Preparation of Silver Antibacterial Agents with Different Forms and Their Effects on the Properties of Water-Based Primer on Tilia europaea Surface
Abstract
:1. Introduction
2. Experimental Materials and Methods
2.1. Experimental Materials
2.2. Preparation of Micron Silver Particles
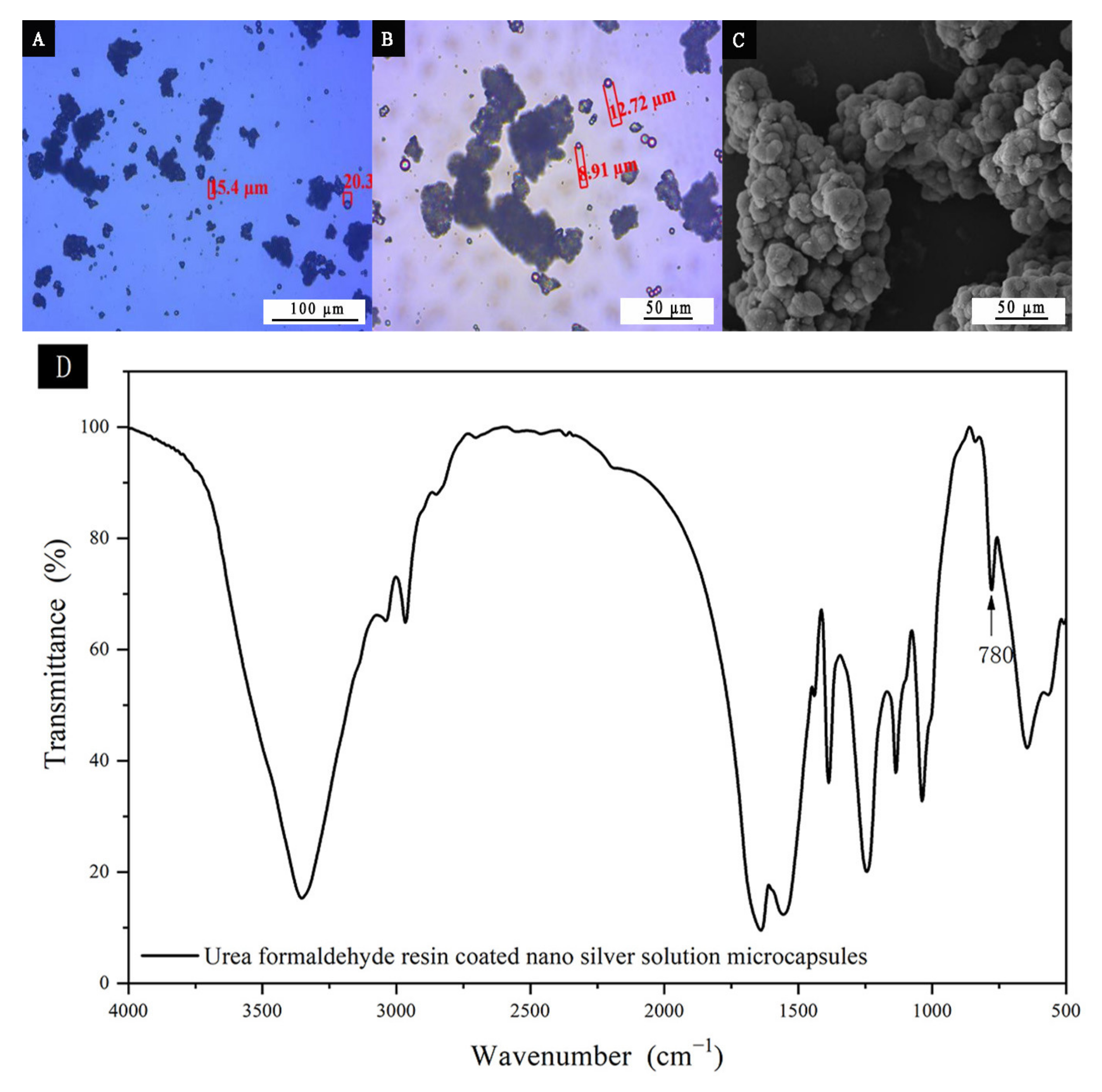
2.3. Preparation of the Microcapsule of Urea Formaldehyde Resin-Coated Nano-Silver Solution
