Electron-Beam Synthesis of Dielectric Coatings Using Forevacuum Plasma Electron Sources (Review)
Abstract
1. Introduction
2. Beam and Ion-Plasma Methods of Dielectric Coating Deposition
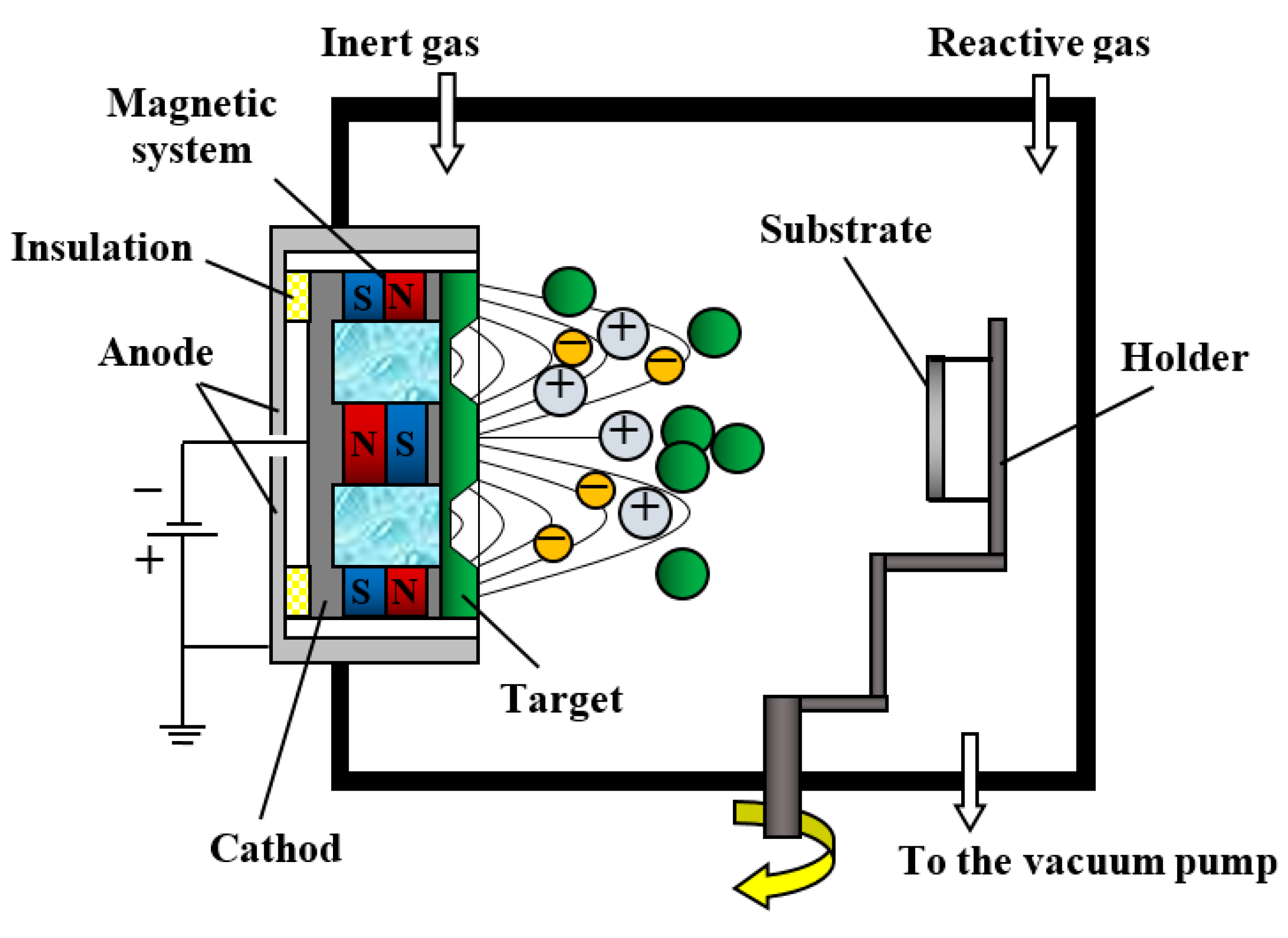
2.1. Magnetron Sputtering
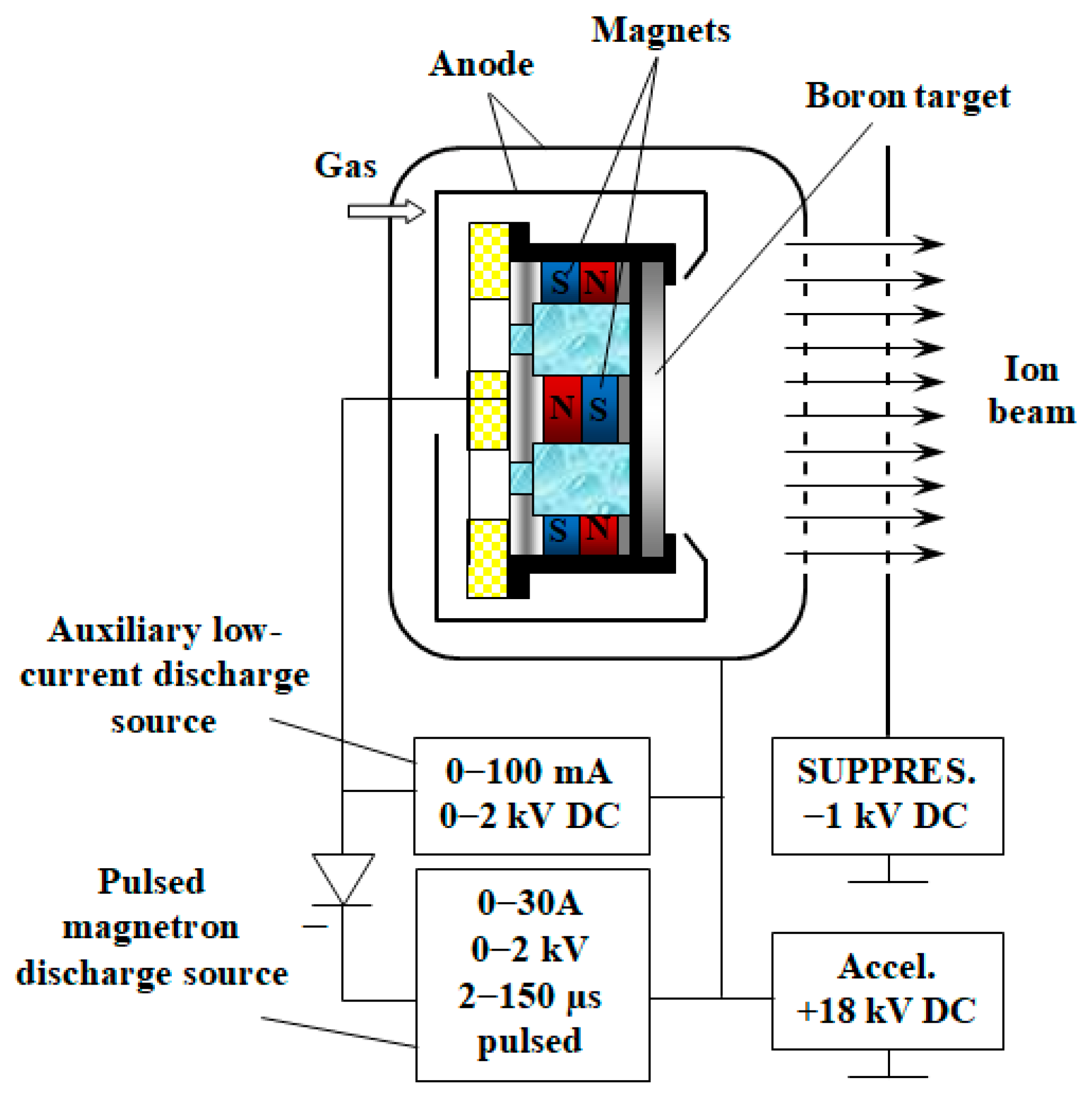
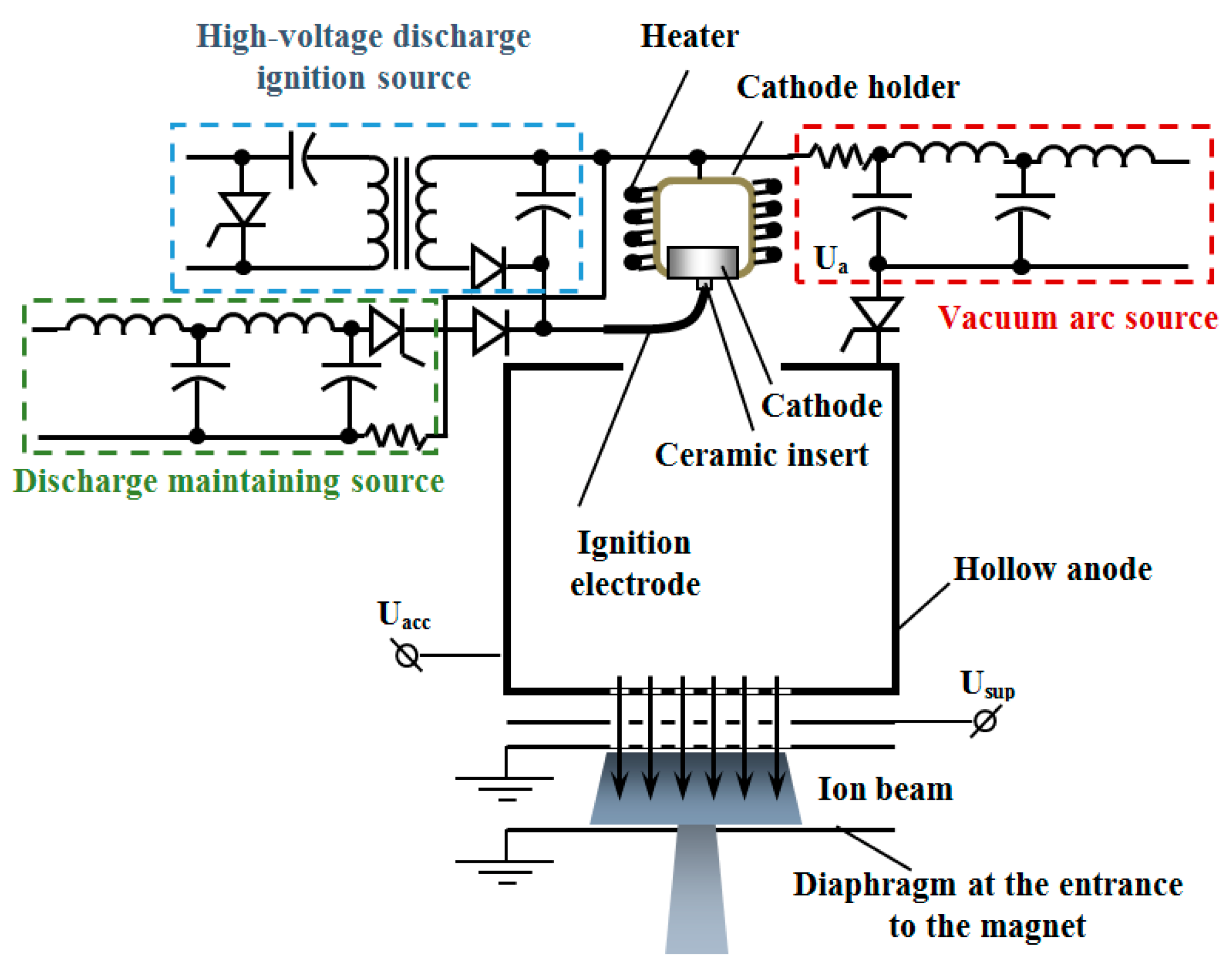
2.2. Cathodic Arc System
2.3. Plasma-Enhanced Chemical Methods
2.4. Laser Methods
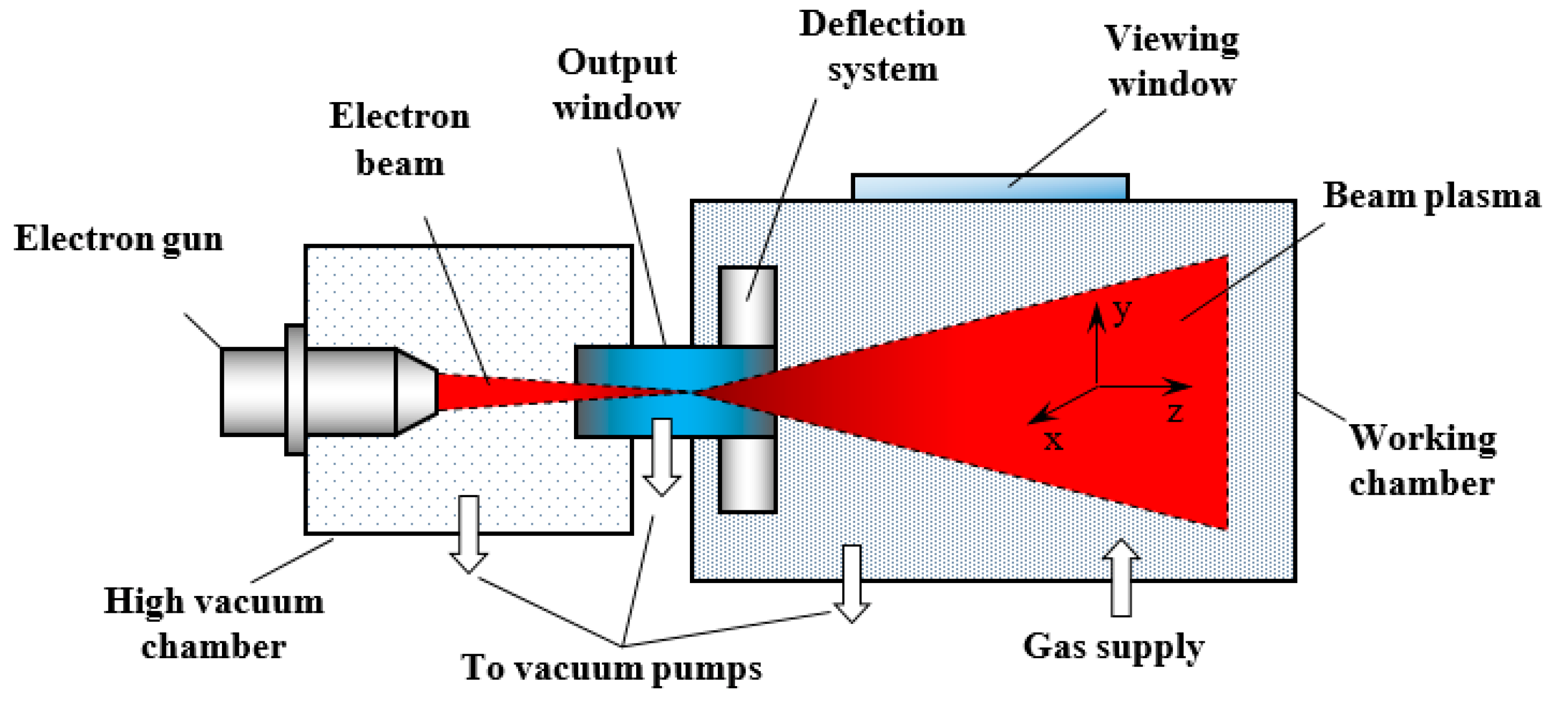
2.5. Electron-Beam Methods
- -
- High electron-beam energy density, up to 10 MW/cm2, at which the temperature attainable in the beam action area is sufficient to melt virtually any material;
- -
- Capability to effectively control the parameters of the electron beam and its position;
- -
- Possibility of obtaining chemically pure and multi-component coatings;
- -
- Simplicity, reliability, and long service life of technological equipment.
