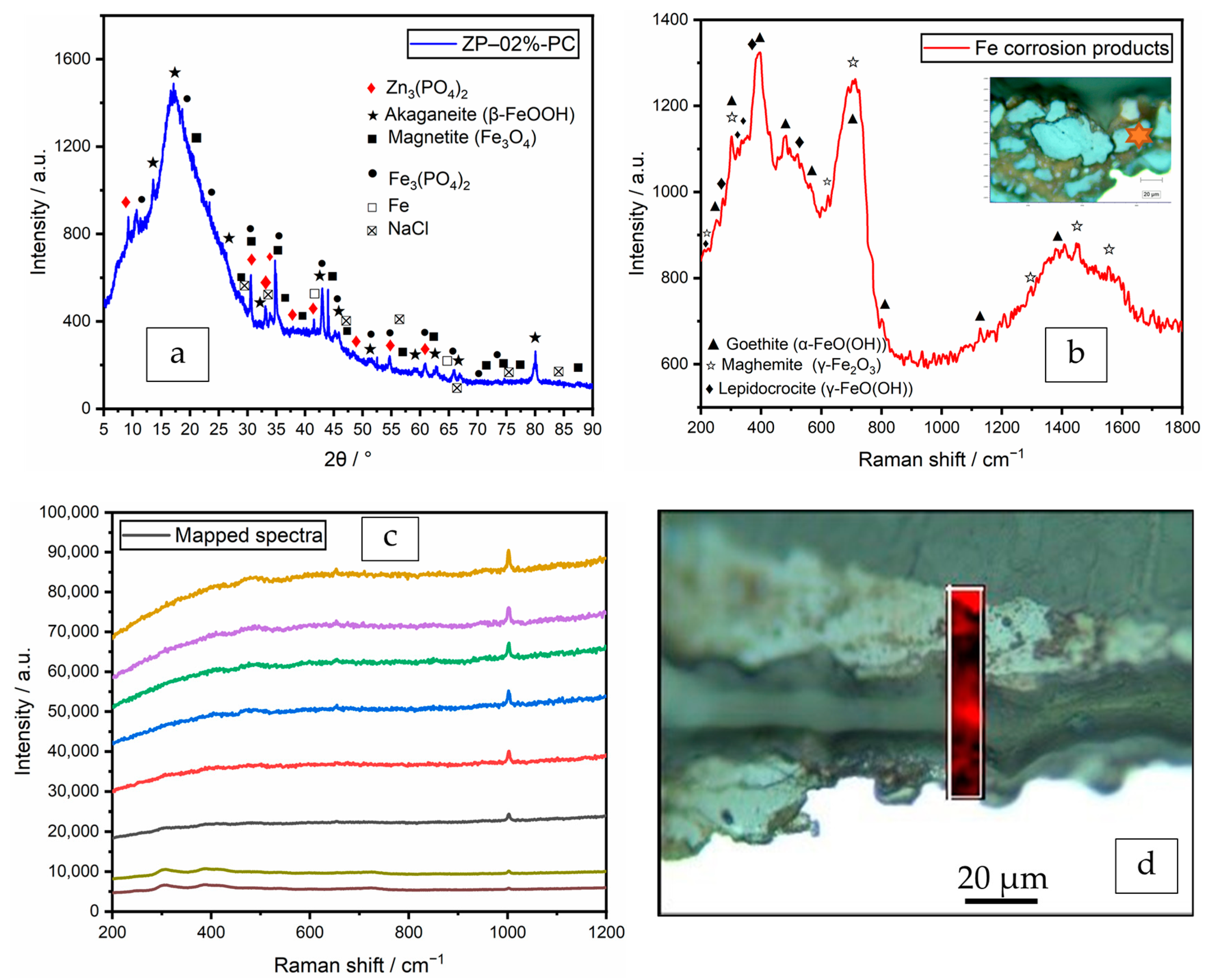
3.5. Confirmation of the Passivation Layer
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sørensen, P.A.; Kiil, S.; Dam-Johansen, K.; Weinell, C.E. Anticorrosive Coatings: A Review. J. Coat. Technol. Res. 2009, 6, 135–176. [Google Scholar] [CrossRef]
- Walter, G.W. A Critical Review of the Protection of Metals by Paints. Corros. Sci. 1986, 26, 27–38. [Google Scholar] [CrossRef]
- Turner, S.; Baskir, J.; Nunez, C. Powder Coatings: A Technology Review. Pollut. Prev. Rev. 1999, 9, 7–21. [Google Scholar]
- Farrell, R. Powder Coatings. Met. Finish. 2010, 108, 100–107. [Google Scholar] [CrossRef]
- Crapper, G. Powder Coatings. In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 10, pp. 541–566. ISBN 9780080878621. [Google Scholar]
- Misev, T.A.; van der Linde, R. Powder Coatings Technology: New Developments at the Turn of the Century. Prog. Org. Coat. 1997, 34, 160–168. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, M.S.; Bhuiyan, M.T.I.; Zhu, J. Green Chemistry for Automotive Coatings: Sustainable Applications; RSC Publications: Cambridge, UK, 2019; pp. 368–394. ISBN 9781782629948. [Google Scholar]
- El-ghaffar, M.A.A.; Abdel-wahab, N.A.; Sanad, M.A.; Sabaa, M.W. Progress in Organic Coatings High Performance Anti-Corrosive Powder Coatings Based on Phosphate Pigments Containing Poly (o-Aminophenol). Prog. Org. Coat. 2015, 78, 42–48. [Google Scholar] [CrossRef]
- Viertel, J.; Neuer, L.; Mauch, B.; Czyborra, T. Project RepaKorr: Development of a Novel Single Coat, Direct to Metal Repair Coating with Outstanding Protection and Colour Retention Performance for Offshore Structures. Mater. Corros. 2017, 68, 1321–1325. [Google Scholar] [CrossRef]
- Oliveira, C.G.; Ferreira, M.G.S. Ranking High-Quality Paint Systems Using EIS.Part I: Intact Coatings. Corros. Sci. 2003, 45, 123–138. [Google Scholar] [CrossRef]
- Croll, S.G. Electrolyte Transport in Polymer Barrier Coatings: Perspectives from Other Disciplines. Prog. Org. Coat. 2018, 124, 41–48. [Google Scholar] [CrossRef]
- Hussain, A.K.; Seetharamaiah, N.; Pichumani, M.; Chakra, C.S. Research Progress in Organic Zinc Rich Primer Coatings for Cathodic Protection of Metals — A Comprehensive Review. Prog. Org. Coat. 2021, 153, 106040. [Google Scholar] [CrossRef]
- Hao, Y.; Liu, F.; Han, E.H.; Anjum, S.; Xu, G. The Mechanism of Inhibition by Zinc Phosphate in an Epoxy Coating. Corros. Sci. 2013, 69, 77–86. [Google Scholar] [CrossRef]
- Jašková, V.; Kalendová, A. Anticorrosive Coatings Containing Modified Phosphates. Prog. Org. Coat. 2012, 75, 328–334. [Google Scholar] [CrossRef]
