Protective Cr Coatings with ZrO2/Cr Multilayers for Zirconium Fuel Claddings
Abstract
:1. Introduction
2. Experimental Details
2.1. Sample Preparation
2.2. Thermal Cycling
2.3. High-Temperature Oxidation in a Water Steam
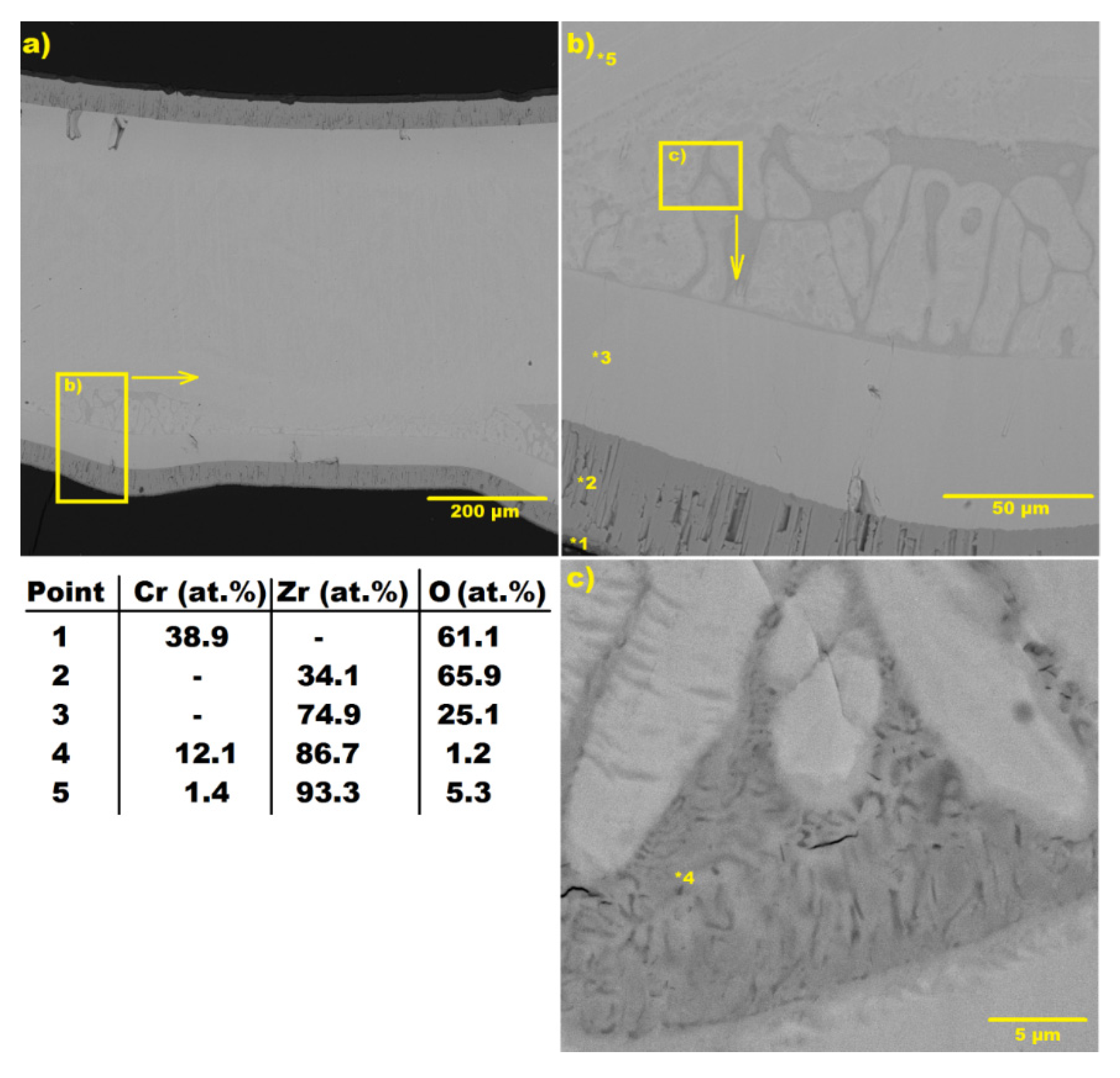
2.4. Sample Characterization
3. Results
3.1. The As-Prepared Coatings
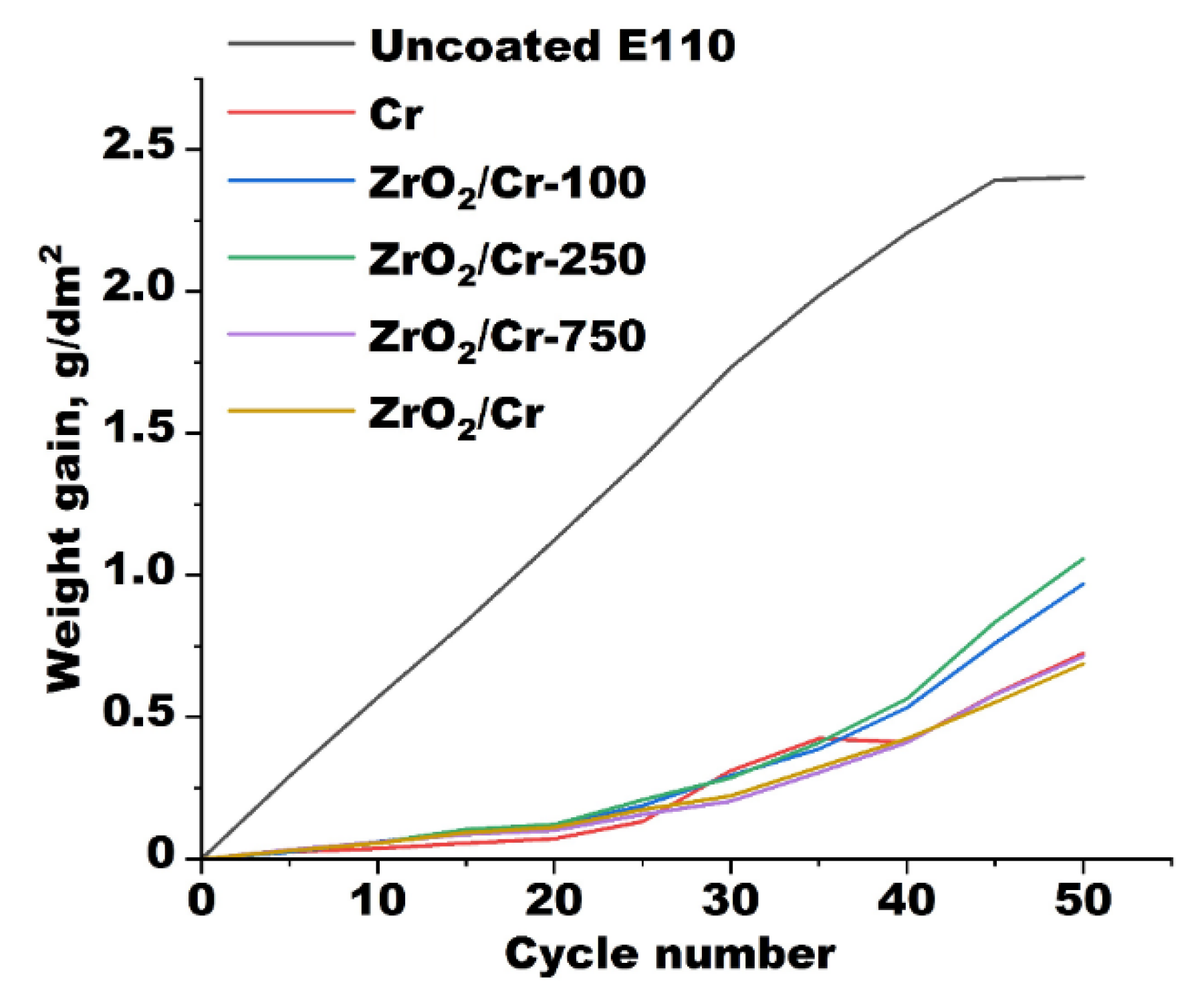
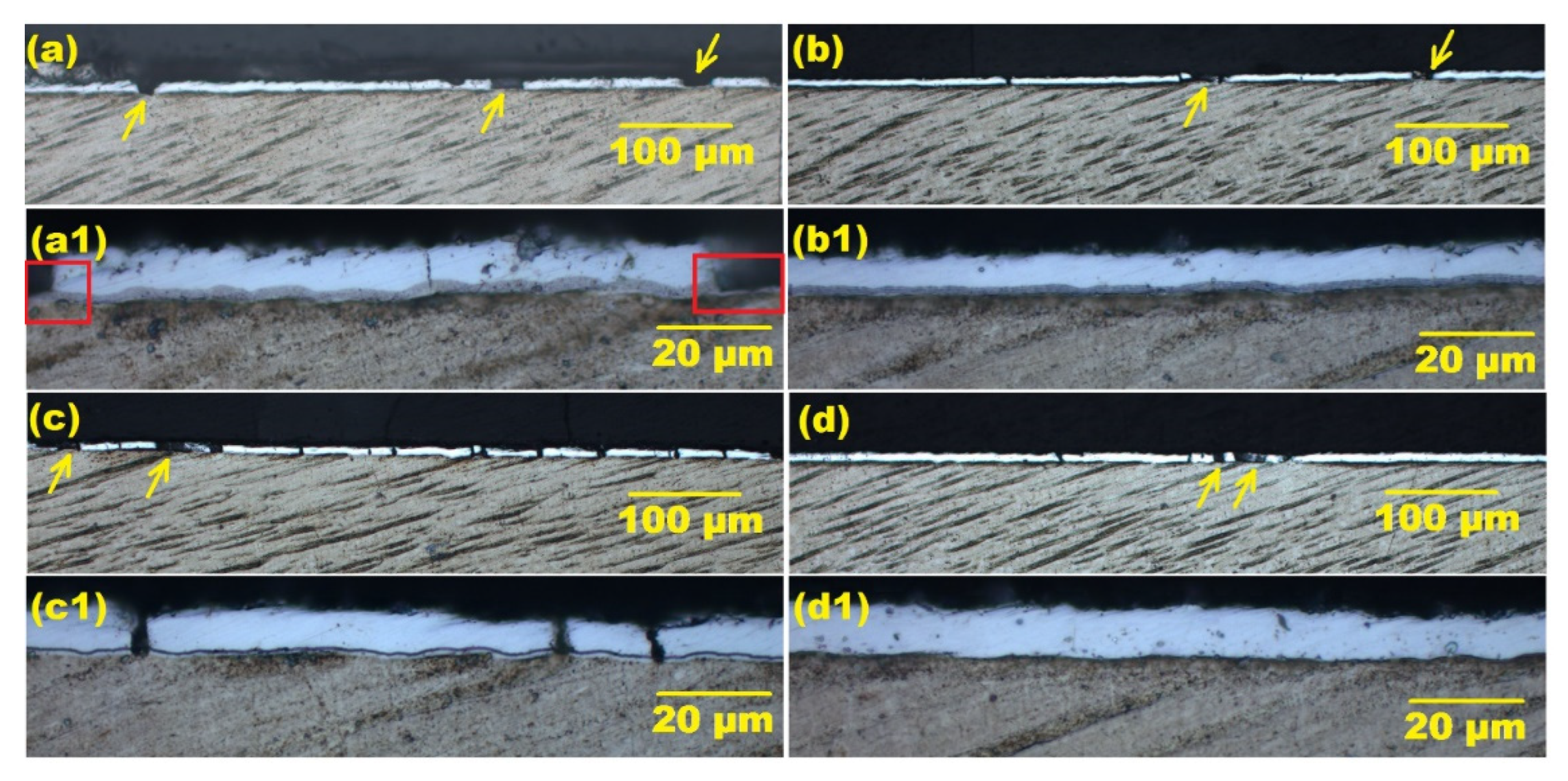
3.2. Thermal Cycling
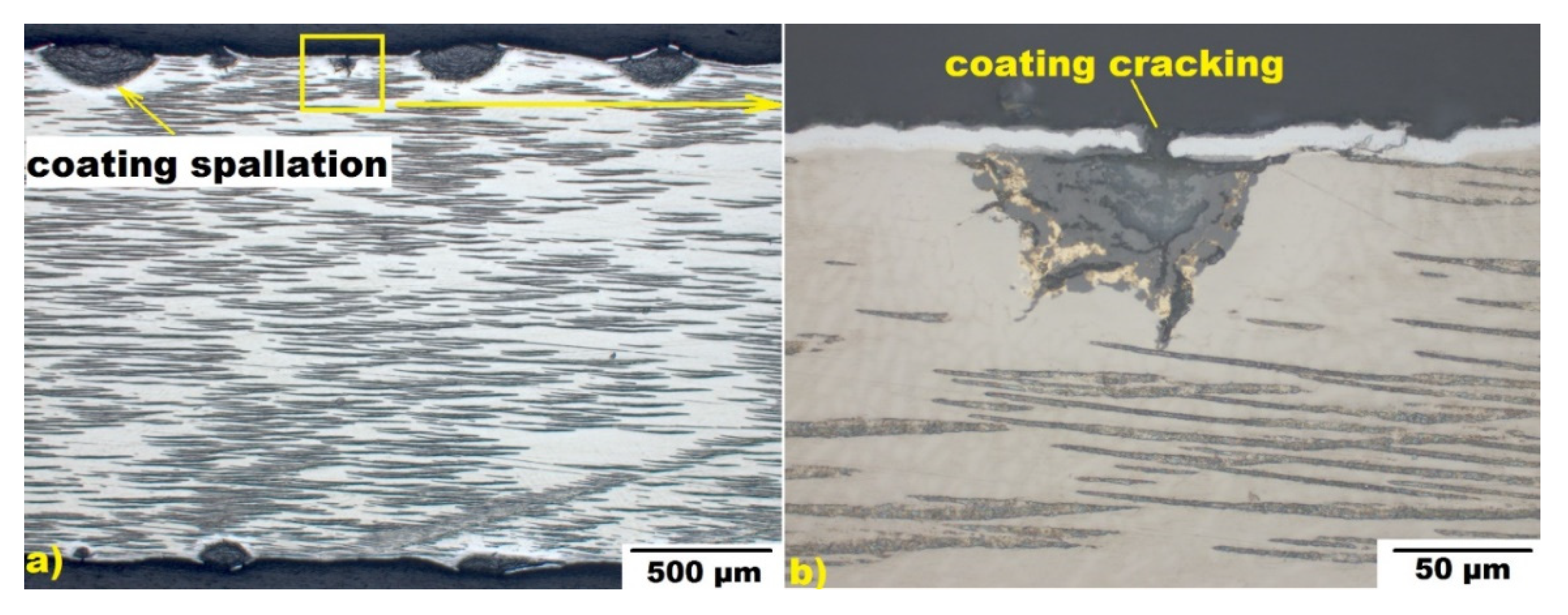
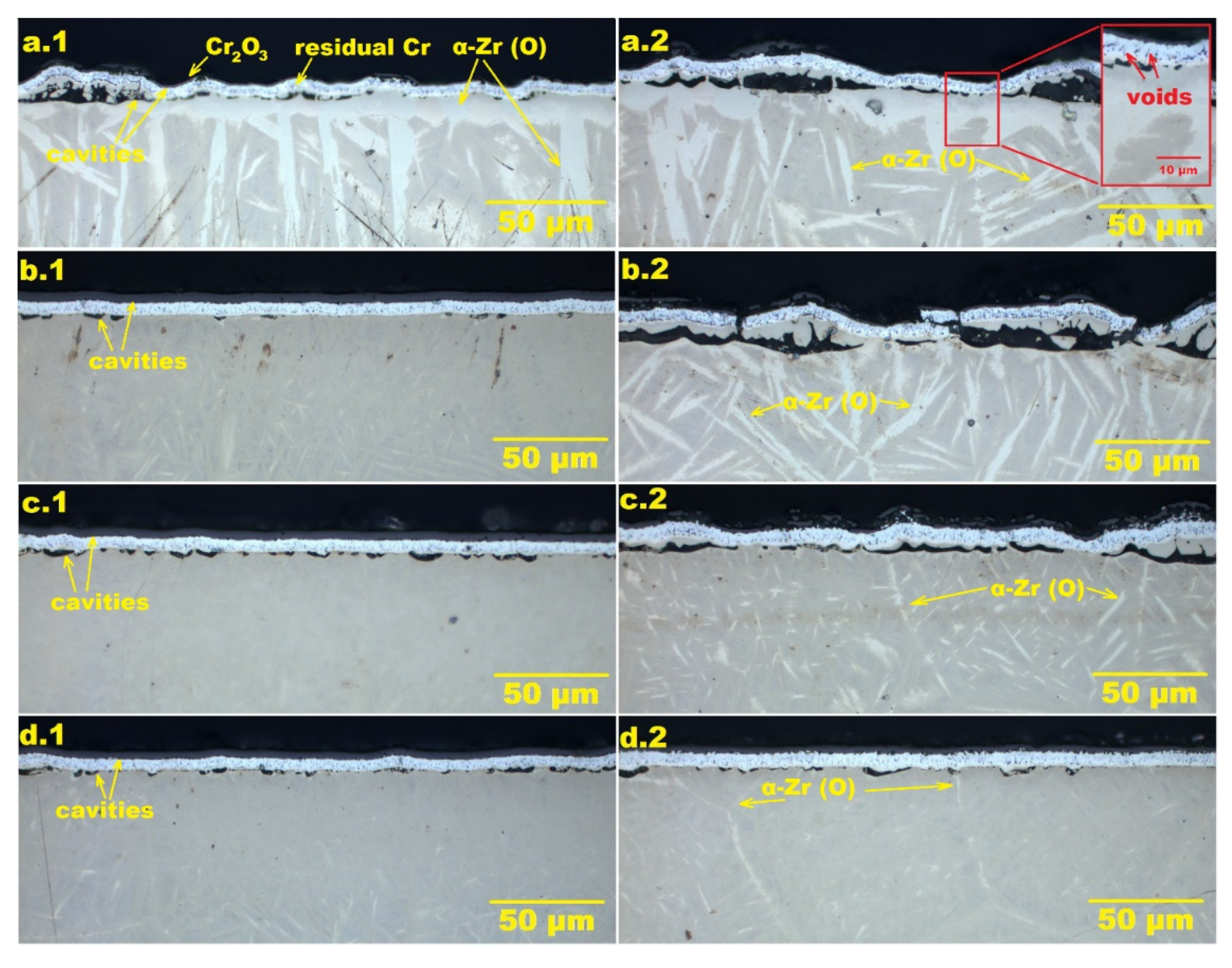
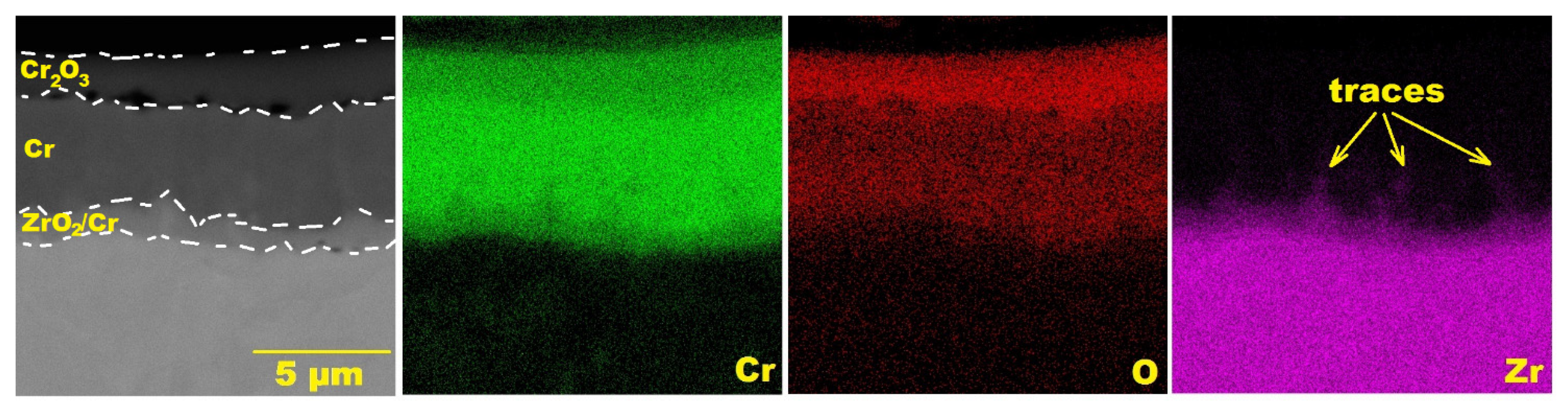
3.3. Steam Oxidation at 1200 °C
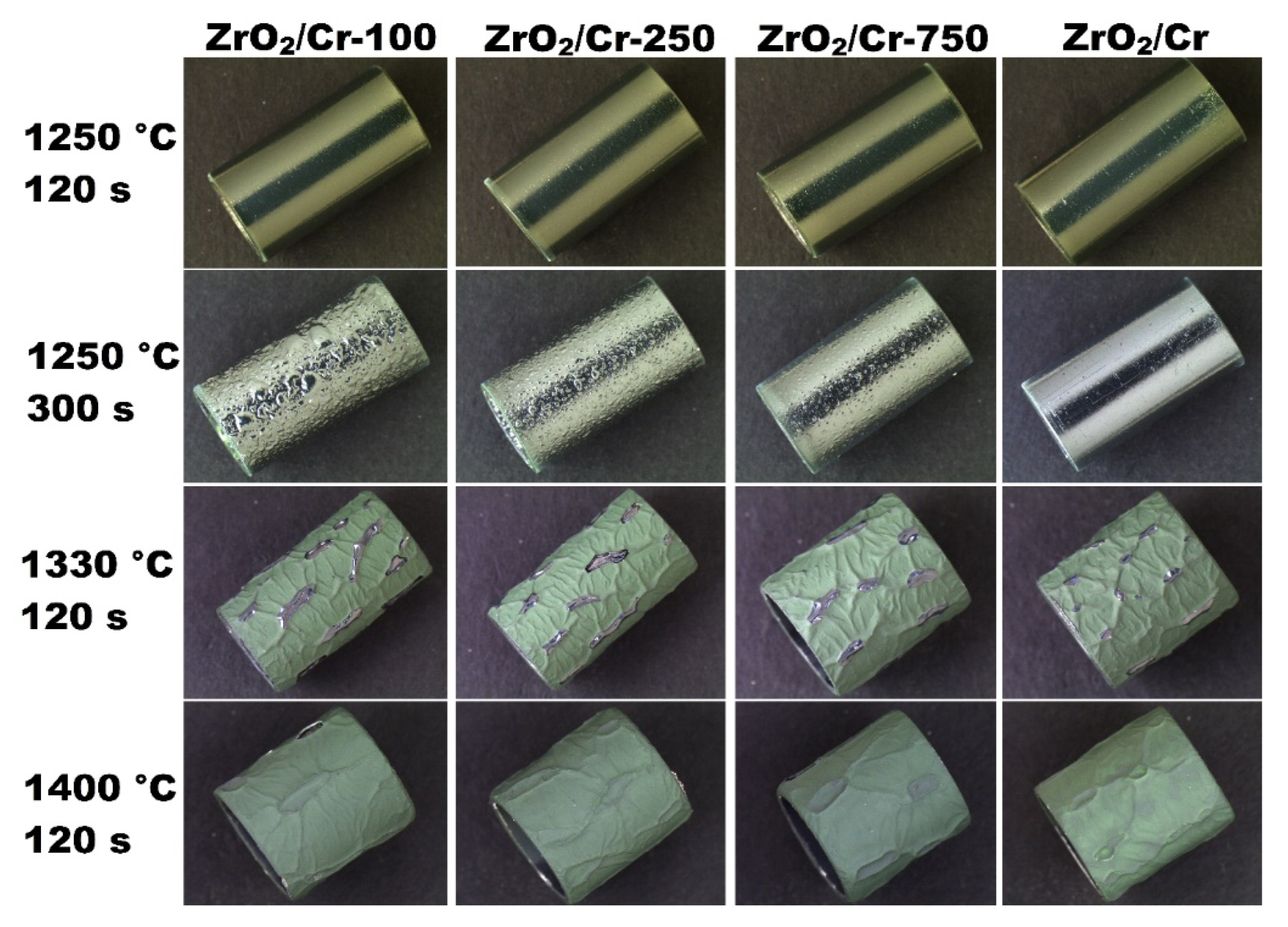
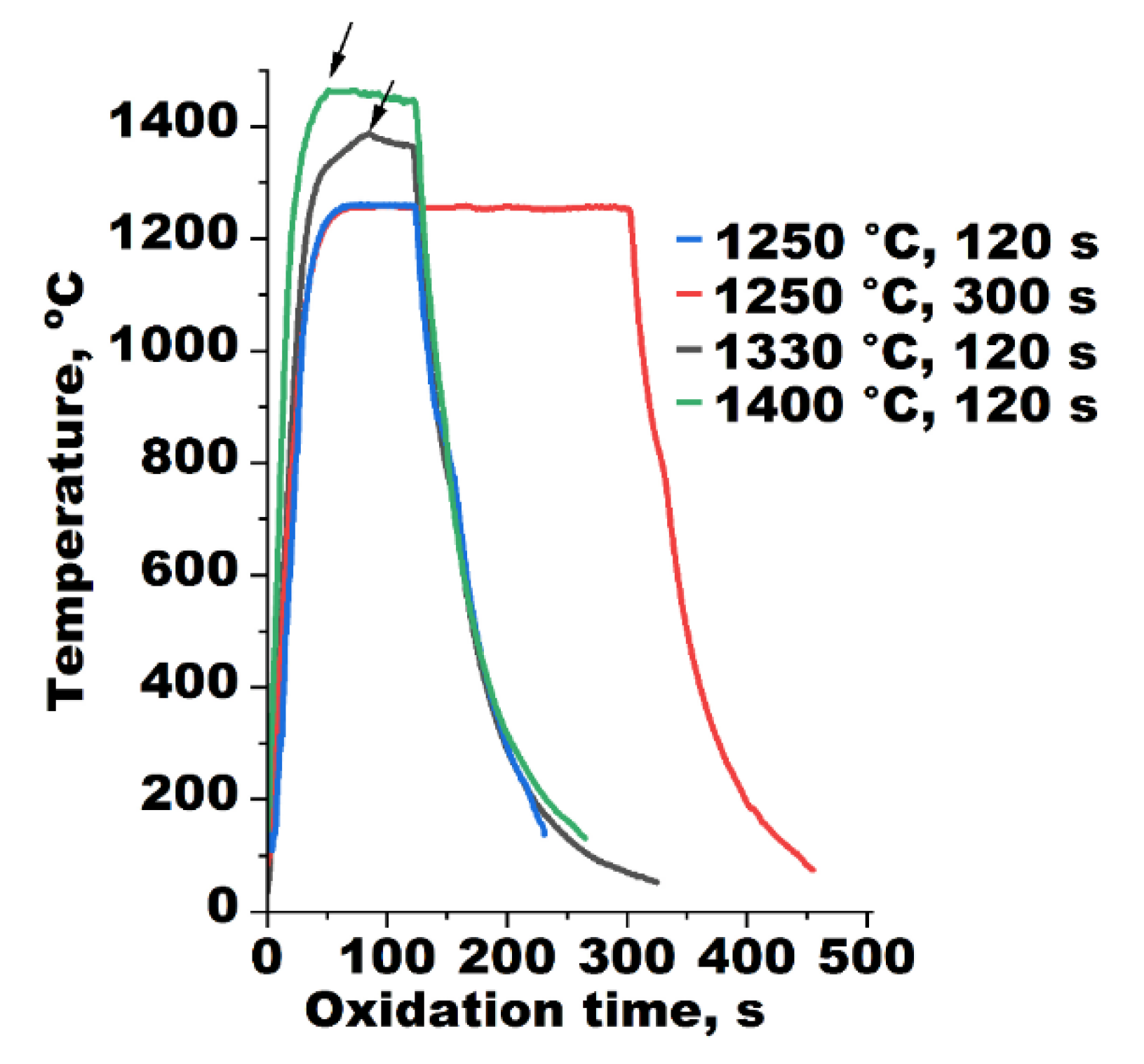
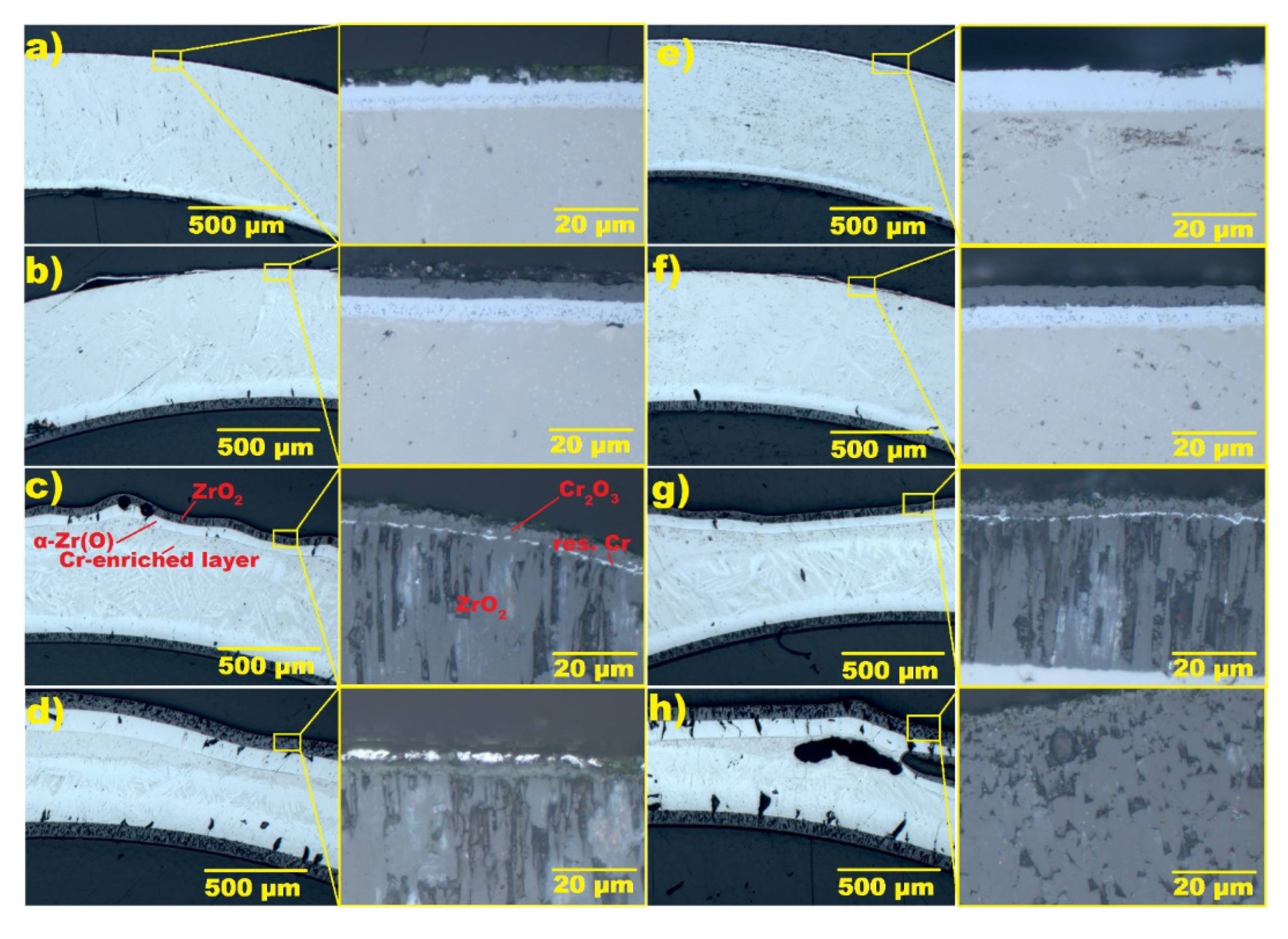
3.4. Steam Oxidation at 1250–1400 °C
3.5. Analysis of the Oxidation Process
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brachet, J.C.; Rouesne, E.; Ribis, J.T.; Guilbert, S.; Urvoy, G. High temperature steam oxidation of chromium-coated zirconium-based alloys: Kinetics and process. Corros. Sci. 2020, 167, 108537. [Google Scholar] [CrossRef]
