The Combined Effect of Hot Water Treatment and Chitosan Coating on Mango (Mangifera indica L. cv. Kent) Fruits to Control Postharvest Deterioration and Increase Fruit Quality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Measurements of Fruit Physical and Chemical Features
2.2.1. Decayed Fruit Percentage
2.2.2. Fruit Firmness
2.2.3. Weight Loss Percentage
2.2.4. Fruit Content of Total Soluble Solids (TSS) and Titratable Acidity (TA)
2.2.5. Vitamin C
2.2.6. Fruit Color Index
2.2.7. Defense-Related Enzymes Activities
2.2.8. Membrane Stability Index Percentage (MSI)
2.3. Statistical Analysis
3. Results
3.1. Effect of Chitosan and Hot Water (HW) Treatments on Fruit Quality
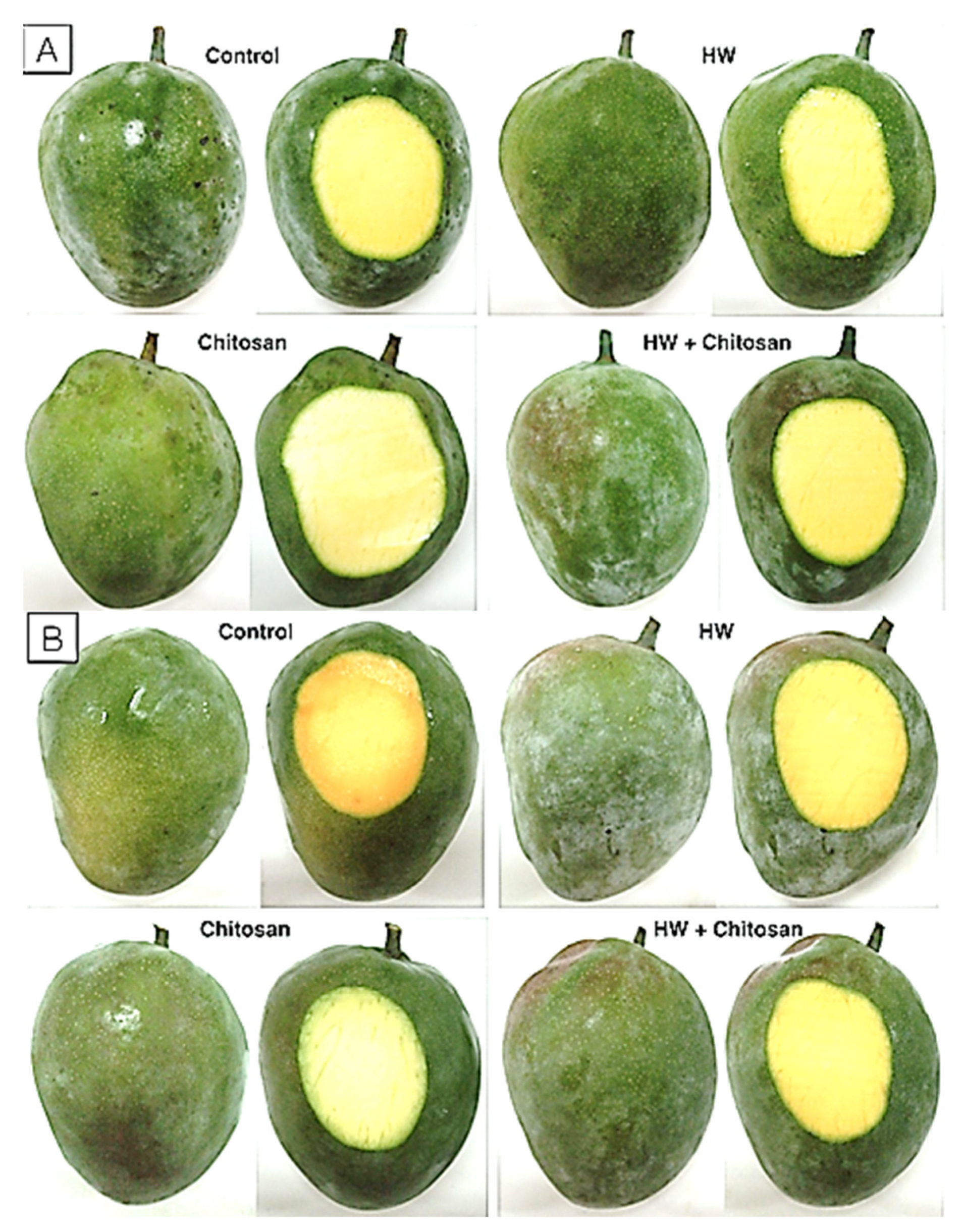
3.1.1. Decay
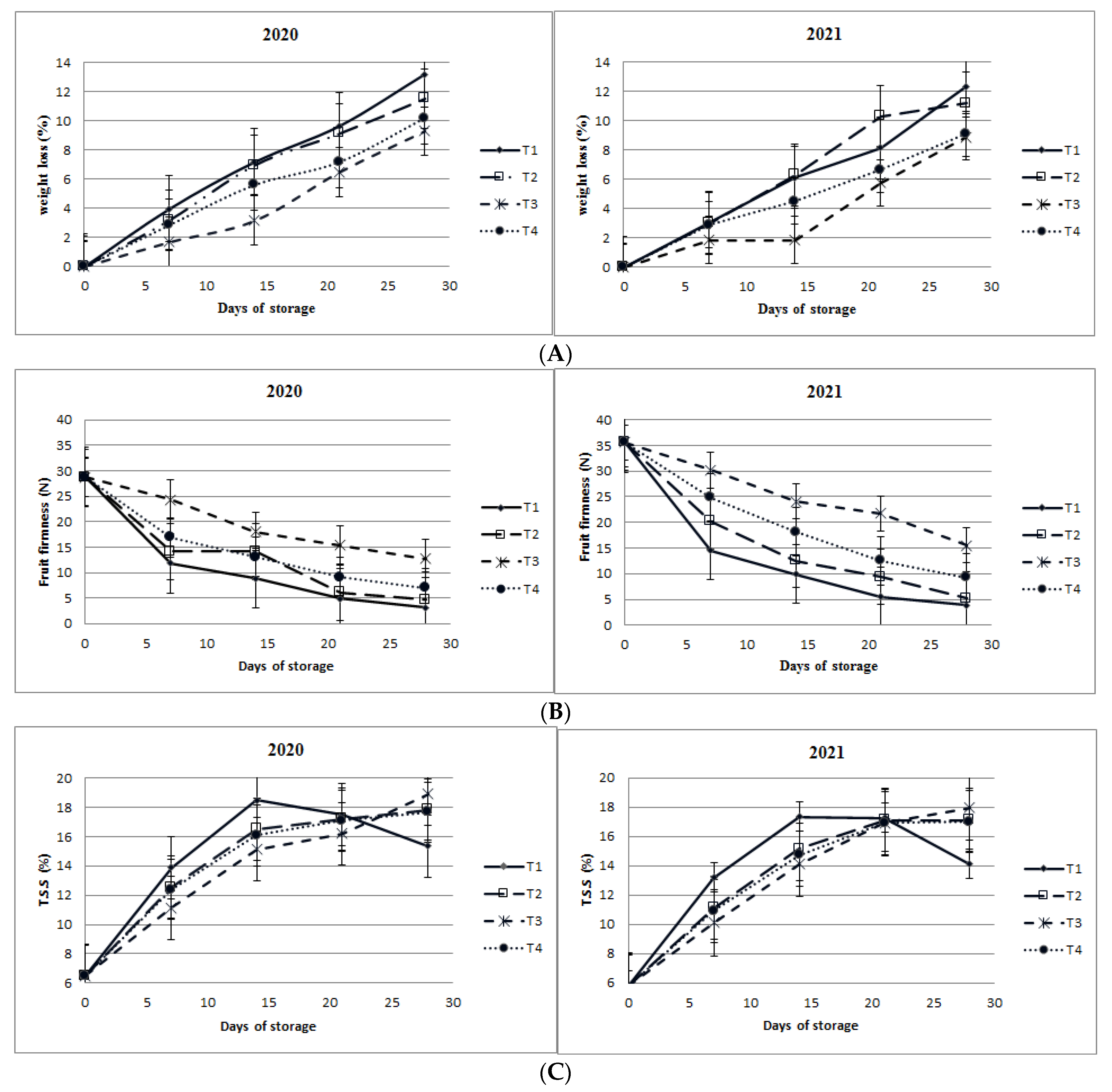
3.1.2. Weight Loss
3.1.3. Firmness
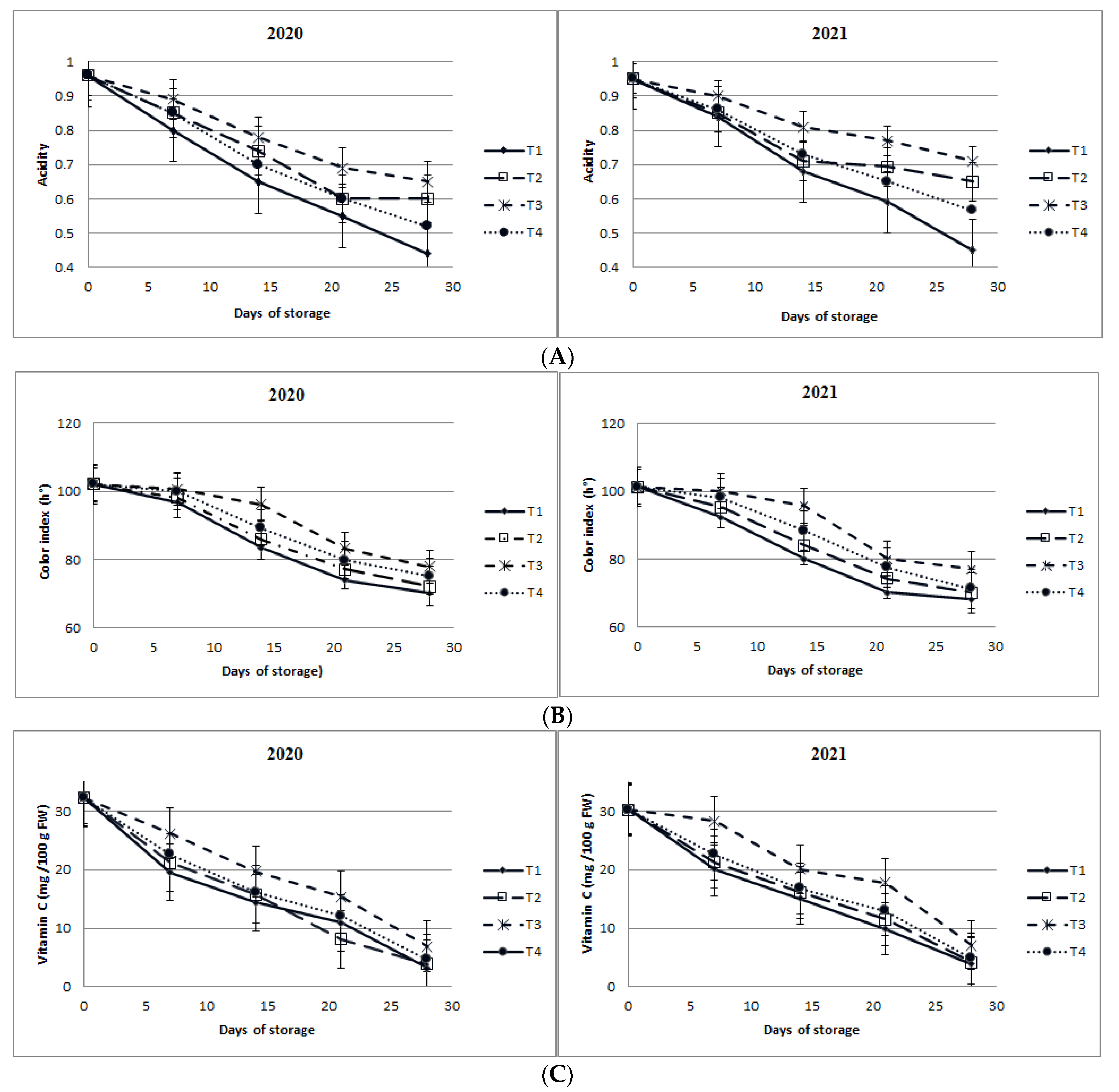
3.1.4. Total Soluble Solids (TSS) and Titratable Acidity (TA)
3.1.5. Changes in Flesh Color (h°)
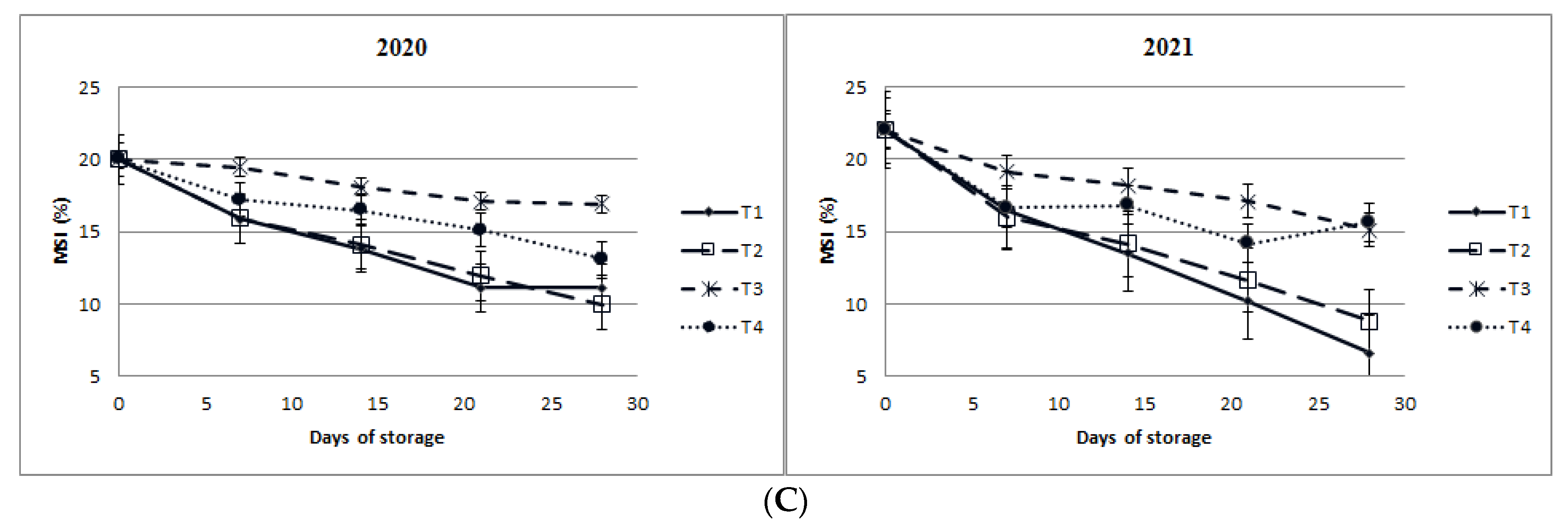
3.1.6. Changes in Vitamin C Content, Peroxidase (POD) Activity, Catalase (CAT) Activity, and Membrane Stability Index Percentage (MSI%)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeng, K.; Cao, J.; Jiang, W. Enhancing disease resistance in harvested mango (Mangifera indica L. cv.‘Matisu’) fruit by salicylic acid. J. Sci. Food Agric. 2006, 86, 694–698. [Google Scholar] [CrossRef]
- Noomhorm, A.; Tiasuwan, N. Controlled atmosphere storage of mango fruit, Mangifera indica L. cv. Rad. J. Food Process Preserv. 1995, 19, 271–281. [Google Scholar] [CrossRef]
- Onyeani, C.; Osunlaja, S. Comparative effect of Nigerian indigenous plants in the control of anthracnose disease of mango fruits. Int. J. Sci. Technol. Res. 2012, 1, 80–85. [Google Scholar]
- Usall, J.; Ippolito, A.; Sisquella, M.; Neri, F. Physical treatments to control postharvest diseases of fresh fruits and vegetables. Postharvest Biol. Technol. 2016, 122, 30–40. [Google Scholar] [CrossRef]
- Hasan, M.U.; Malik, A.U.; Khan, A.S.; Anwar, R.; Muhammad, L.; Amjad, A.; Shah, M.S.; Amin, M. Impact of postharvest hot water treatment on two commercial mango cultivars of Pakistan under simulated air freight conditions for China. Pak. J. Agric. Res. Sci. 2020, 57, 1381–1391. [Google Scholar]
- Dessalegn, Y.; Ayalew, A.; Woldetsadik, K. Integrating plant defense inducing chemical, inorganic salt and hot water treatments for the management of postharvest mango anthracnose. Postharvest Biol. Technol. 2013, 85, 83–88. [Google Scholar] [CrossRef]
