Physical Vapor Deposition Technology in Personal Protective Equipment Production: Improved Antibacterial and Hydrophobic Character of Textiles
Abstract
1. Introduction
2. Materials and Methods
2.1. Coatings Preparation
2.2. Characterization Analysis
3. Results and Discussion
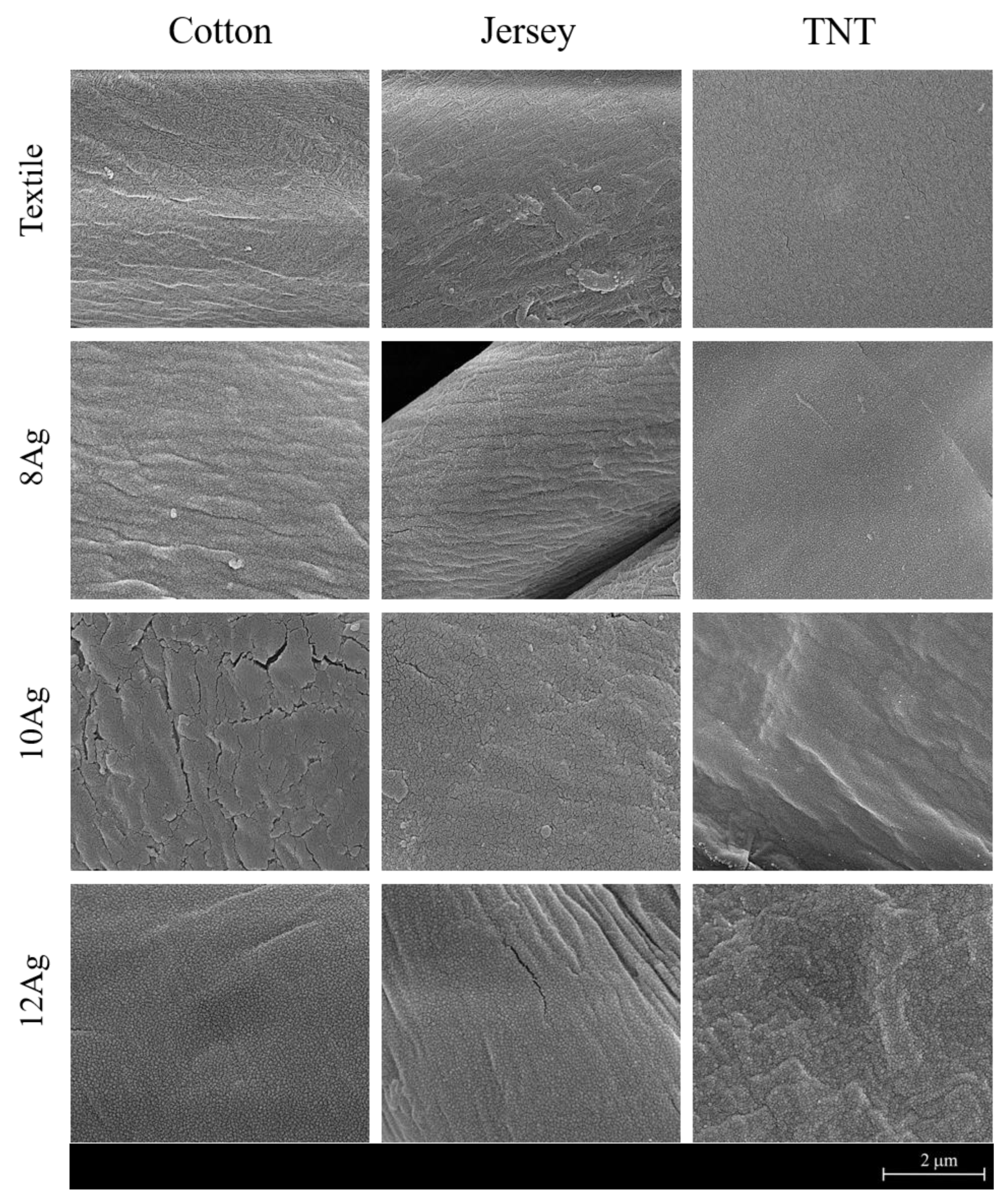
3.1. Chemical and Morphological Analysis
3.2. Wettability
3.3. Cytotoxicity Evaluation
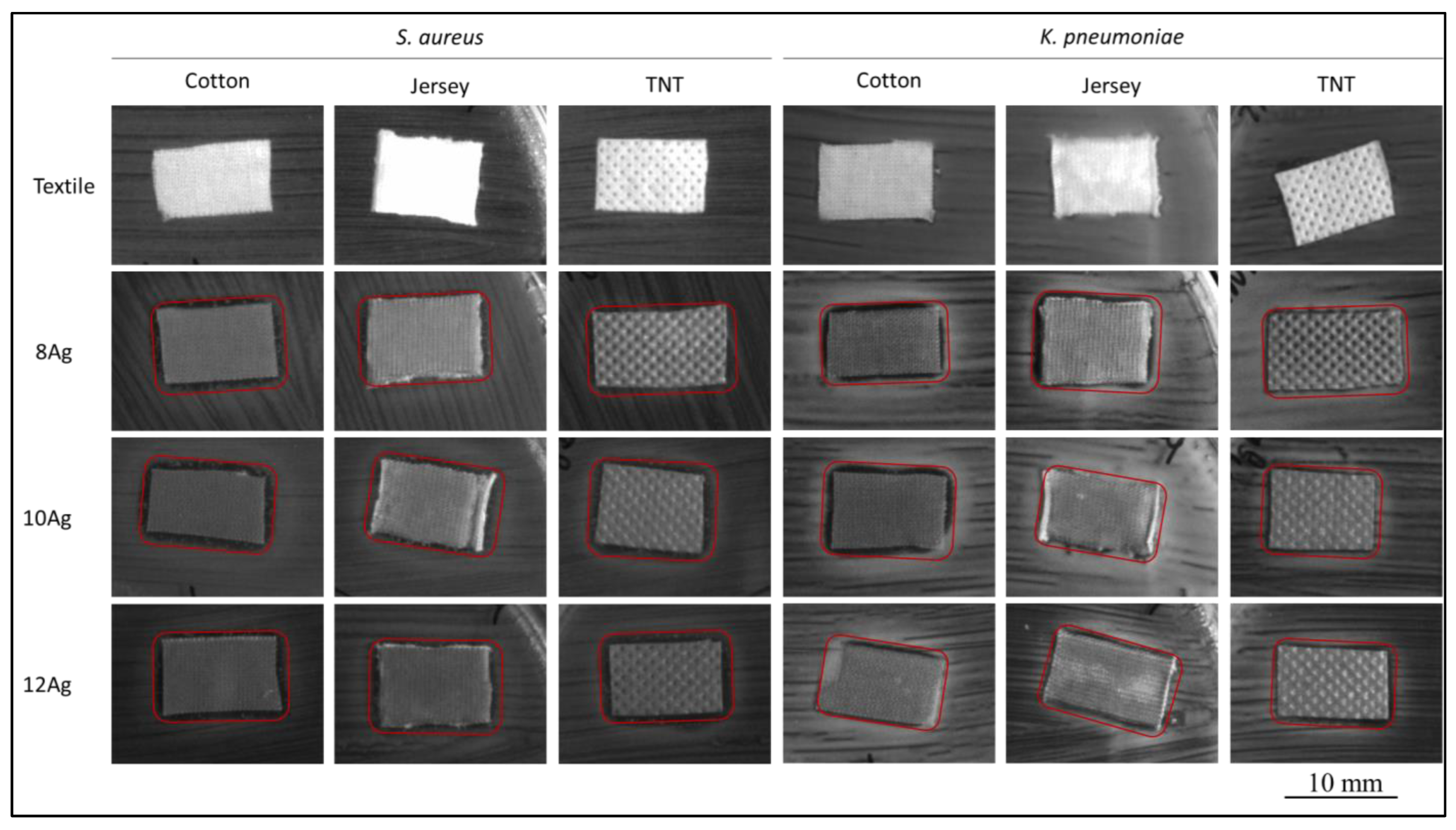
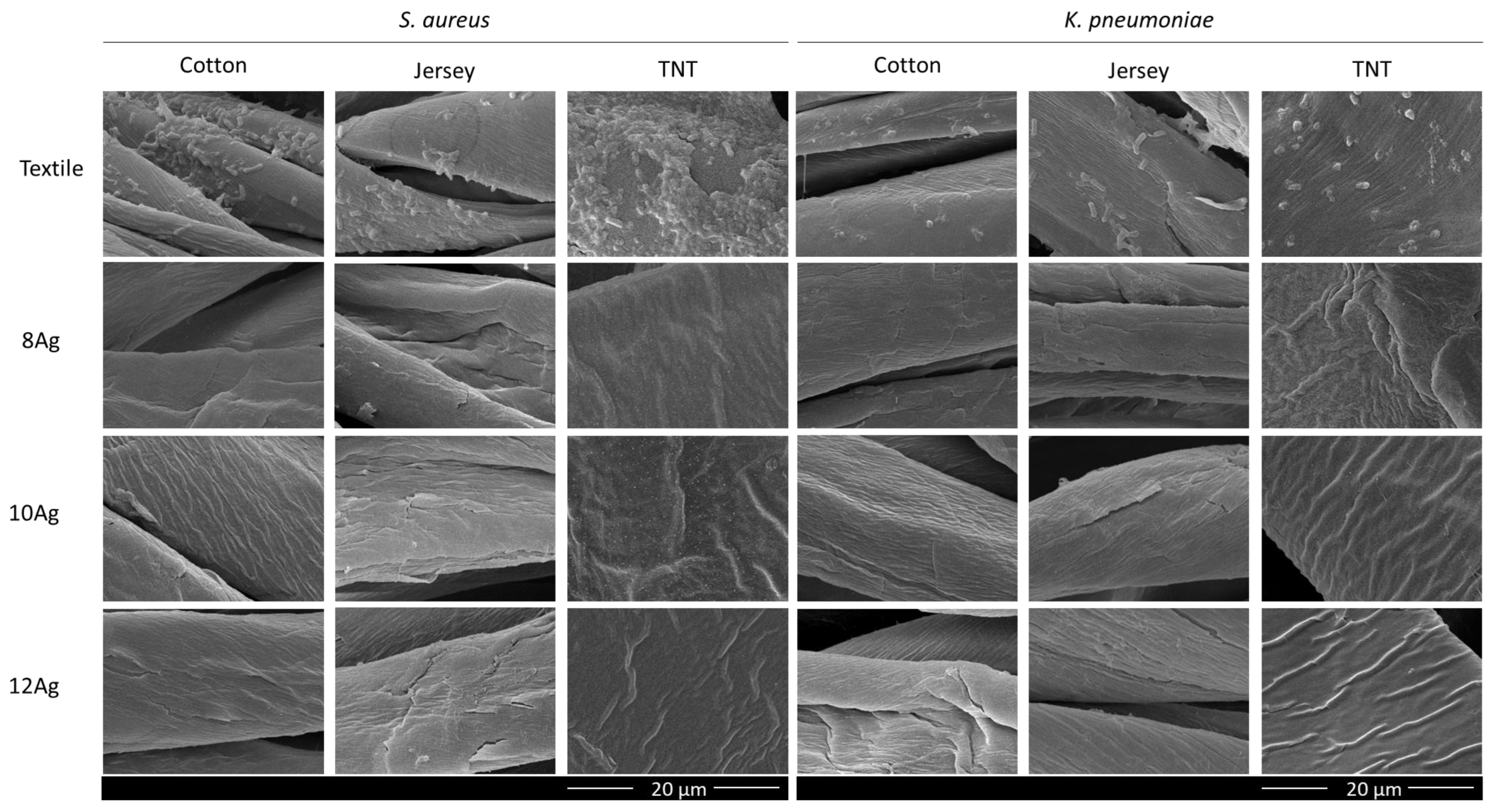
3.4. Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Suetens, C.; Latour, K.; Kärki, T.; Ricchizzi, E.; Kinross, P.; Moro, M.L.; Jans, B.; Hopkins, S.; Hansen, S.; Lyytikäinen, O.; et al. Prevalence of healthcare-associated infections, estimated incidence and composite antimicrobial resistance index in acute care hospitals and long-term care facilities: Results from two European point prevalence surveys, 2016 to 2017. Eurosurveillance 2018, 23, 1800516. [Google Scholar] [CrossRef] [PubMed]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
- Chua, M.H.; Cheng, W.; Goh, S.S.; Kong, J.; Li, B.; Lim, J.Y.C.; Mao, L.; Wang, S.; Xue, K.; Yang, L.; et al. Face Masks in the New COVID-19 Normal: Materials, Testing, and Perspectives. Research 2020, 2020, 7286735. [Google Scholar] [CrossRef] [PubMed]
- Karim, N.; Afroj, S.; Lloyd, K.; Oaten, L.C.; Andreeva, D.V.; Carr, C.; Farmery, A.D.; Kim, I.D.; Novoselov, K.S. Sustainable Personal Protective Clothing for Healthcare Applications: A Review. ACS Nano 2020, 14, 12313–12340. [Google Scholar] [CrossRef]
- O’Dowd, K.; Nair, K.M.; Forouzandeh, P.; Mathew, S.; Grant, J.; Moran, R.; Bartlett, J.; Bird, J.; Pillai, S.C. Face Masks and Respirators in the Fight against the COVID-19 Pandemic: A Review of Current Materials, Advances and Future Perspectives. Materials 2020, 13, 3363. [Google Scholar] [CrossRef]
