Abstract
This study has demonstrated, for the first time, the potential application of coatings to protect bricks or architectures against detrimental atmospheric effects via a self-cleaning approach. In this research, a facile fabrication method was developed to produce amorphous SiO2 particles and their hierarchical structures via applying trimethylchlorosilane (TMCS). They were fully characterized by various surface analytic tools, including a goniometer, SEM, AFM, zeta sizer, and a spectroscopic technique (FTIR), and then applied as super hydrophobic coatings on glass and sand. The characterization results revealed that the SiO2 particles are amorphous, quasi-spherical particles with an average diameter of 250–300 nm, and the hierarchical structures in the film were assembled from building blocks of SiO2 and TMCS. The wettability of the films can be controlled by changing the pH of the SiO2/TCMS dispersion. A super hydrophobic surface with a water contact angle of 165° ± 1° was achieved at the isoelectric point of the films. The obtained translucent super hydrophobic SiO2/TMCS coatings show good self-cleaning performances for glass and sand as construction materials. This study indicated that the superhydrophobic coatings may have potential applications in the protection of buildings and construction architectures in the future.
1. Introduction
In recent times, pollutants from climate and environmental change are causing serious damage to the outdoor surfaces of buildings. These contaminants not only bring damage to the surface, but also remain on the surface of the building [1,2,3,4,5]. If these surfaces are not cleaned over time, they will depreciate the wear resistance of the building and affect the quality of the surface. Cleaning the exterior of a building is not only expensive, but also a very perilous practice. Developing multifunctional super hydrophobic materials is attractive to many researchers, since they hold surface self-cleaning and protection properties, and reduce costs. It is well known that superhydrophobic materials have excellent self-cleaning properties [6,7,8,9,10,11,12], and their practical applications include their use as waterproof, anti-fouling, anti-corrosion [13] and anti-rust materials [14,15]. Among them, corrosion resistance is one of the unique properties of superhydrophobic materials [16,17]. Furthermore, water contact angle (WCA) and roll-off angle (SA) are the two main important parameters used to characterize their superhydrophobic property [18]. A superhydrophobic surface possesses low surface energy and comprises a hierarchical rough structure [19]. As both WCA and SA are greater than 150° and less than 10°, respectively, they become ideal candidates for the self-cleaning application. Therefore, both a high static WCA and a low SA are major requirements for self-cleaning materials [18,20,21,22]. On the self-cleaning superhydrophobic surface, water droplets can easily roll and simultaneously carry away dust/dirt attached to the surface. Moreover, superhydrophobic materials have been developed from various materials, such as carbon nano-structures [22,23,24], silica-based nano-composites [25,26,27,28], fluorinated silanes [29,30,31], siloxane polymers [32,33,34] and two-dimensional (2D) transition metal dichalcogenides [35]. Significant efforts have been made in developing self-cleaning superhydrophobic surfaces [19].
However, protection of concrete is still a challenging task in the structural engineering field, since the desired coating is not only substrate-dependent, but also the cost has to be taken into consideration. The synthesis process of some organic particles is complex, time-consuming, harsh and costly, and can only be prepared in the laboratory [36]. In addition, to produce the self-cleaning hydrophobic coating, some superhydrophobic coatings need further baking and heating treatment after coating on the substrate surface [37,38].
Thus, the development of silica-based coatings is interesting to study since they are cost effective, easy processable materials and they have unique properties, such as excellent chemical inertness, high thermal resistance, strong mechanical strength, and tunable optical properties.
In this study, for the first time, we reported a facile fabrication method of a silica-based superhydrophobic coating system on glass and sand substrates using trimethylcholorsilane-modified SiO2 nanoparticles and tested self-cleaning construction materials. The experimental results showed that the as-prepared superhydrophobic SiO2/trimethylchlorosilane (TMCS) coating had a contact angle up to 165°, with a roll-off angle of less than 10° and demonstrated self-cleaning properties.
2. Materials and Methods
2.1. Materials
Tetraethoxysilane (TEOS, 98%), ethanol (96% purity), trimethylchlorosilane (TMCS, 99%), ammonium hydroxide solution (28% NH3 in H2O, 99.99%), and DI water were used in all the experiments. All the chemicals were purchased from Sigma Aldrich (St. Louis, MO, USA) and were used without further purification.