- Shiesh, C.; Lin, H. Effect of vapor heat and hot water treatments on disease incidence and quality of Taiwan native strain mango fruits. Int. J. Agric. Biol. 2010, 12, 673–678. [Google Scholar]
- Mansour, F.; Abd-El-Aziz, S.; Helal, G. Effect of fruit heat treatment in three mango varieties on incidence of postharvest fungal disease. J. Plant Pathol. 2006, 88, 141–148. Available online: https://www.jstor.org/stable/41998304 (accessed on 1 July 2006).
- Huan, C.; Han, S.; Jiang, L.; An, X.; Yu, M.; Xu, Y.; Ma, R.; Yu, Z. Postharvest hot air and hot water treatments affect the antioxidant system in peach fruit during refrigerated storage. Postharvest Biol. Technol. 2017, 126, 1–14. [Google Scholar] [CrossRef]
- Kim, Y.; Brecht, J.K.; Talcott, S.T. Antioxidant phytochemical and fruit quality changes in mango (Mangifera indica L.) following hot water immersion and controlled atmosphere storage. Food Chem. 2007, 105, 1327–1334. [Google Scholar] [CrossRef]
- Kumar, N.; Neeraj. Polysaccharide-based component and their relevance in edible film/coating: A review. Nutr. Food Sci. 2019, 49, 793–823. [Google Scholar] [CrossRef]
- El-Mohamedy, R.; El-Gamal, N.G.; Bakeer, A. Application of chitosan and essential oils as alternatives fungicides to control green and blue moulds of citrus fruits. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 629–643. [Google Scholar]
- Sivakumar, D.; Bautista-Baños, S. A review on the use of essential oils for postharvest decay control and maintenance of fruit quality during storage. Crop Prot. 2014, 64, 27–37. [Google Scholar] [CrossRef]
- Bautista-Baños, S.; Sivakumar, D.; Bello-Pérez, A.; Villanueva-Arce, R.; Hernández-López, M. A review of the management alternatives for controlling fungi on papaya fruit during the postharvest supply chain. Crop Prot. 2013, 49, 8–20. [Google Scholar] [CrossRef]
- Bambalele, N.L.; Mditshwa, A.; Magwaza, L.S.; Tesfay, S.Z. The Effect of Gaseous Ozone and Moringa Leaf–Carboxymethyl Cellulose Edible Coating on Antioxidant Activity and Biochemical Properties of ‘Keitt’ Mango Fruit. Coatings 2021, 11, 1406. [Google Scholar] [CrossRef]
- Daisy, L.L.; Nduko, J.M.; Joseph, W.M.; Richard, S.M. Effect of edible gum Arabic coating on the shelf life and quality of mangoes (Mangifera indica) during storage. J. Food Sci. Technol. 2020, 57, 79–85. [Google Scholar] [CrossRef]
- Moalemiyan, M.; Ramaswamy, H.S.; Maftoonazad, N. Pectin-based edible coating for shelf-life extension of ataulfo mango. J. Food Process Eng. 2012, 35, 572–600. [Google Scholar] [CrossRef]
