A Facile Nitriding Approach for Improved Impact Wear of Martensitic Cold-Work Steel Using H2/N2 Mixture Gas in an AC Pulsed Atmospheric Plasma Jet
Abstract
1. Introduction
2. Experimental Details
2.1. Materials
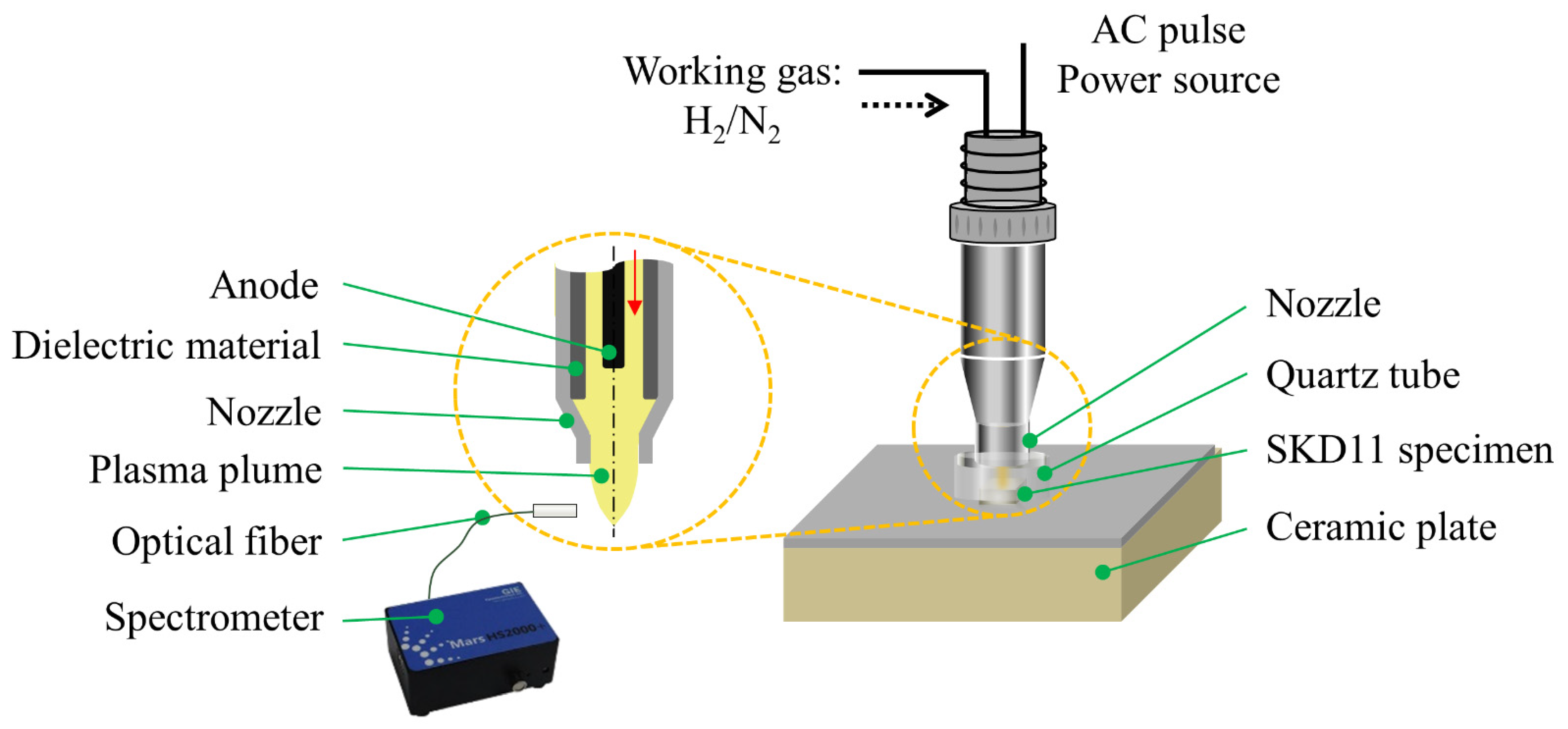
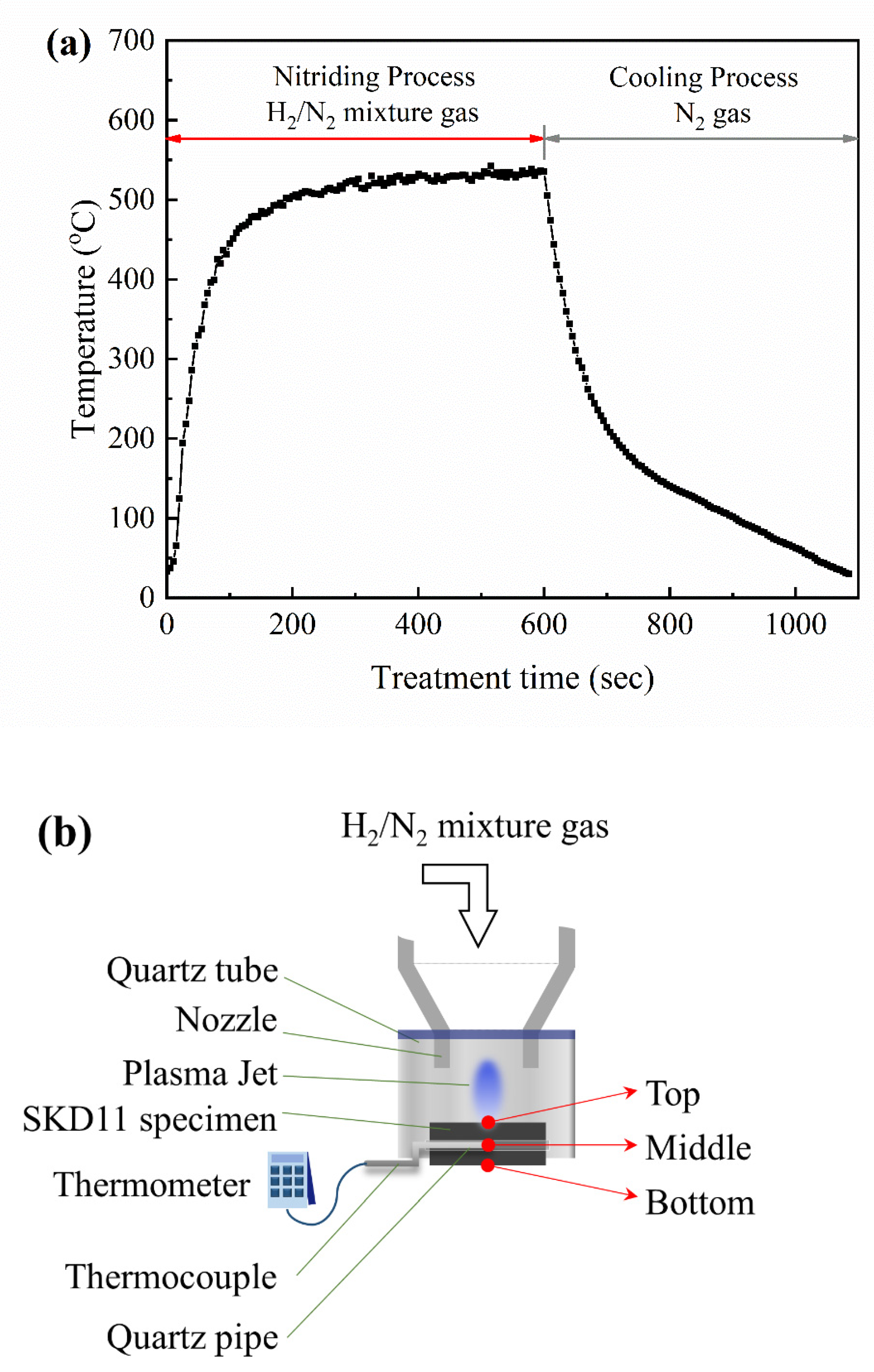
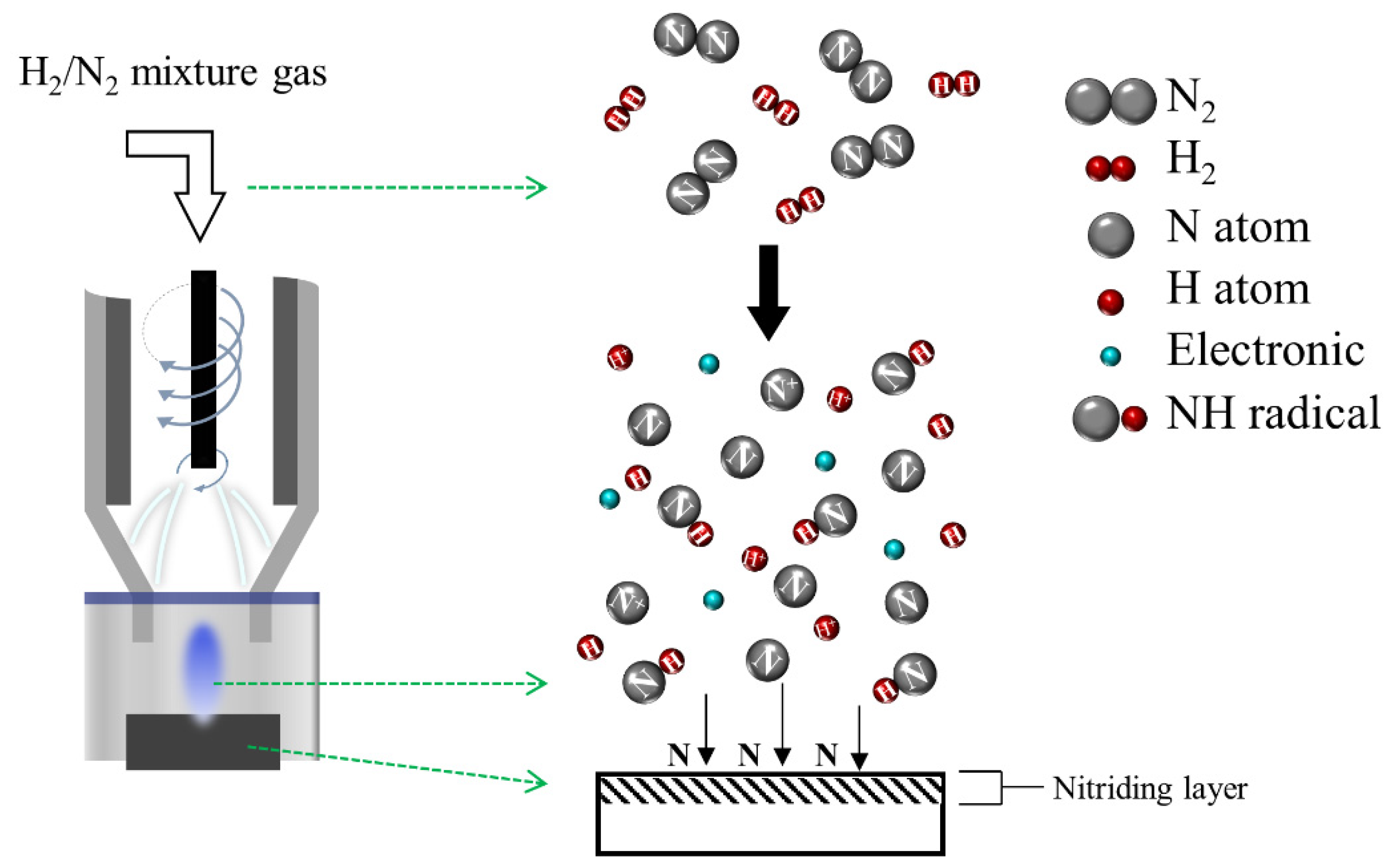
2.2. APPJ System
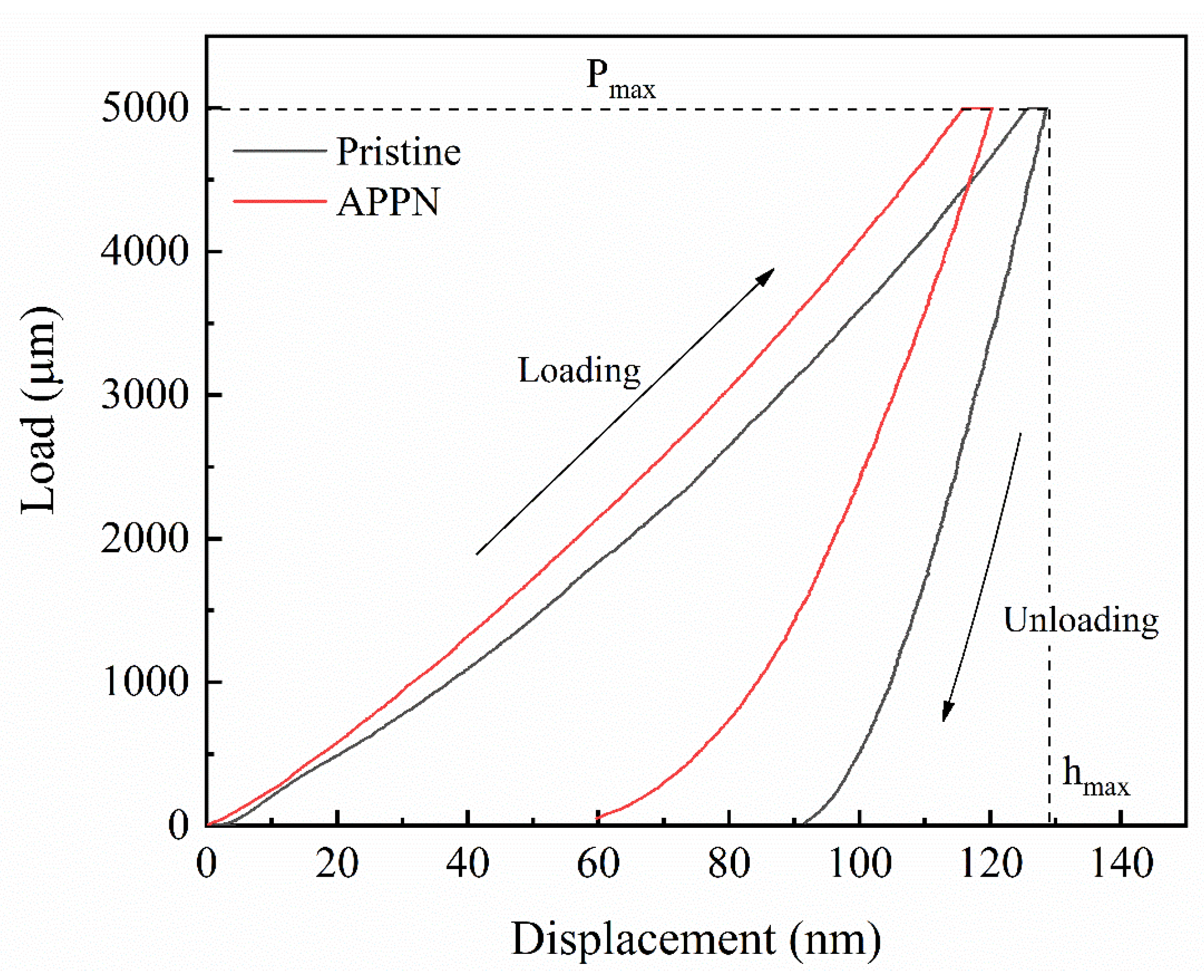
2.3. Materials’ Characterization
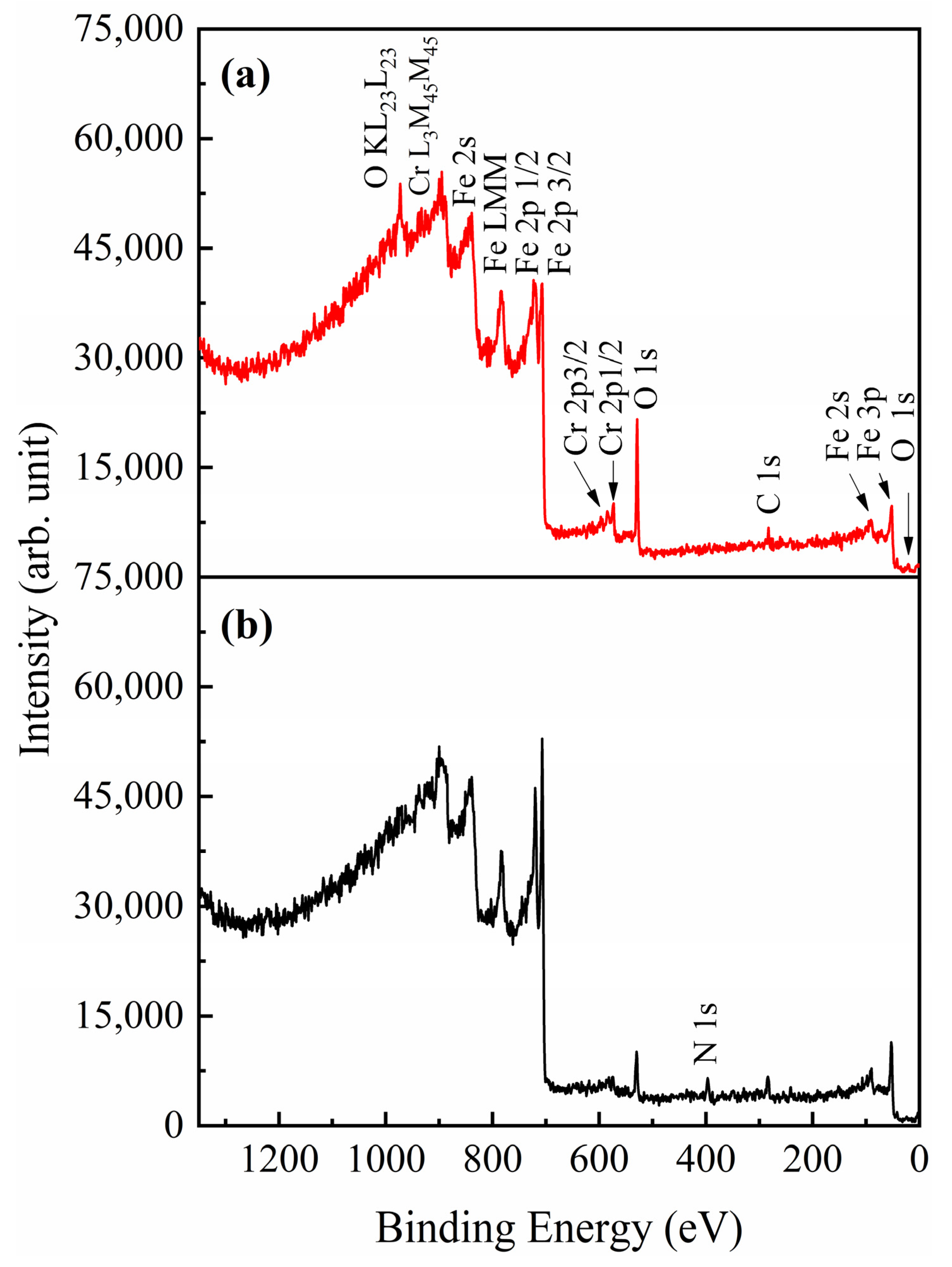
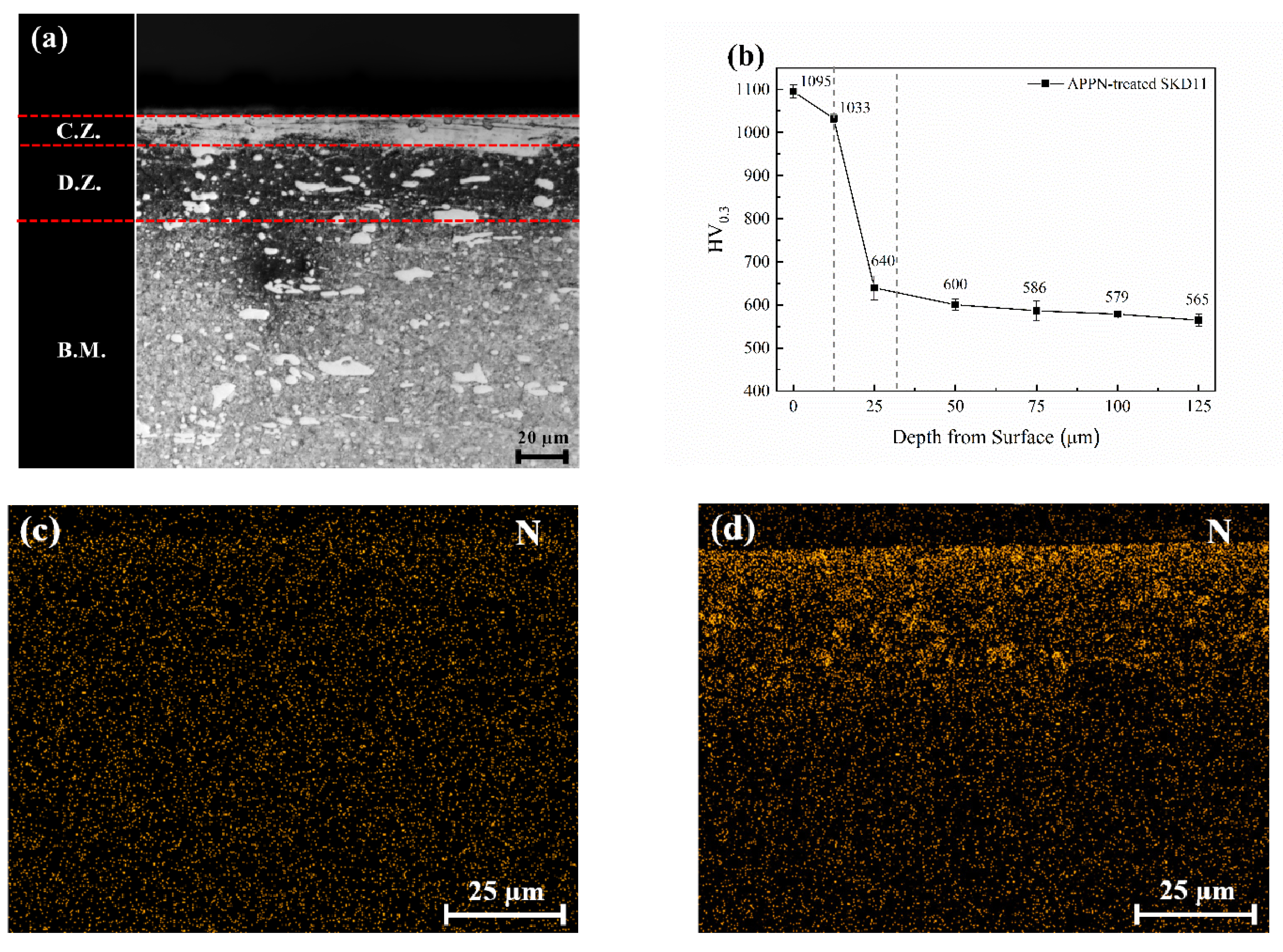
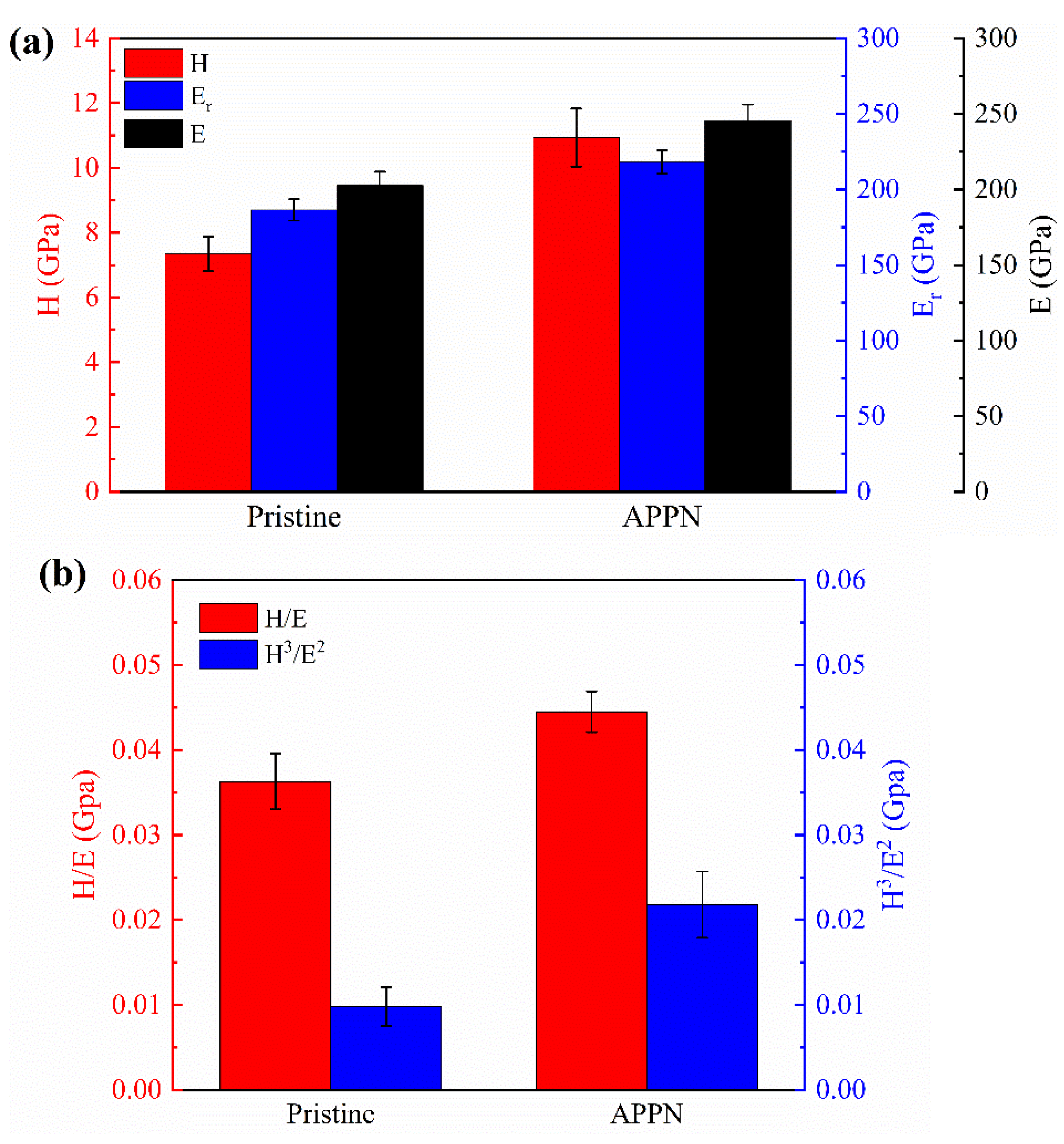
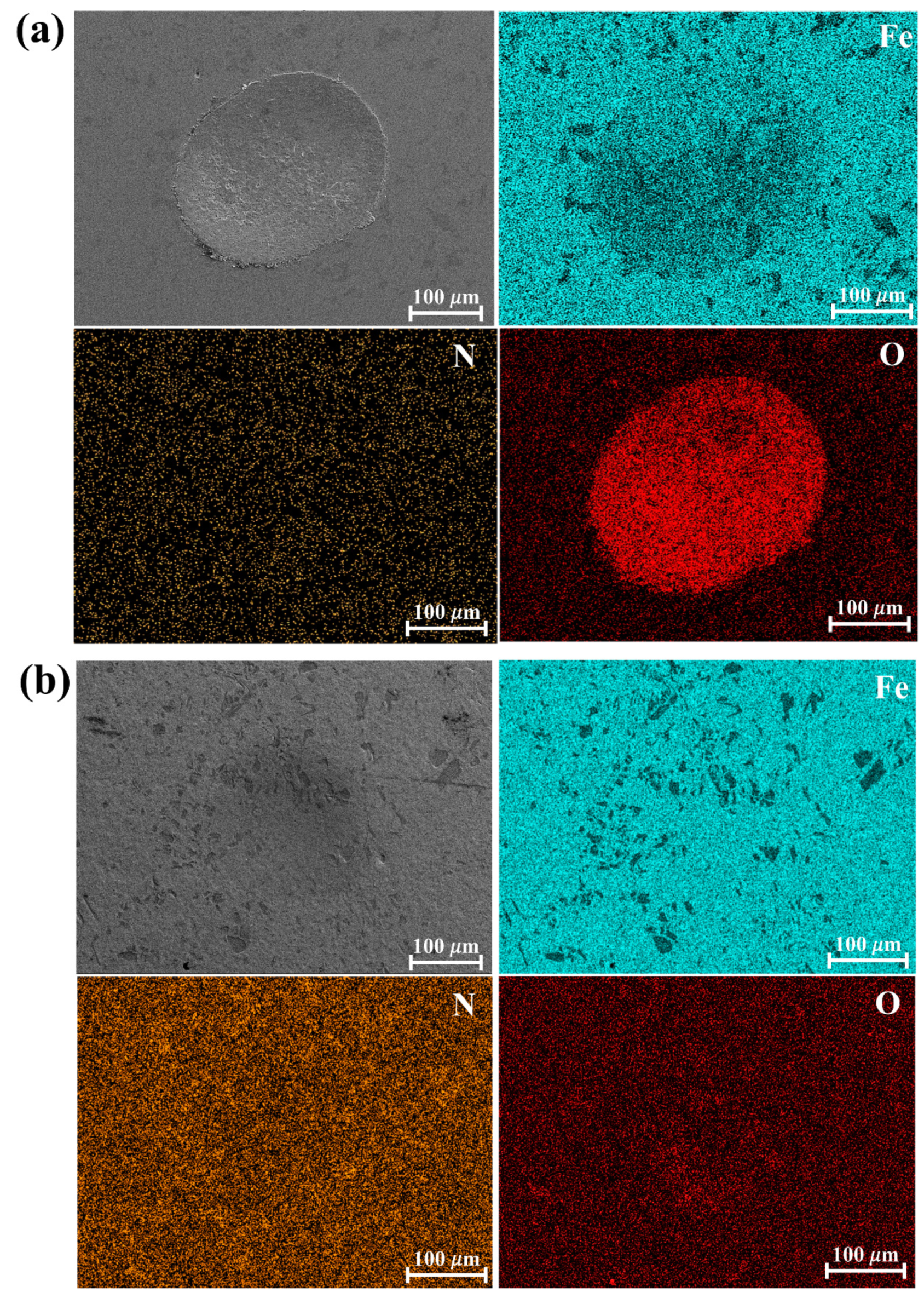
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Groover, M.P. Fundamentals of Modern Manufacturing: Materials, Processes, and Systems, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 99–136. [Google Scholar]
- Koshy, P.; Dewes, R.C.; Aspinwall, D.K. High speed end milling of hardened AISI D2 tool steel (∼ to 58 HRC). J. Mater. Process Technol. 2002, 127, 266–273. [Google Scholar] [CrossRef]
