1. Introduction
Oxide-oxide ceramic matrix composites (CMCs) are materials of interest for replacing metals in some high temperature components in gas-turbine engines for aeronautics and land-based power generation [
1]. The implementation of these CMCs is challenging because combustion environment conditions lead to their erosion and corrosion. In particular, environmental barrier coatings (EBCs) are necessary to prevent these CMCs from reacting with steam and volatilizing in gas-turbine engine atmospheres [
2,
3]. A wide variety of viable EBC candidates have been identified for SiC-based composites, which have been the primary focus of CMC development [
4]. However, those EBC candidates are not compatible with alumina-based CMCs because of differences in the coefficient of thermal expansion (CTE) and chemical stability between the CMC materials. YAlO
3, also known as YAP (yttrium aluminate perovskite), has been proposed for this purpose due to its low water vapor recession rate [
5] and excellent CTE match to alumina [
6,
7], which prolongs the number of thermal cycles to failure. Additionally, Gatzen et al. demonstrated the feasibility of depositing YAP on an alumina CMC via very-low pressure plasma spraying with no pre- or post-processing steps [
7]. This manufacturability is desirable for the longevity of the coatings and is in contrast to other coating/composite pairs that must undergo surface alterations or heat treatments for adequate adhesion [
8].
Similar to other EBC systems [
9,
10,
11,
12], Gatzen found that interactions of a YAP coating with molten airborne calcia-magnesia aluminosilicate (CMAS) deposits led to accelerated degradation. Molten CMAS attack can include (1) grain boundary infiltration of the coating, (2) dissolution of the EBC or underlying component, (3) crystalline reaction products formed from the CMAS and coating constituents, and (4) devitrification of CMAS due to temperature fluctuations during application [
13,
14]. It is the combined effect of these that determines the number of thermal cycles to failure and the mode of failure. The CMAS infiltration and CMAS/EBC reactivity commonly have an inverse relationship, because the interaction can induce (1) solidification of the molten glass and (2) nucleation of certain reaction products, like rare-earth (RE) apatites, that can block CMAS infiltration pathways [
15]. EBC research targets crystallization of rare-earth silicate oxyapatites (commonly Ca
2RE
8(SiO
4)
6O
2), which often nucleate quickly to efficiently slow down CMAS infiltration [
9,
16]. Different RE cations have been explored to optimize the volume of the reaction products and their nucleation rate to minimize the CMAS penetration depth [
17,
18,
19]. The thermodynamic phase equilibrium of different RE-based EBC-CMAS reaction products has been studied extensively through model experiments (typically involving pressed pellets comprised of 1:1 EBC:CMAS by weight), finding that indeed the apatite phase emerges as thermodynamically stable [
20]. Additionally, these studies find that the apatite phase stability and compositional range increases with RE cation size. However, there are conflicting reports about which RE-based EBC is most effective at mitigating CMAS when comparing CMAS penetration rates in porous, polycrystalline coatings. These tests show that the kinetics, particularly the nucleation and growth rate of apatite, play an important role in the coatings’ CMAS-mitigation capabilities. Yet, little is known about the apatite nucleation mechanism or how to control it.
The purpose of this work was to gain insight into the nucleation and growth mechanism of apatite to assess the CMAS-mitigation capabilities of YAP EBCs. To achieve this, we applied CMAS monoliths to YAP single crystals to minimize the complexity of the system and isolate the anisotropic effects. Specifically, the interfacial energy of the CMAS/YAP interface was varied by altering the orientation and surface termination of the YAP single crystal to explore how this interface changes the wettability, EBC dissolution rate, and apatite nucleation rate. Anisotropy in the interfacial energy between CMAS and different YAP termination planes was anticipated based on a computational study of a thermal barrier coating, yttria-stabilized zirconia (YSZ), by Li et al. [
21]. YAP has an orthorhombic (Pnma) structure, which is less symmetric than YSZ, making this a reasonable assumption. Additionally, single crystals are ideal for this study because they do not have grain boundaries or pores that complicate measurements for penetration rates. Our study shows that YAP orientation changes the nature of the CMAS attack, including wettability, the morphology of the apatite layer, and, ultimately, the effectiveness of the physical barrier formed. These findings suggest that the apatite morphology—in addition to the amount of apatite—is a key factor for CMAS-mitigation.
2. Materials and Methods
2.1. CMAS Synthesis
A four-component CMAS glass was synthesized by mixing silica, calcium carbonate, alumina and magnesia with ethanol and zirconia milling media in a rolling mill. The mixture was dried, heated in a Pt crucible at 1550 °C for 4 h (CM 1700-BL box furnace, Bloomfield, IL, USA) and then quenched in air. The composition was selected to be rich in calcia to simulate more corrosive conditions [
22,
23]. The target composition has been commonly employed in other studies in the literature [
24,
25], including one by Kumar et al. to compare 4- and 9-component CMAS attack on TBC materials under the same conditions [
26].