- Prabaharan, M.; Mano, J. Chitosan derivatives bearing cyclodextrin cavitiesas novel adsorbent matrices. Carbohydr. Polym. 2006, 63, 153–166. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S. Totally and partially biodegradable polymer blends based on natural and synthetic macromolecules: Preparation, physical properties, and potential as food packaging materials. J. Macromol. Sci. 1999, 39, 205–271. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Nakayama, A.; Aiba, S.-I. Chitosan and gelatin based edible films: State diagrams, mechanical and permeation properties. Carbohydr. Polym. 1998, 37, 371–382. [Google Scholar] [CrossRef]
- Kumar, N.; Petkoska, A.T.; AL-Hilifi, S.A.; Fawole, O.A. Effect of Chitosan–Pullulan Composite Edible Coating Functionalized with Pomegranate Peel Extract on the Shelf Life of Mango (Mangifera indica). Coatings 2021, 11, 764. [Google Scholar] [CrossRef]
- Basumatary, I.B.; Mukherjee, A.; Katiyar, V.; Kumar, S.; Dutta, J. Chitosan-Based Antimicrobial Coating for Improving Postharvest Shelf Life of Pineapple. Coatings 2021, 11, 1366. [Google Scholar] [CrossRef]
- Chien, P.-J.; Sheu, F.; Yang, F.-H. Effects of edible chitosan coating on quality and shelf life of sliced mango fruit. J. Food Eng. 2007, 78, 225–229. [Google Scholar] [CrossRef]
- Ban, Z.; Wei, W.; Yang, X.; Feng, J.; Guan, J.; Li, L. Combination of heat treatment and chitosan coating to improve postharvest quality of wolfberry (Lycium barbarum). Int. J. Food Sci. 2015, 50, 1019–1025. [Google Scholar] [CrossRef]
- Djioua, T.; Charles, F.; Freire, M., Jr.; Filgueiras, H.; Ducamp-Collin, M.N.; Sallanon, H. Combined effects of postharvest heat treatment and chitosan coating on quality of fresh-cut mangoes (Mangifera indica L.). Int. J. Food Sci. 2010, 45, 849–855. [Google Scholar] [CrossRef] [Green Version]
- Chailoo, M.J.; Asghari, M.R. Hot water and chitosan treatment for the control of postharvest decay in sweet cherry (Prunus avium L.) cv. Napoleon (Napolyon). J. Stored Prod. Postharvest Res. 2011, 2, 135–138. [Google Scholar]
- Vilaplana, R.; Chicaiza, G.; Vaca, C.; Valencia-Chamorro, S. Combination of hot water treatment and chitosan coating to control anthracnose in papaya (Carica papaya L.) during the postharvest period. Crop Prot. 2020, 128, 105007. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Boonyaritthongchai, P.; Buanong, M.; Supapvanich, S.; Wongs-Aree, C. Postharvest Hot Water Treatment Followed by Chitosan-and κ-Carrageenan-Based Composite Coating Induces the Disease Resistance and Preserves the Quality in Dragon Fruit (Hylocereus undatus). Int. J. Fruit Sci. 2020, 20, S2030–S2044. [Google Scholar] [CrossRef]