- Chen, H.; Wang, X.; Zhang, R. Application and development progress of Cr-based surface coatings in nuclear fuel element: I. selection, preparation, and characteristics of coating materials. Coatings 2020, 10, 808. [Google Scholar] [CrossRef]
- Chen, H.; Wang, X.; Zhang, R. Application and development progress of Cr-based surface coating in nuclear fuel elements: II. Current status and shortcomings of performance studies. Coatings 2020, 10, 835. [Google Scholar] [CrossRef]
- Sidelev, D.V.; Kashkarov, E.B.; Syrtanov, M.S.; Krivobokov, V.P. Nickel-chromium (Ni–Cr) coatings deposited by magnetron sputtering for accident tolerant nuclear fuel claddings. Surf. Coat. Technol. 2019, 369, 69–78. [Google Scholar] [CrossRef]
- Kashkarov, E.; Afornu, B.; Sidelev, D.; Krinitcyn, M.; Gouws, V.; Lider, A. Recent advances in protective coatings for accident tolerant Zr-based fuel claddings. Coatings 2021, 11, 557. [Google Scholar] [CrossRef]
- Brachet, J.; Idarraga-Trujillo, I.; Le-Flem, M.; Le-Saux, M.; Vandenberghe, V.; Urvoy, S.; Rouesne, E.; Guilbert, T.; Toffolon-Masclet, C.; Tupin, M.; et al. Early studies on Cr-coated Zircaloy-4 as enhanced accident tolerant nuclear fuel claddings for light water reactors. J. Nucl. Mater. 2019, 517, 268–285. [Google Scholar] [CrossRef]
- Zeng, K.; Hamalainen, M.; Luoma, R. A thermodynamic assessment of the Cr–Zr system. Int. J. Mater. Res. 1993, 94, 23–28. [Google Scholar] [CrossRef]
- Yang, J.; Stegmaier, U.; Tang, C.; Steinbrück, M.; Grobe, M.; Wang, S.; Seifert, H.J. High temperature Cr-Zr interaction of two types of Cr-coated Zr alloys in inert gas environment. J. Nucl. Mater. 2021, 547, 152806. [Google Scholar] [CrossRef]