- Tucker, R.C. ASM Handbook, Volume 5A: Thermal Spray Technology; ASM International: Materials Park, OH, USA, 2013; pp. 14–35. [Google Scholar]
- Wen, D.C. Plasma nitriding of plastic mold steel to increase wear and corrosion properties. Surf. Coat. Technol. 2009, 204, 511–519. [Google Scholar] [CrossRef]
- Zagonel, L.F.; Figueroa, C.A.; Droppa, R., Jr.; Alvarez, F. Influence of the process temperature on the steel microstructure and hardening in pulsed plasma nitriding. Surf. Coat. Technol. 2006, 201, 452–457. [Google Scholar] [CrossRef]
- Yamagata, H. The Science and Technology of Materials in Automotive Engines, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 165–204. [Google Scholar]
- Schaaf, P.; Kasper, J.; Höche, D. Laser gas assisted nitriding of Ti alloys. In Comprehensive Materials Processing-Laser Machining and Surface Treatment; Hashmi, S., Batalha, G.F., VanTyne, C.J., Yilbas, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 9, pp. 261–278. [Google Scholar]
- Aghajani, H.; Behrangi, S. Plasma Nitriding of Steels, 1st ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–67. [Google Scholar]
- Chandler, H. Heat Treater’s Guide: Practices and Procedures for Irons and Steels, 2nd ed.; ASM International: Materials Park, OH, USA, 1994; pp. 27–77. [Google Scholar]
- Zhao, C.; Zha, W.; Zhang, J.; Nie, X. Surface fatigue cracking of plasma nitrided cast iron D6510 under cyclic inclined contact stresses. Int. J. Fatigue. 2019, 124, 10–14. [Google Scholar] [CrossRef]
- Yilmaz, H.; Sadeler, R. Impact wear behavior of ball burnished 316L stainless steel. Surf. Coat. Technol. 2019, 363, 369–378. [Google Scholar] [CrossRef]
- Ko, C.L.; Kuo, Y.L.; Lee, W.J.; Sheng, H.J.; Guo, J.Y. The enhanced abrasion resistance of an anti-fingerprint coating on chrome-plated brass substrate by integrating sputtering and atmospheric pressure plasma jet technologies. Appl. Surf. Sci. 2018, 448, 88–94. [Google Scholar] [CrossRef]