- Naderi, R.; Arman, S.Y.; Fouladvand, S. Investigation on the Inhibition Synergism of New Generations of Phosphate-Based Anticorrosion Pigments. Dye. Pigment. 2014, 105, 23–33. [Google Scholar] [CrossRef]
- Askari, F.; Ghasemi, E.; Ramezanzadeh, B.; Mahdavian, M. Synthesis and Characterization of the Fourth Generation of Zinc Phosphate Pigment in the Presence of Benzotriazole. Dye. Pigment. 2016, 124, 18–26. [Google Scholar] [CrossRef]
- Bastos, A.C.; Ferreira, M.G.S.; Simões, A.M. Comparative Electrochemical Studies of Zinc Chromate and Zinc Phosphate as Corrosion Inhibitors for Zinc. Prog. Org. Coat. 2005, 52, 339–350. [Google Scholar] [CrossRef]
- Darvish, A.; Naderi, R.; Attar, M.M. The Impact of Pigment Volume Concentration on the Protective Performance of Polyurethane Coating with Second Generation of Phosphate Based Anticorrosion Pigment. Prog. Org. Coat. 2014, 77, 1768–1773. [Google Scholar] [CrossRef]
- Alibakhshi, E.; Ghasemi, E.; Mahdavian, M. Optimization of Potassium Zinc Phosphate Anticorrosion Pigment by Taguchi Experimental Design. Prog. Org. Coat. 2013, 76, 224–230. [Google Scholar] [CrossRef]
- Alibakhshi, E.; Ghasemi, E.; Mahdavian, M. Corrosion Inhibition by Lithium Zinc Phosphate Pigment. Corros. Sci. 2013, 77, 222–229. [Google Scholar] [CrossRef]
- Naderi, R.; Mahdavian, M.; Darvish, A. Electrochemical Examining Behavior of Epoxy Coating Incorporating Zinc-Free Phosphate-Based Anticorrosion Pigment. Prog. Org. Coat. 2013, 76, 302–306. [Google Scholar] [CrossRef]
- Puig, M.; Cabedo, L.; Gracenea, J.J.; Jiménez-Morales, A.; Gámez-Pérez, J.; Suay, J.J. Adhesion Enhancement of Powder Coatings on Galvanised Steel by Addition of Organo-Modified Silica Particles. Prog. Org. Coat. 2014, 77, 1309–1315. [Google Scholar] [CrossRef] [Green Version]
- Puig, M.; Gimeno, M.J.; Gracenea, J.J.; Suay, J.J. Anticorrosive Properties Enhancement in Powder Coating Duplex Systems by Means of ZMP Anticorrosive Pigment. Assessment by Electrochemical Techniques. Prog. Org. Coat. 2014, 77, 1993–1999. [Google Scholar] [CrossRef]
- Zubielewicz, M.; Gnot, W. Mechanisms of Non-Toxic Anticorrosive Pigments in Organic Waterborne Coatings. Prog. Org. Coat. 2004, 49, 358–371. [Google Scholar] [CrossRef]
- Fishman, R.S.; Kurtze, D.A.; Bierwagen, G.P. Pigment Inhomogeneity and Void Formation in Organic Coatings. Prog. Org. Coat. 1993, 21, 387–403. [Google Scholar] [CrossRef]