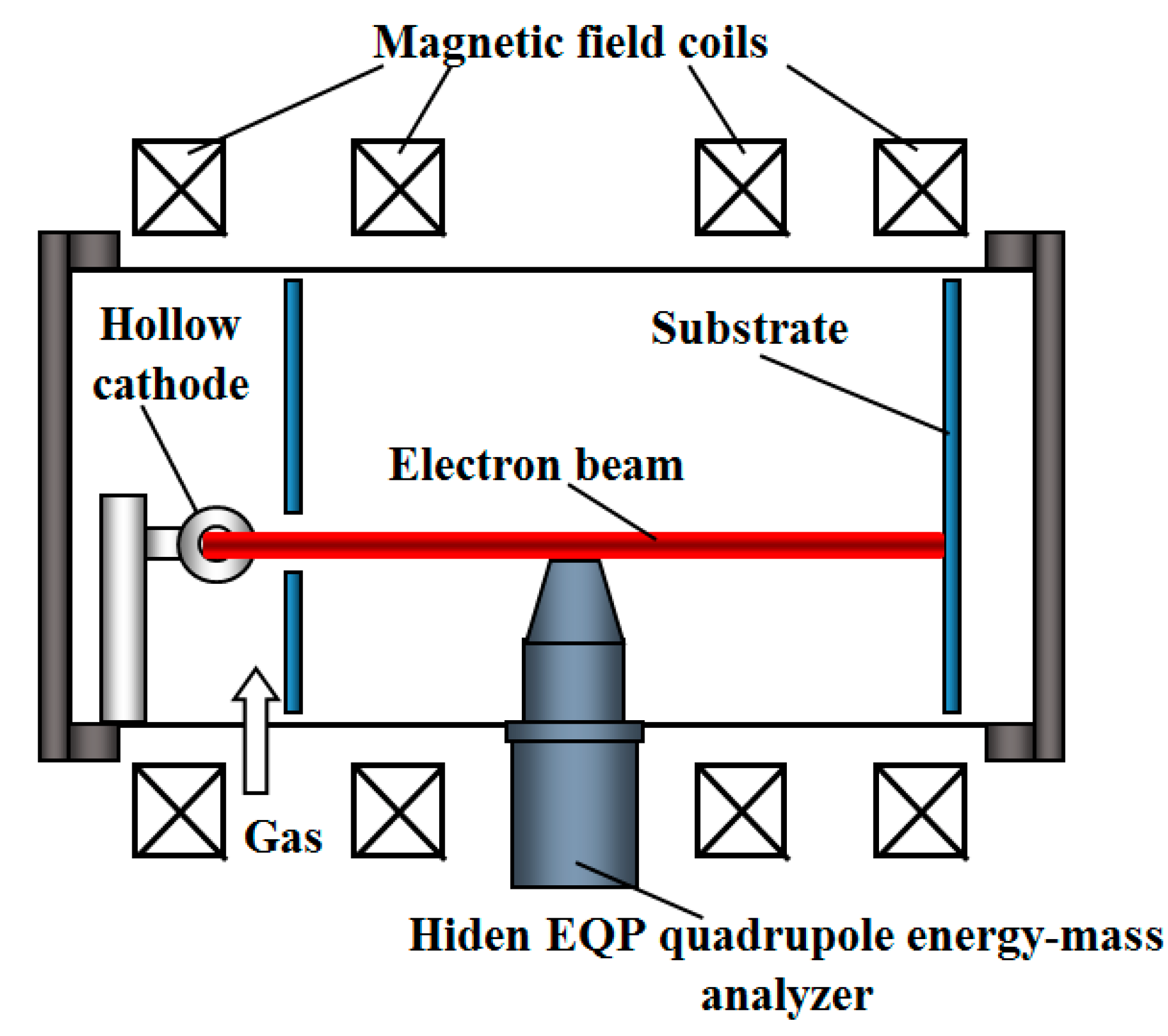
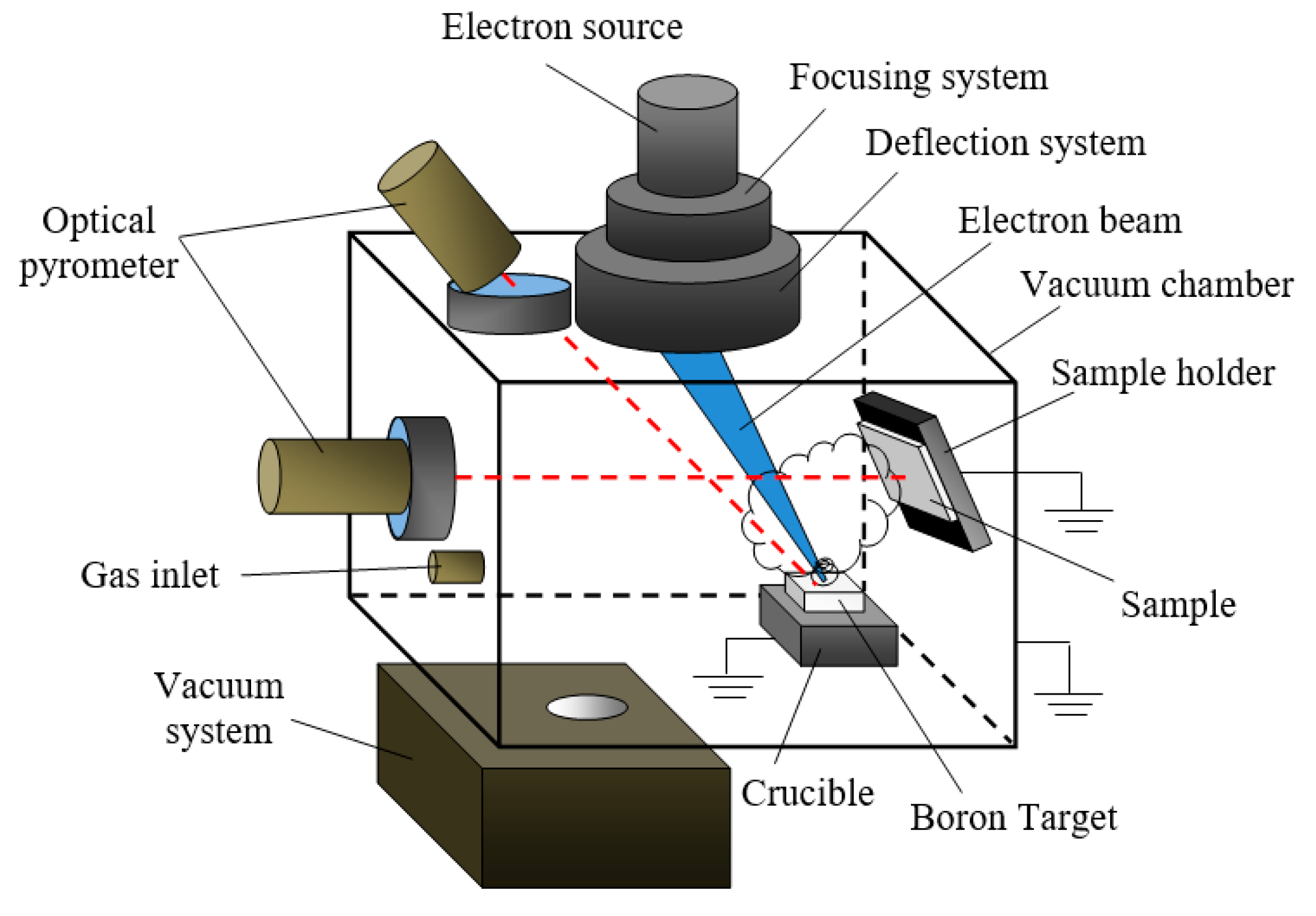
3. Synthesis of Dielectric Coatings using Forevacuum Plasma Electron Sources
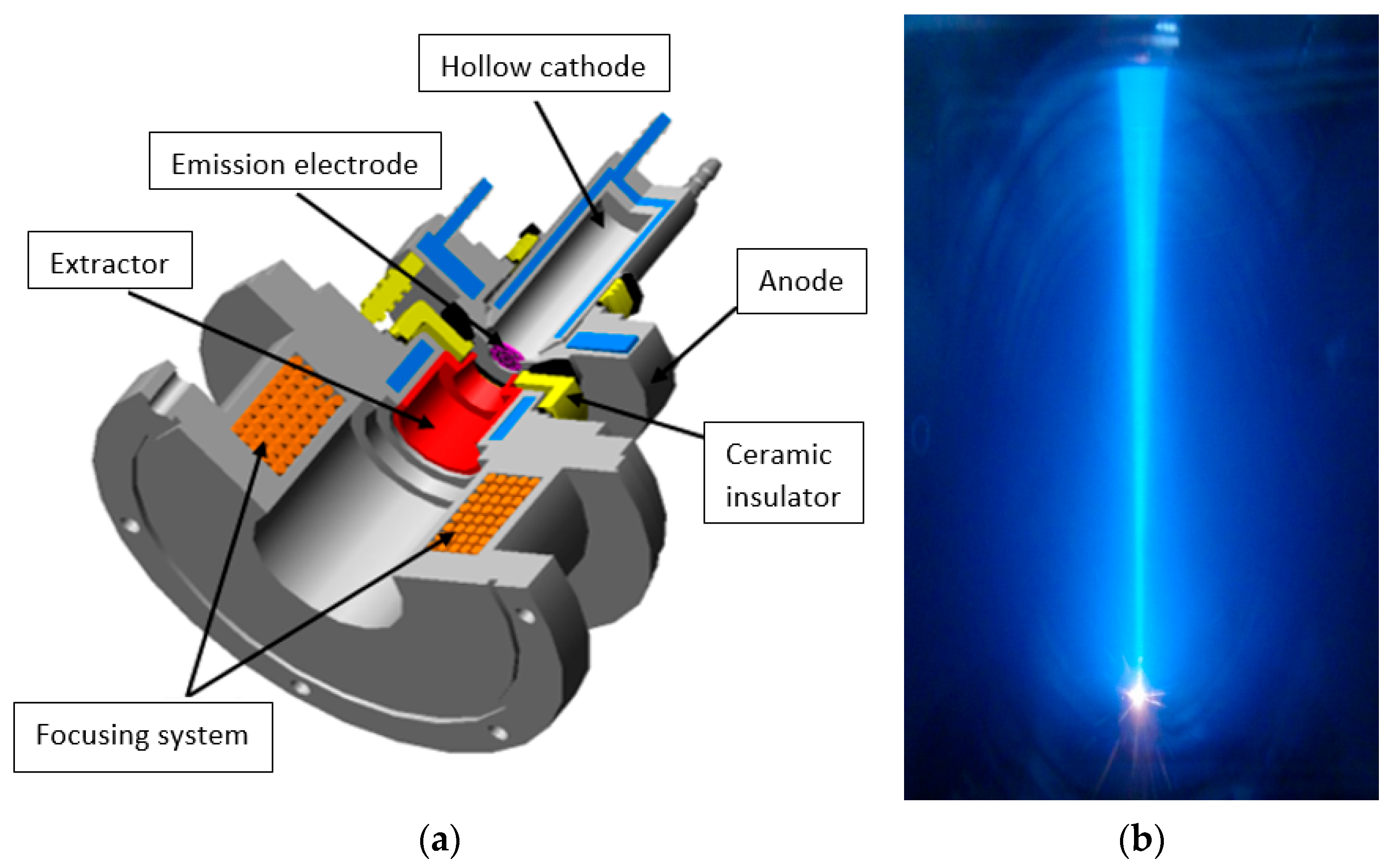
3.1. Forevacuum Plasma Electron Sources: Basic Principles of Operation
- -
- Accelerating voltage up to 30 kV;
- -
- Beam current up to 450 mA;
- -
- Beam power up to 10 kW;
- -
- Power density up to 106 W/cm2;
- -
- Operating pressure up to 100 Pa (argon), 160 Pa (helium).
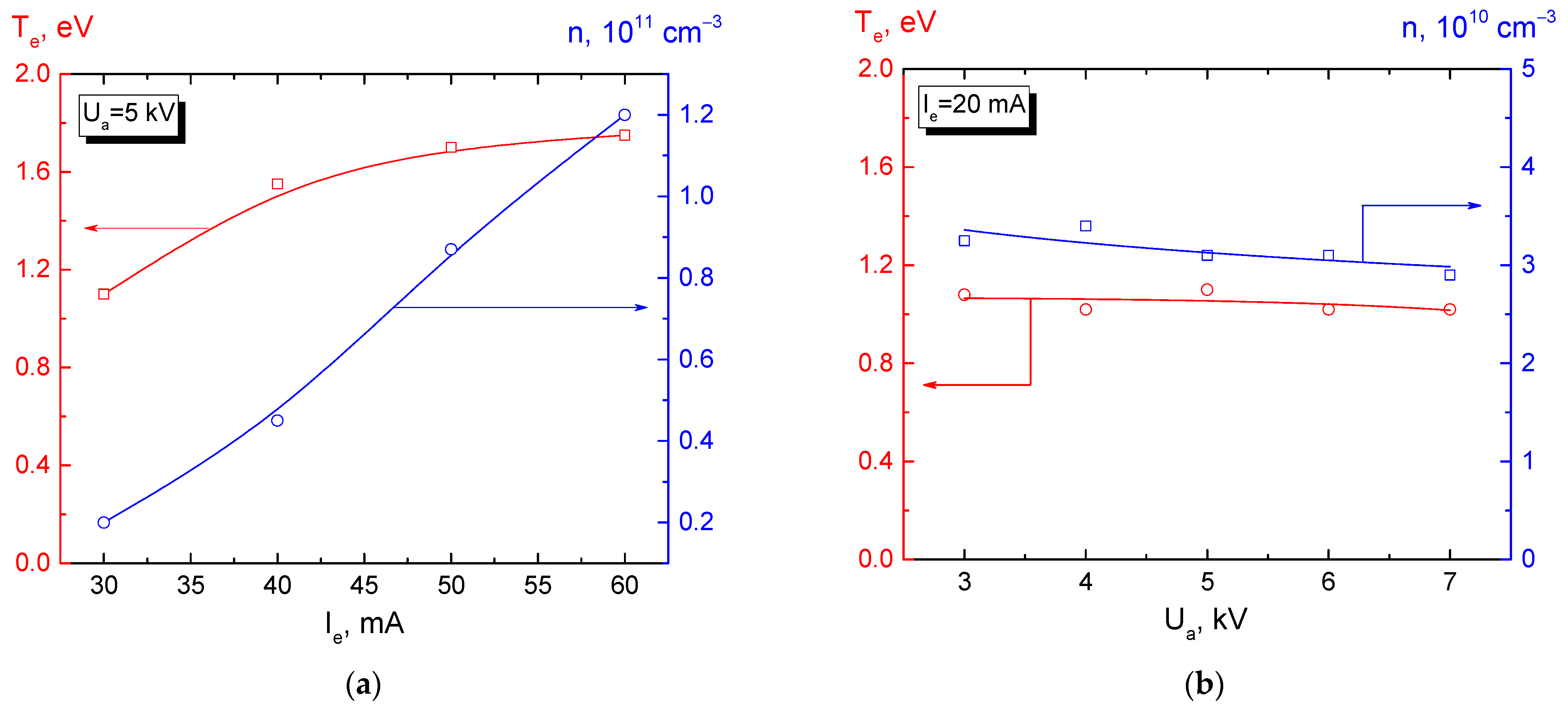
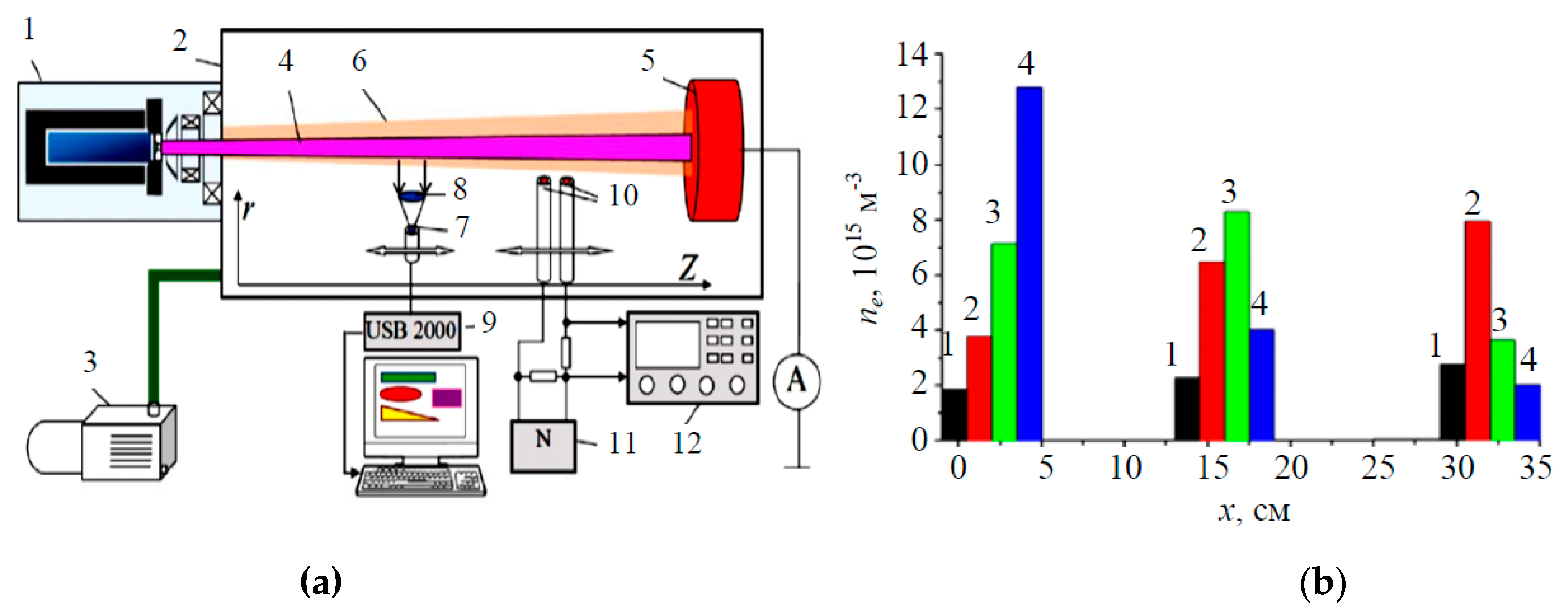
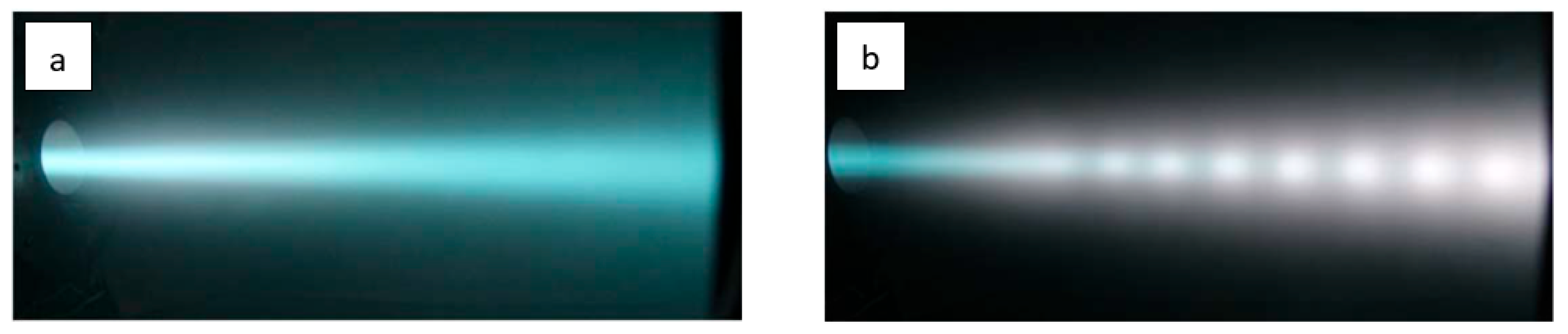
3.2. Beam-Plasma Parameters and Characteristics
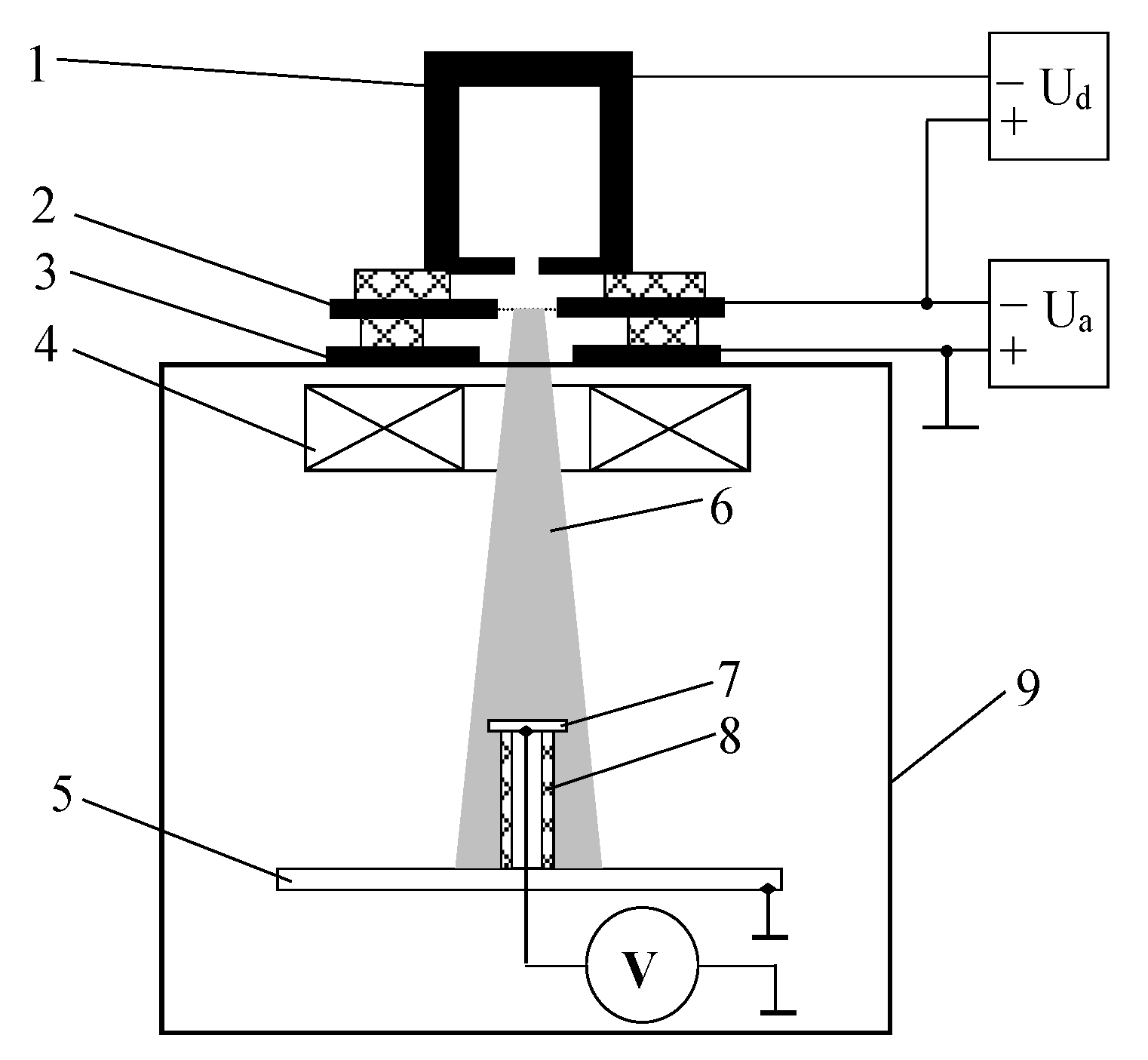
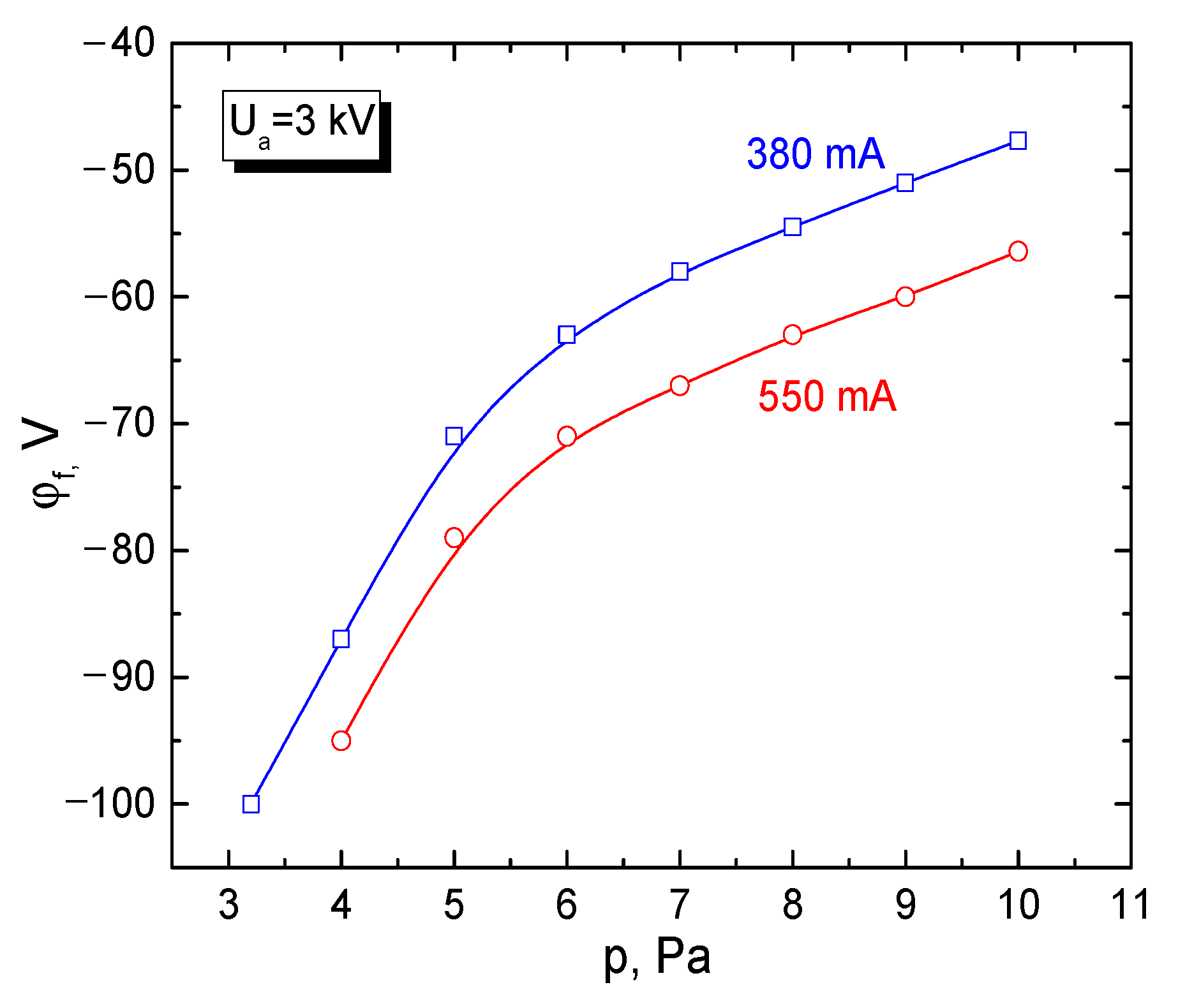
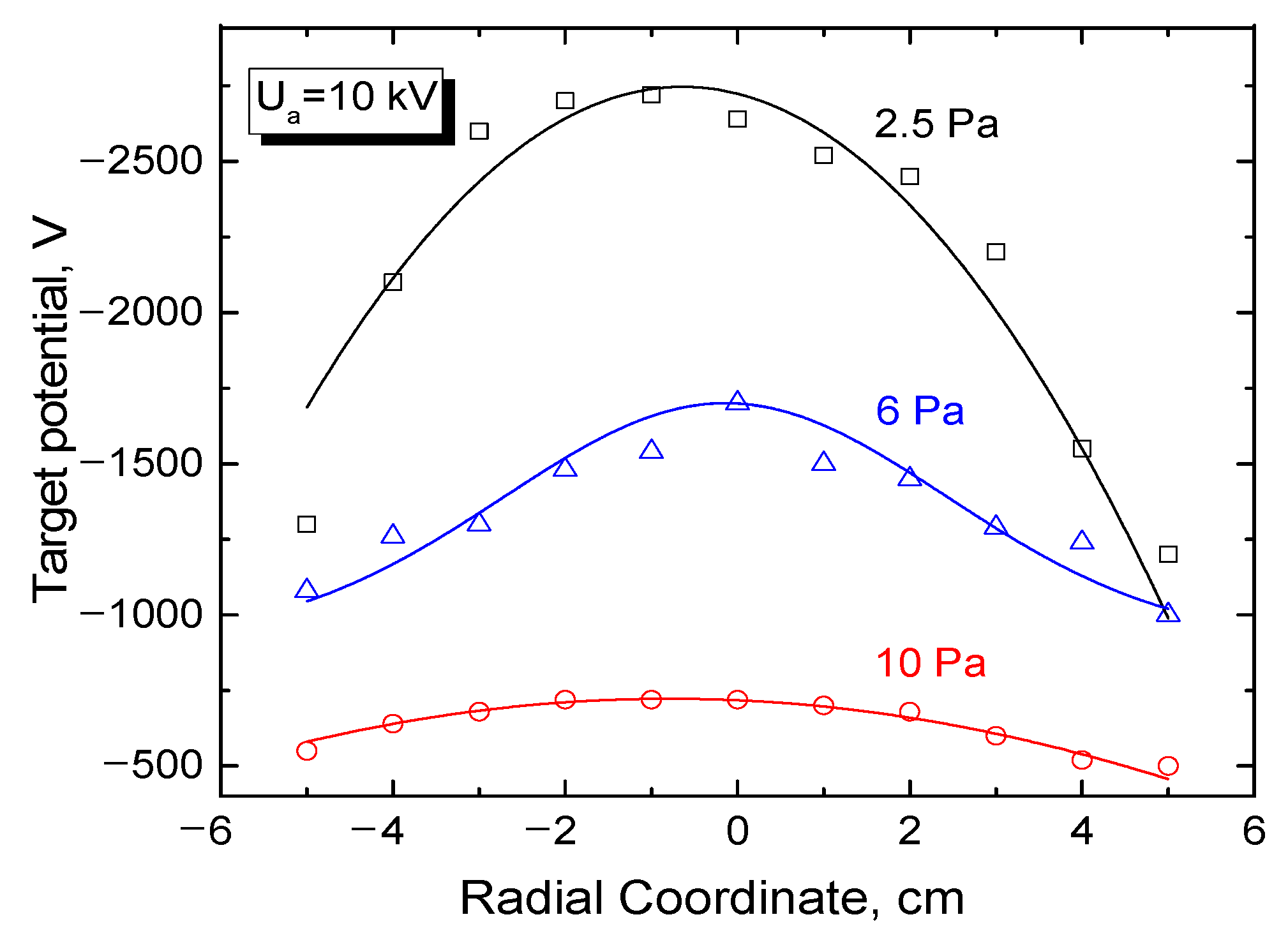
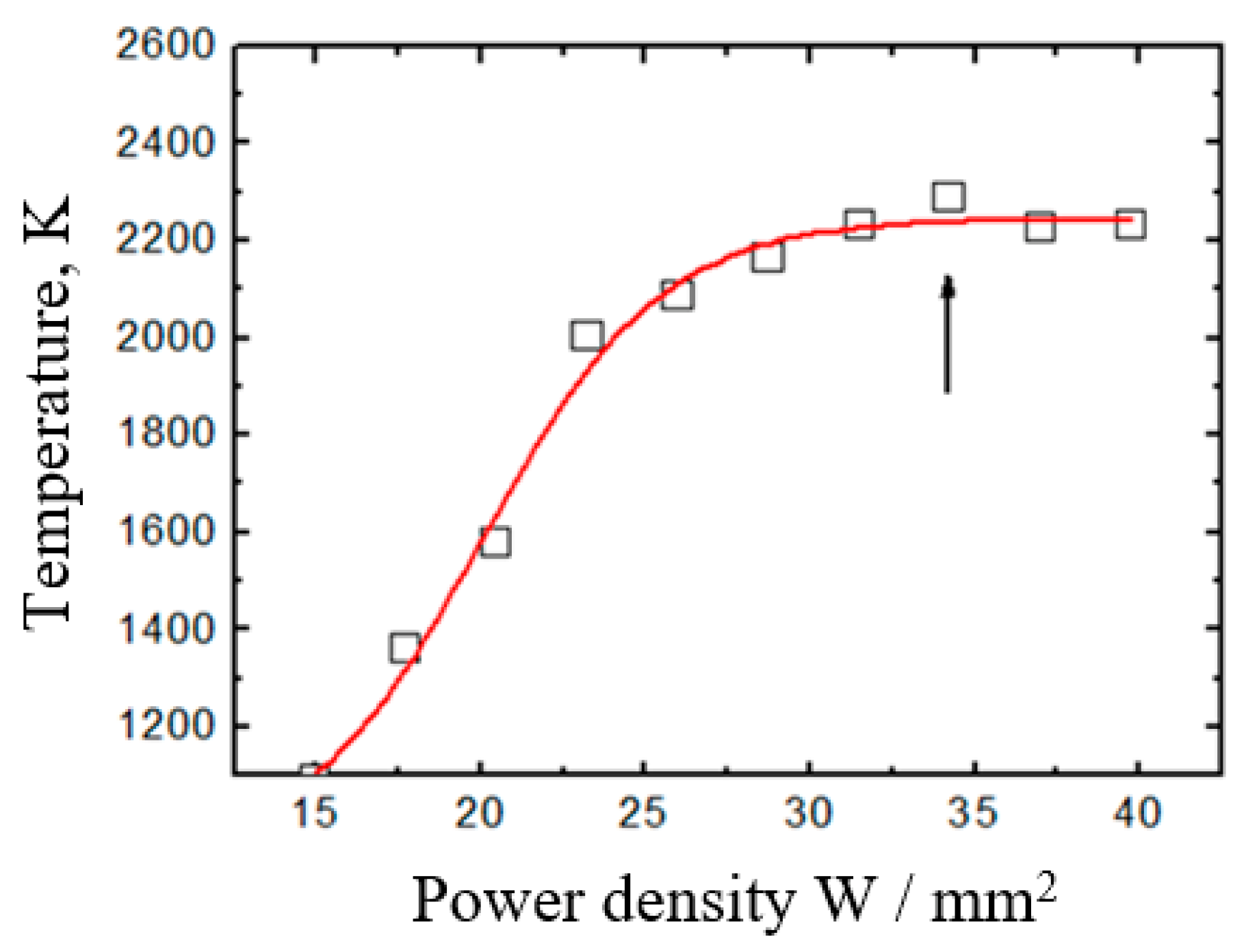
3.3. Potential of a Dielectric Target Irradiated by an Electron Beam in Forevacuum
3.4. Parameters and Characteristics of Dielectric Coatings
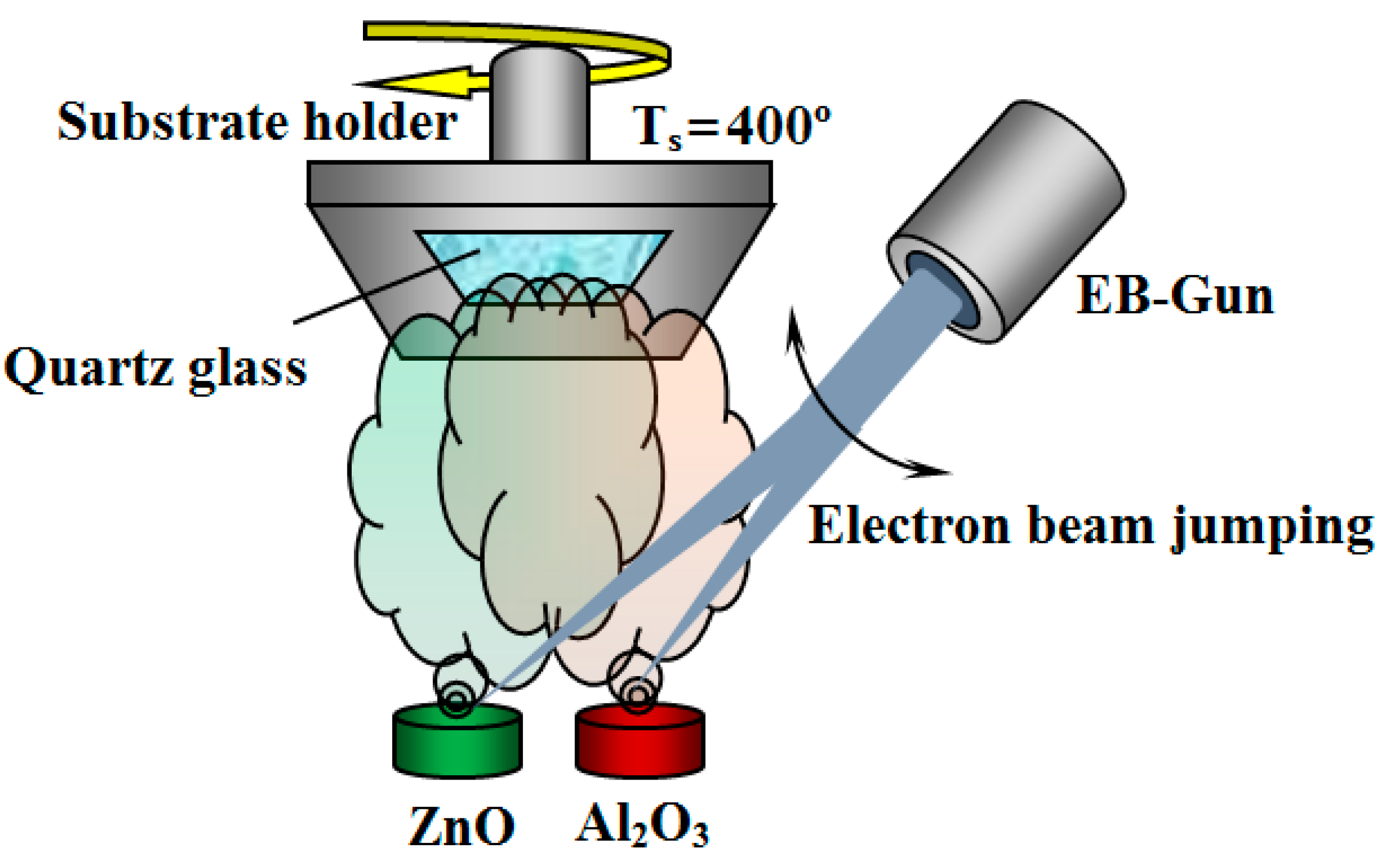
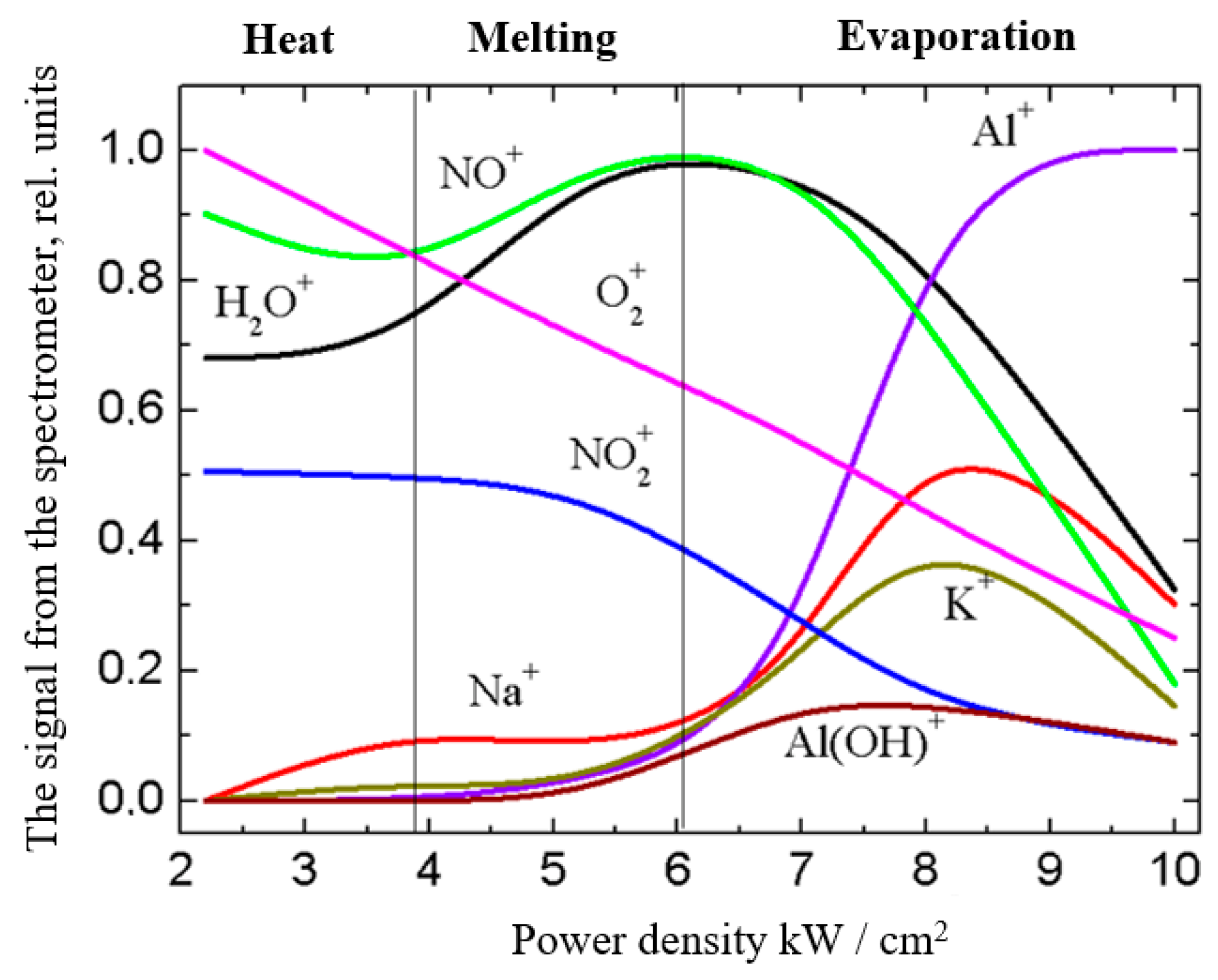
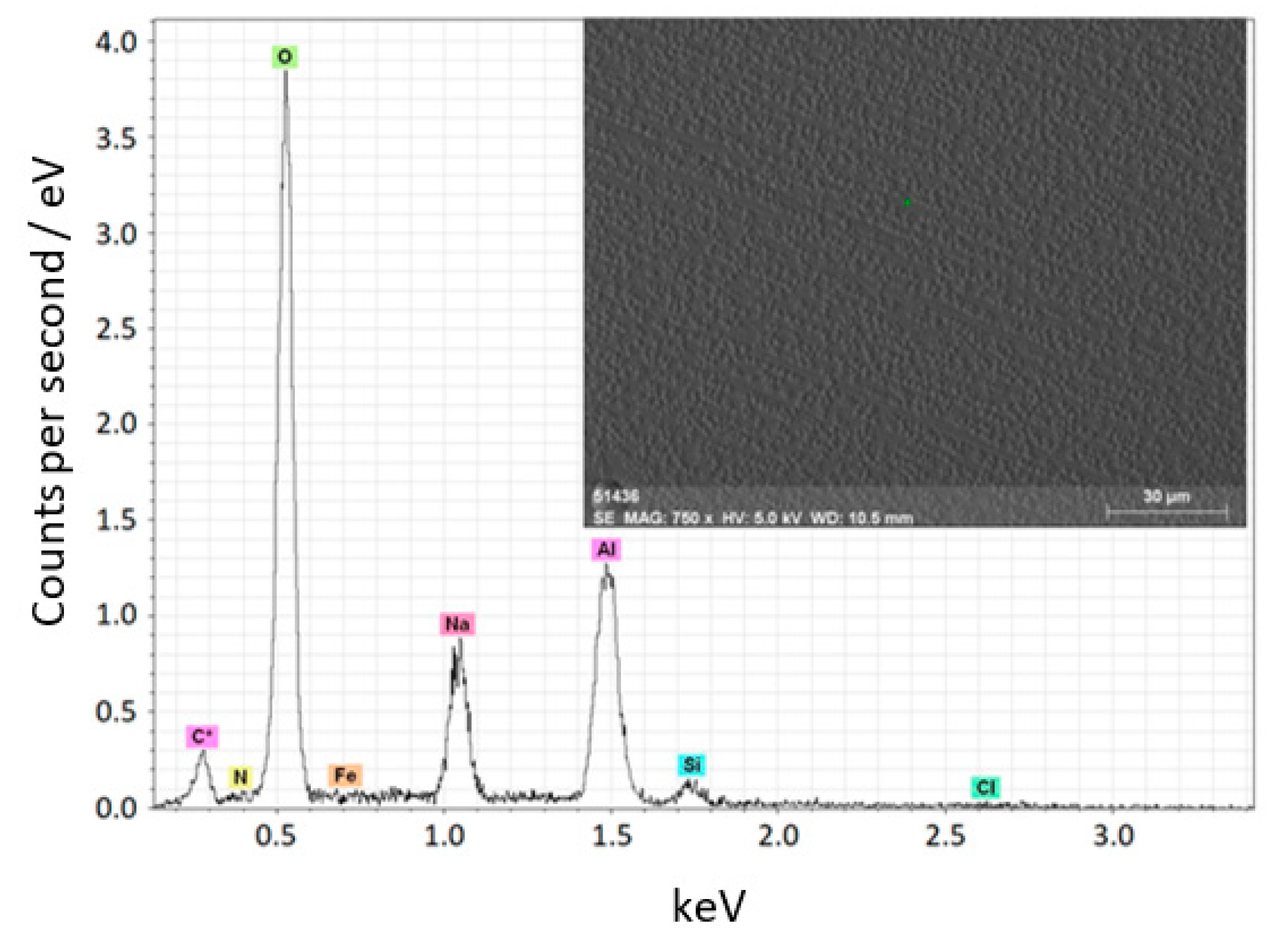
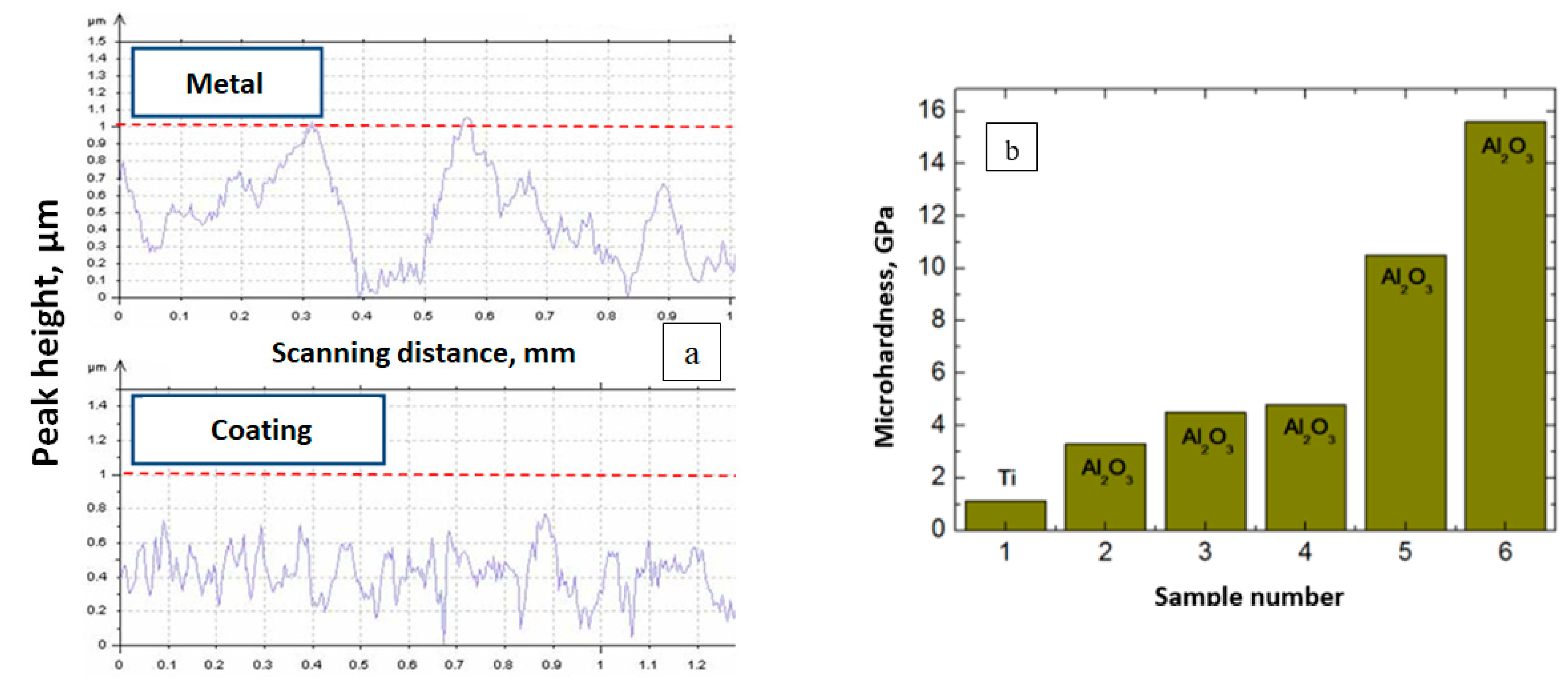
3.4.1. Ceramic Coatings
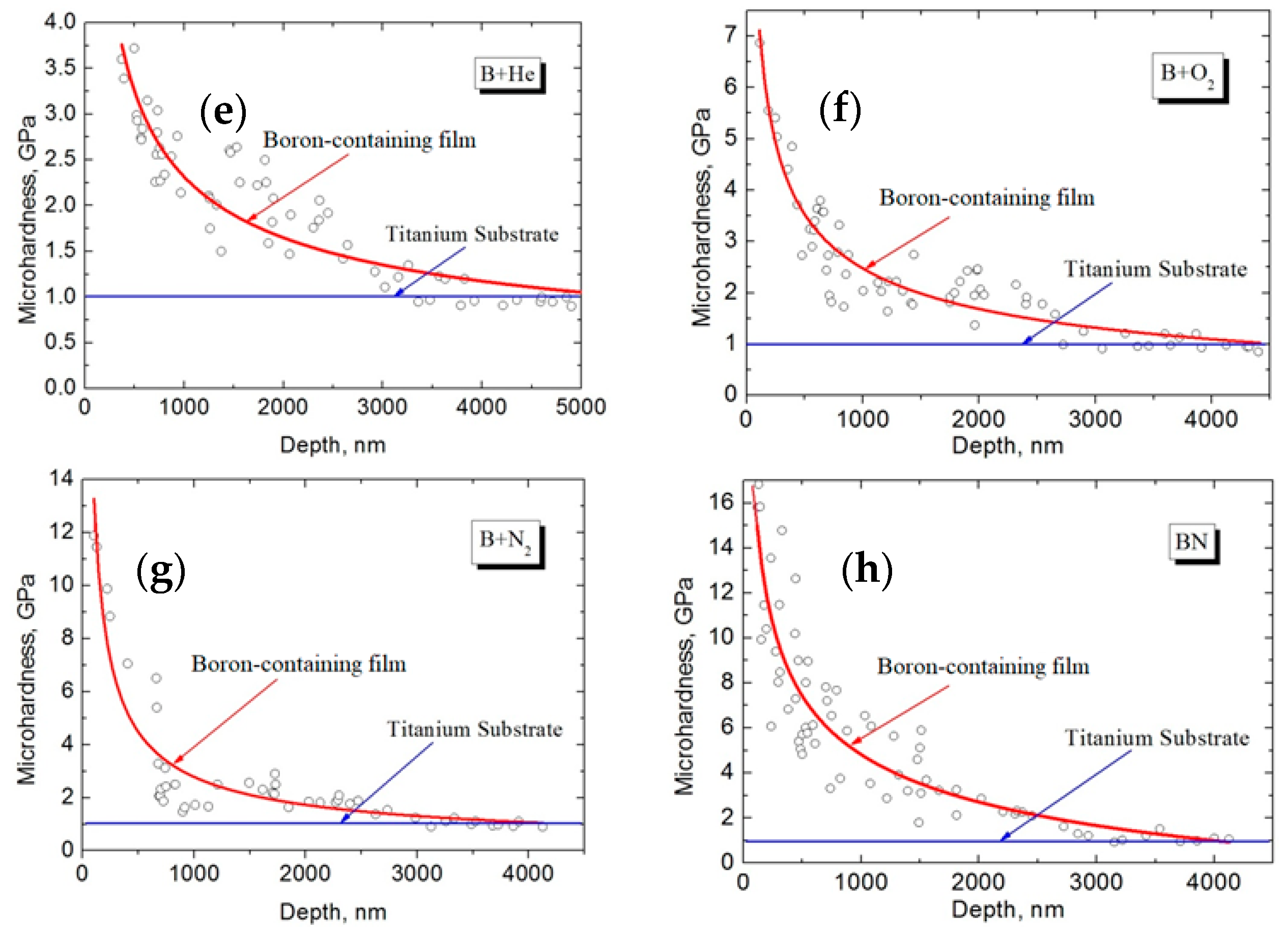
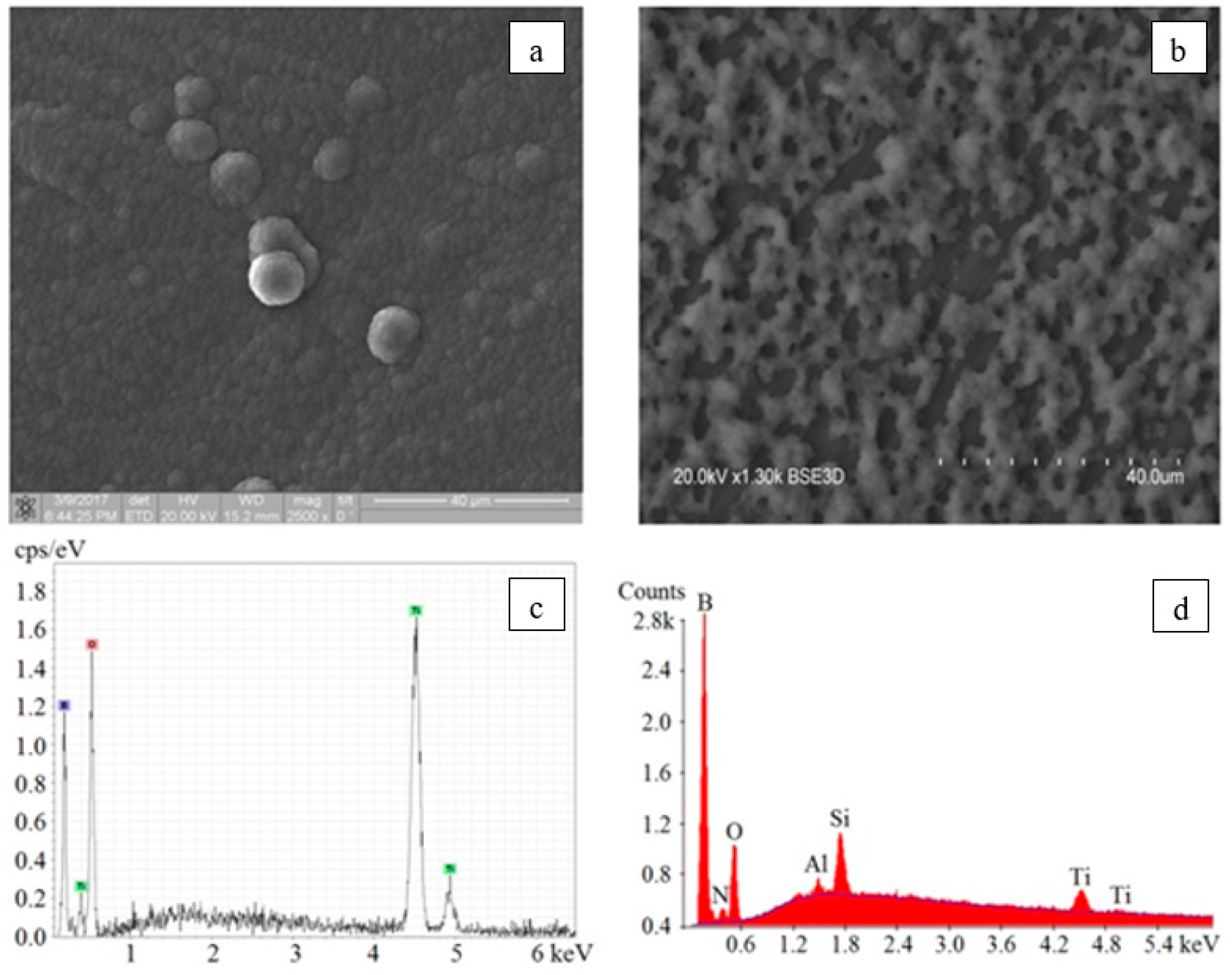
3.4.2. Boron-Containing Coatings
3.4.3. Silicon-Carbon Coatings
| Method | Microhardness | Deposition Time |
|---|---|---|
| Kaufman ion source [145] | 5–12 GPa | 2 h |
| DC PECVD [146] | 9–17 GPa | 6–12 h |
| RF PECVD [147] | 13–18 GPa | 4–8 h |
| Deposition from self-sustained arc discharge plasma with hot cathode [144] | 6.5–20 GPa | 8 h |
| Electron-beam evaporation in forevacuum | 9.5–20 GPa | 10 min |
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Igreja, R.; Dias, C.; Marat-Mendes, J.N. Smart Materials: The Functional Properties of Ceramic/Polymer Composites and their Relation to Sol-Gel Powder Preparation. Key Eng. Mater. 2002, 230–232, 177–180. [Google Scholar] [CrossRef]
- Bolton, W. Materials of Construction: Metals, Alloys, Polymers, Ceramics, Composites: Pocket Guide/Translation from English; “Dodeka, XXI” Publishing House: Moscow, Russia, 2004; 320p. (In Russian) [Google Scholar]
- Garshin, A.P.; Gropyanov, V.M.; Zaycev, G.P.; Semenov, S.S. Ceramics for Mechanical Engineering; Nauchtechlitizdat: Moscow, Russia, 2003; 384p. (In Russian) [Google Scholar]
- Tian, Y.; Bastawros, A.F.; Lo, C.C.H.; Constant, A.P.; Russell, A.M.; Cook, B.A. Superhard self-lubricating AlMgB14 films for microelectromechanical devices. Appl. Phys. Lett. 2003, 83, 2781–2783. [Google Scholar] [CrossRef]
- Labunec, V.F.; Voroshnin, L.G.; Kindarchuk, M.V. Wear-Resistant Boride Coatings; Technica: Kiev, Ukraine, 1989; 158p. (In Russian) [Google Scholar]