- Michael, F.M.; Khalid, M.; Walvekar, R.; Siddiqui, H.; Balaji, A.B. Surface modification techniques of biodegradable and biocompatible polymers. In Biodegradable and Biocompatible Polymer Composites: Processing, Properties and Applications; Shimpi, N.G., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 33–54. [Google Scholar]
- Miyamoto, J.; Inoue, T.; Tokuno, K.; Tsutamori, H.; Abraha, P. Surface modification of tool steel by atmospheric-pressure plasma nitriding using dielectric barrier discharge. Tribol. Online 2016, 11, 460–465. [Google Scholar] [CrossRef]
- Sato, S.; Arai, Y.; Yamashita, N.; Kojyo, A.; Kodama, K.; Ohtsu, N.; Okamoto, Y.; Wagatsuma, K. Surface-nitriding treatment of steels using microwave-induced nitrogen plasma at atmospheric pressure. Appl. Surf. Sci. 2012, 258, 7574–7580. [Google Scholar] [CrossRef]
- Nagamatsu, H.; Ichiki, R.; Yasumatsu, Y.; Inoue, T.; Yoshida, M.; Akamine, S.; Kanazawa, S. Steel nitriding by atmospheric-pressure plasma jet using N2/H2 mixture gas. Surf. Coat. Technol. 2013, 225, 26–33. [Google Scholar] [CrossRef]