- Kalendova, A.; Veselý, D.; Kalenda, P. A Study of the Effects of Pigments and Fillers on the Properties of Anticorrosive Paints. Pigment. Resin Technol. 2006, 35, 83–94. [Google Scholar] [CrossRef]
- Li, W.; Franco, D.C.; Yang, M.S.; Zhu, X.; Zhang, H.; Shao, Y.; Zhang, H.; Zhu, J. Investigation of the Performance of ATH Powders in Organic Powder Coatings. Coatings 2019, 9, 110. [Google Scholar] [CrossRef] [Green Version]
- Schulze, K.A.; Zaman, A.A.; Söderholm, K.J.M. Effect of Filler Fraction on Strength, Viscosity and Porosity of Experimental Compomer Materials. J. Dent. 2003, 31, 373–382. [Google Scholar] [CrossRef]
- Li, W.; Franco, D.C.; Yang, M.S.; Zhu, X.; Zhang, H.; Shao, Y.; Zhang, H.; Zhu, J. Comparative Study of the Performances of Al(OH)3 and BaSO4 in Ultrafine Powder Coatings. Processes 2019, 7, 316. [Google Scholar] [CrossRef] [Green Version]
- Tan, B.; Thomas, N.L. Tortuosity Model to Predict the Combined Effects of Crystallinity and Nano-Sized Clay Mineral on the Water Vapour Barrier Properties of Polylactic Acid. Appl. Clay Sci. 2017, 141, 46–54. [Google Scholar] [CrossRef] [Green Version]
- Walter, G.W. A Critical Review of d.c. Electrochemical Tests for Painted Metals. Corros. Sci. 1986, 26, 39–47. [Google Scholar] [CrossRef]
- Ding, J.; Zhao, H.; Xu, B.; Zhao, X.; Su, S.; Yu, H. Superanticorrosive Graphene Nanosheets through π Deposition of Boron Nitride Nanodots. ACS Sustain. Chem. Eng. 2019, 7, 10900–10911. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, D.; Li, J.; Liu, T.; Pu, J.; Zhao, H.; Wang, L. One-Step Synthesis of Superhydrophobic Polyhedral Oligomeric Silsesquioxane-Graphene Oxide and Its Application in Anti-Corrosion and Anti-Wear Fields. Corros. Sci. 2019, 147, 9–21. [Google Scholar] [CrossRef]
- Pajkossy, T.; Jurczakowski, R. Electrochemical Impedance Spectroscopy in Interfacial Studies. Curr. Opin. Electrochem. 2017, 1, 53–58. [Google Scholar] [CrossRef] [Green Version]
- Murray, J.N. Electrochemical Test Methods for Evaluating Organic Coatings on Metals: An Update. Part, I. Introduction and Generalities Regarding Electrochemical Testing of Organic Coatings. Prog. Org. Coat. 1997, 30, 225–233. [Google Scholar] [CrossRef]
- Margarit-Mattos, I.C.P. EIS and Organic Coatings Performance: Revisiting Some Key Points. Electrochim. Acta 2020, 354, 136725. [Google Scholar] [CrossRef]
- Yang, L.H.; Liu, F.C.; Han, E.H. Effects of P/B on the Properties of Anticorrosive Coatings with Different Particle Size. Prog. Org. Coat. 2005, 53, 91–98. [Google Scholar] [CrossRef]