- Kidin, I.N.; Andryshechkin, V.I.; Volkov, V.A.; Holin, A.S. Electrochemical and Thermal Treatment of Metals and Alloys; Metallurgy: Moscow, Russia, 1978; 320p. (In Russian) [Google Scholar]
- Yushkov, Y.G.; Oks, E.M.; Oskomov, K.V.; Tyunkov, A.V.; Yakovlev, E.V.; Yushenko, A.Y.; Plaskeev, A.A.; Zolotukhin, D.B. On the effect of ceramic target composition on coatings deposited by electron-beam evaporation at forevacuum pressure. Ceram. Int. 2020, 46, 27641–27646. [Google Scholar] [CrossRef]
- Burdovitsin, V.A.; Klimov, A.S.; Medovnik, A.V.; Oks, E.M. Electron beam treatment of non-conducting materials by a fore-pump-pressure plasma-cathode electron beam source. Plasma Sources Sci. Technol. 2010, 19, 055003. [Google Scholar] [CrossRef]
- Burdovitsin, V.; Oks, E.; Zolotukhin, D. Effect of collector potential on the beam-plasma formed by a forevacuum-pressure plasma-cathode electron beam source. J. Phys. D Appl. Phys. 2018, 51, 304006. [Google Scholar] [CrossRef]
- Yushkov, Y.; Oks, E.; Tyunkov, A.; Zolotukhin, D. Alumina coating deposition by electron-beam evaporation of ceramic using a forevacuum plasma-cathode electron source. Ceram. Int. 2019, 45, 9782–9787. [Google Scholar] [CrossRef]
- Tyunkov, A.; Golosov, D.; Zolotukhin, D.; Nikonenko, A.; Oks, E.; Yushkov, Y.; Yakovlev, E. Nitriding of titanium in electron beam excited plasma in medium vacuum. Surf. Coat. Technol. 2020, 383, 125241. [Google Scholar] [CrossRef]
- Zolotukhin, D. Sterilization of a dielectric vessel using a forevacuum plasma electron source. In Proceeding of the Electronic Devices and Control Systems Conference, Tomsk, Russia, 12–14 November 2014; Volume 1, pp. 209–211. (In Russian). [Google Scholar]
- Danilin, V.S.; Syrchin, V.K. Magnetron Sputtering Systems; Radio and Communication: Moscow, Russia, 1982; 72p. (In Russian) [Google Scholar]
- Kelly, P.J.; Arnell, R.D. Magnetron sputtering: A review of recent developments and applications. Vacuum 2000, 56, 159–172. [Google Scholar] [CrossRef]
- Dermel, I.; Shashkeev, K. Peculiarities of magnetron sputtering of metals in the presence of reactive gases. VIAM Rep. 2017, 11, 11. [Google Scholar] [CrossRef]
- Berlin, E.B.; Seidman, L.A. Obtaining Thin Films by Reactive Magnetron Sputtering; Technoshpera: Moscow, Russia, 2014; 256p. (In Russian) [Google Scholar]
- Goncharov, A.A.; Dobrovol’Skii, A.N.; Kostin, E.G.; Petrik, I.S.; Frolova, E.K. Optical, structural, and photocatalytic properties of the nanosized titanium dioxide films deposited in magnetron discharge plasma. Tech. Phys. 2014, 59, 884–891. [Google Scholar] [CrossRef]
- Svadkovsky, I.V. Ion-Plasma Methods of Thin-Film Coatings Formation; Besprint: Minsk, Belarus, 2002; 242p. (In Russian) [Google Scholar]