Table 1 reports the resulting glass composition as determined by X-ray fluorescence (XRF; measured by Eurofins EAG Materials Science, Sunnyvale, CA, USA). The glass was cut into 10 mg cubes with a high-speed diamond-blade saw.
2.2. CMAS-YAP Reaction Studies
Isothermal CMAS-YAP reaction studies were completed on four different orientations of single crystal YAP substrates: (001), (100), (101), and (110), procured from MTI Corporation. (Note that the exact plane termination may vary slightly due to the cut by the manufacturer.) The single side polished single crystals were 5 mm long by 5 mm wide and 500 ± 10 µm thick. The use of YAP single crystals provides observation of the effect of the YAP termination plane on the nucleation and growth of reaction products at the CMAS-YAP interface.
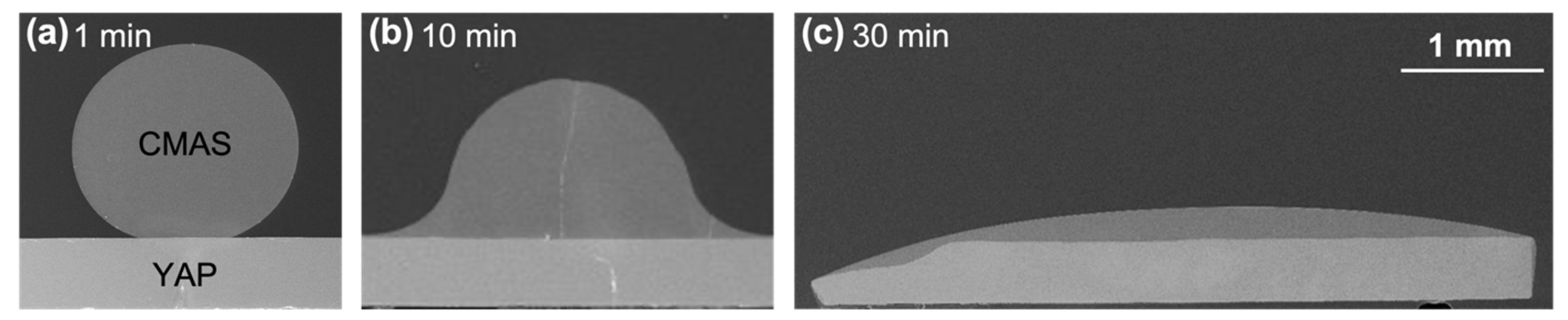
In this study, solid CMAS-glass cubes (1.25 mm side length) were bonded with the YAP single crystals to collect CMAS/YAP contact angles for different substrate orientations. The mass of the CMAS cubes (10 mg) was chosen so that gravitational forces on the droplet upon melting would be negligible and a sessile contact angle could be measured. The CMAS cubes were first bonded to the polished side of each substrate with a heat treatment at 915 °C for 15 min. This pre-attachment process prevented the glass from shifting with the platform vibration of the bottom-loading furnace used for the CMAS/YAP interaction studies. The bonding conditions were tested to ensure no crystallization or melting of the CMAS occurred. After the pre-bonding step, CMAS-YAP samples of each substrate orientation were reacted in air at 1350 °C for dwell durations of 1, 10, 30 and 60 min (CM 1700-BL box furnace) for a total of 16 samples. Samples were inserted when the furnace was at temperature and then air quenched. Based on preliminary data, it was established that 1350 °C was the highest temperature to practically observe the contact angle before reaction products modified the YAP-CMAS interface. Contact angles were measured from the 1-min dwell samples using ImageJ on micrographs collected as described below.
Cross-sections of the reaction regions were prepared for characterization by mounting in epoxy and polishing down to the center of the reaction region to a 250 nm finish using standard metallographical practices. The polished samples were coated with ~1 nm Ir for electron microscopy.
2.3. Characterization by Electron Microscopy and X-ray Diffraction
Secondary electron (SE) images and energy dispersive spectroscopy (EDS) data of all reaction regions were acquired with a Tescan MIRA3 (Kohoutovice, Czech Republic) scanning electron microscope (SEM; 20–30 kV). ImageJ (version 1.51, 2015, National Institutes of Health, Bethesda, MD, USA) was used for extracting metrics from the SEM micrographs. Reaction product amounts were estimated from the 30 and 60 min images by computing the corresponding phase’s average cross-sectional areas and layer thicknesses. Data was collected within a 200 µm radius from the center of the interaction region to exclude edge effects; edges were treated separately, so the measurement does not represent total amount of product formed. Eight images, each with a field of view of 50 µm, were analyzed per sample. Five thickness measurements and one area measurement were recorded per product phase from each image for a total of 40 thickness measurements and 5 area measurements for all reaction products and samples reported. The thickness of YAP dissolved was determined from the images in a similar fashion and by subtracting the original YAP thickness.