- Rashvand, M.; Ranjbar, Z. Effect of Nano-ZnO Particles on the Corrosion Resistance of Polyurethane-Based Waterborne Coatings Immersed in Sodium Chloride Solution via EIS Technique. Prog. Org. Coat. 2013, 76, 1413–1417. [Google Scholar] [CrossRef]
- Croll, S.G. Surface Roughness Profile and Its Effect on Coating Adhesion and Corrosion Protection: A Review. Prog. Org. Coat. 2020, 148, 105847. [Google Scholar] [CrossRef]
- Biris, A.S.; Mazumder, M.K.; Yurteri, C.U.; Sims, R.A.; Snodgrass, J.; De, S. To Gloss and Texture Control of Powder Coated Films. Part. Sci. Technol. 2011, 19, 199–217. [Google Scholar] [CrossRef]
- Kunaver, M.; Klanjšek Gunde, M.; Mozetič, M.; Hrovat, A. The Degree of Dispersion of Pigments in Powder Coatings. Dye. Pigment. 2003, 57, 235–243. [Google Scholar] [CrossRef]
- Seyedmehdi, S.A.; Zhang, H.; Zhu, J. Effect of Nanoclay on Electrical and Mechanical Properties of Polyurethane Conductive Coatings Filled with Nickel-Coated Carbon Fibers. Polym. Eng. Sci. 2013, 54, 1120–1125. [Google Scholar] [CrossRef]
- Askari, F.; Ghasemi, E.; Ramezanzadeh, B.; Mahdavian, M. Mechanistic Approach for Evaluation of the Corrosion Inhibition of Potassium Zinc Phosphate Pigment on the Steel Surface: Application of Surface Analysis and Electrochemical Techniques. Dye. Pigment. 2014, 109, 189–199. [Google Scholar] [CrossRef]
- Mahdavian, M.A.; Attar, M.M. Investigation on Zinc Phosphate Effectiveness at Different Pigment Volume Concentrations via Electrochemical Impedance Spectroscopy. Electrochim. Acta 2005, 50, 4645–4648. [Google Scholar] [CrossRef]
- Bouvet, G.; Nguyen, D.D.; Mallarino, S.; Touzain, S. Analysis of the Non-Ideal Capacitive Behaviour for High Impedance Organic Coatings. Prog. Org. Coat. 2014, 77, 2045–2053. [Google Scholar] [CrossRef]
- Negele, O.; Funke, W. Internal Stress and Wet Adhesion of Organic Coatings. Prog. Org. Coat. 1996, 28, 285–289. [Google Scholar] [CrossRef]
- Lai, Y.M.; Liang, X.F.; Yang, S.Y.; Wang, J.X.; Cao, L.H.; Dai, B. Raman and FTIR Spectra of Iron Phosphate Glasses Containing Cerium. J. Mol. Struct. 2011, 992, 84–88. [Google Scholar] [CrossRef]
- Misawa, T.; Hashimoto, K.; Shimodaira, S. The Mechanism of Formation of Iron Oxide and Oxyhydroxides in Aqueous Solutions at Room Temperature. Corros. Sci. 1974, 14, 131–149. [Google Scholar] [CrossRef]
- Stoch, P.; Stoch, A.; Ciecinska, M.; Krakowiak, I.; Sitarz, M. Structure of Phosphate and Iron-Phosphate Glasses by DFT Calculations and FTIR/Raman Spectroscopy. J. Non-Cryst. Solids 2016, 450, 48–60. [Google Scholar] [CrossRef]

| Additive | Particle Size (D10)/(µm) | Particle Size (D50)/(µm) | Particle Size (D90)/(µm) | Oil Absorption/(g oil/100 g) | pH, 10% in D. I. Water | Solubility in Water /(wt.%) | Density/(g/cm3) |