- Marchenko, V.A. Processes on the target surface during reactive sputtering of vanadium in Ar-O2 media. Proc. RAS Phys. Ser. 2009, 73, 920–923. (In Russian) [Google Scholar]
- Anders, A. Physics of arcing, and implications to sputter deposition. Thin Solid Film. 2006, 502, 22–28. [Google Scholar] [CrossRef]
- Musil, J.; Baroch, P. Discharge in dual magnetron sputtering system. IEEE Trans. Plasma Sci. 2005, 33, 338–339. [Google Scholar] [CrossRef]
- Das, A.; Chikkala, A.K.; Bharti, G.P.; Behera, R.R.; Mamilla, R.S.; Khare, A.; Dobbidi, P. Effect of thickness on optical and microwave dielectric properties of Hydroxyapatite films deposited by RF magnetron sputtering. J. Alloy. Compd. 2018, 739, 729–736. [Google Scholar] [CrossRef]
- Schiller, S.; Goedicke, K.; Reschke, J.; Kirchhoff, V.; Schneider, S.; Milde, F. Pulsed magnetron sputter technology. Surf. Coat. Technol. 1993, 61, 331–337. [Google Scholar] [CrossRef]
- Okojie, J.E.; Golosov, D.A.; Melnikov, S.N.; Zavadski, S.M.; Kolos, V.V.; Poplevka, E.A.; Zhukovich, Y.A. Ferroelectric properties of strontium-bismuth tantalate thin film deposited by rf magnetron sputtering method. Probl. Phys. Math. Tech. 2018, 34, 33–37. (In Russian) [Google Scholar]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Sellers, J. Asymmetric bipolar pulsed DC: The enabling technology for reactive PVD. Surf. Coat. Technol. 1998, 98, 1245–1250. [Google Scholar] [CrossRef]
- Posadowski, W.; Wiatrowski, A.; Dora, J.; Radzimski, Z. Magnetron sputtering process control by medium-frequency power supply parameter. Thin Solid Film. 2008, 516, 4478–4482. [Google Scholar] [CrossRef]
- Vizir, A.; Nikolaev, A.; Oks, E.; Savkin, K.; Shandrikov, M.; Yushkov, G. Boron ion beam generation using a self-sputtering planar magnetron. Rev. Sci. Instrum. 2014, 85, 02C302. [Google Scholar] [CrossRef]
- Vizir, A.; Oks, E.; Yushkov, G.; Shandrikov, M. Magnetron discharge-based boron ion source. In Proceedings of the AIP Conference Proceedings, 17th International Conference on Ion Sources, Geneva, Switzerland, 15–20 September 2017; Volume 2011, p. 090005. [Google Scholar] [CrossRef]
- Anders, A. Tutorial: Reactive high power impulse magnetron sputtering (R-HiPIMS). J. Appl. Phys. 2017, 121, 171101. [Google Scholar] [CrossRef]
- Oks, E.; Tyunkov, A.; Yushkov, Y.; Zolotukhin, D. Synthesis of boron-containing coatings through planar magnetron sputtering of boron targets. Vaccum 2018, 155, 38–42. [Google Scholar] [CrossRef]
- Oks, E.; Anders, A.; Nikolaev, A.; Yushkov, Y. Sputtering of pure boron using a magnetron without a radio-frequency supply. Rev. Sci. Instrum. 2017, 88, 043506. [Google Scholar] [CrossRef] [PubMed]
- Lafferty, J. (Ed.) Vacuum Arcs; Mir: Moscow, Russia, 1982; 432p. (In Russian) [Google Scholar]