2.2. Synthesis of SiO2 Nanoparticles
As in a typical synthesis process, 30 mL of ethanol was mixed with 10 mL of DI water. Later on, 2 mL of TEOS was added drop-wise. The obtained solution was heated at 30 °C and vigorously stirred (700 rpm) for 5 min. Then, a different amount of 0.5 M NH4OH (depending on the pH level) was added and left to react for 1 h to form white turbid silica solution. A schematic illustration of the process is shown in Figure 1.
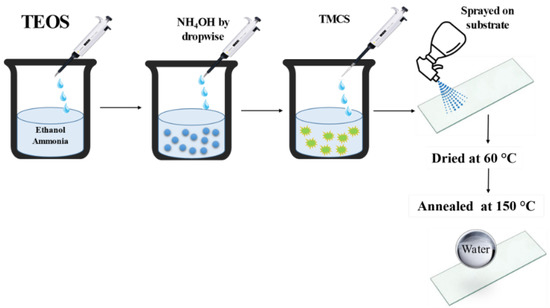
Figure 1.
Preparation of superhydrophobic SiO2/TMCS coatings onto glass.
2.3. Preparation of Superhydrophobic Surfaces
An amount of 25 mL of as-prepared silica solution and 5 mL of TMCS in a volume ratio of 5:1 were stirred at 700 rpm for 5 h to form the colloidal dispersion. The initial pH of the dispersion was 2.8 and different pH levels were achieved using 0.5 M NH4OH. The dispersion with a pH range from 2 to 12 was used for the coating and the contact angles were measured for each pH level after spraying. The hydrophobic colloidal dispersion was sprayed onto substrates with a household sprayer. After coating, the substrates were dried under ambient conditions for 1 h, and then heated at 150 °C for 1h with an increase of 1 °C/min in order to remove the residual solvent.
2.4. Characterization
A zeta sizer (Malvern Zetasizer Nano ZS, Worcestershire, UK) was applied to measure the zeta (ζ) potential of the colloidal particles. The formation of silica particles and their modification were investigated in powder form using an FT-IR spectrometer (ANicolet iS10, Thermo Scientific, Waltham, MA, USA). Contact angles (OCA) of water droplets on the glass and sand were measured by a goniometer (Dataphysics OCA 15Pro, Filderstadt, Germany). Scanning electron microscopy (SEM) (Zeiss Auriga Crossbeam 540, Carl Zeiss, Oberkochen, Germany) and X-ray diffraction (XRD, SmartLab, Rigaku, Tokyo, Japan) were utilized to investigate the morphology and crystal structure of nano-SiO2 and functionalized SiO2/TMCS.
3. Results and Discussion
In this study, to develop a superhydrophobic self-cleaning coating for the exterior of buildings or construction, silica-based particle systems were proposed based on the following advantages: (1) abundance on earth; (2) cost-effectiveness; (3) easily processed; and (4) similar nature to buildings. For this purpose, hydrophobic-silica particle coatings were developed to test their self-cleaning performance on glass and sand.
3.1. Fabrication of Superhydrophobic SiO2/TMCS Coating
Pure SiO2 NPs were synthesized by hydrolysis of TEOS in the presence of ammonia aqua solution using the Stober method [34], and then TMCS was introduced onto the surface of silica particles. Superhydrophobic properties were achieved by substitution of hydroxyl groups on the surface of the SiO2 NPs with methyl groups from TMCS (Figure 2).
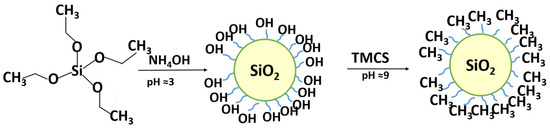
Figure 2.
Process of formation of superhydrophobic SiO2/TMCS coating.
The zeta potential, which depends on the surface charge, is important for the stability of nanoparticles in suspension. When the pH decreases, the particles tend to form agglomerates due to the absence of electrostatic stabilization of the nanoparticles that keeps them apart. As a result, silica nanoparticles functionalized with TMCS dispersion showed some precipitates. When the pH is at about 9, it reaches the isoelectrical point, where the negative and positive charges are equal on the surface of the silica particles. As the pH value increases from 9, the Z potential becomes more and more positive, indicating the increase in positive charge on the particle surface. As a result, the repulsion forces overcome the attractive forces between the nanoparticles and result in electrostatic stabilization [39]. In addition, the isoelectric point (IEP) of the as-synthesized bare silica NPs was found at pH = 2–3 (Figure 3a). This might be a feature of the condensation reaction of the silicic acids (Si(OH)4). As the pH increases from 3, the negative charges increase, the repulsion forces between nanoparticles also increase and suspension tends to stabilize [40].