- Patnaik, P. A comprehensive Guide to the Hazardous Properties of Chemical Substances, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2007; pp. 402–410. [Google Scholar]
- Kajdas, C.; Wilusz, E.; Harvey, S. Encyclopedia of Tribology, 1st ed.; Elsevier: Amsterdam, The Netherlands, 1990; pp. 2420–2424. [Google Scholar]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Knotek, O.; Bosserhoff, B.; Schrey, A.; Leyendecker, T.; Lemmer, O.; Esser, S. A new technique for testing the impact load of thin films: The coating impact test. Surf. Coat. Technol. 1992, 54, 102–107. [Google Scholar] [CrossRef]
- Jcpds Card No: 65-7296. International Centre for Diffraction Data. Available online: https://www.icdd.com/ (accessed on 1 August 2021).
- Jcpds Card No: 88-2323. International Centre for Diffraction Data. Available online: https://www.icdd.com/ (accessed on 1 August 2021).
- Jcpds Card No: 22-0211. International Centre for Diffraction Data. Available online: https://www.icdd.com/ (accessed on 1 August 2021).
- Jcpds Card No: 06-0091. International Centre for Diffraction Data. Available online: https://www.icdd.com/ (accessed on 1 August 2021).
- Jcpds Card No: 77-2006. International Centre for Diffraction Data. Available online: https://www.icdd.com/ (accessed on 1 August 2021).
- Jcpds Card No: 11-0065. International Centre for Diffraction Data. Available online: https://www.icdd.com/ (accessed on 1 August 2021).
- Díaz-Guillén, J.C.; Díaz-Guillén, J.A.; Granda-Gutiérrez, E.E.; Díaz-Guillén, M.R.; González-Albarrán, M.A. Electrochemical corrosion performance of AISI D2 tool steel surface hardened by pulsed plasma nitriding. Int. J. Electrochem. Sci. 2013, 8, 973–982. [Google Scholar]
- Conci, M.D.; Bozzi, A.C.; Franco Jr, A.R. Effect of plasma nitriding potential on tribological behaviour of AISI D2 cold-worked tool steel. Wear 2014, 317, 188–193. [Google Scholar] [CrossRef]
- Chastain, J.; King, R.C., Jr. Handbook of X-ray Photoelectron Spectroscopy, 1st ed.; Perkin-Elmer: Eden Prairie, MN, USA, 1992; pp. 40–86. [Google Scholar]