- Barvinok, V.A.; Bogdanovich, V.I. Physical Foundations and Mathematical Modeling of Vacuum Ion-Plasma Spraying Processes; Mashinostroenie: Moscow, Russia, 1999; 309p. (In Russian) [Google Scholar]
- Maksimov, Y.V.; Vereschaka, A.S.; Vereschaka, A.A.; Kudrov, A.S.; Lytkin, D.N.; Shegai, D.L.; Bulycheva, A.I. Development and research of multilayer composite coatings with a nanodispersed structure, deposited on cutting tools using assisted cathode-vacuum-arc processes. Proc. MSTU 2013, 15, 73–81. [Google Scholar]
- Valuev, V.P.; Valueva, T.V.; Lisenkov, A.A.; Sanchugov, E.L. Technological use of vacuum arc discharge. Instrum. Technol. 2011, 1, 16–27. (In Russian) [Google Scholar]
- Udris, Y.Y. Spattering of Drops by the Cathode Spot of a Mercury Arc; Leningrad, GEI: Moscow, Russia, 1958; pp. 107–128. (In Russian) [Google Scholar]
- Anders, A.; Anders, S.; Brown, I.G. Transport of vacuum arc plasmas through magnetic macroparticle filters. Plasma Sources Sci. Technol. 1995, 4, 1–12. [Google Scholar] [CrossRef]
- Boxman, R.; Goldsmith, S. Macroparticle contamination in cathodic arc coatings: Generation, transport and control. Surf. Coat. Technol. 1992, 52, 39–50. [Google Scholar] [CrossRef]
- Leng, Y.X.; Huang, N.; Yang, P.; Chen, J.Y.; Sun, H.; Wang, J.; Wan, G.J.; Leng, Y.; Chu, P.K. Influence of oxygen pressure on the properties and biocompatibility of titanium oxide fabricated by metal plasma ion implantation and deposition. Thin Solid Film. 2002, 420–421, 408. [Google Scholar] [CrossRef]
- Lucenko, A.N.; Muboyadjyan, S.A.; Budinovsky, S.A. Industrial technological processes of ionic surface treatment. Aviat. Mater. Technol. 2005, 1, 30–40. (In Russian) [Google Scholar]
- Martin, P.; Bendavid, A.; Cairney, J.; Hoffman, M. Nanocomposite Ti–Si–N, Zr–Si–N, Ti–Al–Si–N, Ti–Al–V–Si–N thin film coatings deposited by vacuum arc deposition. Surf. Coat. Technol. 2005, 200, 2228–2235. [Google Scholar] [CrossRef]
- Bobrov, G.V.; Ilyin, A.A. Inorganic Coating (Theory, Technology, Equipment); Intermet: Moscow, Russia, 2004; 624p. (In Russian) [Google Scholar]
- Şilik, E.; Pat, S.; Özen, S.; Mohammadigharehbagh, R.; Yudar, H.H.; Musaoğlu, C.; Korkmaz, Ş. Electrochromic properties of TiO2 thin films grown by thermionic vacuum arc method. Thin Solid Film. 2017, 640, 27–32. [Google Scholar] [CrossRef]
- Bendavid, A.; Martin, P.; Jamting, Å; Takikawa, H. Structural and optical properties of titanium oxide thin films deposited by filtered arc deposition. Thin Solid Film. 1999, 355-356, 6–11. [Google Scholar] [CrossRef]
- Yordanov, R.; Boyadjiev, S.; Georgieva, V. Characterization of RF and DC magnetron reactive sputtered TiO2 thin films for gas sensors. Dig. J. Nanomater. Biostructures 2014, 9, 467. Available online: https://chalcogen.ro/467_Yordanov.pdf (accessed on 1 December 2021).