- Morais, D.; Guedes, R.; Lopes, M. Antimicrobial Approaches for Textiles: From Research to Market. Materials 2016, 9, 498. [Google Scholar] [CrossRef]
- Guo, Z.D.; Wang, Z.Y.; Zhang, S.F.; Li, X.; Li, L.; Li, C.; Cui, Y.; Fu, R.B.; Dong, Y.Z.; Chi, X.Y.; et al. Aerosol and Surface Distribution of Severe Acute Respiratory Syndrome Coronavirus 2 in Hospital Wards, Wuhan, China, 2020. Emerg. Infect. Dis. 2020, 56, 1586. [Google Scholar] [CrossRef]
- Bshena, O.; Heunis, T.; Dicks, L.; Klumperman, B. Antimicrobial fibers: Therapeutic possibilities and recent advances. Future Med. Chem. 2011, 3, 1821–1847. [Google Scholar] [CrossRef]
- Windler, L.; Height, M.; Nowack, B. Comparative evaluation of antimicrobials for textile applications. Environ. Int. 2013, 53, 62–73. [Google Scholar] [CrossRef]
- Bhandari, V.; Jose, S.; Badanayak, P.; Sankaran, A.; Anandan, V. Antimicrobial Finishing of Metals, Metal Oxides, and Metal Composites on Textiles: A Systematic Review. Ind. Eng. Chem. Res. 2022, 61, 86–101. [Google Scholar] [CrossRef]
- Antunes, J.; Moreira, I.; Gomes, F.; Cunha, F.; Henriques, M.; Fangueiro, R. Recent Trends in Protective Textiles against Biological Threats: A Focus on Biological Warfare Agents. Polymers 2022, 14, 1599. [Google Scholar] [CrossRef] [PubMed]
- Andra, S.; Balu, S.; Jeevanandam, J.; Muthalagu, M. Emerging nanomaterials for antibacterial textile fabrication. Naunyn-Schmiedebergs Arch. Pharmacol. 2021, 394, 1355–1382. [Google Scholar] [CrossRef] [PubMed]
- Raza, Z.; Taqi, M.; Tariq, M. Antibacterial agents applied as antivirals in textile-based PPE: A narrative review. J. Text. Inst. 2022, 113, 515–526. [Google Scholar] [CrossRef]
- Li, G.; Liu, H.; Li, T.; Wang, J. Surface modification and functionalization of silk fibroin fibers/fabric toward high performance applications. Mater. Sci. Eng. C-Mater. Biol. Appl. 2012, 32, 627–636. [Google Scholar] [CrossRef]
- Wang, H.; Wei, Q.; Gao, W. Sputter Deposition of Antibacterial Nano-Silver on PLA Nonwoven Medical Dressings. AATCC Rev. 2009, 9, 34–36. [Google Scholar]
