Electrodeposition of Copper Oxides as Cost-Effective Heterojunction Photoelectrode Materials for Solar Water Splitting
Abstract
:1. Introduction
2. Experimental Details
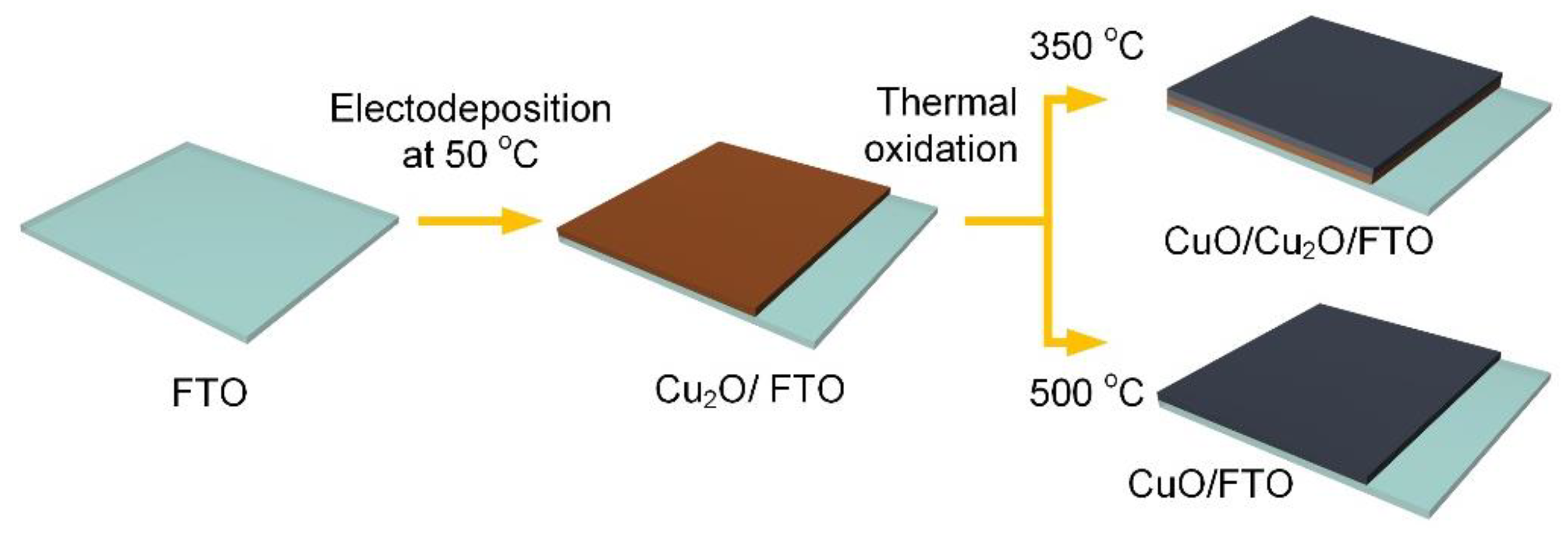
2.1. Fabrication of Copper Oxide Thin Films
2.2. Materials Characterization
3. Results and Discussion
3.1. Morphology and Optical Properties of Copper Oxide Thin Films
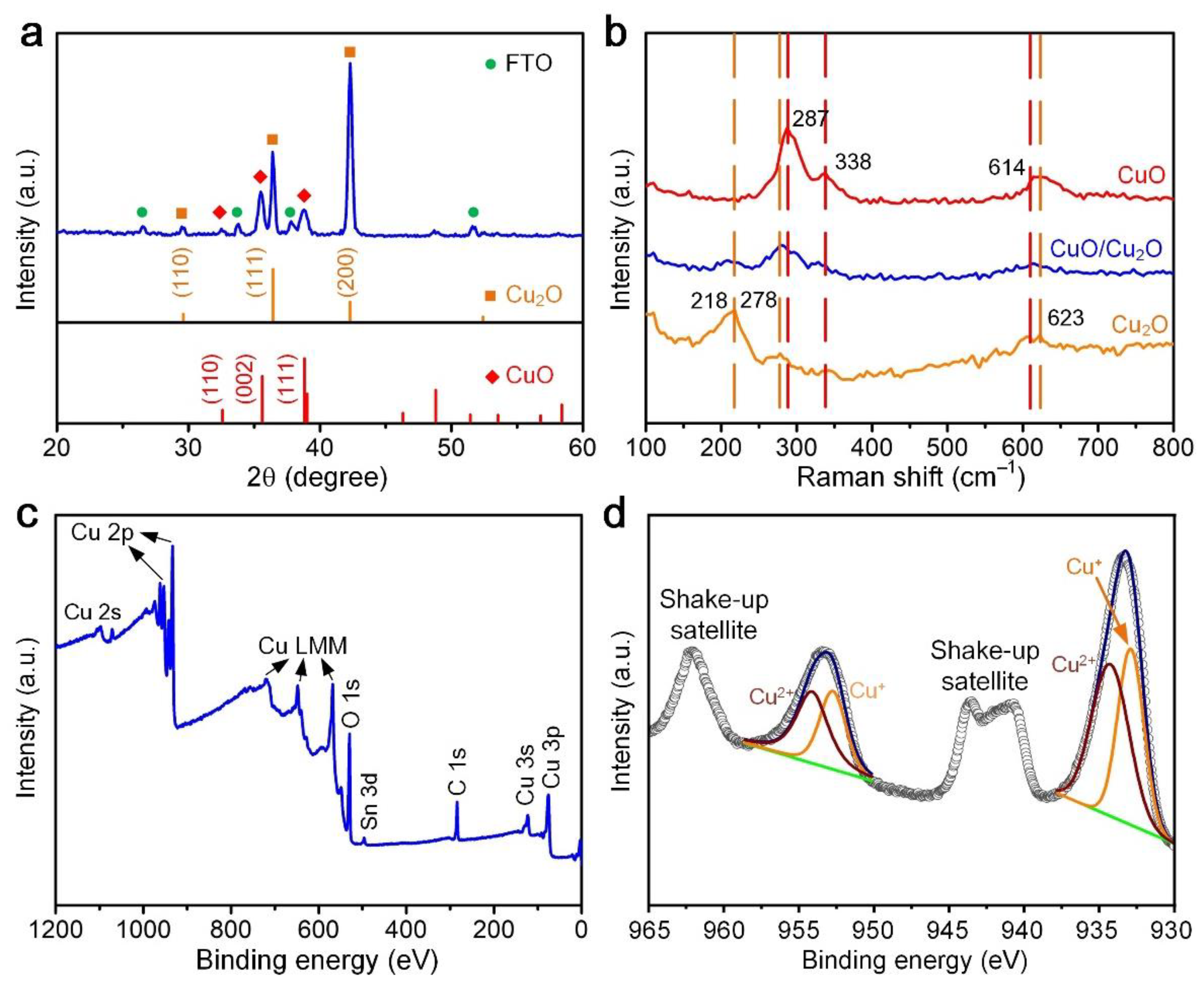
3.2. Characterization of Copper Oxide Thin Films Using XRD, Raman Spectroscopy, and XPS
3.3. Electrochemical Measurements of Copper Oxide Thin Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Ruijven, B.J.; De Cian, E.; Wing, I.S. Amplification of future energy demand growth due to climate change. Nat. Commun. 2019, 10, 2762. [Google Scholar] [CrossRef] [Green Version]
- Abdin, Z.; Alim, M.A.; Saidur, R.; Islam, M.R.; Rashmi, W.; Mekhilef, S.; Wadi, A. Solar energy harvesting with the application of nanotechnology. Renew. Sust. Energ. Rev. 2013, 26, 837–852. [Google Scholar] [CrossRef]
- Guo, C.X.; Guai, G.H.; Li, C.M. Graphene based materials: Enhancing solar energy harvesting. Adv. Energy Mater. 2011, 1, 448–452. [Google Scholar] [CrossRef]
- Chang, S.-Y.; Cheng, P.; Li, G.; Yang, Y. Transparent polymer photovoltaics for solar energy harvesting and beyond. Joule 2018, 2, 1039–1054. [Google Scholar] [CrossRef] [Green Version]
- Minggu, L.J.; Daud, W.R.W.; Kassim, M.B. An overview of photocells and photoreactors for photoelectrochemical water splitting. Int. J. Hydrog. Energy 2010, 35, 5233–5244. [Google Scholar] [CrossRef]
- Ahmad, H.; Kamarudin, S.K.; Minggu, L.J.; Kassim, M. Hydrogen from photo-catalytic water splitting process: A review. Renew. Sust. Energ. Rev. 2015, 43, 599–610. [Google Scholar] [CrossRef]
- Lin, H.; Huang, C.P.; Li, W.; Ni, C.; Shah, S.I.; Tseng, Y.-H. Size dependency of nanocrystalline TiO2 on its optical property and photocatalytic reactivity exemplified by 2-chlorophenol. Appl. Catal. B 2006, 68, 1–11. [Google Scholar] [CrossRef]
- Yamada, Y.; Kanemitsu, Y. Determination of electron and hole lifetimes of rutile and anatase TiO2 single crystals. Appl. Phys. Lett. 2012, 101, 133907. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Ju, Y.; Toku, Y.; Morita, Y. Synthesis of a single-crystal Fe2O3 nanowire array based on stress-induced atomic diffusion used for solar water splitting. R. Soc. Open Sci. 2018, 5, 172126. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Jang, J.-W.; Lee, J.S. Key strategies to advance the photoelectrochemical water splitting performance of α-Fe2O3 photoanode. ChemCatChem 2019, 11, 157–179. [Google Scholar] [CrossRef]