- Michau, A.; Ougier, M.; Maskrot, H.; Brachet, J.-C.; Guilbert, T.; Palancher, H.; Bischoff, J.; Pouillier, E. Interlayers for Cr-coated nuclear fuel claddings: Preliminary study of Mo. In Proceedings of the NuMat 2020, Ghent, Belgium, 26 October 2018; Volume 1. [Google Scholar] [CrossRef]
- Syrtanov, M.S.; Kashkarov, E.B.; Abdulmenova, A.V.; Sidelev, D.V. High-temperature oxidation of Zr-1Nb zirconium alloy with protective Cr/Mo coating. Surf. Coat. Technol. 2020, 439, 128459. [Google Scholar] [CrossRef]
- Kalin, B.A.; Yashin, A.S.; Dzhumaev, P.S.; Safonov, D.A.; Korenevsky, E.L.; Fedorov, D.A.; Novikov, V.V.; Kuznetsov, V.I.; Fedotov, P.V.; Krivobokov, V.P.; et al. Features of creating wear-resistant anti-corrosion coatings with a barrier layer on fragments of fuel claddings from E110 o.ch. IOP Conf. Series Mater. Sci. Eng. 2020, 1005, 012009. [Google Scholar] [CrossRef]
- Wang, X.; Guan, H.; Liao, Y.; Zhu, M.; Xu, C.; Jin, X.; Liao, B.; Xue, W.; Zhang, Y.; Bai, G.; et al. Enhancement of high temperature steam oxidation resistance of Zr–1Nb alloy with ZrO2/Cr bilayer coating. Corros. Sci. 2021, 187, 109494. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; Han, X.; Fenga, W.; Zhou, X.; Peng, S.; Zhang, H. Oxidation resistance improvement of Zr-4 alloy in 1000 °C steam environment using ZrO2/FeCrAl bilayer coating. Surf. Coat. Technol. 2018, 349, 807–815. [Google Scholar] [CrossRef]
- Krejci, J.; Kabatova, J.; Manoch, F.; Koci, J.; Cvrcek, L.; Malek, J.; Krum, S.; Sutta, P.; Bublikova, P.; Halodova, P. Development and testing of multicomponent fuel cladding with enhanced accidental performance. Nucl. Eng. Technol. 2020, 597, 597–609. [Google Scholar] [CrossRef]
- Pan, X.; Qiu, L.; Hu, X.; Jiang, H. Corrosion and wear properties of Cr coating and ZrO2/Cr bilayer coating on Zr-4 alloy. Coatings 2022, 12, 1281. [Google Scholar] [CrossRef]
- Liu, J.; Hao, Z.; Cui, Z.; Ma, D.; Lu, J.; Cui, Y.; Li, C.; Liu, W.; Xie, S.; Huang, P.; et al. Investigation of the oxidation mechanisms of superlattice Cr-CrN/TiSiN-Cr multilayer coatings on Zircaloy substrates under high-temperature steam atmospheres. Corros. Sci. 2021, 192, 109782. [Google Scholar] [CrossRef]