- Chen, P.; PM, C.; Mellenthin, W.M. Effects of harvest date on ripening capacity and postharvest life of “D’Anjou” pears. J. Am. Soc. Hortic. Sci. 1981, 106, 38–42. [Google Scholar]
- Ranganna, S. Manual of Analysis of Fruit and Vegetable Products; Tata McGraw-Hill Education: New York, NY, USA, 1977; p. 634. [Google Scholar]
- McGuire, R.G. Reporting of objective color measurements. HortScience 1992, 27, 1254–1255. [Google Scholar] [CrossRef] [Green Version]
- Voss, D.H. Relating colorimeter measurement of plant color to the Royal Horticultural Society Colour Chart. HortScience 1992, 27, 1256–1260. [Google Scholar] [CrossRef]
- Ebiloma, U.; Arogba, S.; Aminu, O. Some activities of peroxydase from mango (Mangifera indica L. Var. Mapulehu) kernel. Int. J. Biol. Chem. 2011, 5, 200–206. [Google Scholar] [CrossRef]
- Beers, R.F.; Sizer, I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [CrossRef]
- Sairam, R.K.; Rao, K.V.; Srivastava, G. Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci. 2002, 163, 1037–1046. [Google Scholar] [CrossRef]
- Snedecor, G.; Cochran, W. Statistical Methods, 6th ed.; Iowa State University Press: Ames, IA, USA, 1990; p. 507. [Google Scholar]
- Lo’ay, A.A.; Dawood, H. Minimize browning incidence of banana by postharvest active chitosan/PVA Combines with oxalic acid treatment to during shelf-life. Sci. Hortic. 2017, 226, 208–215. [Google Scholar] [CrossRef]
- Lo’ay, A.A.; Dawood, H. Active chitosan/PVA with ascorbic acid and berry quality of ‘Superior seedless’ grapes. Sci. Hortic. 2017, 224, 286–292. [Google Scholar] [CrossRef]
- Lo’ay, A.A.; Taher, M.A. Influence of edible coatings chitosan/PVP blending with salicylic acid on biochemical fruit skin browning incidence and shelf life of guava fruits cv. ‘Banati’. Scientia Horticulturae 2018, 235, 424–436. [Google Scholar] [CrossRef]
- Lo’ay, A.A.; El-Khateeb, A. Impact of chitosan/PVA with salicylic acid, cell wall degrading enzyme activities and berries shattering of ‘Thompson seedless’ grape vines during shelf life. Sci. Hortic. 2018, 238, 281–287. [Google Scholar] [CrossRef]
- El Hadrami, A.; Adam, L.R.; El Hadrami, I.; Daayf, F. Chitosan in plant protection. Mar. Drugs 2010, 8, 968–987. [Google Scholar] [CrossRef]
- Zeng, K.; Deng, Y.; Ming, J.; Deng, L. Induction of disease resistance and ROS metabolism in navel oranges by chitosan. Sci. Hortic. 2010, 126, 223–228. [Google Scholar] [CrossRef]