- Dannenberg, R.; Greene, P. Reactive sputter deposition of titanium dioxide. Thin Solid Film. 2000, 360, 122–127. [Google Scholar] [CrossRef]
- Gushenets, V.; Oks, E.; Bugaev, A. Characteristics of a Pulsed Vacuum Arc Discharge with Pure Boron Cathode. In Proceedings of the 2018 28th International Symposium on Discharges and Electrical Insulation in Vacuum (ISDEIV), Greifswald, Germany, 23–28 September 2018; Volume 2, pp. 423–426. [Google Scholar]
- Gushenets, V.; Oks, E.M.; Bugaev, A.S. A pulsed vacuum arc ion source with a pure boron cathode. In Proceedings of the AIP Conference Proceedings, CERN, Geneva, Switzerland, 15–20 October 2017; Volume 2011, p. 090006. [Google Scholar]
- Frolova, V.P.; Vizir, A.V.; Nikolaev, A.G.; Yushkov, G.Y.; Oks, E.M. Vacuum arc with boron-containing cathode. In Proceedings of the International Symposium on Discharges and Electrical Insulation in Vacuum (ISDEIV), Greifswald, Switzerland, 23–28 September 2018; pp. 317–320. [Google Scholar] [CrossRef]
- Bugaev, A.; Frolova, V.; Gushenets, V.; Nikolaev, A.; Oks, E.; Savkin, K.; Shandrikov, M.; Vizir, A.; Yushkov, A.; Kadlubovich, B.; et al. Generation of boron ion beams by vacuum arc and planar magnetron ion sources. Rev. Sci. Instrum. 2019, 90, 103302. [Google Scholar] [CrossRef]
- Plasma-Enhanced Chemical Vapor Deposition: Where we are and the Outlook for the Future/Yasaman Hamedani. IntechOpen Chapter. 2015. Available online: https://www.intechopen.com/chapters/51808 (accessed on 1 December 2021).
- Schuegraf, K.K. (Ed.) Handbook of Thin-Film Deposition Processes and Techniques; Noyes Publications: Park Ridge, NJ, USA, 1988; p. 413. [Google Scholar] [CrossRef]
- Hellmich, A.; Jung, T.; Kielhorn, A.; Rißland, M. CVD-processes by hollow cathode glow discharge. Surf. Coat. Technol. 1998, 98, 1541–1546. [Google Scholar] [CrossRef]
- Xie, J.; Komvopoulos, K. Thermal stability of ultrathin amorphous carbon films synthesized by plasma-enhanced chemical vapor deposition and filtered cathodic vacuum arc. Philos. Mag. 2017, 97, 820–832. [Google Scholar] [CrossRef]
- Röhlecke, S.; Tews, R.; Kottwitz, A.; Schade, K. High rate PECVD of a-Si alloys on large areas. Surf. Coat. Technol. 1995, 74–75, 259–263. [Google Scholar] [CrossRef]
- Zhivotov, V.K.; Rusanov, V.D.; Fridman, A.A. Plasma-Chemical Processes in Microwave Discharge. Chem. Plasma 1984, 11, 200–246. (In Russian) [Google Scholar]
- Ivanov, A.A.; Leiman, V.G. Electron Beams in Plasma Chemistry; Atomizdat: Moscow, Russia, 1978; Volume 5, pp. 176–221. (In Russian) [Google Scholar]
- Cho, N.; Kim, Y.; Lim, J.; Hong, C.; Sul, Y.; Kim, C. Laser annealing effect of SiC films prepared by PECVD (plasma enhanced chemical vapor deposition). Thin Solid Film. 2002, 409, 1–7. [Google Scholar] [CrossRef]
- Marclay, E.; Webb, D.; Buchmann, P.; Vettiger, P. Broadband low-reflectivity coating for semiconductor power lasers by ion-beam and PECVD deposition. Appl. Surf. Sci. 1989, 43, 43–46. [Google Scholar] [CrossRef]
- Tyukavkin, A.V. Decelleration of the electron beams in gaseous media. Proc. MGUL 2000, 2, 83–87. [Google Scholar]
- Ankudinov, S.N.; Dmitriev, A.L.; Cherepanov, L.N. Method for Deposition Vacuum Coatings in Holes. Russian Patent RU 2211258 C2, 27 August 2003. (In Russian). [Google Scholar]
- Vasilieva, T.; Lysenko, S. Factors responsible for biomaterials modification in the electron-beam plasma. J. Phys. Conf. Ser. 2007, 63, 012033. [Google Scholar] [CrossRef]
- Wallis, G.; Sauer, K.; Sünder, D.; E Rosinskiĭ, S.; A Rukhadze, A.; Rukhlin, V.G. Injection of high-current relativistic electron beams into plasma and gas. Sov. Phys. Uspekhi 1975, 17, 492–506. [Google Scholar] [CrossRef]
- Ivanov, Y.F.; Filimonov, S.Y.; Denisova, Y.A.; Ivanova, O.V.; Ikonnikova, I.A.; Budovskikh, E.A.; Gromov, V.E. Steel 45 Surface Modification by a Combined Electron-Ion-Plasma Method. High Temp. Mater. Process. Int. Q. High-Technol. Plasma Process. 2015, 19, 29–36. [Google Scholar] [CrossRef]
- Lee, W.-S.; Chae, K.-W.; Eun, K.; Baik, Y.-J. Generation of pulsed direct-current plasma above 100 torr for large area diamond deposition. Diam. Relat. Mater. 2001, 10, 2220–2224. [Google Scholar] [CrossRef]
- Obizhaev, D.Y. The Structure and Properties of Functional Layers of Silicon Nitride at Different Stages of Their Formation in the Technology of Devices of Nano- and Microsystem Technology: Abstract on Candidate of Science Dissertation; “MATI”—Moscow State Aviation Technological University Press: Moscow, Russia, 2007; 22p. (In Russian) [Google Scholar]
- Azema, N.; Durand, J.; Berjoan, R.; Dupuy, C.; Cot, L. Oxidation stages of aluminium nitride thin films obtained by plasma-enhanced chemical vapour deposition (PECVD). J. Eur. Ceram. Soc. 1991, 8, 291–298. [Google Scholar] [CrossRef]
- Chryssou, C.; Pitt, C. Al2O3 thin films by plasma-enhanced chemical vapour deposition using trimethyl-amine alane (TMAA) as the Al precursor. Appl. Phys. A 1997, 65, 469–475. [Google Scholar] [CrossRef]
- Vasilieva, T.M. Production of bioactive compounds and materials on the basis of processes stimulated by beam-plasma action on the substance. In Proceedings of the VIII International Symposium on Theoretical and Applied Plasma Chemistry, Ivanovo, Russia, 10–15 September 2018; Ivanovo State University of Chemistry and Technology: Ivanovo, Russia, 2018; 168p. (In Russian) [Google Scholar]
- Martinu, L.; Zabeida, O.; Klemberg-Sapieha, J. Plasma-Enhanced Chemical Vapor Deposition of Functional Coatings; Elsevier Inc.: Amsterdam, The Netherlands, 2010; pp. 392–465. [Google Scholar]
- Jiang, K. Al2O3 Thin Films: Relation between Structural Evolution, Mechanical Properties, and Stability: Materials Chemistry Dissertation; 2011. Available online: https://d-nb.info/1019852542/34 (accessed on 1 December 2021).
- Podsvirov, O.A.; Karasev, P.A.; Vinogradov, A.Y. Mechanical stresses in diamond-like films: The role of deposition conditions and ion irradiation. Surface 2010, 3, 81–84. (In Russian) [Google Scholar]
- Batanov, G.M.; Borzosekov, V.; Golberg, D.; Iskhakova, L.D.; Kolik, L.V.; Konchekov, E.; Kharchev, N.K.; Letunov, A.A.; Malakhov, D.; Milovich, F.; et al. Microwave method for synthesis of micro- and nanostructures with controllable composition during gyrotron discharge. J. Nanophotonics 2016, 10, 012520. [Google Scholar] [CrossRef]
- Blackwell, D.D.; Walton, S.G.; Leonhardt, D.; Murphy, D.P.; Fernsler, R.F.; Amatucci, W.E.; Meger, R.A. Probe diagnostic development for electron beam produced plasmas. J. Vac. Sci. Technol. A 2001, 19, 1330–1335. [Google Scholar] [CrossRef]
- Leonhardt, D.; Muratore, C.; Walton, S.; Blackwell, D.; Fernsler, R.; Meger, R. Generation of electron-beam produced plasmas and applications to surface modification. Surf. Coat. Technol. 2004, 177–178, 682–687. [Google Scholar] [CrossRef]
- Pyachin, S.A.; Pugachevsky, S.A. New Technologies for Obtaining Functional Nanomaterials: Laser Ablation, Electrospark Exposure; Publishing House of Material Science Institute of of Khabarovsk Scientific Centre: Khabarovsk, Russia, 2013; 38p. (In Russian) [Google Scholar]
- Ion, J.C. Laser Processing of Engineering Materials: Principles, Procedure and Industrial Application; Elsevier Butterworth–Heinemann: Burlington, NJ, USA, 2005; 57p, Available online: https://www.elsevier.com/books/laser-processing-of-engineering-materials/ion/978-0-7506-6079-2 (accessed on 1 December 2021).
- Chucharkin, M.L.; Porokhov, N.V.; Kalabukhov, A.S.; Snigiryov, O.V.; Rusanov, S.Y.; Kashin, V.V.; Tsvetkov, V.B.; Vinkler, D. High-temperature superconducting films on faceted monocrystalline filaments. Radioelectron. J. 2013, 2, 1. (In Russian) [Google Scholar]
- Veiko, V.; Skvortsov, A.; Huynh, C.T.; Petrov, A. Laser ablation of monocrystalline silicon under pulsed-frequency fiber laser. Sci. Tech. J. Inf. Technol. Mech. Opt. 2015, 15, 426–434. [Google Scholar] [CrossRef][Green Version]
- Mesa, M.; Oliete, P.; Pastor, J.Y.; Martín, A.; Llorca, J. Mechanical properties up to 1900 K of Al2O3/Er3Al5O12/ZrO2 eutectic ceramics grown by the laser floating zone method. J. Eur. Ceram. Soc. 2014, 34, 2081–2087. [Google Scholar] [CrossRef]
- Kononenko, T.V.; Konov, V.; Lubnin, E.N.; Dausinger, F. Pulsed laser deposition of hard carbon coatings at atmospheric pressure. Quantum Electron. 2003, 33, 189–191. [Google Scholar] [CrossRef]
- Arakelyan, S.M.; Prokoshev, V.G.; Abramov, D.V.; Kucherik, A.O. Laser Nanostructuring of Materials: Methods of Implementation and Diagnostics: A Tutorial; Vladimir State University Press: Vladimir, Russia, 2010; 140p. (In Russian) [Google Scholar]
- Bashkova, I.O.; Reshetnikov, S.M.; Gilmutdinov, F.Z.; Kharanzhevskiy, E.V. Physical and chemical properties of nano-dimensional oxide coatings deposited on the zirconium alloy by high-speed laser synthesis. Chem. Phys. Mesoscopy 2018, 20, 85–95. (In Russian) [Google Scholar]
- White, R.A.; Fusaro, R.; Jones, M.G.; Solomon, H.D.; Milian-Rodriguez, R.R. Underwater Cladding with Laser Beam and Plasma Arc Welding. Weld. J. 1997, 76, 57–61. [Google Scholar]
- Ismail, R.A.; Zaidan, S.A.; Kadhim, R.M. Preparation and characterization of aluminum oxide nanoparticles by laser ablation in liquid as passivating and anti-reflection coating for silicon photodiodes. Appl. Nanosci. 2017, 7, 477–487. [Google Scholar] [CrossRef]
- Bulaev, S.A. The essence of pulsed laser deposition in vacuum as a method for producing films of nanometer thicknesses. Proc. Kazan Technol. Univ. 2014, 17, 25–28. (In Russian) [Google Scholar]
- Nevolin, V.N.; Fominskii, V.Y.; Gnedovets, A.G.; Romanov, R.I. Pulsed laser deposition of thin-film coatings using an antidroplet shield. Tech. Phys. 2009, 54, 1681–1688. [Google Scholar] [CrossRef]
- Pugachevskii, M.A.; Zavodinskii, V.G.; Kuz’Menko, A.P. Dispersion of zirconium dioxide by pulsed laser radiation. Tech. Phys. 2011, 56, 254–258. [Google Scholar] [CrossRef]
- Somonov, V.V. Laser Technological Complex “SKAT-301”: Laboratory Manual; St Petersburg University Press: Saint Petersburg, Russia, 2011. (In Russian) [Google Scholar]
- Kim, J.H.; Cho, H.; Pham, T.V.; Hwang, J.H.; Ahn, S.; Jang, S.G.; Lee, H.; Park, C.; Kim, C.S.; Kim, M.J. Dual growth mode of boron nitride nanotubes in high temperature pressure laser ablation. Sci. Rep. 2019, 9, 15674. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Boyen, H.-G.; Yin, H.; Ziemann, P.; Banhart, F. Microstructure of the intermediate turbostratic boron nitride layer. Diam. Relat. Mater. 2005, 14, 1474–1481. [Google Scholar] [CrossRef]
- See Website for Additional Details. Available online: https://caeonline.com/buy/lasers/lambda-physik-lpx-300/9177297 (accessed on 1 December 2021).