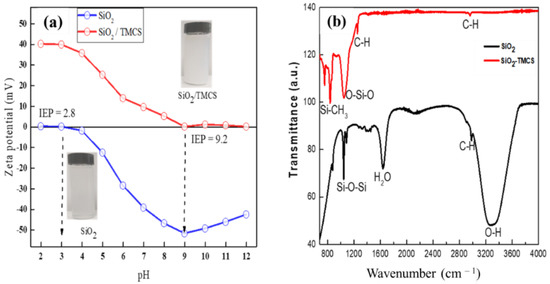
Figure 3.
Isoelectric point (a) and FTIR (b) of SiO2 NPs and SiO2/TMCS film.
As shown in Figure 3b, broad absorption bands at 3400 cm−1 and 1620 cm−1 corresponded to the –OH groups adsorbed at the SiO2 surface in the FTIR spectrum. The broad absorption bands at around 3400 cm−1 and 1600cm−1 corresponded to the –OH groups adsorbed at the SiO2 surface, which disappeared after applying TMCS on the surface of SiO2 and after annealing (Figure 3b, red line). The absorption peaks at around 1099 cm−1 were assigned to the Si–O–Si asymmetric stretching and bending vibrations. Likewise, the characteristic peaks at 2972 cm−1 and at 1392 cm−1 were assigned to C–H of the CH3 antisymmetric stretching vibration to the symmetric vibration and in-plane vibration of the –CH3 group, respectively. It indicated the presence of CH3 (hydrophobic functional group) on the surface of SiO2 particles. Moreover, the peak at 765 cm−1 that corresponded to the symmetric stretching vibration of the Si–CH3 group showed the hydrophobic modification of the silica particles. The reaction between SiO2 particles and TMCS was completed through the formation of the Si–O–Si bonds.
3.2. Investigation of Surface Morphology of SiO2/TMCS Coating
The morphology of the bare SiO2 NPs and the SiO2 modified with TMCS coating onto glass was examined by SEM and AFM techniques. According to the SEM images (Figure 4a), bare SiO2 NPs had a spherical monodisperse uniform shape and were 15–20 nm in size, and also had an amorphous structure with a wide peak at 2θ = 22.5° (Figure 4c). However, after the SiO2 particles were modified by TMCS (Figure 4b,d), the surface of the materials became rough. Moreover, SiO2 modified with TMCS particles formed a uniformly distributed film on the glass substrate, with sizes ranging from 100 to 300 nm and exhibited a rough surface covered by the low surface free energy of methyl groups (Si–CH3) at 765cm−1, as confirmed by FTIR (Figure 3b).
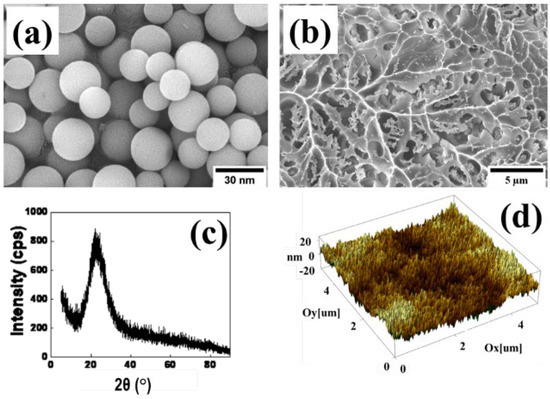
Figure 4.
SiO2 NPs (a) SEM and (c) XRD; and SiO2/TMCS coatings (b) SEM and (d) AFM.
Particles of different sizes and shapes in the system resulted in the rough surface of the coatings, which is beneficial for the formation of the superhydrophobic properties. The rough surface structure is a crucial parameter to create superhydrophobic properties [41,42,43,44].