- Vilaplana, R.; Hurtado, G.; Valencia-Chamorro, S. Hot water dips elicit disease resistance against anthracnose caused by Colletotrichum musae in organic bananas (Musa acuminata). LWT-Food Sci. Technol. 2018, 95, 247–254. [Google Scholar] [CrossRef]
- Wijeratnam, R.W.; Hewajulige, I.; Abeyratne, N. Postharvest hot water treatment for the control of Thielaviopsis black rot of pineapple. Postharvest Biol. Technol. 2005, 36, 323–327. [Google Scholar] [CrossRef]
- Lo’ay, A.A.; Mostafa, N.A.; Al-Qahtani, S.M.; Al-Harbi, N.A.; Hassan, S.; Abdein, M.A. Influence of the Position of Mango Fruit on the Tree (Mangifera indica L. CV. ‘Zibda’) on Chilling Sensitivity and Antioxidant Enzyme Activity. Horticulturae 2021, 7, 515. [Google Scholar] [CrossRef]
- Lo’ay, A.A.; Taher, M.A. Effectiveness salicylic acid blending in chitosan/PVP biopolymer coating on antioxidant enzyme activities under low storage temperature stress of ‘Banati’ guava fruit. Sci. Hortic. 2018, 238, 343–349. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, Q.; Cao, J.; Jiang, W. Effects of chitosan coating on postharvest quality of mango (Mangifera indica L. cv. Tainong) fruits. J. Food Process. Preserv. 2008, 32, 770–784. [Google Scholar] [CrossRef]
- Yin, C.; Huang, C.; Wang, J.; Liu, Y.; Lu, P.; Huang, L. Effect of chitosan-and alginate-based coatings enriched with cinnamon essential oil microcapsules to improve the postharvest quality of mangoes. Materials 2019, 12, 2039. [Google Scholar] [CrossRef] [Green Version]
- Yu, K.; Xu, J.; Zhou, L.; Zou, L.; Liu, W. Effect of Chitosan Coatings with Cinnamon Essential Oil on Postharvest Quality of Mangoes. Foods 2021, 10, 3003. [Google Scholar] [CrossRef]
- Muy Rangel, D.; Espinoza Valenzuela, B.; Siller Cepeda, J.; Sañudo Barajas, J.A.; Valdez Torres, B.; Osuna Enciso, T. Efecto del 1-metilciclopropeno (1-MCP) y de una película comestible sobre la actividad enzimática y calidad poscosecha del mango’Ataulfo’. Fitotec. Mex. 2009, 32, 53–60. [Google Scholar] [CrossRef]
- Kerch, G.; Korkhov, V. Effect of storage time and temperature on structure, mechanical and barrier properties of chitosan-based films. Eur. Food Res. Technol. 2011, 232, 17–22. [Google Scholar] [CrossRef]
- Qiuping, Z.; Wenshui, X. Effect of 1-methylcyclopropene and/or chitosan coating treatments on storage life and quality maintenance of Indian jujube fruit. LWT-Food Sci. Technol. 2007, 40, 404–411. [Google Scholar] [CrossRef]
- Fawaz, S.A. Physiological studies on mango fruits handling. FAO 2000, 33, 137–149. [Google Scholar]