Selected area electron diffraction (SAED) patterns, EDS data and high-angle annular dark field (HAADF) images of the reaction products were acquired with Talos F200i S/TEM (FEI, Hillsboro, OR, USA) and Themis Z S/TEM (FEI, Hillsboro, OR, USA) transmission electron microscopes (TEM). The TEM lamellae analyzed were extracted from the 30-min (101) sample interaction region by the in situ lift out technique using a focused ion beam (NanoLab 600 and G4, FEI, Hillsboro, OR, USA).
The 10-min (001) sample was scanned in Bragg–Brentano geometry with an X’Pert MPD diffractometer (Malvern Panalytical, Malvern, UK) to identify potentially crystalline phases within the bulk of CMAS droplets. A thin layer of the modeling clay used for mounting onto the bulk holder was extended over the YAP single crystal to protect the detector from potential burnout. The clay was scanned separately to identify its contribution to the diffraction pattern.
4. Discussion
4.1. Influence of CMAS/YAP Interfacial Energy on Reaction Behavior
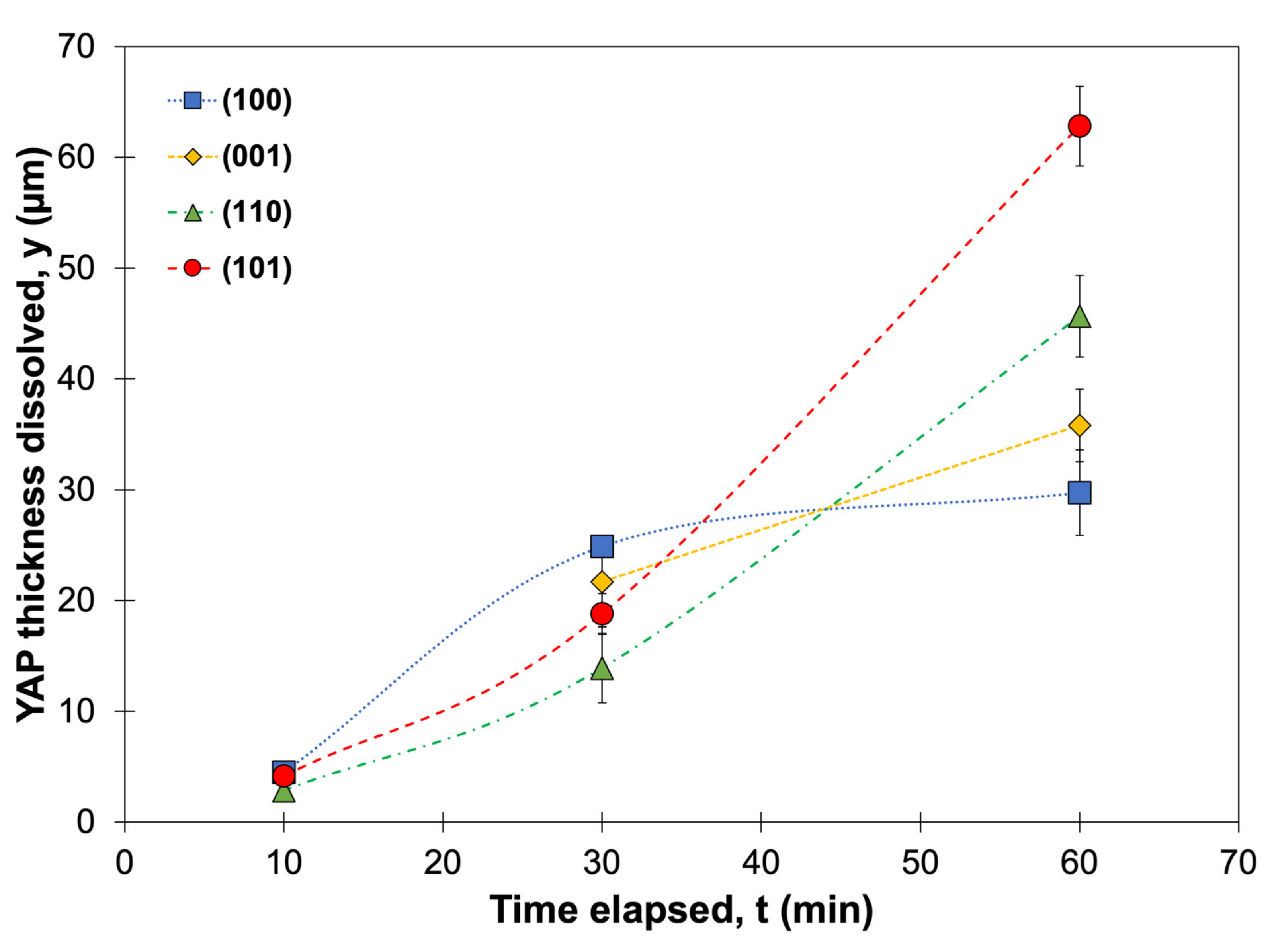
The contact angles reported in
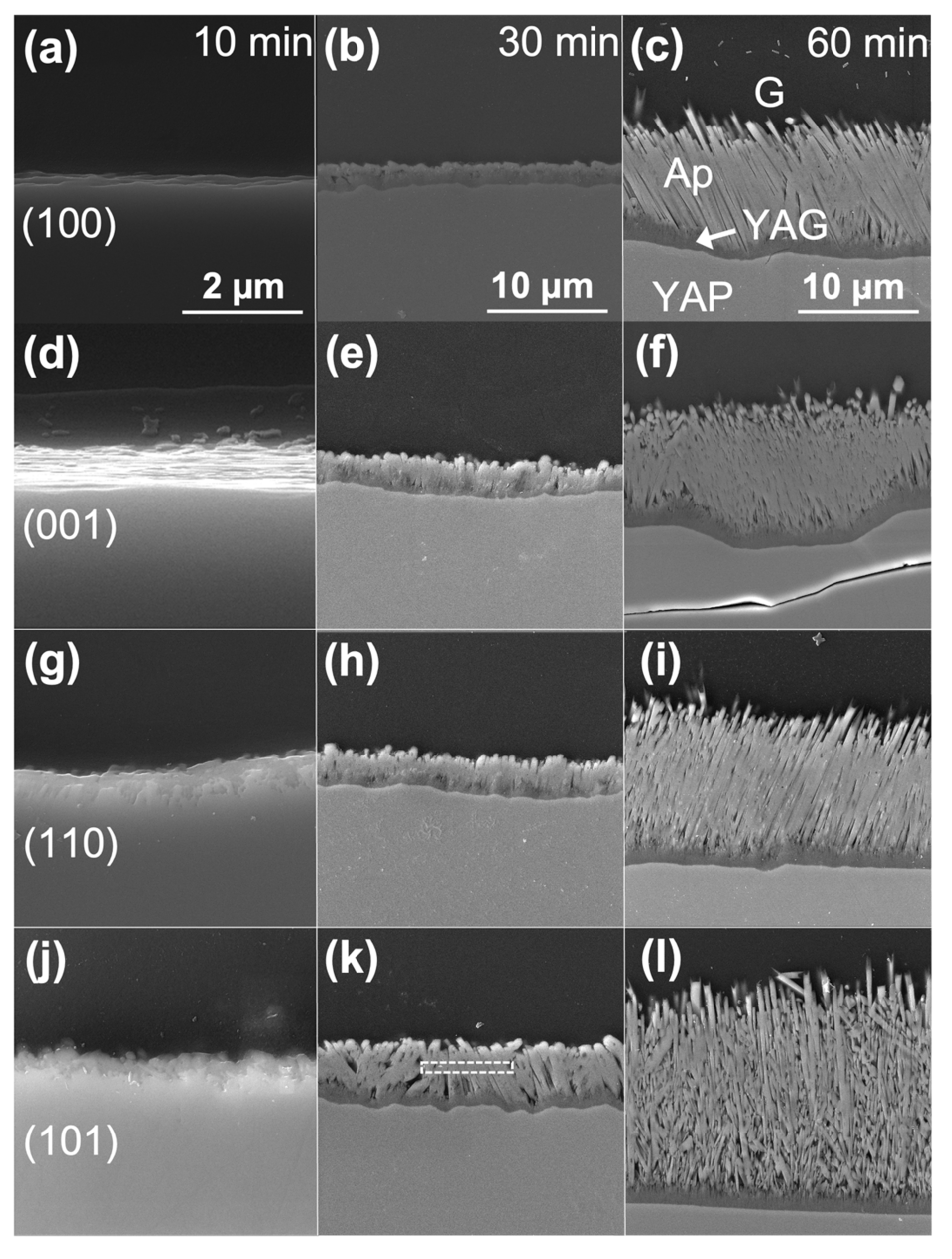
Table 2 indicate that the interfacial energy between CMAS and the YAP substrates decreased in the order: (101), (110), (001), (100). The initial formation of apatite after 10 min reflected these contact angles; apatite formed first on the substrates with the highest contact angle, (110) and (101), as seen in
Figure 8. This apatite formation could imply that a higher interfacial energy (1) promotes dissolution or (2) lowers the activation energy barrier for preferable heterogeneous nucleation. The YAP thickness after CMAS attack also correlated with the contact angle, where substrates with larger contact angles dissolved more over time. This dissolution behavior suggests that YAP (100) consumption slows down between 30 and 60 min. After 60 min, differences in the apatite layer’s cross-sectional area were insignificant for all substrates except (101), which showed a less dense apatite structure and continual consumption of the YAP substrate. Thus, the apatite layer on YAP (101) was less effective at blocking CMAS than the other orientations despite forming readily in the interaction region.