- Moskalioviene, T.; Galdikas, A. The effect of hydrogen on plasma nitriding of austenitic stainless steel: Kinetic modeling. Met. Mater. Trans. A 2015, 46, 5588–5595. [Google Scholar] [CrossRef]
- Naeem, M.; Iqbal, J.; Abrar, M.; Khan, K.H.; Díaz-Guillén, J.C.; Lopez-Badillo, C.M.; Shafiq, M.; Zaka-ul-Islam, M.; Zakaullah, M. The effect of argon admixing on nitriding of plain carbon steel in N2 and N2-H2 plasma. Surf. Coat. Technol. 2018, 350, 48–56. [Google Scholar] [CrossRef]
- Dunlap, R.A. The symmetry and packing fraction of the body centered tetragonal structure. Eur. J. Phys. 2017, 3, 17–24. [Google Scholar]
- Bhadeshia, H.; Honeycombe, R. Steels: Microstructure and Properties, 4th ed.; Butterworth-Heinemann Elsevier Ltd.: Oxford, UK, 2017; pp. 1–30. [Google Scholar]
- Pye, D. Practical Nitriding and Ferritic Nitrocarburizing, 1st ed.; ASM International: Materials Park, OH, USA, 2003; pp. 1–37. [Google Scholar]
- NIST Atomic Spectra Database. Available online: https://www.nist.gov/pml/atomic-spectra-database (accessed on 12 May 2014).