- Sharp, I.D.; Cooper, J.K.; Toma, F.M.; Buonsanti, R. Bismuth vanadate as a platform for accelerating discovery and development of complex transition-metal oxide photoanodes. ACS Energy Lett. 2017, 2, 139–150. [Google Scholar] [CrossRef]
- Butler, K.T.; Dringoli, B.J.; Zhou, L.; Rao, P.M.; Walsh, A.; Titova, L.V. Ultrafast carrier dynamics in BiVO4 thin film photoanode material: Interplay between free carriers, trapped carriers and low-frequency lattice vibrations. J. Mater. Chem. 2016, 4, 18516–18523. [Google Scholar] [CrossRef] [Green Version]
- Bocarsly, A.B.; Bookbinder, D.C.; Dominey, R.N.; Lewis, N.S.; Wrighton, M.S. Photoreduction at illuminated p-type semiconducting silicon photoelectrodes. Evidence for Fermi level pinning. J. Am. Chem. Soc. 1980, 102, 3683–3688. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, T.; Gong, J. Single-crystal silicon-based electrodes for unbiased solar water splitting: Current status and prospects. Chem. Soc. Rev. 2019, 48, 2158–2181. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Hu, Z.; Jimmy, C.Y.; Wang, J. Room temperature synthesis of a highly active Cu/Cu2O photocathode for photoelectrochemical water splitting. J. Mater. Chem. A 2016, 4, 13736–13741. [Google Scholar] [CrossRef]
- Kim, D.S.; Kim, Y.B.; Choi, J.H.; Suh, H.W.; Lee, H.H.; Lee, K.W.; Jung, S.H.; Kim, J.J.; Deshpande, N.G.; Cho, H.K. Toward simultaneous achievement of outstanding durability and photoelectrochemical reaction in Cu2O photocathodes via electrochemically designed resistive switching. Adv. Energy Mater. 2021, 11, 2101905. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, L.; Schülli, T.U.; Bai, Y.; Monny, S.A.; Du, A.; Wang, L. Identifying copper vacancies and their role in the CuO based photocathode for water splitting. Angew. Chem. 2019, 131, 17768–17773. [Google Scholar] [CrossRef]
- Li, J.; Jin, X.; Li, R.; Zhao, Y.; Wang, X.; Liu, X.; Jiao, H. Copper oxide nanowires for efficient photoelectrochemical water splitting. Appl. Catal. B 2019, 240, 1–8. [Google Scholar] [CrossRef]
- Paracchino, A.; Laporte, V.; Sivula, K.; Grätzel, M.; Thimsen, E. Highly active oxide photocathode for photoelectrochemical water reduction. Nat. Mater. 2011, 10, 456–461. [Google Scholar] [CrossRef]
- Paracchino, A.; Brauer, J.C.; Moser, J.-E.; Thimsen, E.; Graetzel, M. Synthesis and characterization of high-photoactivity electrodeposited Cu2O solar absorber by photoelectrochemistry and ultrafast spectroscopy. J. Phys. Chem. C 2012, 116, 7341–7350. [Google Scholar] [CrossRef]
- Zhang, Z.; Dua, R.; Zhang, L.; Zhu, H.; Zhang, H.; Wang, P. Carbon-layer-protected cuprous oxide nanowire arrays for efficient water reduction. ACS Nano 2013, 7, 1709–1717. [Google Scholar] [CrossRef]