|---|---|---|---|---|---|---|---|
| HALOX® CZ-170 | 0.57 | 2.48 | 6.56 | 43.5 | 10.0 | 0.02 | 3.6 |
| Coating Binder | Component | Composition | Content/(wt. %) |
|---|---|---|---|
| Polyester/TGIC (PC) | resin | carboxylated polyester | 90.78 |
| curing agent | TGIC | 6.75 | |
| flow and leveling agent | polyacrylate with silica | 1.65 | |
| degassing agent | benzoin | 0.82 | |
| pigment | N/A | 0.00 | |
| Polyester/TGIC (PB) | resin | carboxylated polyester | 90.04 |
| curing agent | TGIC | 6.70 | |
| flow and leveling agent | polyacrylate with silica | 1.64 | |
| degassing agent | benzoin | 0.82 | |
| pigment | high color carbon black for automotive coatings | 0.80 | |
| Epoxy/DICY (EB) | resin | epoxy, epoxide equivalent weight (EEW) = 730~820 g/eq | 89.00 |
| curing agent | DICY | 7.73 | |
| flow and leveling agent | polyacrylate with silica | 1.65 | |
| degassing agent | benzoin | 0.82 | |
| pigment | high color carbon black for automotive coatings | 0.80 |
| Coating Systems | Formula Code | ZP/(wt. %) | Filler BaSO4/(wt. %) | Binder/(wt. %) |
|---|---|---|---|---|
| Polyester/TGIC clearcoat (PC) | Control-PC | 0.0 | 0.0 | 100.0 |
| ZP–02%-PC | 2.0 | 0.0 | 98.0 | |
| ZP–04%-PC | 4.0 | 0.0 | 96.0 | |
| ZP–08%-PC | 8.0 | 0.0 | 92.0 | |
| ZP–16%-PC | 16.0 | 0.0 | 84.0 | |
| Polyester/TGIC with filler BaSO4 (PB) | Control-PB | 0.0 | 15.0 | 85.0 |
| ZP–02%-PB | 2.0 | 14.7 | 83.3 | |
| ZP–04%-PB | 4.0 | 14.4 | 81.6 | |
| ZP–08%-PB | 8.0 | 13.8 | 78.2 | |
| ZP–16%-PB | 16.0 | 12.6 | 71.4 | |
| Epoxy/DICY with filler BaSO4 (EB) | Control-EB | 0.0 | 15.0 | 85.0 |
| ZP–02%-EB | 2.0 | 14.7 | 83.3 | |
| ZP–04%-EB | 4.0 | 14.4 | 81.6 | |
| ZP–08%-EB | 8.0 | 13.8 | 78.2 | |
| ZP–16%-EB | 16.0 | 12.6 | 71.4 |
| Time | CPEcoat | Rpore | CPEpass | Rpass | CPEdl | Rct | χ2 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Days | Qcoat/ Ω−1∙cm−2∙sα | αcoat | Ω∙cm2 | Qpass/ Ω−1∙cm−2∙sα | αpass | Ω∙cm2 | Qdl/ Ω−1∙cm−2∙sα | αdl | Ω∙cm2 | - |
| 0 | 5.47 × 10−10 | 0.844 | 1.29 × 105 | 7.45 × 10−9 | 0.618 | 5.11 × 107 | 3.26 × 10−4 | |||
| 1 | 1.62 × 10−8 | 0.626 | 1.49 × 104 | 2.34 × 10−6 | 0.371 | 2.20 × 107 | 7.66 × 10−6 | 0.935 | 8.46 × 106 | 7.40 × 10−5 |
| 3 | 2.97 × 10−8 | 0.592 | 1.55 × 104 | 2.16 × 10−6 | 0.403 | 2.69 × 105 | 3.99 × 10−7 | 0.853 | 7.93 × 106 | 9.89 × 10−5 |
| 5 | 3.81 × 10−8 | 0.570 | 1.12 × 104 | 3.21 × 10−6 | 0.379 | 1.46 × 105 | 7.05 × 10−7 | 0.840 | 4.32 × 106 | 7.85 × 10−5 |