- Xiao, W.; Chen, H.; Liu, X.; Tang, D.; Deng, H.; Zou, S.; Ren, Y.; Zhou, X.; Lei, M. Thermal shock resistance of TiN-, Cr-, and TiN/Cr-coated zirconium alloy. J. Nucl. Mater. 2019, 526, 151777. [Google Scholar] [CrossRef]
- Sidelev, D.V.; Ruchkin, S.E.; Syrtanov, M.S.; Kashkarov, E.B.; Shelepov, I.A.; Malgin, A.G.; Polunin, K.K.; Stoykov, K.V.; Mokrushin, A.A. Protective Cr coatings with CrN/Cr multilayers for zirconium fuel claddings. Surf. Coat. Technol. 2022, 433, 128131. [Google Scholar] [CrossRef]
- Sidelev, D.V.; Syrtanov, M.S.; Ruchkin, S.E.; Pirozhkov, A.V.; Kashkarov, E.B. Protection of Zr alloy under high-temperature air oxidation: A multilayer coating approach. Coatings 2021, 11, 227. [Google Scholar] [CrossRef]
- Syrtanov, M.; Pirozhkov, A.; Sidelev, D. In-situ phase transformations in CrN/Cr-coated E110 alloy under high temperature. Eng. Mater. 2022, 910, 940–946. [Google Scholar] [CrossRef]
- Li, Z.; Liu, C.; Chen, Q.; Yang, J.; Liu, J.; Yang, H.; Zhang, W.; Zhang, R.; He, L.; Long, J.; et al. Microstructure, high-temperature corrosion and steam oxidation properties of Cr/CrN multilayer coatings prepared by magnetron sputtering. Corros. Sci. 2021, 191, 109755. [Google Scholar] [CrossRef]
- Xiang, Y.; Liu, C.; Li, Z.; Liu, H.; Yang, J.; Yang, H.; Zhang, R.; He, L.; Liu, J.; Long, J.; et al. Interface stability and microstructural evolution of the (Cr/CrN)24-coated zirconium alloy under different thermal shock temperatures. Surf. Coat. Technol. 2022, 429, 127947. [Google Scholar] [CrossRef]
- Procedure for Conducting Oxidation and Post-Quench Ductility Tests with Zirconium-Based Cladding Alloys; Argonne National Laboratory: Lemont, IL, USA, 2009. Available online: http://pbadupws.nrc.gov/docs/ML0909/ML090900841.pdf (accessed on 1 April 2021).