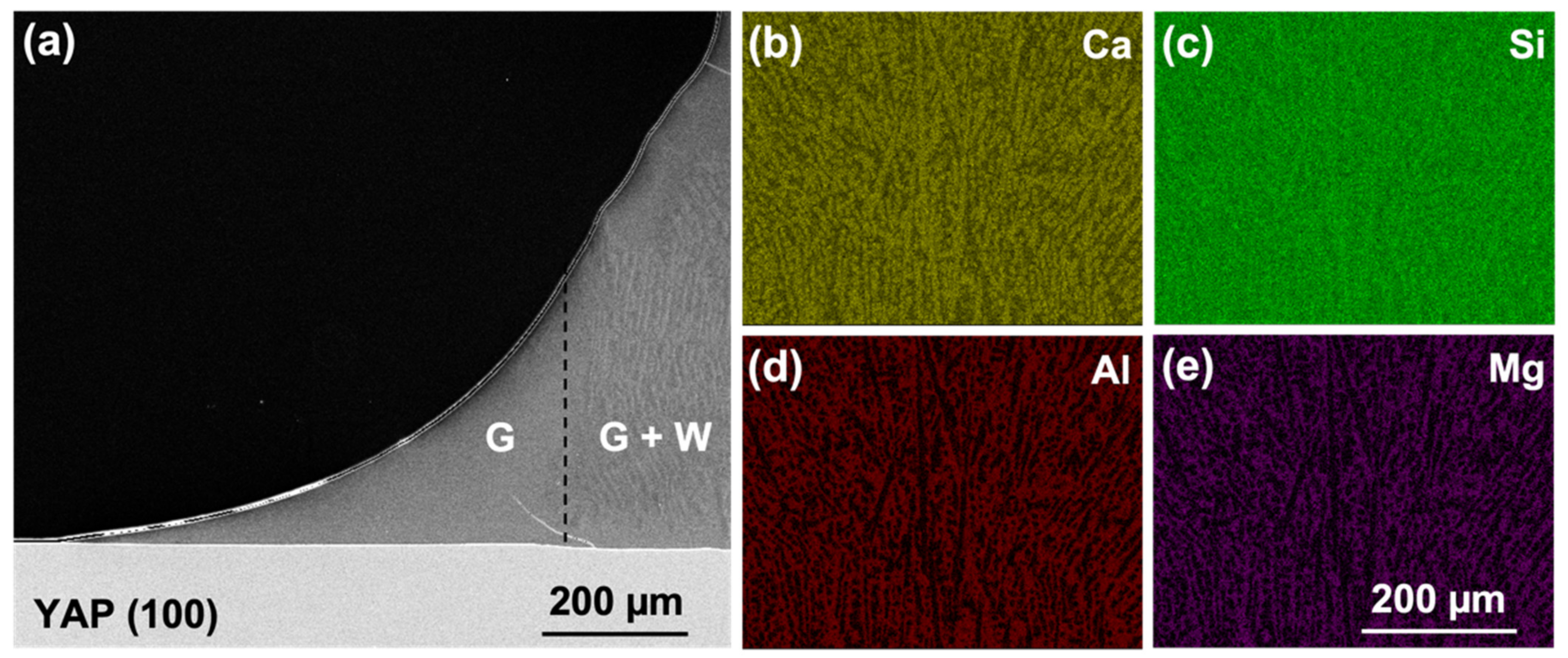
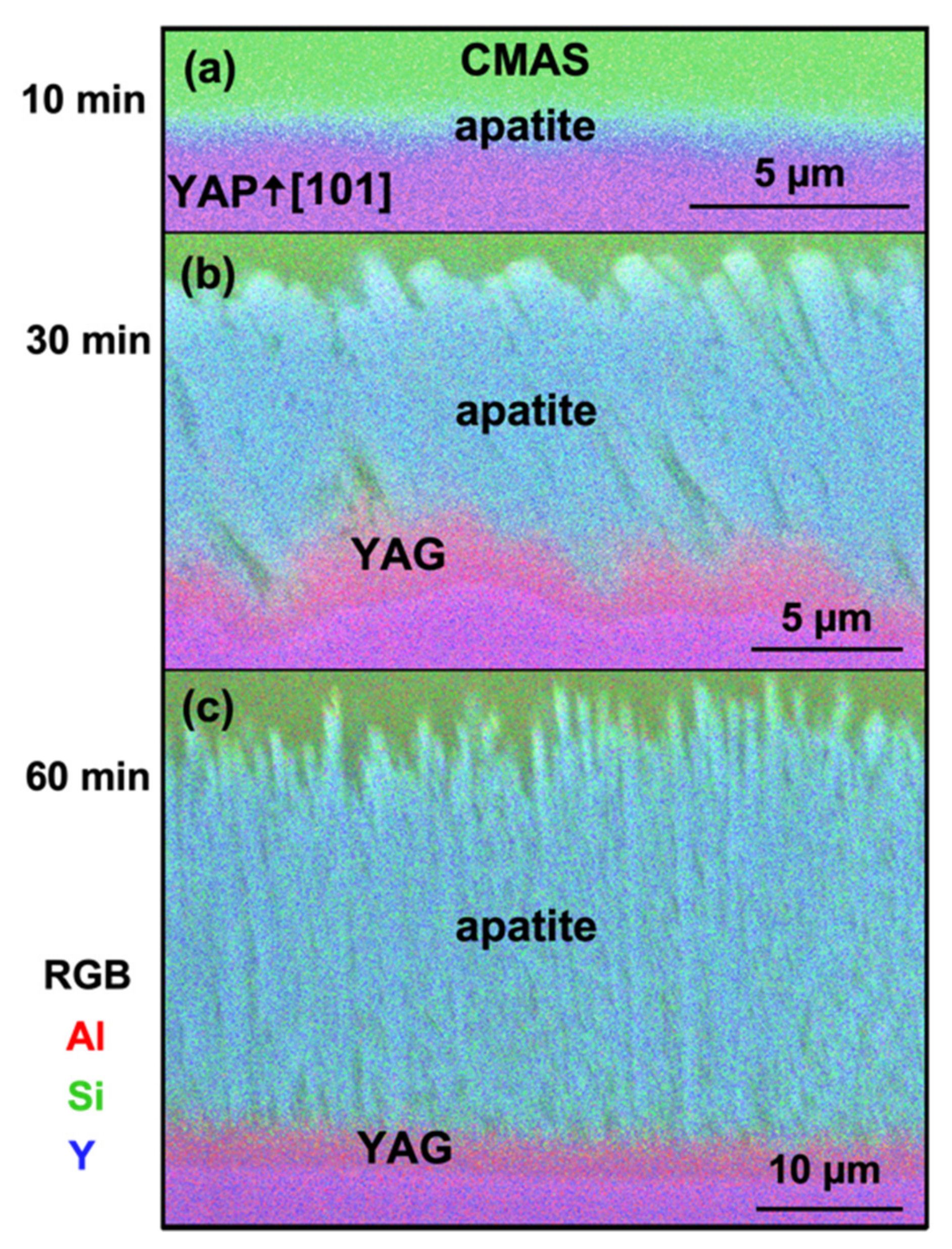
The formation of YAG may limit the effect of the YAP orientation on the CMAS attack behavior after prolonged exposures. A 2 µm-thick YAG layer consistently formed at the CMAS attack front, effectively blocking the CMAS from direct contact with the YAP substrates. Such behavior made any original interfacial energy or orientation relationship of the YAP irrelevant as the reaction proceeds. The concurrent change in the CMAS wetting behavior (
Figure 1) during apatite and YAG nucleation provided additional support that the YAP/CMAS interfacial energy did not significantly contribute to the reaction products in the center, where the CMAS loading was high.
Additionally, this finding has important implications regarding the approach to mitigate attack with CMAS-phobic coatings. Here, significant CMAS wetting of two common reaction products, apatite and YAG, eliminated any potential benefits of the non-wetting YAP. Thus, candidate CMAS-phobic coatings may also become ineffectual if they form similar reaction products.
4.2. Role of YAG in Apatite Growth
For all substrate orientations, YAG was present on the YAP surface, either beneath apatite (
Figure 5) or nearby (
Figure 9b). However, it was not considered a barrier to CMAS because large YAP recession rates were observed when YAG was present without apatite (e.g., edge of (110) YAP shown in the inset of
Figure 9b). Furthermore, YAG formation did not consume the CMAS constituents. This finding agrees with the observations by Eils et al., who found garnet (albeit a different composition) nucleation sluggish and, thus, less effective at mitigating further CMAS penetration compared to apatite [
30].