| Time | CPEcoat | Rpore | CPEpass | Rpass | CPEdl | Rct | χ2 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Days | Qcoat/ Ω−1∙cm−2∙sα | αcoat | Ω∙cm2 | Qpass/ Ω−1∙cm−2∙sα | αpass | Ω∙cm2 | Qdl/ Ω−1∙cm−2∙sα | αdl | Ω∙cm2 | - |
| 0 | 1.12 × 10−10 | 0.962 | 1.61 × 106 | - | - | - | 3.89 × 10−10 | 0.671 | 9.85 × 107 | 8.30 × 10−5 |
| 1 | 2.66 × 10−10 | 0.903 | 6.12 × 104 | 2.06 × 10−7 | 0.491 | 1.12 × 105 | 3.24 × 10−8 | 0.718 | 3.22 × 107 | 2.63 × 10−4 |
| 3 | 2.71 × 10−10 | 0.900 | 1.03 × 105 | 1.81 × 10−7 | 0.514 | 1.17 × 107 | 1.43 × 10−6 | 0.949 | 4.04 × 106 | 2.76 × 10−4 |
| 5 | 1.97 × 10−10 | 0.924 | 1.12 × 105 | 2.73 × 10−7 | 0.457 | 4.22 × 106 | 9.07 × 10−7 | 0.770 | 8.44 × 106 | 5.62 × 10−4 |
| Time | CPEcoat | Rpore | CPEpass | Rpass | CPEdl | Rct | χ2 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Days | Qcoat/ Ω−1∙cm−2∙sα | αcoat | Ω∙cm2 | Qpass/ Ω−1∙cm−2∙sα | αpass | Ω∙cm2 | Qdl/ Ω−1∙cm−2∙sα | αdl | Ω∙cm2 | - |
| 0 | 2.34 × 10−9 | 0.772 | 4.44 × 107 | - | - | - | 1.42 × 10−7 | 1.000 | 6.84 × 106 | 1.19 × 10−3 |
| 1 | 1.30 × 10−8 | 0.634 | - | 3.51 × 10−6 | 0.290 | - | 6.17 × 10−8 | 0.958 | 4.18 × 106 | 1.56 × 10−4 |
| 3 | 6.87 × 10−10 | 0.658 | - | 2.98 × 10−6 | 0.346 | 1.53 × 105 | 3.09 × 10−7 | 0.905 | 3.54 × 106 | 3.20 × 10−4 |
| 5 | 1.19 × 10−9 | 0.650 | - | 4.59 × 10−6 | 0.352 | 1.55 × 105 | 4.42 × 10−7 | 0.894 | 3.72 × 106 | 4.33 × 10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Huang, J.; Chen, J.; Noël, J.J.; Barker, I.; Henderson, J.D.; He, P.; Zhang, H.; Zhang, H.; Zhu, J. A Comparative Study on the Anti-Corrosive Performance of Zinc Phosphate in Powder Coatings. Coatings 2022, 12, 217. https://doi.org/10.3390/coatings12020217
Yang S, Huang J, Chen J, Noël JJ, Barker I, Henderson JD, He P, Zhang H, Zhang H, Zhu J. A Comparative Study on the Anti-Corrosive Performance of Zinc Phosphate in Powder Coatings. Coatings. 2022; 12(2):217. https://doi.org/10.3390/coatings12020217
Chicago/Turabian StyleYang, Shuai (Marshall), Jinbao Huang, Jian Chen, James Joseph Noël, Ivan Barker, Jeffrey Daniel Henderson, Ping He, Haiping Zhang, Hui Zhang, and Jesse Zhu. 2022. "A Comparative Study on the Anti-Corrosive Performance of Zinc Phosphate in Powder Coatings" Coatings 12, no. 2: 217. https://doi.org/10.3390/coatings12020217
APA StyleYang, S., Huang, J., Chen, J., Noël, J. J., Barker, I., Henderson, J. D., He, P., Zhang, H., Zhang, H., & Zhu, J. (2022). A Comparative Study on the Anti-Corrosive Performance of Zinc Phosphate in Powder Coatings. Coatings, 12(2), 217. https://doi.org/10.3390/coatings12020217