3.3. Contact Angle (OCA) Study
The wettability of glass and sand coated with SiO2/TMCS was studied by using a goniometer (an OSA-15EC instrument). Figure 5 shows the contact angles of water droplets on the SiO2 modified with TMCS superhydrophobic materials after being applied to the surface of glass and sand. Glass surfaces (Figure 5a,b) treated with SiO2/TMCS slurry produced a superhydrophobic effect with a contact angle above 150°. The reason for the superhydrophobic coatings is the formation of the rough surface at IEP due to the van der Waals attraction.
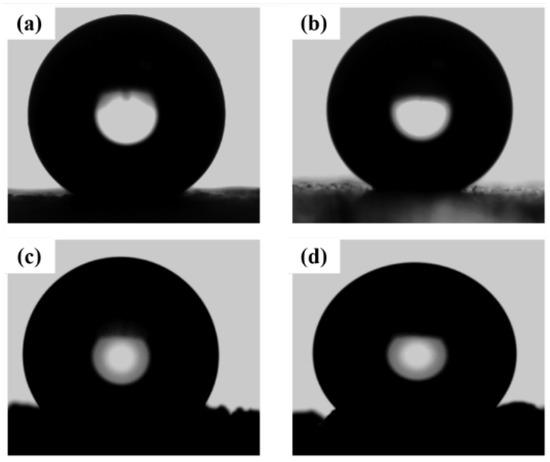
Figure 5.
Water contact angle measurement of glass ((a) 159, (b) 165) and sand ((c) 150, (d) 148) coating with SiO2/TMCS, respectively.
Figure 6 shows the effect of the pH of the SiO2/TMCS dispersion on the contact angle. The wettability of coated glass was increased with the increasing pH of SiO2, reaching a superhydrophobic state with a contact angle of 152° at pH = 7, 159° at pH = 8, and 165° at pH = 9. This increase in contact angle can be explained by an increase in surface roughness, due to the formation of a network of silica particles via Vander Waals attraction at the IEP point. However, after pH = 9, the contact angle sharply decreased, reaching 129°. The diagram shows that, despite the influence of the pH of SiO2/TMCS dispersion, all samples showed a hydrophobic state.
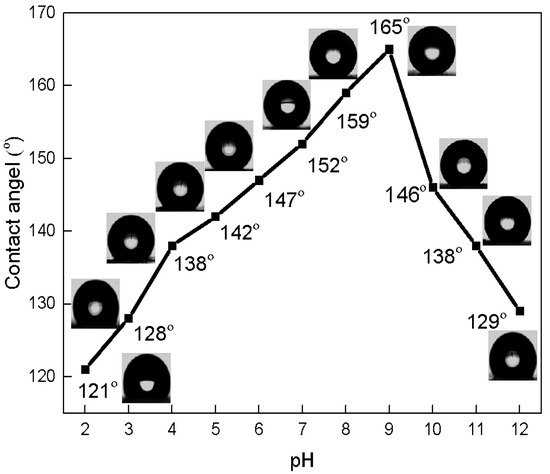
Figure 6.
Dependence of contact angle on pH of SiO2/TMCS.
The high contact angle of hydrophobized sand (151°) and glass (165°) confirmed the formation of superhydrophobic coatings (Figure 7). To evaluate the anti-icing properties of the SiO2/TMCS coating, surface-coated brick and concrete were placed in a freezer at −30°C, then water was dropped over them. The water contact angle was 135°. An image of the experiment is given in the Supporting Information (Figures S1 and S2). It can be observed from the image that the SiO2/TMCS coating can be applied in extreme cold temperatures for the purpose of coating building materials. Fluidity testing of the SiO2/TMCS coating on the brick can also be observed in the video in the supporting information. It can be observed that hydrophobic surfaces do not easily become wet in contact with water. The phenomenon is due to unbalanced molecular forces at the water/solids interface, which causes surface tension. Low surface energy resists wetting [45,46].
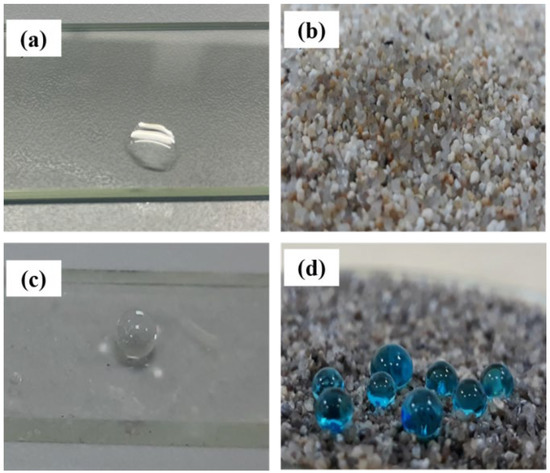
Figure 7.