- Abd Jelil, R. A review of low-temperature plasma treatment of textile materials. J. Mater. Sci. 2015, 50, 5913–5943. [Google Scholar] [CrossRef]
- Shahidi, S.; Moazzenchi, B.; Ghoranneviss, M. A review-application of physical vapor deposition (PVD) and related methods in the textile industry. Eur. Phys. J. Appl. Phys. 2015, 71, 31302. [Google Scholar] [CrossRef]
- Tan, X.; Liu, J.; Niu, J.; Tian, J. Recent Progress in Magnetron Sputtering Technology Used on Fabrics. Materials 2018, 11, 1953. [Google Scholar] [CrossRef]
- Liu, Y.; Leng, J.; Wu, Q.; Zhang, S.; Teng, X. Investigation on the properties of nano copper matrix composite via vacuum arc melting method. Mater. Res. Express 2017, 4, 106512. [Google Scholar] [CrossRef]
- Scholz, J.; Nocke, G.; Hollstein, F.; Weissbach, A. Investigations on fabrics coated with precious metals using the magnetron sputter technique with regard to their anti-microbial properties. Surf. Coat. Technol. 2005, 192, 252–256. [Google Scholar] [CrossRef]
- Wei, Q.; Xu, Y.; Wang, Y. 3–Textile surface functionalization by physical vapor deposition (PVD). In Surface Modification of Textiles; Wei, Q., Ed.; Woodhead Publishing: Sawston, UK, 2009; pp. 58–90. [Google Scholar]
- Al-Jumaili, A.; Alancherry, S.; Bazaka, K.; Jacob, M. Review on the Antimicrobial Properties of Carbon Nanostructures. Materials 2017, 10, 1066. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, M.; Mozafari, M. Biological Response to Carbon-Family Nanomaterials: Interactions at the Nano-Bio Interface. Front. Bioeng. Biotechnol. 2019, 7, 4. [Google Scholar] [CrossRef]
- Hauert, R. An overview on the tribological behavior of diamond-like carbon in technical and medical applications. Tribol. Int. 2004, 37, 991–1003. [Google Scholar] [CrossRef]
- Narayan, R.J. Laser processing of diamondlike carbon thin films for medical prostheses. Int. Mater. Rev. 2006, 51, 127–143. [Google Scholar] [CrossRef]