2.4. Preparation of Waterborne Wood Antibacterial Paint Film
2.5. Testing and Characterization
3. Results and Discussion
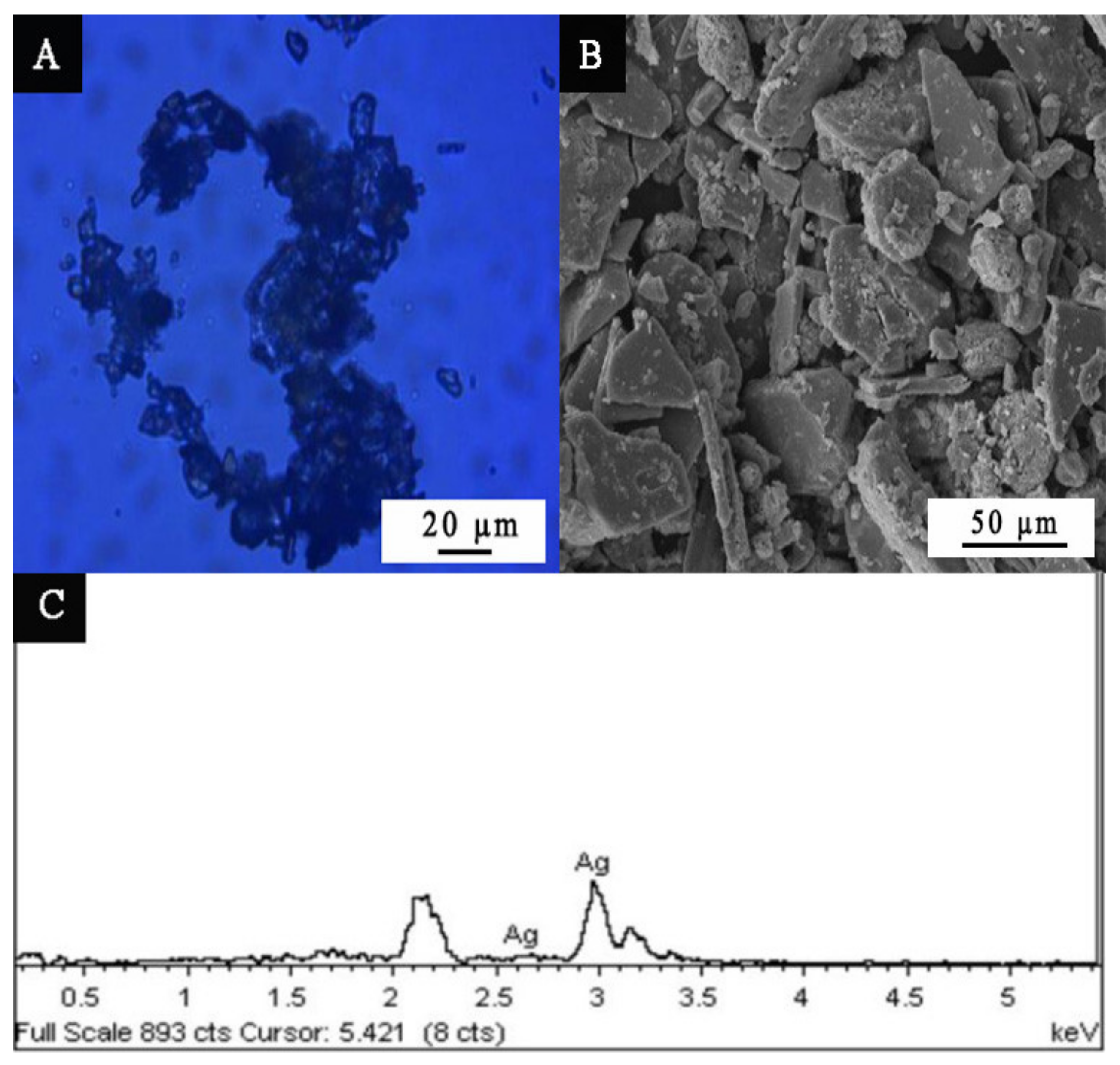