Optical images of the wetting states of water droplets on the glass before (a) and after treatment (c) and sand before (b) and after treatment (d).
3.4. Self-Cleaning Study of Construction Materials
Self-cleaning is a crucial property of superhydrophobic materials for practical applications. A series of experiments were conducted to demonstrate the self-cleaning properties of the coated glass. The experiments were executed following two methods, as shown in Figure 8. In the first experiment, blue color water droplets (contaminated water) were rolled from glass substrates, and the result showed no remains of tailings (Figure 8a,b). In the second experiment, dust from the sands was applied as dirt onto a superhydrophobic glass substrate for visualization of the self-cleaning process. Then, the process was observed to determine the capability of water droplets to remove the dust particles (Figure 8c,d).
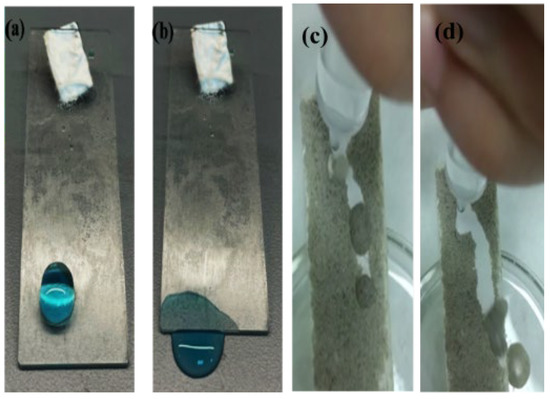
Figure 8.
Optical images of the sliding water droplets (a,b) on the superhydrophobic surface and self-cleaning behavior of the superhydrophobic glass surfaces (c,d).
These results indicate that the coated glass substrates have good self-cleaning capabilities because the dyed water and dust did not remain on the coated glass slide after the removal of dirt by rolling water droplets. SiO2 modified with TMCS coatings possesses superhydrophobic properties due to the surface roughness by creating the hierarchical rough structure and low surface free energy of methyl groups. These properties could maintain the water droplets in the ‘solid–vapor–liquid phase’ through forming a high-water contact angle (90° < θ < 150°) and a low-water roll-off angle (10°). Consequently, the two mentioned characteristics of the as-prepared superhydrophobic glass caused the water droplets to easily roll off over the modified surface without moistening the surface, meaning that contaminants will be easier to remove.
4. Conclusions
Bare SiO2 nanoparticles and SiO2 modified with TMCS nanocomposites were successfully synthesized, characterized and used as self-cleaning coatings. The results showed that nano-sized SiO2 particles had spherical, monodisperse and uniform shapes. XRD analysis of SiO2 indicates a wide peak located approximately at 2θ = 22.5°, which suggests an amorphous structure. The SEM results indicate that, after SiO2 particles were modified with TMCS, the surface of the coatings became rough. It was found that the coatings demonstrated excellent superhydrophobic and self-cleaning properties. The highest contact angle was recorded as 165° at pH = 9. The proposed coatings may have potential applications in the protection of buildings and constructions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/coatings12101422/s1, Figure S1: Optical images of the wetting states of water droplets on the brick at 30 °C; Figure S2: Optical images of the wetting states of water droplets on the concrete at 30 °C; Figure S3: Fluidity test on the concrete; Figure S4: Self-cleaning test on the concrete.