- Shim, J.W.; Bae, I.H.; Jeong, M.H.; Park, D.S.; Lim, K.S.; Kim, J.U.; Kim, M.K.; Kim, J.H.; Sim, D.S. Effects of a Titanium Dioxide Thin Film for Improving the Biocompatibility of Diamond-Like Coated Coronary Stents. Met. Mater. Int. 2020, 26, 1455–1462. [Google Scholar] [CrossRef]
- Bito, K.; Hasebe, T.; Maegawa, S.; Maeda, T.; Matsumoto, T.; Suzuki, T.; Hotta, A. In vitro basic fibroblast growth factor (bFGF) delivery using an antithrombogenic 2-methacryloyloxyethyl phosphorylcholine (MPC) polymer coated with a micropatterned diamond-like carbon (DLC) film. J. Biomed. Mater. Res. Part A 2017, 105, 3384–3391. [Google Scholar] [CrossRef]
- Carvalho, I.; Curado, M.; Palacio, C.; Carvalho, S.; Cavaleiro, A. Ag release from sputtered Ag/a:C nanocomposite films after immersion in pure water and NaCl solution. Thin Solid Film. 2019, 671, 85–94. [Google Scholar] [CrossRef]
- Manninen, N.; Calderon, S.; Carvalho, I.; Henriques, M.; Cavaleiro, A.; Carvalho, S. Antibacterial Ag/a-C nanocomposite coatings: The influence of nano-galvanic a-C and Ag couples on Ag ionization rates. Appl. Surf. Sci. 2016, 377, 283–291. [Google Scholar] [CrossRef]
- Virk, R.K.; Ramaswamy, G.N.; Bourham, M.; Bures, B.L. Plasma and Antimicrobial Treatment of Nonwoven Fabrics for Surgical Gowns. Text. Res. J. 2004, 74, 1073–1079. [Google Scholar] [CrossRef]
- Manninen, N.; Galindo, R.; Benito, N.; Figueiredo, N.; Cavaleiro, A.; Palacio, C.; Carvalho, S. Ag-Ti(C, N)-based coatings for biomedical applications: Influence of silver content on the structural properties. J. Phys. D-Appl. Phys. 2011, 44, 375501. [Google Scholar] [CrossRef]
- Manninen, N.K.; Ribeiro, F.; Escudeiro, A.; Polcar, T.; Carvalho, S.; Cavaleiro, A. Influence of Ag content on mechanical and tribological behavior of DLC coatings. Surf. Coat. Technol. 2013, 232, 440–446. [Google Scholar] [CrossRef]