- Guo, X.; Diao, P.; Xu, D.; Huang, S.; Yang, Y.; Jin, T.; Wu, Q.; Xiang, M.; Zhang, M. CuO/Pd composite photocathodes for photoelectrochemical hydrogen evolution reaction. Int. J. Hydrog. Energy. 2014, 39, 7686–7696. [Google Scholar] [CrossRef]
- Chiang, C.-Y.; Chang, M.-H.; Liu, H.-S.; Tai, C.Y.; Ehrman, S. Process intensification in the production of photocatalysts for solar hydrogen generation. Ind. Eng. Chem. Res. 2012, 51, 5207–5215. [Google Scholar] [CrossRef]
- Wijesundera, R.P. Fabrication of the CuO/Cu2O heterojunction using an electrodeposition technique for solar cell applications. Semicond. Sci. Technol. 2010, 25, 045015. [Google Scholar] [CrossRef]
- Matsumura, H.; Fujii, A.F.A.; Kitatani, T.K.T. Properties of high-mobility Cu2O films prepared by thermal oxidation of Cu at low temperatures. Jpn. J. Appl. Phys. 1996, 35, 5631. [Google Scholar] [CrossRef]
- Xing, H.; E, L.; Guo, Z.; Zhao, D.; Liu, Z. Enhancement in the charge transport and photocorrosion stability of CuO photocathode: The synergistic effect of spatially separated dual-cocatalysts and p-n heterojunction. Chem. Eng. J. 2020, 394, 124907. [Google Scholar] [CrossRef]
- Jian, J.; Kumar, R.; Sun, J. Cu2O/ZnO p–n junction decorated with NiOx as a protective layer and cocatalyst for enhanced photoelectrochemical water splitting. ACS Appl. Energy Mater. 2020, 3, 10408–10414. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, D.; Wu, Q.; Diao, P. Cu2O/CuO bilayered composite as a high-efficiency photocathode for photoelectrochemical hydrogen evolution reaction. Sci. Rep. 2016, 6, 35158. [Google Scholar] [CrossRef] [Green Version]
- Bae, H.; Burungale, V.; Na, W.; Rho, H.; Kang, S.H.; Ryu, S.-W.; Ha, J.-S. Nanostructured CuO with a thin g-C3N4 layer as a highly efficient photocathode for solar water splitting. RSC Adv. 2021, 11, 16083–16089. [Google Scholar] [CrossRef]
- Mary, A.S.; Murugan, C.; Pandikumar, A. Uplifting the charge carrier separation and migration in Co-doped CuBi2O4/TiO2 pn heterojunction photocathode for enhanced photoelectrocatalytic water splitting. J. Colloid Interface Sci. 2022, 608, 2482–2492. [Google Scholar] [CrossRef]
- Baek, S.K.; Kim, J.S.; Yun, Y.D.; Kim, Y.B.; Cho, H.K. Cuprous/cupric heterojunction photocathodes with optimal phase transition interface via preferred orientation and precise oxidation. ACS Sustain. Chem. Eng. 2018, 6, 10364–10373. [Google Scholar] [CrossRef]
- Ma, M.; Lei, E.; Zhao, D.; Xin, Y.; Wu, X.; Meng, Y.; Liu, Z. The p-n heterojunction of BiVO4/Cu2O was decorated by plasma Ag NPs for efficient photoelectrochemical degradation of Rhodamine B. Colloids Surf. A Physicochem. Eng. Asp. 2022, 633, 127834. [Google Scholar] [CrossRef]