- Fallik, E.; Lurie, S. Thermal control of fungi in the reduction of postharvest decay. In Heat Treatment for Postharvest Pest Control: Theory and Practice; CABI: Wallingford, UK, 2007. [Google Scholar] [CrossRef]
- Kittur, F.; Saroja, N.; Tharanathan, R. Polysaccharide-based composite coating formulations for shelf-life extension of fresh banana and mango. Eur. Food Res. Technol. 2001, 213, 306–311. [Google Scholar] [CrossRef]
- Hojo, R.H.; São José, A.R.; Hojo, E.T.D.; Alves, J.F.T.; Rebouças, T.N.H.; Dias, N.O. Quality of ‘tommy atkins’ mangoes in post-harvest with calcium choride spray use in the pre-harvest period. Rev. Bras. Frutic. 2009, 31, 62–70. [Google Scholar] [CrossRef]
- Jongsri, P.; Wangsomboondee, T.; Rojsitthisak, P.; Seraypheap, K. Effect of molecular weights of chitosan coating on postharvest quality and physicochemical characteristics of mango fruit. LWT-Food Sci. Technol. 2016, 73, 28–36. [Google Scholar] [CrossRef]
- Amin, U.; Khan, M.K.I.; Khan, M.U.; Ehtasham Akram, M.; Pateiro, M.; Lorenzo, J.M.; Maan, A.A. Improvement of the Performance of Chitosan—Aloe vera Coatings by Adding Beeswax on Postharvest Quality of Mango Fruit. Foods 2021, 10, 2240. [Google Scholar] [CrossRef] [PubMed]
- Wang, C. Postharvest techniques for reducing low temperature injury in chilling sensitive commodities. In Improving Postharvest Technologies of Fruits Vegetables and Ornamentals; Artes, F., Gil, M.I., Conesa, M.A., Eds.; U.S. Department of Agriculture: Washington, DC, USA, 2000. [Google Scholar]
- Wang, J.; Wang, B.; Jiang, W.; Zhao, Y. Quality and shelf life of mango (Mangifera Indica L. cv. ‘Tainong’) coated by using chitosan and polyphenols. Food Sci. Technol. Int. 2007, 13, 317–322. [Google Scholar] [CrossRef]
- Medeiros, B.G.d.S.; Pinheiro, A.C.; Carneiro-da-Cunha, M.G.; Vicente, A.A. Development and characterization of a nanomultilayer coating of pectin and chitosan–Evaluation of its gas barrier properties and application on ‘Tommy Atkins’ mangoes. J. Food Eng. 2012, 110, 457–464. [Google Scholar] [CrossRef] [Green Version]
- Cissé, M.; Polidori, J.; Montet, D.; Loiseau, G.; Ducamp-Collin, M.N. Preservation of mango quality by using functional chitosan-lactoperoxidase systems coatings. Postharvest Biol. Technol. 2015, 101, 10–14. [Google Scholar] [CrossRef]
- Goulao, L.F.; Oliveira, C.M. Cell wall modifications during fruit ripening: When a fruit is not the fruit. Trends Food Sci. Technol. 2008, 19, 4–25. [Google Scholar] [CrossRef] [Green Version]