- Harsha, K.S. Principles of Vapor Deposition of Thin Films; Elsevier: London, UK, 2006; p. 400. [Google Scholar]
- Paperny, V. Sources of Multiply Charged Metal Ions: Vacuum Discharge or Laser Produced Plasma? Springer: Singapore, 2002; Volume 88, pp. 27–37. [Google Scholar]
- Devoino, O.G.; Okovity, V.V. Composite powder based on zirconium dioxide, partially stabilized with cerium oxide. Sci. Tech. 2013, 6, 3–8. (In Russian) [Google Scholar]
- Sidqi, N.; Clark, C.; Buller, G.S.; Thalluri, G.K.V.V.; Mitrofanov, J.; Noblet, Y. Comparative study of dielectric coating materials for micro-cavity applications. Opt. Mater. Express 2019, 9, 3452–3468. [Google Scholar] [CrossRef]
- A Moncrieff, D.; Barker, P.R.; E Robinson, V.N. Electron scattering by gas in the scanning electron microscope. J. Phys. D: Appl. Phys. 1979, 12, 481–488. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Kuroyama, T.; Okuhara, Y.; Matsubara, H. Properties of Al doped zinc oxide films prepared by electron beam-PVD. J. Ceram. Soc. Jpn. 2013, 121, 981–983. [Google Scholar] [CrossRef][Green Version]
- Burdovitsin, V.A.; Klimov, A.S.; Medovnik, A.V.; Oks, E.M.; Yushkov, Y.G. Fore-Vacuum Plasma Electron Sources; Tomsk University Press: Tomsk, Russia, 2014; 288p. (In Russian) [Google Scholar]
- Oks, E. Plasma Cathode Electron Sources: Physics, Technology, Applications; Wiley—VCH Verlag GmbH & CO. KGaA: Weinheim, Germany, 2006; 171p. [Google Scholar]
- Zenin, A.A.; Klimov, A.; Burdovitsin, V.A.; Oks, E. Generating stationary electron beams by a forevacuum plasma source at pressures up to 100 Pa. Tech. Phys. Lett. 2013, 39, 454–456. [Google Scholar] [CrossRef]
- Zhirkov, I.S.; Burdovitsin, V.A.; Oks, E.; Osipov, I.V. Discharge initiation in a hollow-cathode plasma source of electrons. Tech. Phys. 2006, 51, 1379–1382. [Google Scholar] [CrossRef]
- Goebel, D.M.; Watkins, R.M. High current, low pressure plasma cathode electron gun. Rev. Sci. Instrum. 2000, 71, 388–398. [Google Scholar] [CrossRef]
- Bakeev, I.Y.; Klimov, A.; Oks, E.M.; Zenin, A. Generation of high-power-density electron beams by a forevacuum-pressure plasma-cathode electron source. Plasma Sources Sci. Technol. 2018, 27, 075002. [Google Scholar] [CrossRef]
- Burdovitsin, V.A.; Goreev, A.K.; Klimov, A.; Zenin, A.A.; Oks, E. Expansion of the working range of forevacuum plasma electron sources toward higher pressures. Tech. Phys. 2012, 57, 1101–1105. [Google Scholar] [CrossRef]
- Lock, E.; Petrovykh, D.; Mack, P.; Carney, T.; White, R.G.; Walton, S.G.; Fernsler, R.F. Surface Composition, Chemistry, and Structure of Polystyrene Modified by Electron-Beam-Generated Plasma. Langmuir 2010, 26, 8857–8868. [Google Scholar] [CrossRef]
- Klimov, A.S.; Medovnik, A.V.; Yushkov, Y.G.; Tyunkov, A.V.; Zenin, A.A.; Kazakov, A.V.; Zolotukhin, D.B. Application of Forevacuum Plasma Electron Sources for Processing Dielectrics; TUSUR Press: Tomsk, Russia, 2017; 188p. (In Russian) [Google Scholar]
- Lock, E.; Fernsler, R.F.; Walton, S.G. Experimental and theoretical evaluations of electron temperature in continuous electron beam generated plasmas. Plasma Sources Sci. Technol. 2008, 17, 025009. [Google Scholar] [CrossRef]
- Zolotukhin, D.B. Parameters and Characteristics of a Beam Plasma Generated in the Forevacuum Pressure Range by an Electron Source with a Plasma Cathode, Abstract for Candidate of Science Dissertation; TUSUR Press: Tomsk, Russia, 2016; 22p. (In Russian) [Google Scholar]
- Golant, V.E.; Zhilinsky, A.P.; Sakharov, I.E. Plasma Physics Basics; Atomizadat: Moscow, Russia, 1977; 384p. (In Russian) [Google Scholar]
- Zolotukhin, D.B.; Klimov, A.S.; Zenin, A.A. Parameters of the plasma generated by an electron beam in a fore-vacuum. Proc. TUSUR 2013, 30, 79–82. (In Russian) [Google Scholar]
- Zolotukhin, D.B.; Burdovitsin, V.A. Monte Carlo simulation of elastic and inelastic scattering of an electron beam in a gas. Proc. TUSUR 2012, 26, 55–58. (In Russian) [Google Scholar]
- Manheimer, W.M.; Fernsler, R.F.; Lampe, M.; A Meger, R. Theoretical overview of the large-area plasma processing system (LAPPS). Plasma Sources Sci. Technol. 2000, 9, 370–386. [Google Scholar] [CrossRef]
- Zolotukhin, D.B. Numerical Simulation of the Dependences of the Parameters of the Beam Plasma on the Beam Current and Gas Pressure in the Forevacuum. Proceedings of XI International Conference of Students and Young Scientists “Prospects of Fundamental Sciences Development”; Tomsk Polytechnic University Press: Tomsk, Russia, 2014; pp. 79–81. (In Russian) [Google Scholar]
- Burdovitsin, V.A.; Oks, E.M.; Fedorov, M.V. Parameters of the Plasma Sheet Generated by a Sheet Beam in the Range of Forvacuum Pressures. Russ. Phys. J. 2004, 47, 310–314. [Google Scholar] [CrossRef]
- Klimov, A.S.; Oks, E.M.; Zenin, A.A. Generation of broad electron beams by fore-vacuum plasma sources based on a discharge with an extended hollow cathode. J. High. Educ. Inst. Phys. 2017, 60, 37. (In Russian) [Google Scholar]
- Klimov, A.S.; Zenin, A.A.; Zolotukhin, D.B.; Tyunkov, A.V.; Yushkov, Y.G. Plasma generation with electron sources at the medium gas pressures. Adv. Appl. Phys. 2019, 7, 249–259. (In Russian) [Google Scholar]
- Klimov, A.S.; Zenin, A.A.; Oks, E.M.; Prech, K. Specifics of the focused electron beam transport in the forevacuum range of pressure. Phys. Plasmas 2018, 25, 113103. [Google Scholar] [CrossRef]
- Pillai, S.D.; Shayanfar, S. Electron beam processing of fresh produce—A critical review. Radiat. Phys. Chem. 2018, 143, 85–88. [Google Scholar] [CrossRef]
- Frank, N.W. Introduction and historical review of electron beam processing for environmental pollution control. Radiat. Phys. Chem. 1995, 45, 989–1002. [Google Scholar] [CrossRef]
- Dunn, D.A.; Nichparenko, W.; Simpson, J.E.; Thomassen, K.I. Oscillations and Noise Generation in a Beam-Generated Plasma. J. Appl. Phys. 1965, 36, 3273. [Google Scholar] [CrossRef]
- Zolotukhin, D.; Burdovitsin, V.A.; Oks, E. Generation of a beam plasma by a forevacuum electron source in a space bounded by dielectric walls. Tech. Phys. 2015, 60, 772–774. [Google Scholar] [CrossRef]
- Zolotukhin, D.B.; A Burdovitsin, V.; Oks, E.M. Generation of uniform electron beam plasma in a dielectric flask at fore-vacuum pressures. Plasma Sources Sci. Technol. 2015, 25, 15001. [Google Scholar] [CrossRef]
- Zolotukhin, D.B.; Burdovitsini, V.A.; Oks, E.M.; Tyunkov, A.V.; Yushkov, Y.G. Properties of beam plasma generated in the insulated metal cavity in the fore-vacuum pressure range. Proc. TUSUR 2017, 20, 42–45. (In Russian) [Google Scholar] [CrossRef]
- Zolotukhin, D.B.; Burdovitsin, V.A.; Oks, E.M. On the role of secondary electrons in beam plasma generation inside a dielectric flask by fore-vacuum plasma-cathode electron source. Phys. Plasmas 2017, 24, 093502. [Google Scholar] [CrossRef]
- Zolotukhin, D.; Burdovitsin, V.; Oks, E.; Tyunkov, A.; Yushkov, Y. Sterilization of dielectric containers using a fore-vacuum pressure plasma-cathode electron source. J. Phys. Conf. Ser. 2015, 652, 012044. [Google Scholar] [CrossRef]
- Klimov, A.S.; Barkov, A.V. Behavior of Ceramic Materials during Their Treatment of Electron Beam, Solid State Physics: Proceedings of XI Russian Scientific Students Conference; Tomsk State University: Tomsk, Russia, 2008; pp. 167–170. (In Russian) [Google Scholar]
- Klimov, A.S.; Goreev, A.K. On the Mechanism of Establishing the Potential of an Electronic Collector in the Forevacuum Pressure Range. Proceedings of XVI International Scientific-Practical Conference of Students, PhD Students and Young Scientists “Modern Technics and Technologies”; Tomsk Polytechnic University Press: Tomsk, Russia, 2010; Volume 3, pp. 35–36. (In Russian) [Google Scholar]
- Burdovitsin, V.A.; Medovnik, A.V.; Oks, E.; Skrobov, E.V.; Yushkov, Y. Potential of a dielectric target during its irradiation by a pulsed electron beam in the forevacuum pressure range. Tech. Phys. 2012, 57, 1424–1429. [Google Scholar] [CrossRef]
- Martens, V.Y. Potential of insulated electrode in a system “plasma, electron flow”. Tech. Phys. 1996, 66, 70–76. (In Russian) [Google Scholar]
- Klimov, A.S. Generation of Electron Beams in the Fore-Vacuum Pressure Range Based on Plasma-Emission Discharge Systems with a Hollow Cathode. Ph.D. Thesis, Tomsk State University of Control Systems and Radioelectronics, Tomsk, Russia, 23 December 2016. (In Russian). [Google Scholar]
- Zolotukhin, D.B.; Tyunkov, A.V.; Yushkov, Y.G. Distribution of potential upon the surface of non-conductive boron-containing target during irradiation by an electron beam in the fore-vacuum. Appl. Phys. 2017, 6, 39–43. (In Russian) [Google Scholar]
- Yushkov, Y.; Zolotukhin, D.B.; Oks, E.M.; Tyunkov, A. Different stages of electron-beam evaporation of ceramic target in medium vacuum. J. Appl. Phys. 2020, 127, 113303. [Google Scholar] [CrossRef]
- Oks, E.; Tyunkov, A.; Yushkov, Y.; Zolotukhin, D. Ceramic coating deposition by electron beam evaporation. Surf. Coat. Technol. 2017, 325, 1–6. [Google Scholar] [CrossRef]
- Yushkov, Y.; Oks, E.; Tyunkov, A.; Yushenko, A.; Zolotukhin, D. Electron-Beam Deposition of Aluminum Nitride and Oxide Ceramic Coatings for Microelectronic Devices. Coatings 2021, 11, 645. [Google Scholar] [CrossRef]
- Tyunkov, A.; Burdovitsin, V.; Oks, E.; Yushkov, Y.; Zolotukhin, D. An experimental test-stand for investigation of electron-beam synthesis of dielectric coatings in medium vacuum pressure range. Vacuum 2019, 163, 31–36. [Google Scholar] [CrossRef]
- Yushkov, Y.G.; Tyunkov, A.V.; Oks, E.M.; Zolotukhin, D.B. Electron beam evaporation of boron at forevacuum pressures for plasma-assisted deposition of boron-containing coatings. J. Appl. Phys. 2016, 120, 233302. [Google Scholar] [CrossRef]
- Yushkov, Y.; Oks, E.; Tyunkov, A.; Corbella, C.; Zolotukhin, D. Deposition of boron-containing coatings by electron-beam evaporation of boron-containing targets. Ceram. Int. 2019, 46, 4519–4525. [Google Scholar] [CrossRef]
- Bugaev, A.S.; Vizir, A.V.; Gushenets, V.I.; Nikolaev, A.G.; Oks, E.M.; Savkin, K.P.; Yushkov, Y.G.; Tyunkov, A.; Frolova, V.P.; Shandrikov, M.V.; et al. Generation of Boron Ions for Beam and Plasma Technologies. Russ. Phys. J. 2019, 62, 1117–1122. [Google Scholar] [CrossRef]
- Zolotukhin, D.; Tyunkov, A.; Yushkov, Y. Synthesis of the boron-based coating in beam plasma using fore-vacuum electron source. In Proceedings of the AIP Conference Proceedings, Tomsk, Russia, 25–28 April 2017; Volume 1899, p. 040004. [Google Scholar]
- Kržan, B.; Novotny-Farkas, F.; Vižintin, J. Tribological behavior of tungsten-doped DLC coating under oil lubrication. Tribol. Int. 2009, 42, 229–235. [Google Scholar] [CrossRef]
- Grigoryev, M.N.; Mikhailova, T.S.; Myasoedova, T.N. Production of silicon-carbon films on conductive and dielectric substrates by the method of electrochemical deposition, Proceedings of Southern Federal University. Tech. Sci. 2018, 201, 56–66. (In Russian) [Google Scholar]
- Grenadyorov, A.S.; Oskomov, K.V.; Solov’Ev, A.A.; Rabotkin, S. Deposition of silicon–carbon coatings from the plasma of a non-self-sustained arc discharge with a heated cathode. Tech. Phys. 2016, 61, 690–695. [Google Scholar] [CrossRef]
- Krivobokov, V.P.; Sochugov, N.S.; Soloviev, A.A. Plasma Coatings (Methods and Equipment): A Tutorial; Tomsk Polytechnic University Press: Tomsk, Russia, 2008; 104p. [Google Scholar]
- See Website for Additional Information on the Equipment for Micro- and Nano-Electronics. Available online: http://www.cryosystems-mve.ru/lm/equipment/t-o/cvd/ (accessed on 1 December 2021).