- Hossain, R.; Nekouei, R.K.; Al Mahmood, A.; Sahajwalla, V. Value-added fabrication of NiO-doped CuO nanoflakes from waste flexible printed circuit board for advanced photocatalytic application. Sci. Rep. 2022, 12, 12171. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Dong, P.-H.; Hsu, Y.-K. Defective indium tin oxide forms an ohmic back contact to an n-type Cu2O photoanode to accelerate charge-transfer kinetics for enhanced low-bias photoelectrochemical water splitting. ACS Appl. Mater. Interfaces 2021, 13, 38375–38383. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Song, Y.; Cui, A.; Mu, X.; Li, L.; Han, L.; Shan, G.; Liu, H. Adenine-functionalized graphene oxide as a charge transfer layer to enhance activity and stability of Cu2O photocathode for CO2 reduction reaction. Appl. Surf. Sci. 2022, 591, 153197. [Google Scholar] [CrossRef]
- Chiang, C.-Y.; Aroh, K.; Franson, N.; Satsangi, V.R.; Dass, S.; Ehrman, S. Copper oxide nanoparticle made by flame spray pyrolysis for photoelectrochemical water splitting—Part II. Photoelectrochemical study. Int. J. Hydrog. Energy 2011, 36, 15519–15526. [Google Scholar] [CrossRef]
- Kalanur, S.S.; Lee, Y.J.; Seo, H. Enhanced and stable photoelectrochemical H2 production using a engineered nano multijunction with Cu2O photocathode. Mater. Today Chem. 2022, 26, 101031. [Google Scholar] [CrossRef]
- Du, F.; Chen, Q.-Y.; Wang, Y.-H. Effect of annealing process on the heterostructure CuO/Cu2O as a highly efficient photocathode for photoelectrochemical water reduction. J. Phys. Chem. Solids 2017, 104, 139–144. [Google Scholar] [CrossRef]
- Jamali, S.; Moshaii, A.; Mohammadian, N. Improvement of photoelectrochemical and stability properties of electrodeposited Cu2O thin films by annealing processes. Phys. Status Solidi. A 2017, 214, 1700380. [Google Scholar] [CrossRef]
- Chang, C.-J.; Lai, C.-W.; Jiang, W.-C.; Li, Y.-S.; Choi, C.; Yu, H.-C.; Chen, S.-J.; Choi, Y. Fabrication and characterization of P-type semiconducting copper oxide-based thin-film photoelectrodes for solar water splitting. Coatings 2022, 12, 1206. [Google Scholar] [CrossRef]
- Kyesmen, P.I.; Nombona, N.; Diale, M. Heterojunction of nanostructured α-Fe2O3/CuO for enhancement of photoelectrochemical water splitting. J. Alloys Compd. 2021, 863, 158724. [Google Scholar]
- Ma, J.; Wang, Q.; Li, L.; Zong, X.; Sun, H.; Tao, R.; Fan, X. Fe2O3 nanorods/CuO nanoparticles pn heterojunction photoanode: Effective charge separation and enhanced photoelectrochemical properties. J. Colloid Interface Sci. 2021, 602, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Meng, F.; Zhang, L.; Zhang, D.; Wei, S.; Qi, K.; Fan, J.; Zhang, H.; Cui, X. CuO/ZnO heterojunction nanoarrays for enhanced photoelectrochemical water oxidation. Appl. Surf. Sci. 2019, 469, 276–282. [Google Scholar] [CrossRef]