Author Contributions
Data curation, R.K., A.T. and A.K.; Investigation, A.K., T.Z., A.S., N.A., Y.K. and O.T.; Methodology, A.S., Z.T., N.M., K.K. and O.T.; Project administration, O.T.; Resources, R.K.; Writing—original draft, A.K. and M.N.; Writing—review and editing, Z.T. and O.T. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to acknowledge the support of the Ministry of Education and Science of the Republic of Kazakhstan under Grant No. AP08052848.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Belfiore, C.M.; Barca, D.; Bonazza, A.; Comite, V.; La Russa, M.F.; Pezzino, A.; Ruffolo, S.A.; Sabbioni, C. Application of spectrometric analysis to the identification of pollution sources causing cultural heritage damage. Environ. Sci. Pollut. Res. 2013, 20, 8848–8859. [Google Scholar] [CrossRef]
- Akhmetzhan, A.; Myrzakhmetova, N.; Amangeldi, N.; Kuanyshova, Z.; Akimbayeva, N.; Dosmaganbetova, S.; Toktarbay, Z.; Longinos, S.N. A Short Review on the N,N-Dimethylacrylamide-Based Hydrogels. Gels 2021, 7, 234. [Google Scholar] [CrossRef]
- Kronlund, D.; Bergbreiter, A.; Lindén, M.; Grosso, D.; Smått, J.-H. Hydrophobization of marble pore surfaces using a total immersion treatment method—Influence of co-solvents and temperature on fluorosurfactant vesicle behavior. Colloids Surf. A Physicochem. Eng. Asp. 2015, 483, 104–111. [Google Scholar] [CrossRef]
- Abdiyev, K.Z.; Maric, M.; Orynbayev, B.Y.; Toktarbay, Z.; Zhursumbaeva, M.B.; Seitkaliyeva, N.Z. Flocculating properties of 2-acrylamido-2-methyl-1-propane sulfonic acid-co-allylamine polyampholytic copolymers. Polym. Bull. 2022, 1–16. [Google Scholar] [CrossRef]
- Xue, C.-H.; Jia, S.-T.; Zhang, J.; Ma, J.-Z. Large-area fabrication of superhydrophobic surfaces for practical applications: An overview. Sci. Technol. Adv. Mater. 2010, 11, 033002. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Li, S.; Wang, Y.; Han, Z.; Ren, L. One-step method for fabrication of biomimetic superhydrophobic surface on aluminum alloy. Colloids Surf. A Physicochem. Eng. Asp. 2015, 466, 125–131. [Google Scholar] [CrossRef]
- Nuraje, N.; Asmatulu, R.; Cohen, R.E.; Rubner, M.F. Durable Antifog Films from Layer-by-Layer Molecularly Blended Hydrophilic Polysaccharides. Langmuir 2011, 27, 782–791. [Google Scholar] [CrossRef]
- Erbil, H.Y. Practical Applications of Superhydrophobic Materials and Coatings: Problems and Perspectives. Langmuir 2020, 36, 2493–2509. [Google Scholar] [CrossRef]
- Zhao, S.; Liang, Y.; Yang, Y.; Huang, J.; Guo, Z.; Liu, W. A robust surface with superhydrophobicity and underwater superoleophobicity for on-demand oil/water separation. Nanoscale 2021, 13, 15334–15342. [Google Scholar] [CrossRef]
- Kudaibergenova, R.; Ualibek, O.; Sugurbekov, E.; Demeuova, G.; Frochot, C.; Acherar, S.; Sugurbekova, G. Reduced graphene oxide-based superhydrophobic magnetic nanomaterial as high selective and recyclable sorbent for oil/organic solvent wastewater treatment. Int. J. Environ. Sci. Technol. 2022, 19, 8491–8506. [Google Scholar] [CrossRef]
- Nuraje, N.; Khan, W.; Ceylan, M.; Lei, Y.; Asmatulu, R. Superhydrophobic electrospun nanofibers. J. Mater. Chem. A 2013, 1, 1929. [Google Scholar] [CrossRef]
- Asmatulu, R.; Ceylan, M.; Nuraje, N. Study of superhydrophobic electrospun nanocomposite fibers for energy systems. Langmuir 2011, 27, 504–507. [Google Scholar] [CrossRef]
- Jishnu, A.; Jayan, J.S.; Saritha, A.; Sethulekshmi, A.S.; Venu, G. Superhydrophobic graphene-based materials with self-cleaning and anticorrosion performance: An appraisal of neoteric advancement and future perspectives. Colloids Surf. A Physicochem. Eng. Asp. 2020, 606, 125395. [Google Scholar] [CrossRef]