It is unclear how apatite grows after the formation of the YAG layer; YAG’s columnar morphology may provide a direct diffusion pathway between CMAS and YAP to facilitate the reaction, or YAG may be forming and dissolving at a steady state rate to maintain equilibrium in the system. Regardless, YAP recession and apatite growth is not stymied by the formation of YAG. Yet, YAG appears to influence the growth rate and final morphology of the apatite grains and, thus, apatite’s effectiveness at mitigating CMAS.
The effect of YAG on apatite morphology was most obvious by comparing the center and edges of the interaction region for the (110) YAP after 30 min. In the center of the reaction region, small-diameter apatite rods were found on the surface of the YAG layer. The rods also contained embedded YAG particles (
Figure 7), suggesting a heterogeneous nucleation relationship. In contrast, YAG was not found between the large apatite crystals and YAP substrate at the edges of the interaction region. There, apatite quickly formed directly on to the (110) YAP surface, and its growth was facilitated by rapid consumption of the YAP nearby. YAG then formed within depressions of the YAP substrate that were unprotected by apatite. These observations suggest that when the apatite layer forms on top of the YAG, it is a more effective barrier to CMAS than when apatite nucleates directly on to the YAP surface (discussed below). Similarly, Zhong et al. found that the formation of garnet in Yb–silicate EBCs benefitted the formation of a dense apatite layer [
31]. Therefore, insight into the YAG/apatite orientation relationships will help determine if the YAG layer can be engineered to optimize the apatite morphology for blocking CMAS. The relationship between YAG and YAP is outside the scope of this paper but is part of ongoing work.
4.3. Effect of Apatite Nucleation and Growth on YAP Recession
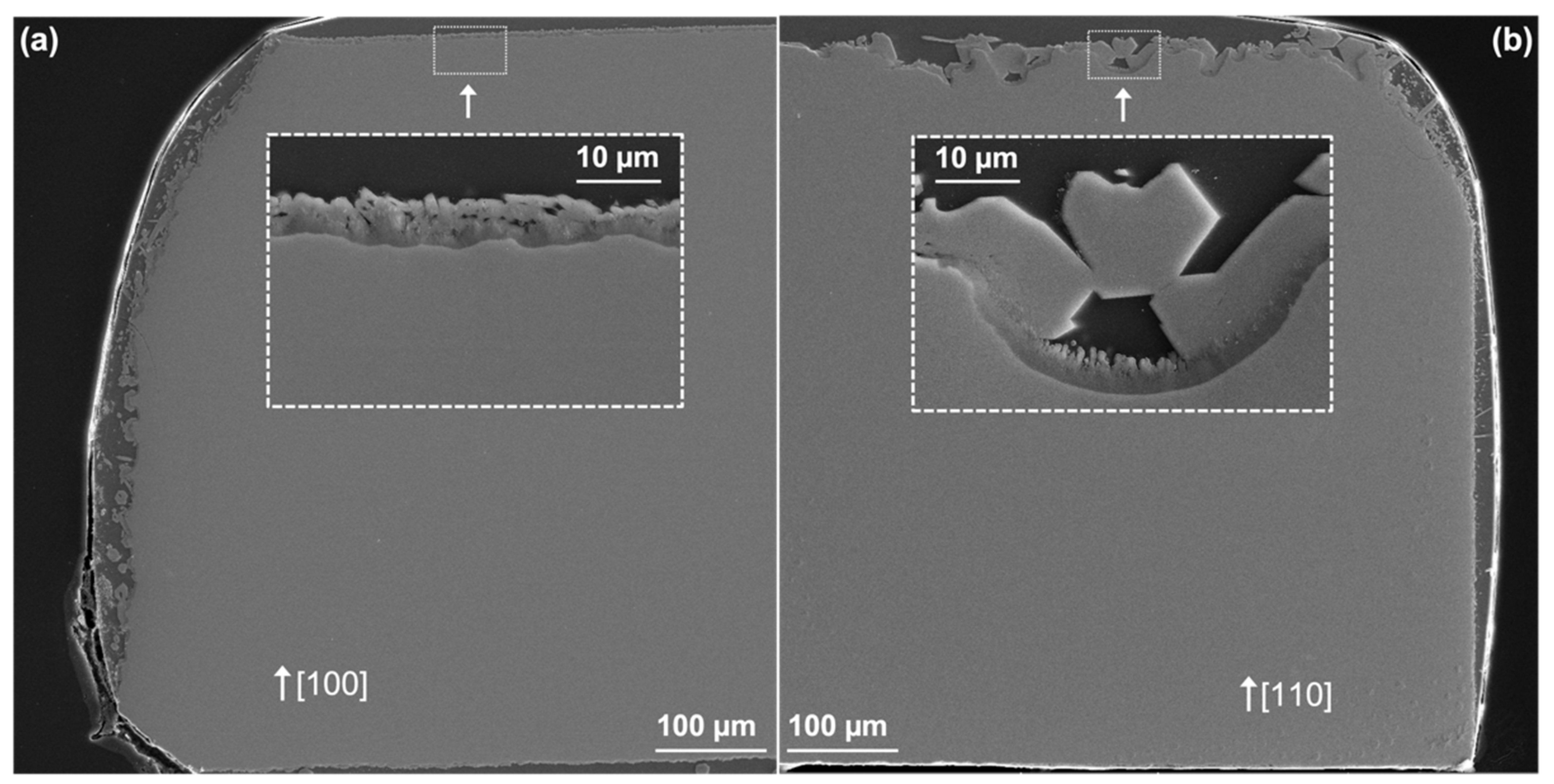
The CMAS loading rate affected the composition, morphology, and nucleation rate of the reaction products as seen by comparing the edges and center of the CMAS attack region. Specifically, apatite nucleation was faster for particular YAP orientations near the edges. This effect provided an opportunity for direct observations of the apatite nucleation behavior without YAG present at the interface. Therefore, this region was used to evaluate the effect of apatite nucleation on CMAS mitigation. It is important to note that the CMAS exposure time was not known along the edges and was likely to be different between substrates because the CMAS spreading rate was uncontrolled.