- Jabeen, S.; Sherazi, T.A.; Ullah, R.; Naqvi, S.A.R.; Rasheed, M.A.; Ali, G.; Shah, A.U.; Khan, Y. Electrodeposition-assisted formation of anodized TiO2–CuO heterojunctions for solar water splitting. Appl. Nanosci. 2021, 11, 79–90. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, H.; Liu, Z. Highly efficient photocatalyst based on all oxides WO3/Cu2O heterojunction for photoelectrochemical water splitting. Appl. Catal. B 2017, 201, 84–91. [Google Scholar] [CrossRef]
- Alizadeh, M.; Tong, G.B.; Qadir, K.W.; Mehmood, M.S.; Rasuli, R. Cu2O/InGaN heterojunction thin films with enhanced photoelectrochemical activity for solar water splitting. Renew. Energy 2020, 156, 602–609. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, J.; Yang, S.; Xu, Y.; Cai, X.; Li, X.; Zhang, X.; Peng, F.; Fang, Y. Electrodeposition of Cu2O/g-C3N4 heterojunction film on an FTO substrate for enhancing visible light photoelectrochemical water splitting. Chin. J. Catal. 2017, 38, 365–371. [Google Scholar] [CrossRef]
- De Jongh, P.E.; Vanmaekelbergh, D.; Kelly, J.J. Cu2O: Electrodeposition and characterization. Chem. Mater. 1999, 11, 3512–3517. [Google Scholar] [CrossRef]
- Jiao, X.; Chen, Z.; Li, X.; Sun, Y.; Gao, S.; Yan, W.; Wang, C.; Zhang, Q.; Lin, Y.; Luo, Y.; et al. Defect-mediated electron–hole separation in one-unit-cell ZnIn2S4 layers for boosted solar-driven CO2 reduction. J. Am. Chem. Soc. 2017, 139, 7586–7594. [Google Scholar] [CrossRef]
- Di, J.; Xia, J.; Chisholm, M.F.; Zhong, J.; Chen, C.; Cao, X.; Dong, F.; Chi, Z.; Chen, H.; Weng, Y.-X.; et al. Defect-tailoring mediated electron–hole separation in single-unit-cell Bi3O4Br nanosheets for boosting photocatalytic hydrogen evolution and nitrogen fixation. Adv. Mater. 2019, 31, 1807576. [Google Scholar] [CrossRef]
- Wang, L.; Lee, C.-Y.; Schmuki, P. Solar water splitting: Preserving the beneficial small feature size in porous α-Fe2O3 photoelectrodes during annealing. J. Mater. Chem. A 2013, 1, 212–215. [Google Scholar] [CrossRef]
- Jayathilaka, C.; Kapaklis, V.; Siripala, W.; Jayanetti, S. Improved efficiency of electrodeposited p-CuO/n-Cu2O heterojunction solar cell. Appl. Phys. Express. 2015, 8, 065503. [Google Scholar] [CrossRef]
- De Los Santos Valladares, L.; Salinas, D.H.; Dominguez, A.B.; Najarro, D.A.; Khondaker, S.I.; Mitrelias, T.; Barnes, C.H.W.; Aguiar, J.A.; Majima, Y. Crystallization and electrical resistivity of Cu2O and CuO obtained by thermal oxidation of Cu thin films on SiO2/Si substrates. Thin Solid Film 2012, 520, 6368–6374. [Google Scholar] [CrossRef]
- Eskandari, A.; Sangpour, P.; Vaezi, M.R. Hydrophilic Cu2O nanostructured thin films prepared by facile spin coating method: Investigation of surface energy and roughness. Mater. Chem. Phys. 2014, 147, 1204–1209. [Google Scholar] [CrossRef]