- Zhao, X.; Abutalip, M.; Afroz, K.; Nuraje, N. Hydrophobically modified polycarboxybetaine: From living radical polymerization to self-assembly. Langmuir 2018, 35, 1606–1612. [Google Scholar] [CrossRef]
- Wang, Z.; Paul, S.; Stein, L.H.; Salemi, A.; Mitra, S. Recent Developments in Blood-Compatible Superhydrophobic Surfaces. Polymers 2022, 14, 1075. [Google Scholar] [CrossRef]
- Zhang, Z.-Q.; Wang, L.; Zeng, M.-Q.; Zeng, R.-C.; Lin, C.-G.; Wang, Z.-L.; Chen, D.-C.; Zhang, Q. Corrosion resistance and superhydrophobicity of one-step polypropylene coating on anodized AZ31 Mg alloy. J. Magnes. Alloys 2021, 9, 1443–1457. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, W.; Sun, F.; Zhang, P.; He, Y.; Wang, X.; Luo, D.; Ma, W.; Sergio, G.-C. Construction of a superhydrophobic coating using triethoxyvinylsilane-modified silica nanoparticles. Surf. Eng. 2019, 35, 418–425. [Google Scholar] [CrossRef]
- Liu, H. Robust translucent superhydrophobic PDMS/PMMA film by facile one-step spray for self-cleaning and efficient emulsion separation. Chem. Eng. J. 2017, 330, 26–35. [Google Scholar] [CrossRef]
- Xiang, T.; Lv, Z.; Wei, F.; Liu, J.; Dong, W.; Li, C.; Zhao, Y.; Chen, D. Superhydrophobic Civil Engineering Materials: A Review from Recent Developments. Coatings 2019, 9, 753. [Google Scholar] [CrossRef]
- Yang, C.; Hao, P.; He, F. Effect of upper contact line on sliding behavior of water droplet on superhydrophobic surface. Sci. Bull. 2009, 54, 727–731. [Google Scholar] [CrossRef]
- Yi, M.; Liu, L.; Wu, L.; Li, X. Research on sliding angles of water droplets on the hierarchical structured superhydrophobic surfaces. Appl. Phys. A 2020, 126, 47. [Google Scholar] [CrossRef]
- Xiang, S.; Liu, W. Self-Healing Superhydrophobic Surfaces: Healing Principles and Applications. Adv. Mater. Interfaces 2021, 8, 2100247. [Google Scholar] [CrossRef]
- Longinos, S.K.; Longinou, D.-D.; Celebi, E.; Toktarbay, Z.; Parlaktuna, M. Kinetic study of methane hydrate formation with the use of a surface baffle. React. Kinet. Mech. Catal. 2021, 134, 75–86. [Google Scholar] [CrossRef]
- Abdelmagid, G.; Yilbas, B.S.; Al-Sharafi, A.; Al-Qahtani, H.; Al-Aqeeli, N. Water droplet on inclined dusty hydrophobic surface: Influence of droplet volume on environmental dust particles removal. RSC Adv. 2019, 9, 3582–3596. [Google Scholar] [CrossRef]
- Sun, C.; Lyu, Q.; Si, Y.; Tong, T.; Lin, L.-C.; Yang, F.; Tang, C.Y.; Dong, Y. Superhydrophobic Carbon Nanotube Network Membranes for Membrane Distillation: High-Throughput Performance and Transport Mechanism. Environ. Sci. Technol. 2022, 56, 5775–5785. [Google Scholar] [CrossRef]
- Chen, X.; Yang, M.; Zhao, X.; Hu, D.; Liu, W.; Ma, W. Tailoring superhydrophobic PDMS/CeFe2O4/MWCNTs nanocomposites with conductive network for highly efficient microwave absorption. Chem. Eng. J. 2022, 432, 134226. [Google Scholar] [CrossRef]
- Zulkharnay, R.; Ualibek, O.; Toktarbaiuly, O.; May, P.W. Hydrophobic behaviour of reduced graphene oxide thin film fabricated via electrostatic spray deposition. Bull. Mater. Sci. 2021, 44, 112. [Google Scholar] [CrossRef]
- Ibrahim, S.; Sultan, M. Superhydrophobic Coating Polymer/Silica Nanocomposites: Part I Synthesis and Characterization as Eco-Friendly Coating. Silicon 2020, 12, 805–811. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Lei, Q.; Wu, Y.; Li, W. Fabrication of superhydrophobic composite coating based on fluorosilicone resin and silica nanoparticles. R. Soc. Open Sci. 2018, 5, 180598. [Google Scholar] [CrossRef]