- Lo’ay, A.A.; Doaa, M. The potential of vine rootstocks impacts on ‘Flame Seedless’ bunches behavior under cold storage and antioxidant enzyme activity performance. Sci. Hortic. 2020, 260, 108844. [Google Scholar] [CrossRef]
- El-Banna, M.; Lo’ay, A. Evaluation berries shattering phenomena of ‘Flame seedless’ vines grafted on different rootstocks during shelf life. Sci. Hortic. 2019, 246, 51–56. [Google Scholar] [CrossRef]
- Lo’ay, A.A.; Rabie, M.M.; Alhaithloul, H.A.S.; Alghanem, S.M.S.; Ibrahim, A.M.; Abdein, M.A.; Abdelgawad, Z.A. On the Biochemical and Physiological Responses of ‘Crimson Seedless’ Grapes Coated with an Edible Composite of Pectin, Polyphenylene Alcohol, and Salicylic Acid. Horticulturae 2021, 7, 498. [Google Scholar] [CrossRef]
- Lo’ay, A.A.; EL-Ezz, S.F.A. Performance of ‘Flame seedless’ grapevines grown on different rootstocks in response to soil salinity stress. Sci. Hortic. 2021, 275, 109704. [Google Scholar] [CrossRef]
- Lo’ay, A.A.; Ameer, N. Performance of calcium nanoparticles blending with ascorbic acid and alleviation internal browning of ‘Hindi Be-Sennara’mango fruit at a low temperature. Sci. Hortic. 2019, 254, 199–207. [Google Scholar] [CrossRef]

| Treatments | Storage Period (Days) | |||||
|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | 28 | Mean | |
| Season 2020 | ||||||
| Control | 0.00 h | 0.00 h | 8.96 d | 17.5 c | 30.20 a | 11.33 a |
| Hot water (HW) | 0.00 h | 0.00 h | 0.00 h | 6.13 e | 19.35 b | 5.09 b |
| 1% chitosan | 0.00 h | 0.00 h | 0.00 h | 0.00 h | 3.68 f | 0.73 c |
| HW + 1% chitosan | 0.00 h | 0.00 h | 0.00 h | 0.00 h | 2.43 g | 0.48 c |
| Mean | 0.00 d | 0.00 d | 2.24 c | 5.90 b | 13.91 a | – |
| Treatments | Storage Period (Days) | |||||
|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | 28 | Mean | |
| Season 2021 | ||||||
| Control | 0.00 h | 0.00 h | 7.58 d | 15.66 c | 28.32 a | 10.31 a |
| Hot water (HW) | 0.00 h | 0.00 h | 0.00 h | 6.30 e | 21.50 b | 5.56 b |
| 1% chitosan | 0.00 h | 0.00 h | 0.00 h | 0.00 h | 4.13 g | 0.82 c |
| HW + 1% chitosan | 0.00 h | 0.00 h | 0.00 h | 0.00 h | 5.12 f | 1.02 c |
| Mean | 0.00 d | 0.00 d | 1.89 c | 5.49 b | 14.76 a | – |
| Season 2020 | Fruit Firmness (N) | Weight Loss (%) | TSS (%) | Acidity (%) | Ascorbic Acid (mg/100 g FW) | Color Index (h°) | Peroxidase Activity(Units/mg FW) | Catalase Activity (Units/mg FW) | MSI (%) |
| Treatments (T) | 38.00 *** | 38.20 *** | 3.17 * | 969.00 *** | 21 *** | 121.40 *** | 199.80 *** | 3.39 * | 199.80 *** |