- Afanasiev, V.P.; Terukov, E.I.; Sherchenkov, A.A. Thin-Film Solar Elements Based on Silicon, 2nd ed.; Saint Petersburg Electrotechnical University ‘LETI’ Press: Saint Petersburg, Russia, 2011; 168p. (In Russian) [Google Scholar]
- Cheng, Y.-T.; Cheng, C.-M. Relationships between hardness, elastic modulus, and the work of indentation. Appl. Phys. Lett. 1998, 73, 614–616. [Google Scholar] [CrossRef]














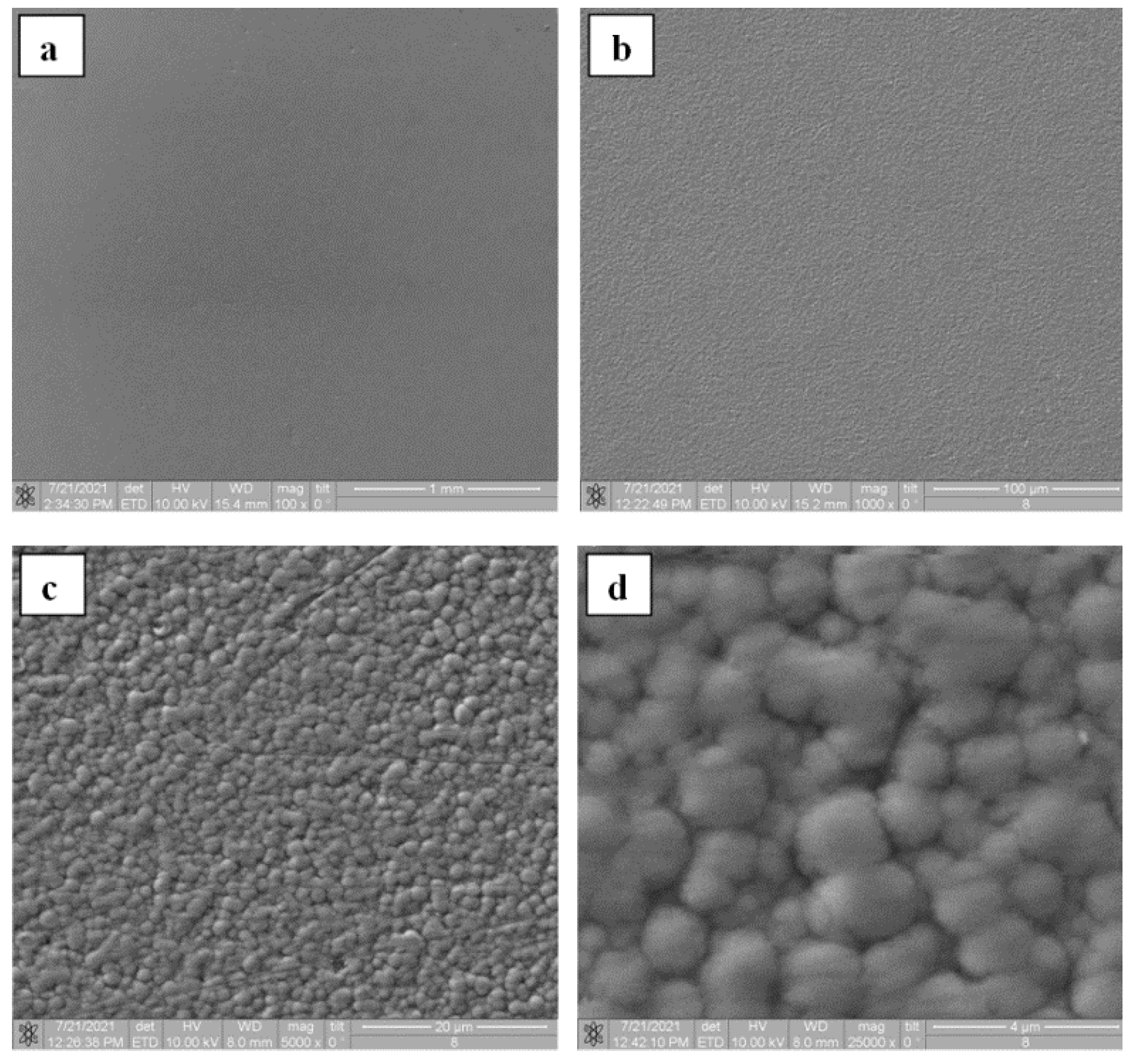
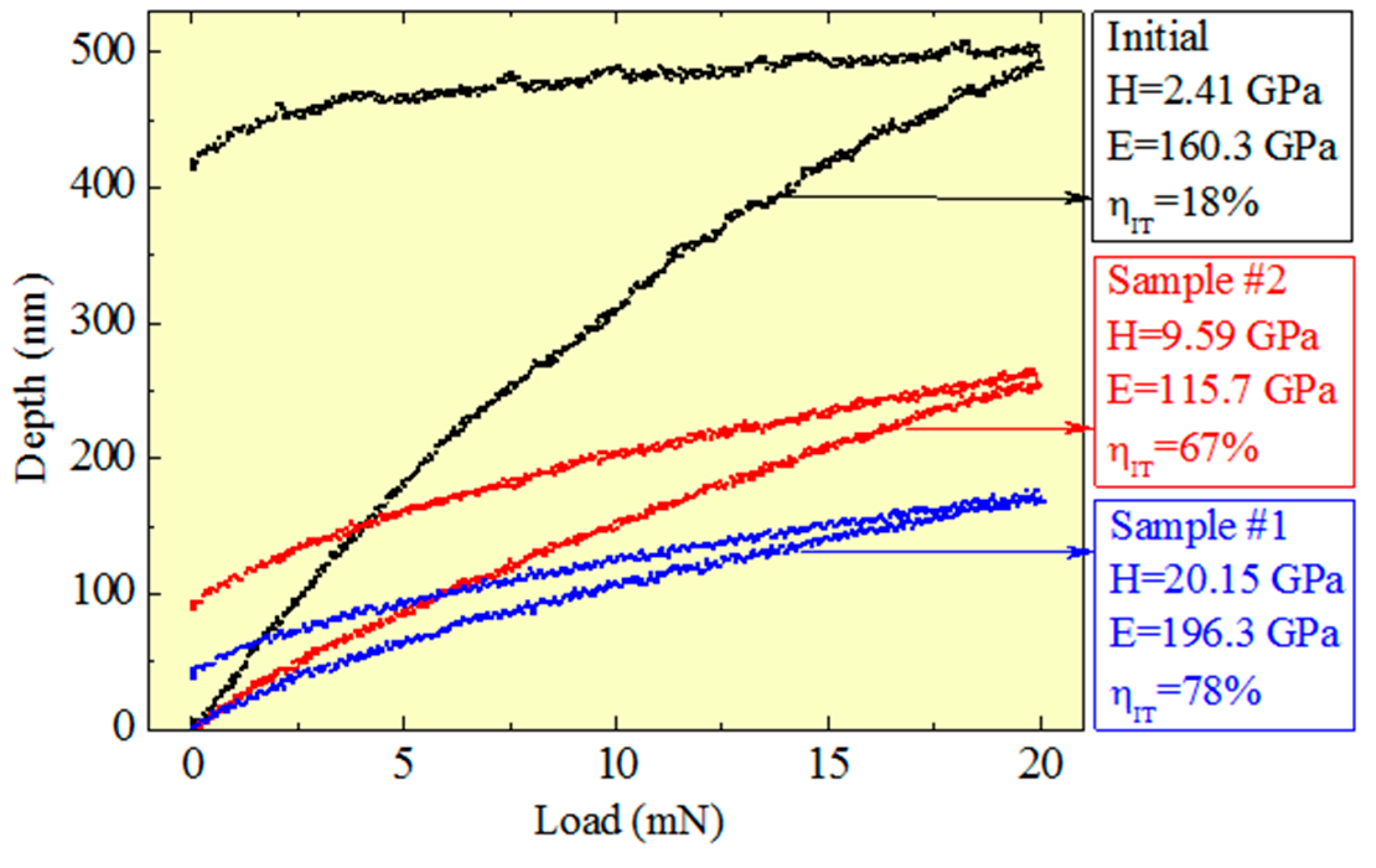

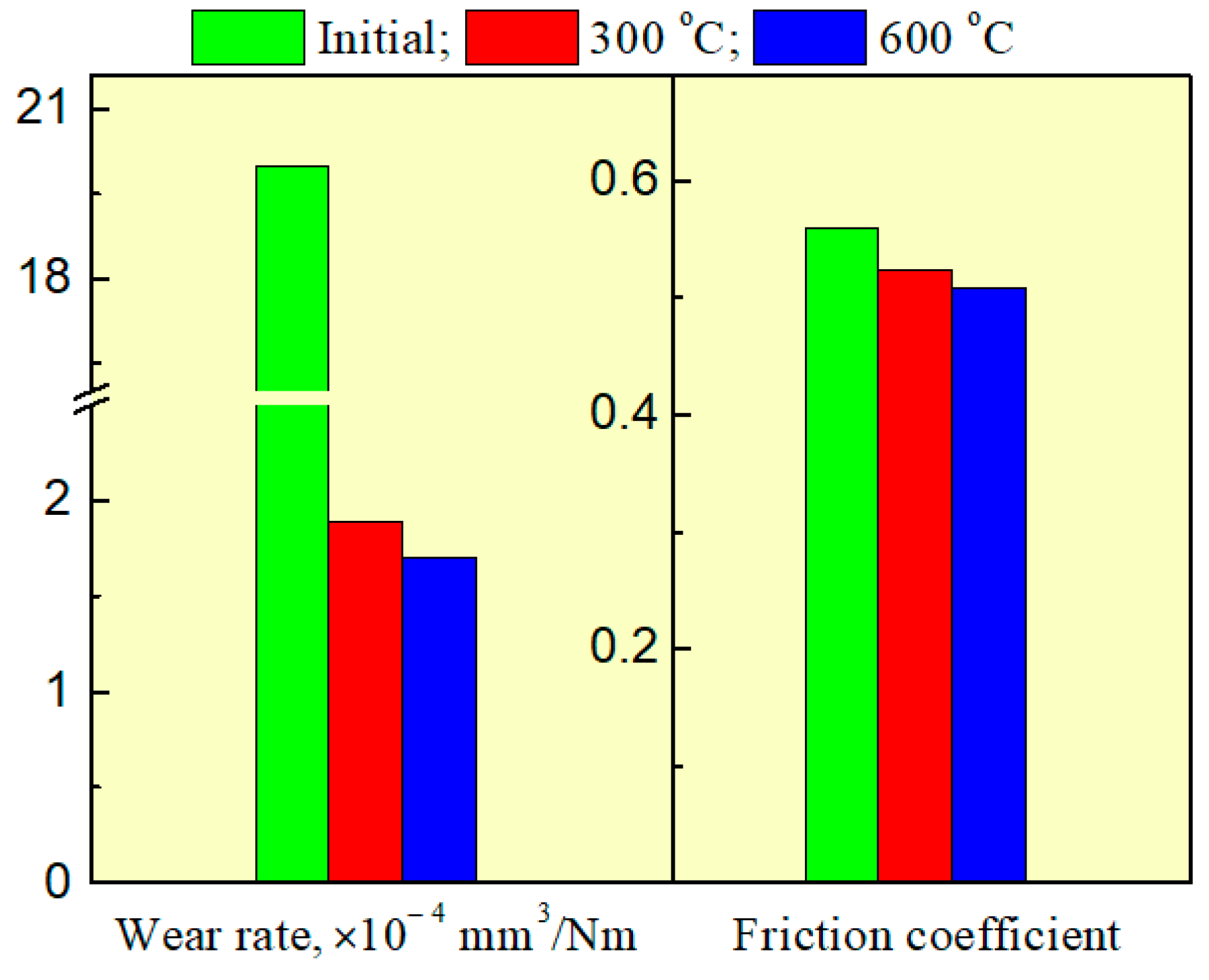
| Process Parameter | SiO2 | Si3N4 |
|---|---|---|
| Substrate temperature, °C | 350 | 400 |
| Gas flow ratio | N2O/SiH4/N2:75/1/75 | NH3/SiH4/N2:60/1/44 |
| Pressure, Torr | 0.25 | 0.35 |
| Deposition rate, nm/min | 50 | 20 |
| Electron-beam parameters | 4.7 kV, 16 mA/cm2 | 2.3 kV, 13 mA/cm2 |
| Method | Advantages | Drawbacks |
|---|---|---|
| Reactive magnetron sputtering Deposition rate—up to 15 nm/min | Good adhesion of coatings, controllability of the structure of coatings, the ability to coat the large-area surfaces | Poisoning of the electrodes of the discharge system, low utilization of the cathode material, low productivity in the deposition of dielectric coatings. |
| RF magnetron sputtering Deposition rate—up to 50 nm/min | Ability to work directly with the dielectric targets, no disadvantages of reactive magnetron sputtering | The complexity of matching the output parameters of the RF generator with the parameters of the discharge system, high operating pressures affecting the quality of the coatings obtained. |
| Vacuum arc method Deposition rate—up to 100 nm/min | Good adhesion of coatings, relative simplicity of technical implementation, effective ionic cleaning of products before application, high properties of coatings | The presence of a droplet fraction of the metal phase in the coating, relatively high deposition temperatures |
| Plasma-chemical (PECVD) method Deposition rate—up to 50 nm/min | Relatively low process temperature, large range of formed coatings | Difficulty in controlling the thickness of coatings, as well as the composition of gases and vapors during their decomposition; the need for complex and expensive differential pumping systems |
| Laser methods Deposition rate—up to 50 nm/min | Obtaining coatings of complex compounds, high purity of coatings | The complexity of technical implementation; most of the energy of laser radiation is lost when the laser radiation interacts with the vapor of the evaporated target |
| Electron-beam method Deposition rates: Up to 50 nm/min—pure dielectric coatings Up to 1000 nm/min—deposition of dielectric coatings in a reaction gas media | Effective control of the parameters of the electron beam and its position, high purity of coatings; practically all supplied energy is transformed into the energy of the electron beam | The problem of charging the surface of non-conductive products, which requires special efforts to neutralize the charge; it is also difficult to ensure uniformity of coating. |
| Sample 1 | Sample 2 | Sample 3 | Sample 4 |
|---|---|---|---|
| Coating Thickness, µm | |||
| 2.44 | 1.78 | 1.82 | 1.98 |
| Deposition Rate, nm/min | |||
| 1218 | 888 | 912 | 990 |
| Physical Parameter | VK-94-1 Ceramics | Coating |
|---|---|---|
| Dielectric constant at 20 °C and 10 kHz/10 MHz | 10.3/10.3 | 9/7.8 |
| Dielectric loss tangent tgδ·10−4 at 20 °C and 1 MHz | 6 | 5.9 |
| Volume resistivity, Ω·cm | 1013 | 1.2 × 1012 |
| Amplitude | Coating | |||
|---|---|---|---|---|
| B + He | B + O2 | +N2 | BN | |
| Root mean square roughness Sq, µm | 0.41 | 0.403 | 0.498 | 0.711 |
| Arithmetic roughness Sa, µm | 0.325 | 0.312 | 0.391 | 0.497 |
| Maximum surface relief height Sz, µm | 2.5 | 2.94 | 3.71 | 7.89 |
| The highest peak Sp, µm | 1.49 | 1.41 | 1.5 | 4.49 |
| The deepest depression Sv, µm | 1.08 | 1.7 | 3.93 | 3.69 |
| Asymmetry Ssk | 0.413 | –0.128 | 3.32 | 1.02 |
| Kurtosis Sku | 3.08 | 3.61 | 3.71 | 8.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yushkov, Y.G.; Oks, E.M.; Tyunkov, A.V.; Zolotukhin, D.B. Electron-Beam Synthesis of Dielectric Coatings Using Forevacuum Plasma Electron Sources (Review). Coatings 2022, 12, 82. https://doi.org/10.3390/coatings12010082
Yushkov YG, Oks EM, Tyunkov AV, Zolotukhin DB. Electron-Beam Synthesis of Dielectric Coatings Using Forevacuum Plasma Electron Sources (Review). Coatings. 2022; 12(1):82. https://doi.org/10.3390/coatings12010082
Chicago/Turabian StyleYushkov, Yury G., Efim M. Oks, Andrey V. Tyunkov, and Denis B. Zolotukhin. 2022. "Electron-Beam Synthesis of Dielectric Coatings Using Forevacuum Plasma Electron Sources (Review)" Coatings 12, no. 1: 82. https://doi.org/10.3390/coatings12010082
APA StyleYushkov, Y. G., Oks, E. M., Tyunkov, A. V., & Zolotukhin, D. B. (2022). Electron-Beam Synthesis of Dielectric Coatings Using Forevacuum Plasma Electron Sources (Review). Coatings, 12(1), 82. https://doi.org/10.3390/coatings12010082