- Polunin, K.K.; Mokrushin, A.A.; Urusov, A.A. Study of high temperature oxidation of zirconium fuel element claddings with chromiferous coatings. Mater. Sci. Eng. 2020, 1005, 012002. [Google Scholar] [CrossRef]
- Gong, W.; Zhang, H.; Wu, C.; Tian, H.; Wang, X. The role of alloying elements in the initiation of nanoscale porosity in oxide films formed on zirconium alloys. Corros. Sci. 2013, 77, 391–396. [Google Scholar] [CrossRef]
- Pawlewicz, W.T.; Hays, D.D. Microstructure control for sputter-deposited ZrO2, ZrO2•CaO and ZrO2•Y2O3. Thin Solid Film. 1982, 94, 31–45. [Google Scholar] [CrossRef]
- Ribis, J.; Wu, A.; Brachet, J.-C.; Barceló, F.; Arnal, B. Atomic-scale interface structure of a Cr-coated Zircaloy-4 material. J. Mater. Sci. 2018, 53, 9879–9895. [Google Scholar] [CrossRef]
- Brachet, J.C.; Guilbert, T.; Saux, M.L.; Rousselot, J.; Nony, G.; Toffolon-Masclet, C.; Michau, A.; Schuster, F.; Palancher, H.; Bischoff, J.; et al. Behavior of Cr-coated M5 claddings during and after high temperature steam oxidation from 800 °C up to 1500 °C (LOss-of-coolant accident & design extension conditions). In Proceedings of the WRFPM/TOPFUEL, Prague, Czech Republic, 30 September 2018; Volume 30. [Google Scholar]
- Duan, Z.; Yang, H.; Satoh, Y.; Murakami, K.; Kano, S.; Zhao, Z.; Shen, J.; Abe, H. Current status of materials development of nuclear fuel cladding tubes for light water reactors. Nucl. Eng. Des. 2017, 316, 131–150. [Google Scholar] [CrossRef]
- Abriata, J.P.; Garces, J.; Versaci, R. The O–Zr (oxygen-zirconium) system. Bull. Alloy Phase Diagr. 1986, 7, 116–124. [Google Scholar] [CrossRef]
- Liu, J.; Tang, C.; Steinbrück, M.; Yang, J.; Stegmaier, U.; Große, M.; Yun, D.; Seifert, H.J. Transient experiments on oxidation and degradation of Cr-coated Zircaloy in steam up to 1600 °C. Corros. Sci. 2021, 192, 109805. [Google Scholar] [CrossRef]
- Barin, I. Thermochemical Data of Pure Substances; VCH: Weinheim, Germany, 1989. [Google Scholar]
- Han, X.; Xue, J.; Peng, S.; Zhang, H. An interesting oxidation phenomenon of Cr coatings on Zry-4 substrates in high temperature steam environment. Corros. Sci. 2019, 156, 117–124. [Google Scholar] [CrossRef]
- Liu, J.; Steinbrück, M.; Große, M.; Stegmaier, U.; Tang, C.; Yun, D.; Yang, Y.; Cui, Y.; Seifert, H.S. Systematic investigations on the coating degradation mechanism during the steam oxidation of Cr-coated Zry-4 at 1200 °C. Corros. Sci. 2022, 202, 110310. [Google Scholar] [CrossRef]






| Sample | Barrier Layer | h(Cr), µm | Ub, V | js, mA/cm2 | t, h | Ts, °C | |
|---|---|---|---|---|---|---|---|
| N | h, nm | ||||||
| ZrO2/Cr | 1 | 1500 | 8.5 ± 0.2 | −50 | 72 | 6.9 | 346 |
| ZrO2/Cr-100 | 30 | 100 | 6.9 ± 0.2 | 72 | 6.6 | 343 | |
| ZrO2/Cr-250 | 12 | 250 | 7.0 ± 0.1 | 71 | 6.7 | 348 | |
| ZrO2/Cr-750 | 4 | 750 | 6.9 ± 0.1 | 73 | 6.8 | 345 | |
| Sample | Cr [18] | ZrO2/Cr | ZrO2/Cr-100 | ZrO2/Cr-250 | ZrO2/Cr-750 |
|---|---|---|---|---|---|
| Lc, mm−1 | 4.6 | 2.0 | 2.1 | 9.3 | 15.1 |
| Ls, mm−1 | - | 1.2 | 5.0 | 3.5 | 2.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sidelev, D.V.; Ruchkin, S.E.; Shelepov, I.A.; Saburov, N.S.; Malgin, A.G.; Polunin, K.K.; Stoykov, K.V.; Mokrushin, A.A. Protective Cr Coatings with ZrO2/Cr Multilayers for Zirconium Fuel Claddings. Coatings 2022, 12, 1409. https://doi.org/10.3390/coatings12101409
Sidelev DV, Ruchkin SE, Shelepov IA, Saburov NS, Malgin AG, Polunin KK, Stoykov KV, Mokrushin AA. Protective Cr Coatings with ZrO2/Cr Multilayers for Zirconium Fuel Claddings. Coatings. 2022; 12(10):1409. https://doi.org/10.3390/coatings12101409
Chicago/Turabian StyleSidelev, Dmitrii Vladimirovich, Sergey Evgeneyvich Ruchkin, Ivan Andreevich Shelepov, Nikolay Sergeevich Saburov, Andrey Gennadievich Malgin, Kirill Konstantinovich Polunin, Kirill Vicheslavovich Stoykov, and Andrey Andreevich Mokrushin. 2022. "Protective Cr Coatings with ZrO2/Cr Multilayers for Zirconium Fuel Claddings" Coatings 12, no. 10: 1409. https://doi.org/10.3390/coatings12101409
APA StyleSidelev, D. V., Ruchkin, S. E., Shelepov, I. A., Saburov, N. S., Malgin, A. G., Polunin, K. K., Stoykov, K. V., & Mokrushin, A. A. (2022). Protective Cr Coatings with ZrO2/Cr Multilayers for Zirconium Fuel Claddings. Coatings, 12(10), 1409. https://doi.org/10.3390/coatings12101409