- Manninen, N.K.; Galindo, R.E.; Carvalho, S.; Cavaleiro, A. Silver surface segregation in Ag-DLC nanocomposite coatings. Surf. Coat. Technol. 2015, 267, 90–97. [Google Scholar] [CrossRef]
- Calderon, S.; Ferreri, I.; Henriques, M.; De Hosson, J.; Cavaleiro, A.; Carvalho, S. Nano-galvanic coupling for enhanced Ag+ release in ZrCN-Ag films: Antibacterial application. Surf. Coat. Technol. 2016, 298, 1–6. [Google Scholar] [CrossRef]
- Carvalho, I.; Faraji, M.; Ramalho, A.; Carvalho, A.; Carvalho, S.; Cavaleiro, A. Ex-vivo studies on friction behaviour of ureteral stent coated with Ag clusters incorporated in a:C matrix. Diam. Relat. Mater. 2018, 86, 1–7. [Google Scholar] [CrossRef]
- Carvalho, I.; Dias, N.; Henriques, M.; Calderon, V.; Ferreira, P.; Cavaleiro, A.; Carvalho, S. Antibacterial Effects of Bimetallic Clusters Incorporated in Amorphous Carbon for Stent Application. ACS Appl. Mater. Interfaces 2020, 12, 24555–24563. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, I.; Rodrigues, L.; Lima, M.J.; Carvalho, S.; Cruz, S.M.A. Overview on the Antimicrobial Activity and Biocompatibility of Sputtered Carbon-Based Coatings. Processes 2021, 9, 1428. [Google Scholar] [CrossRef]
- Marques, S.; Carvalho, I.; Leite, T.; Henriques, M.; Carvalho, S. Antimicrobial TiN-Ag Coatings in Leather Insole for Diabetic Foot. Materials 2022, 15, 2009. [Google Scholar] [CrossRef]
- Pokhrel, L.; Jacobs, Z.; Dikin, D.; Akula, S. Five nanometer size highly positive silver nanoparticles are bactericidal targeting cell wall and adherent fimbriae expression. Sci. Rep. 2022, 12, 1–11. [Google Scholar] [CrossRef]
- Ferdous, Z.; Nemmar, A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef]
- Hadrup, N.; Sharma, A.; Loeschner, K. Toxicity of silver ions, metallic silver, and silver nanoparticle materials after in vivo dermal and mucosal surface exposure: A review. Regul. Toxicol. Pharmacol. 2018, 98, 257–267. [Google Scholar] [CrossRef]