| Storage period (S) | 414.30 *** | 416.30 *** | 505 *** | 8362.10 *** | 541.50 *** | 1755.10 *** | 771.80 *** | 73.11 *** | 771.80 *** |
| T X S | 3.74 *** | 3.70 *** | 7.77 *** | 118.80 *** | 2.32 * | 13.70 *** | 58.80 *** | 7.80 *** | 58.80 *** |
| Season 2021 | Fruit Firmness (N) | Weight Loss (%) | TSS (%) | Acidity (%) | Ascorbic Acid (mg/100 g FW) | Color Index (h°) | Peroxidase Activity (O.D) | Catalase Activity | MSI (%) |
| Treatments (T) | 158.40 *** | 4.25 * | 2.77 ns | 389.20 *** | 191.30 *** | 332.80 *** | 52.70 *** | 25.26 *** | 52.70 *** |
| Storage period (S) | 600.10 *** | 90.87 *** | 701.28 *** | 2349.80 *** | 3115.30 *** | 3395.80 *** | 67.30 *** | 37.51 *** | 67.30 *** |
| T X S | 12.30 *** | 2.89 ** | 13.19 *** | 61.90 *** | 18.80 *** | 32.58 *** | 13.80 *** | 2.90 ** | 13.80 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalil, H.A.; Abdelkader, M.F.M.; Lo’ay, A.A.; El-Ansary, D.O.; Shaaban, F.K.M.; Osman, S.O.; Shenawy, I.E.; Osman, H.-E.H.; Limam, S.A.; Abdein, M.A.; et al. The Combined Effect of Hot Water Treatment and Chitosan Coating on Mango (Mangifera indica L. cv. Kent) Fruits to Control Postharvest Deterioration and Increase Fruit Quality. Coatings 2022, 12, 83. https://doi.org/10.3390/coatings12010083
Khalil HA, Abdelkader MFM, Lo’ay AA, El-Ansary DO, Shaaban FKM, Osman SO, Shenawy IE, Osman H-EH, Limam SA, Abdein MA, et al. The Combined Effect of Hot Water Treatment and Chitosan Coating on Mango (Mangifera indica L. cv. Kent) Fruits to Control Postharvest Deterioration and Increase Fruit Quality. Coatings. 2022; 12(1):83. https://doi.org/10.3390/coatings12010083
Chicago/Turabian StyleKhalil, Hoda A., Mohamed F. M. Abdelkader, A. A. Lo’ay, Diaa O. El-Ansary, Fatma K. M. Shaaban, Samah O. Osman, Ibrahim E. Shenawy, Hosam-Eldin Hussein Osman, Safaa A. Limam, Mohamed A. Abdein, and et al. 2022. "The Combined Effect of Hot Water Treatment and Chitosan Coating on Mango (Mangifera indica L. cv. Kent) Fruits to Control Postharvest Deterioration and Increase Fruit Quality" Coatings 12, no. 1: 83. https://doi.org/10.3390/coatings12010083
APA StyleKhalil, H. A., Abdelkader, M. F. M., Lo’ay, A. A., El-Ansary, D. O., Shaaban, F. K. M., Osman, S. O., Shenawy, I. E., Osman, H.-E. H., Limam, S. A., Abdein, M. A., & Abdelgawad, Z. A. (2022). The Combined Effect of Hot Water Treatment and Chitosan Coating on Mango (Mangifera indica L. cv. Kent) Fruits to Control Postharvest Deterioration and Increase Fruit Quality. Coatings, 12(1), 83. https://doi.org/10.3390/coatings12010083