- Masudy-Panah, S.; Moakhar, R.S.; Chua, C.S.; Kushwaha, A.; Wong, T.I.; Dalapati, G.K. Rapid thermal annealing assisted stability and efficiency enhancement in a sputter deposited CuO photocathode. RSC Adv. 2016, 6, 29383–29390. [Google Scholar] [CrossRef] [Green Version]
- Raship, N.A.; Sahdan, M.Z.; Adriyanto, F.; Nurfazliana, M.F.; Bakri, A.S. Effect of annealing temperature on the properties of copper oxide films prepared by dip coating technique. AIP Conf. Proc. 2017, 1788, 030121. [Google Scholar]
- Makuła, P.; Pacia, M.; Macyk, W. How to correctly determine the band gap energy of modified semiconductor photocatalysts based on UV—Vis spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [Green Version]
- Ju, S.; Kang, H.; Jun, J.; Son, S.; Park, J.; Kim, W.; Lee, H. Periodic micropillar-patterned FTO/BiVO4 with superior light absorption and separation efficiency for efficient PEC performance. Small 2021, 17, 2006558. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yu, Y.; Shen, X.; Hu, X. A Cu2O/Cu/carbon cloth as a binder-free electrode for non-enzymatic glucose sensors with high performance. New J. Chem. 2020, 44, 1993–2000. [Google Scholar] [CrossRef]
- Wang, Y.; Steigert, A.; Yin, G.; Parvan, V.; Klenk, R.; Schlatmann, R.; Lauermann, I. Cu2O as a potential intermediate transparent conducting oxide layer for monolithic perovskite-CIGSe tandem solar cells. Phys. Status Solidi C 2017, 14, 1700164. [Google Scholar]
- Fentahun, D.A.; Tyagi, A.; Singh, S.; Sinha, P.; Mishra, A.; Danayak, S.; Kumar, R.; Kar, K.K. Tunable optical and electrical properties of p-type Cu2O thin films. J. Mater. Sci. Mater. 2021, 32, 11158–11172. [Google Scholar] [CrossRef]
- Kshirsagar, J.M.; Shrivastava, R.; Adwani, P.S. Preparation and characterization of copper oxide nanoparticles and determination of enhancement in critical heat flux. Therm. Sci. 2017, 21, 233–242. [Google Scholar] [CrossRef] [Green Version]
- Jeong, D.; Jo, W.; Jeong, J.; Kim, T.; Han, S.; Son, M.-K.; Jung, H. Characterization of Cu2O/CuO heterostructure photocathode by tailoring CuO thickness for photoelectrochemical water splitting. RSC Adv. 2022, 12, 2632–2640. [Google Scholar] [CrossRef]
- Attou, L.; Jaber, B.; Ez-Zahraouy, H. Effect of annealing temperature on structural, optical and photocatalytic properties of CuO nanoparticles. Mediterr. J. Chem. 2018, 7, 308–316. [Google Scholar] [CrossRef]
- Al-Jawhari, H.A. A review of recent advances in transparent p-type Cu2O-based thin film transistors. Mater. Sci. Semicond. Process. 2015, 40, 241–252. [Google Scholar] [CrossRef]
- Balık, M.; Bulut, V.; Erdogan, I.Y. Optical, structural and phase transition properties of Cu2O, CuO and Cu2O/CuO: Their photoelectrochemical sensor applications. Int. J. Hydrog. Energy 2019, 44, 18744–18755. [Google Scholar] [CrossRef]