| Sample | JC of C (W/mm2) | JC of Ag (W/mm2) | Deposition Time (min) | Deposition Rate (nm/min) | Ag (at. %) |
|---|---|---|---|---|---|
| 0Ag | 67 × 10−3 | - | 3.0 | 17 | 0 |
| 8Ag | 3 × 10−3 | 2.7 | 19 | 8 | |
| 10Ag | 5 × 10−3 | 2.7 | 19 | 10 | |
| 12Ag | 7 × 10−3 | 2.7 | 19 | 12 |
| Sample | Substrate/Water Contact Angle (θ) | ||
|---|---|---|---|
| Cotton | Jersey | TNT | |
| Textile | 62° (±7) | 128° (±3) | 126° (±4) |
| 0Ag | 117° (±5) | 127° (±4) | 120° (±2) |
| 8Ag | 108° (±5) | 108° (±14) | 99° (±4) |
| 10Ag | 122° (±2) | 95° (±2) | 110° (±2) |
| 12Ag | 126° (±3) | 120° (±10) | 121° (±3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antunes, J.; Matos, K.; Carvalho, I.; Carvalho, S.; Ferreira, F.; Cruz, S.M.A. Physical Vapor Deposition Technology in Personal Protective Equipment Production: Improved Antibacterial and Hydrophobic Character of Textiles. Coatings 2022, 12, 1399. https://doi.org/10.3390/coatings12101399
Antunes J, Matos K, Carvalho I, Carvalho S, Ferreira F, Cruz SMA. Physical Vapor Deposition Technology in Personal Protective Equipment Production: Improved Antibacterial and Hydrophobic Character of Textiles. Coatings. 2022; 12(10):1399. https://doi.org/10.3390/coatings12101399
Chicago/Turabian StyleAntunes, José, Karim Matos, Isabel Carvalho, Sandra Carvalho, Fábio Ferreira, and Sandra M. A. Cruz. 2022. "Physical Vapor Deposition Technology in Personal Protective Equipment Production: Improved Antibacterial and Hydrophobic Character of Textiles" Coatings 12, no. 10: 1399. https://doi.org/10.3390/coatings12101399
APA StyleAntunes, J., Matos, K., Carvalho, I., Carvalho, S., Ferreira, F., & Cruz, S. M. A. (2022). Physical Vapor Deposition Technology in Personal Protective Equipment Production: Improved Antibacterial and Hydrophobic Character of Textiles. Coatings, 12(10), 1399. https://doi.org/10.3390/coatings12101399