- Kim, G.-H.; Kang, D.-H.; Jung, B.-N.; Shim, J.-K. Fabrication and Characterization of Hydrophobic Cellulose Nanofibrils/Silica Nanocomposites with Hexadecyltrimethoxysilane. Polymers 2022, 14, 833. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, Q. Preparation and Properties of Hydrophobically Modified Nano-SiO2 with Hexadecyltrimethoxysilane. ACS Omega 2021, 6, 9764–9770. [Google Scholar] [CrossRef]
- Cai, Y.; Li, J.; Yi, L.; Yan, X.; Li, J. Fabricating superhydrophobic and oleophobic surface with silica nanoparticles modified by silanes and environment-friendly fluorinated chemicals. Appl. Surf. Sci. 2018, 450, 102–111. [Google Scholar] [CrossRef]
- Jeong, H.; Baek, S.; Han, S.; Jang, H.; Rockson, T.K.; Lee, H.S. Chemically Robust Superhydrophobic Poly(vinylidene fluoride) Films with Grafting Crosslinkable Fluorinated Silane. Macromol. Res. 2018, 26, 493–499. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Kim, H.; Nam, K.; Lee, D.Y. Fabrication of Robust Superhydrophobic Surfaces with Dual-Curing Siloxane Resin and Controlled Dispersion of Nanoparticles. Polymers 2020, 12, 1420. [Google Scholar] [CrossRef] [PubMed]
- Hou, B.; Li, X.; Ma, X.; Du, C.; Zhang, D.; Zheng, M.; Xu, W.; Lu, D.; Ma, F. The cost of corrosion in China. npj Mater. Degrad. 2017, 1, 4. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, X.; Bao, Y.; Liu, J. A facile spraying method for fabricating superhydrophobic leather coating. Colloids Surf. A Physicochem. Eng. Asp. 2015, 472, 21–25. [Google Scholar] [CrossRef]
- Ogihara, H.; Xie, J.; Okagaki, J.; Saji, T. Simple Method for Preparing Superhydrophobic Paper: Spray-Deposited Hydrophobic Silica Nanoparticle Coatings Exhibit High Water-Repellency and Transparency. Langmuir 2012, 28, 4605–4608. [Google Scholar] [CrossRef]
- Sikora, A.; Shard, A.G.; Minelli, C. Size and ζ-Potential Measurement of Silica Nanoparticles in Serum Using Tunable Resistive Pulse Sensing. Langmuir 2016, 32, 2216–2224. [Google Scholar] [CrossRef]
- Xu, P.; Wang, H.; Tong, R.; Du, Q.; Zhong, W. Preparation and morphology of SiO2/PMMA nanohybrids by microemulsion polymerization. Colloid Polym. Sci. 2006, 284, 755–762. [Google Scholar] [CrossRef]
- Boinovich, L.; Emelyanenko, A. Principles of Design of Superhydrophobic Coatings by Deposition from Dispersions. Langmuir 2009, 25, 2907–2912. [Google Scholar] [CrossRef]
- Ding, K.; Wang, C.; Li, S.; Zhang, X.; Lin, N. Large-area cactus-like micro-/nanostructures with anti-reflection and superhydrophobicity fabricated by femtosecond laser and thermal treatment. Surf. Interfaces 2022, 33, 102292. [Google Scholar] [CrossRef]
- Wang, P.; Li, C.; Zhang, D. Recent advances in chemical durability and mechanical stability of superhydrophobic materials: Multi-strategy design and strengthening. J. Mater. Sci. Technol. 2022, 129, 40–69. [Google Scholar] [CrossRef]
- Song, Y.; Wang, C.; Dong, X.; Yin, K.; Zhang, F.; Xie, Z.; Chu, D.; Duan, J. Controllable superhydrophobic aluminum surfaces with tunable adhesion fabricated by femtosecond laser. Opt. Laser Technol. 2018, 102, 25–31. [Google Scholar] [CrossRef]
- Yessimova, O.; Kumargaliyeva, S.; Kerimkulova, M.; Mussabekov, K.; Toktarbay, Z. Wetting ability of a phytopreparation and their associates with polyelectrolytes. Rasayan J. Chem. 2020, 13, 481–487. [Google Scholar] [CrossRef]
- Toktarbaiuly, O.; Syrlybekov, A.; Mauit, O.; Kurbanova, A.; Sugurbekova, G.; Shvets, I. Magnetic and electronic properties of Fe3O4/PtSe2/Fe3O4 junctions. Mater. Today Proc. 2022, 49, 2469–2473. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).