- Mao, Y.; He, J.; Sun, X.; Li, W.; Lu, X.; Gan, J.; Liu, Z.; Gong, L.; Chen, J.; Liu, P.; et al. Electrochemical synthesis of hierarchical Cu2O stars with enhanced photoelectrochemical properties. Electrochim. Acta 2012, 62, 1–7. [Google Scholar] [CrossRef]
- Patil, P.; Nakate, U.T.; Nakate, Y.T.; Ambare, R.C. Acetaldehyde sensing properties using ultrafine CuO nanoparticles. Mediterr. J. Chem. 2019, 101, 76–81. [Google Scholar] [CrossRef]
- Xu, L.; Li, J.; Sun, H.; Guo, X.; Xu, J.; Zhang, H.; Zhang, X. In situ growth of Cu2O/CuO nanosheets on Cu coating carbon cloths as a binder-free electrode for asymmetric supercapacitors. Front. Chem. 2019, 7, 420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansen, R.J.J.; van Bekkum, H. XPS of nitrogen-containing functional groups on activated carbon. Carbon 1995, 33, 1021–1027. [Google Scholar] [CrossRef]
- Wang, P.; Liu, Z.; Han, C.; Ma, X.; Tong, Z.; Tan, B. Cu2O/CuO heterojunction formed by thermal oxidation and decorated with Pt co-catalyst as an efficient photocathode for photoelectrochemical water splitting. J. Nanoparticle Res. 2021, 23, 268. [Google Scholar] [CrossRef]
- Liu, S.; Hou, H.; Liu, X.; Duan, J.; Yao, Y.; Liao, Q. High-performance hierarchical cypress-like CuO/Cu2O/Cu anode for lithium ion battery. Ionics 2017, 23, 1075–1082. [Google Scholar] [CrossRef]
- Tariq, M.; Afzal, N.; Rafique, M. Enhanced photodetection performance of sputtered cupric oxide thin film through annealing process. Opt. Quantum Electron. 2021, 53, 685. [Google Scholar] [CrossRef]
- Wang, P.; Ng, Y.H.; Amal, R. Embedment of anodized p-type Cu2O thin films with CuO nanowires for improvement in photoelectrochemical stability. Nanoscale 2013, 5, 2952–2958. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, A.F.; Sun, Q.; Wang, T.; Zeng, H. Cu nanoparticles/fluorine-doped tin oxide (FTO) nanocomposites for photocatalytic H2 evolution under visible light irradiation. Catalysts 2017, 7, 385. [Google Scholar] [CrossRef] [Green Version]
- Gelderman, K.; Lee, L.; Donne, S.W. Flat-band potential of a semiconductor: Using the Mott–Schottky equation. J. Chem. Educ. 2007, 84, 685. [Google Scholar] [CrossRef]
- Bengas, R.; Lahmar, H.; Redha, K.M.; Mentar, L.; Azizi, A.; Schmerber, G.; Dinia, A. Electrochemical synthesis of n-type ZnS layers on p-Cu2O/n-ZnO heterojunctions with different deposition temperatures. RSC Adv. 2019, 9, 29056–29069. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Zhang, Z.; Yong, K. Synergistic doping effects of a ZnO: N/BiVO4: Mo bunched nanorod array photoanode for enhancing charge transfer and carrier density in photoelectrochemical systems. Nanoscale 2018, 10, 20256–20265. [Google Scholar] [CrossRef] [PubMed]