The substrate orientation appeared to change the morphology and size of the apatite formed. For the (110) and (101), we observed large apatite crystals (up to 50 µm in diameter) at 10 min. These apatite crystals were significantly larger than any found in the center of the reaction region even at longer exposures. These apatite crystals were assumed to form their Wulff shape in the form of a hexagonal rod, agreeing with previous observations [
28,
29]. However, the growth of these crystals appeared faster than their nucleation rate, resulting in fast dissolution of nearby YAP (i.e., deeper CMAS penetration). Therefore, while apatite formed quickly and was effective for protecting the YAP directly below, no continuous apatite layer was formed to create an effective barrier for mitigating CMAS penetration on the (110)- and (101)-oriented substrates.
After 30 min in the low-index systems, small apatite crystals covered the surface effectively to create a CMAS barrier that, presumably, prevented their own growth. Although there were many crystals comprising this barrier, the lack of apatite in the early time steps, both in the center of the reaction region and on the edges, suggests that the nucleation rate was suppressed for these substrates. Additionally, the slow wetting behavior observed in
Figure 8 also supports the suppression of reaction products in these samples because CMAS wetting is associated with apatite formation. This slow nucleation behavior may be due to an orientation relationship that facilitates apatite densification/coalescence or due to the lower CMAS/YAP interfacial energy for these substrate orientations (see
Section 4.1). Regardless of the cause, the slow nucleation behavior of the (100) was the most beneficial for mitigating CMAS as it created an effective apatite barrier.
This finding is surprising in that it suggests that fast nucleation and growth of apatite may not always be beneficial for mitigating CMAS. Here, the nucleation of apatite was much slower than the growth rate, leading to fast consumption of the YAP substrate to facilitate apatite coarsening. This behavior demonstrates a kinetic approach to improve CMAS mitigation: increase the number of apatite nucleation sites to increase apatite density or suppress the apatite crystals’ growth rate. The preferred nucleation sites for apatite on YAP and YAG must be known to achieve this goal and is the focus of future work.
5. Conclusions
This model study evaluated the effects of apatite nucleation and growth behavior on the CMAS mitigation capabilities of the promising EBC candidate, YAP. Four YAP substrates with different crystallographic orientations were exposed to a four-component CMAS at 1350 °C for 1, 10, 30 and 60 min. The apatite and YAG formation behavior varied with CMAS loading. In the center of the reaction region, where the CMAS loading was highest, a thin YAG layer was found between the apatite layer and YAP substrate. The YAG layer appeared to nullify the impact of the YAP orientation on the apatite morphology. In regions with low CMAS loading (i.e., edge of reaction region), apatite formed rapidly on the YAP substrates with orientations found to have greater EBC/CMAS interfacial energy (e.g., (110) and (101)). These apatite crystals nucleated directly on to the YAP surface without any perceivable YAG layer. However, the growth of these rapidly formed apatite crystals was facilitated by dissolution of the nearby YAP substrate, resulting in deeper CMAS penetration compared with the other YAP substrate orientations. These observations suggest that CMAS mitigation is suppressed when the apatite growth rate is faster than the nucleation rate. Effective CMAS mitigation strategies may include increasing the number of nucleation sites and/or decreasing the apatite growth rate. Additionally, this study shows that secondary phases like YAG may be useful to control the nucleation and growth of apatite for better protection against CMAS.