- Brühl, S.; Russell, M.; Gómez, B.; Grigioni, G.; Feugeas, J.; Ricard, A. A study by emission spectroscopy of the N2 active species in pulsed dc discharges. J. Phys. D Appl. Phys. 1997, 30, 2917–2922. [Google Scholar] [CrossRef]
- Lamichhane, P.; Ghimire, B.; Mumtaz, S.; Paneru, R.; Ki, S.H.; Choi, E.H. Control of hydrogen peroxide production in plasma activated water by utilizing nitrification. J. Phys. D Appl. Phys. 2019, 52, 26520601–26520609. [Google Scholar] [CrossRef]
- Corujeira Gallo, S.; Charitidis, C.; Dong, H. Surface functionalization of carbon fibers with active screen plasma. J. Vac. Sci. Technol. A. 2017, 35, 021404. [Google Scholar] [CrossRef]
- Hsu, C.C.; Yang, Y.J. The increase of the jet size of an atmospheric-pressure plasma jet by ambient air control. IEEE Trans. Plasma Sci. 2010, 38, 496–499. [Google Scholar] [CrossRef]
- Petitjean, L.; Ricard, A. Emission spectroscopy study of N2-H2 glow discharge for metal surface nitriding. J. Phys. D Appl. Phys. 1984, 17, 919. [Google Scholar] [CrossRef]
- Priest, J.M.; Baldwin, M.J.; Fewell, M.P. The action of hydrogen in low-pressure rf-plasma nitriding. Surf. Coat. Technol. 2001, 145, 152–163. [Google Scholar] [CrossRef]
- Martinez, H.; Yousif, F.B. Electrical and optical characterization of pulsed plasma of N2-H2. Eur. Phys. J. D 2008, 46, 493–498. [Google Scholar] [CrossRef]
- Selwyn, G.S.; Herrmann, H.W.; Park, J.; Henins, I. Materials processing using an atmospheric pressure, rf-generated plasma source. Contrib. Plasma Phys. 2001, 41, 610–619. [Google Scholar] [CrossRef]
- Zheng, Q.; Shimizu, T.; Yang, M. Finite element analysis of springback behavior in resistance heating assisted microbending process. Mech. Eng. J. 2015, 2, 14-00413–14-00414. [Google Scholar] [CrossRef][Green Version]
- Chen, J.S.; Yu, C.; Lu, H. Phase stability, magnetism, elastic properties and hardness of binary iron nitrides from first principles. J. Alloy Compd. 2015, 625, 224–230. [Google Scholar] [CrossRef]
- Santos, E., Jr.; Nascimento, K.D.; Camargo, S.S., Jr. Relation between in-vitro wear and nanomechanical properties of commercial light-cured dental composites coated with surface sealants. Mat. Res. 2013, 16, 1148–1155. [Google Scholar] [CrossRef]
- Tsui, T.; Pharr, G.; Oliver, W.; Bhatia, C.; White, R.; Anders, S.; Anders, A.; Brown, I. Nanoindentation and nanoscratching of hard carbon coatings for magnetic disks. Mater. Res. Soc. Symp. Proc. 1995, 383, 447–452. [Google Scholar] [CrossRef]



| Fe | Cr | C | Mo | Si | Mn | V | Co | P | S |
|---|---|---|---|---|---|---|---|---|---|
| Bal. | 11.2 | 1.68–1.41 | 0.53 | 0.34 | 0.30 | 0.191 | 0.06 | 0.02 | 0.006 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.-Y.; Kuo, Y.-L.; Wang, H.-P. A Facile Nitriding Approach for Improved Impact Wear of Martensitic Cold-Work Steel Using H2/N2 Mixture Gas in an AC Pulsed Atmospheric Plasma Jet. Coatings 2021, 11, 1119. https://doi.org/10.3390/coatings11091119
Guo J-Y, Kuo Y-L, Wang H-P. A Facile Nitriding Approach for Improved Impact Wear of Martensitic Cold-Work Steel Using H2/N2 Mixture Gas in an AC Pulsed Atmospheric Plasma Jet. Coatings. 2021; 11(9):1119. https://doi.org/10.3390/coatings11091119
Chicago/Turabian StyleGuo, Jhao-Yu, Yu-Lin Kuo, and Hsien-Po Wang. 2021. "A Facile Nitriding Approach for Improved Impact Wear of Martensitic Cold-Work Steel Using H2/N2 Mixture Gas in an AC Pulsed Atmospheric Plasma Jet" Coatings 11, no. 9: 1119. https://doi.org/10.3390/coatings11091119
APA StyleGuo, J.-Y., Kuo, Y.-L., & Wang, H.-P. (2021). A Facile Nitriding Approach for Improved Impact Wear of Martensitic Cold-Work Steel Using H2/N2 Mixture Gas in an AC Pulsed Atmospheric Plasma Jet. Coatings, 11(9), 1119. https://doi.org/10.3390/coatings11091119