- Radecka, M.; Rekas, M.; Trenczek-Zajac, A.; Zakrzewska, K. Importance of the band gap energy and flat band potential for application of modified TiO2 photoanodes in water photolysis. J. Power Sources 2008, 181, 46–55. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, Y.; Wang, P. Hierarchical top-porous/bottom-tubular TiO2 nanostructures decorated with Pd nanoparticles for efficient photoelectrocatalytic decomposition of synergistic pollutants. ACS Appl. Mater. Interfaces 2012, 4, 990–996. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, P. Highly stable copper oxide composite as an effective photocathode for water splitting via a facile electrochemical synthesis strategy. J. Mater. Chem. A 2012, 22, 2456–2464. [Google Scholar] [CrossRef]
- Sivula, K.; Le Formal, F.; Grätzel, M. Solar water splitting: Progress using hematite (α-Fe2O3) photoelectrodes. ChemSusChem 2011, 4, 432–449. [Google Scholar] [CrossRef] [PubMed]
- Annamalai, A.; Subramanian, A.; Kang, U.; Park, H.; Choi, S.H.; Jang, J.S. Activation of hematite photoanodes for solar water splitting: Effect of FTO deformation. J. Phys. Chem. C 2015, 119, 3810–3817. [Google Scholar] [CrossRef]


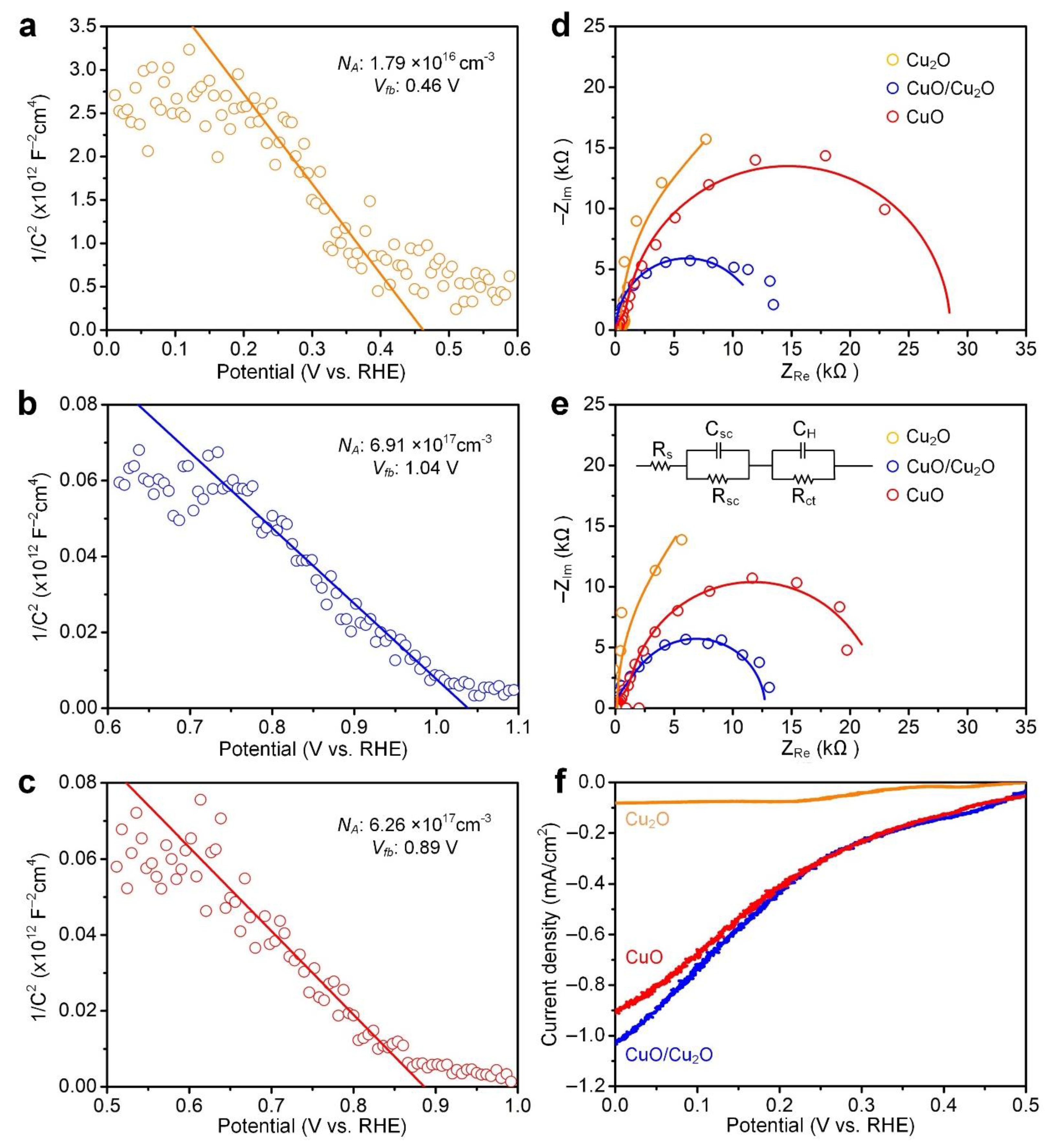
| Thin Film | NA (cm−3) | Vfb (V) | Photocurrent Density (mA/cm2) |
|---|---|---|---|
| Cu2O | 1.79 × 1016 | 0.46 | 0.08 |
| CuO/Cu2O | 6.91 × 1017 | 1.04 | 1.03 |
| CuO | 6.26 × 1017 | 0.89 | 0.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, T.-H.; Liu, B.-J.; Lin, Y.-W.; Li, Y.-S.; Lai, C.-W.; Lan, Y.-P.; Choi, C.; Chang, H.-C.; Choi, Y. Electrodeposition of Copper Oxides as Cost-Effective Heterojunction Photoelectrode Materials for Solar Water Splitting. Coatings 2022, 12, 1839. https://doi.org/10.3390/coatings12121839
Yin T-H, Liu B-J, Lin Y-W, Li Y-S, Lai C-W, Lan Y-P, Choi C, Chang H-C, Choi Y. Electrodeposition of Copper Oxides as Cost-Effective Heterojunction Photoelectrode Materials for Solar Water Splitting. Coatings. 2022; 12(12):1839. https://doi.org/10.3390/coatings12121839
Chicago/Turabian StyleYin, Tai-Hsin, Bu-Jine Liu, Yu-Wei Lin, Yi-Syuan Li, Chih-Wei Lai, Yu-Pin Lan, Changsik Choi, Han-Chen Chang, and YongMan Choi. 2022. "Electrodeposition of Copper Oxides as Cost-Effective Heterojunction Photoelectrode Materials for Solar Water Splitting" Coatings 12, no. 12: 1839. https://doi.org/10.3390/coatings12121839
APA StyleYin, T.-H., Liu, B.-J., Lin, Y.-W., Li, Y.-S., Lai, C.-W., Lan, Y.-P., Choi, C., Chang, H.-C., & Choi, Y. (2022). Electrodeposition of Copper Oxides as Cost-Effective Heterojunction Photoelectrode Materials for Solar Water Splitting. Coatings, 12(12), 1839. https://doi.org/10.3390/coatings12121839