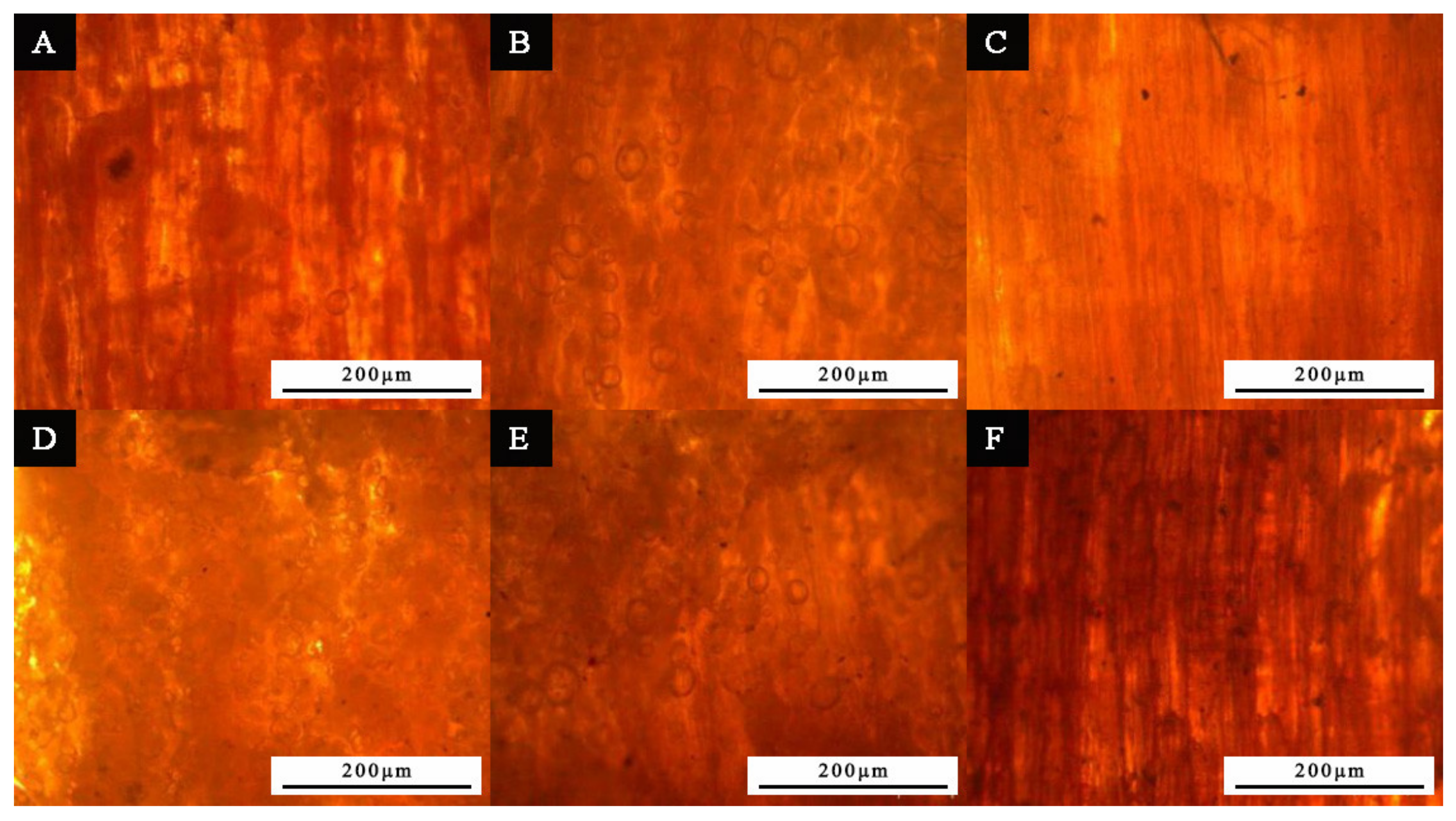
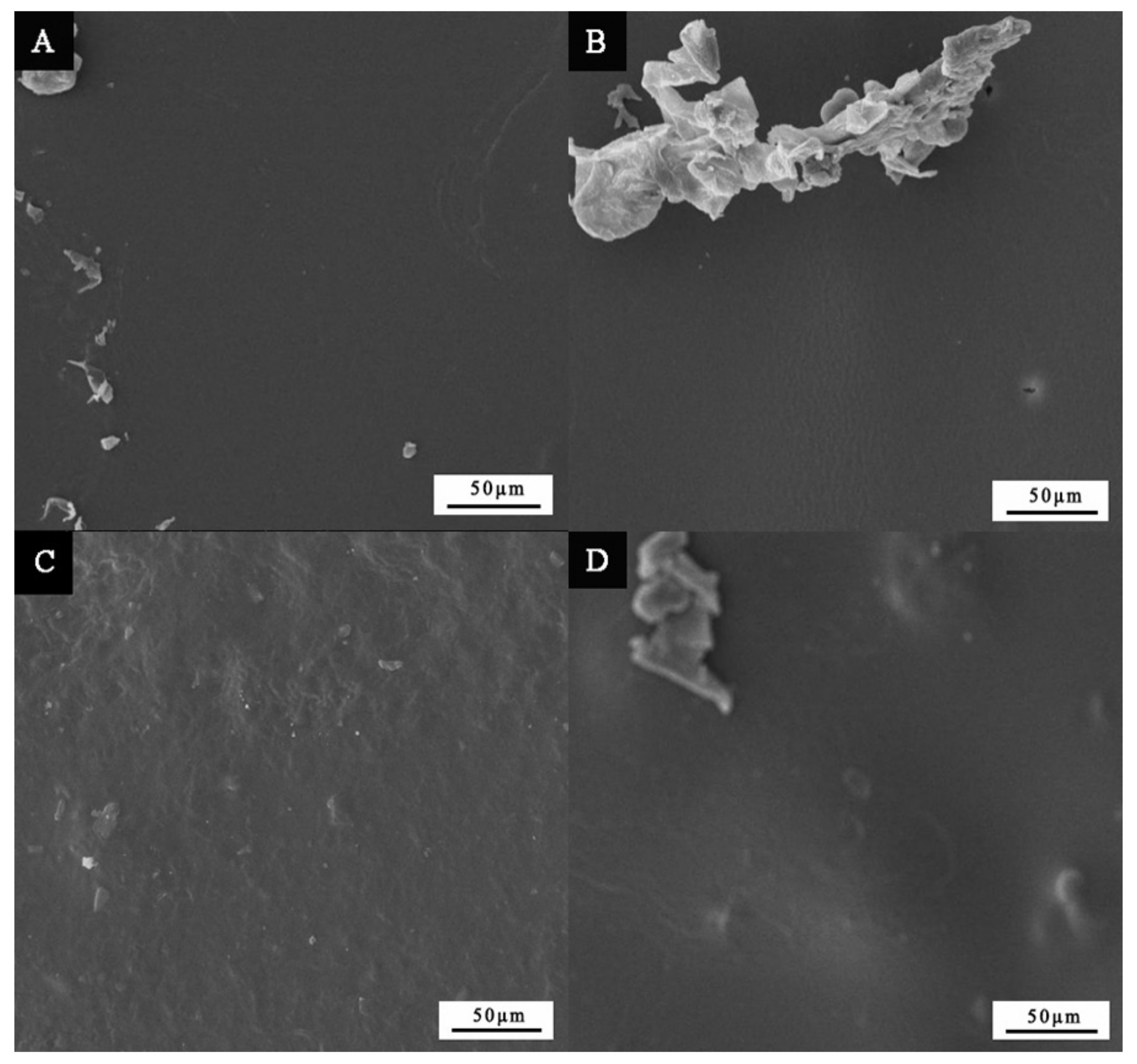
3.1. Microstructure Analysis
3.2. Color Difference, Gloss, Adhesion, and Impact Resistance of Antibacterial Coatings
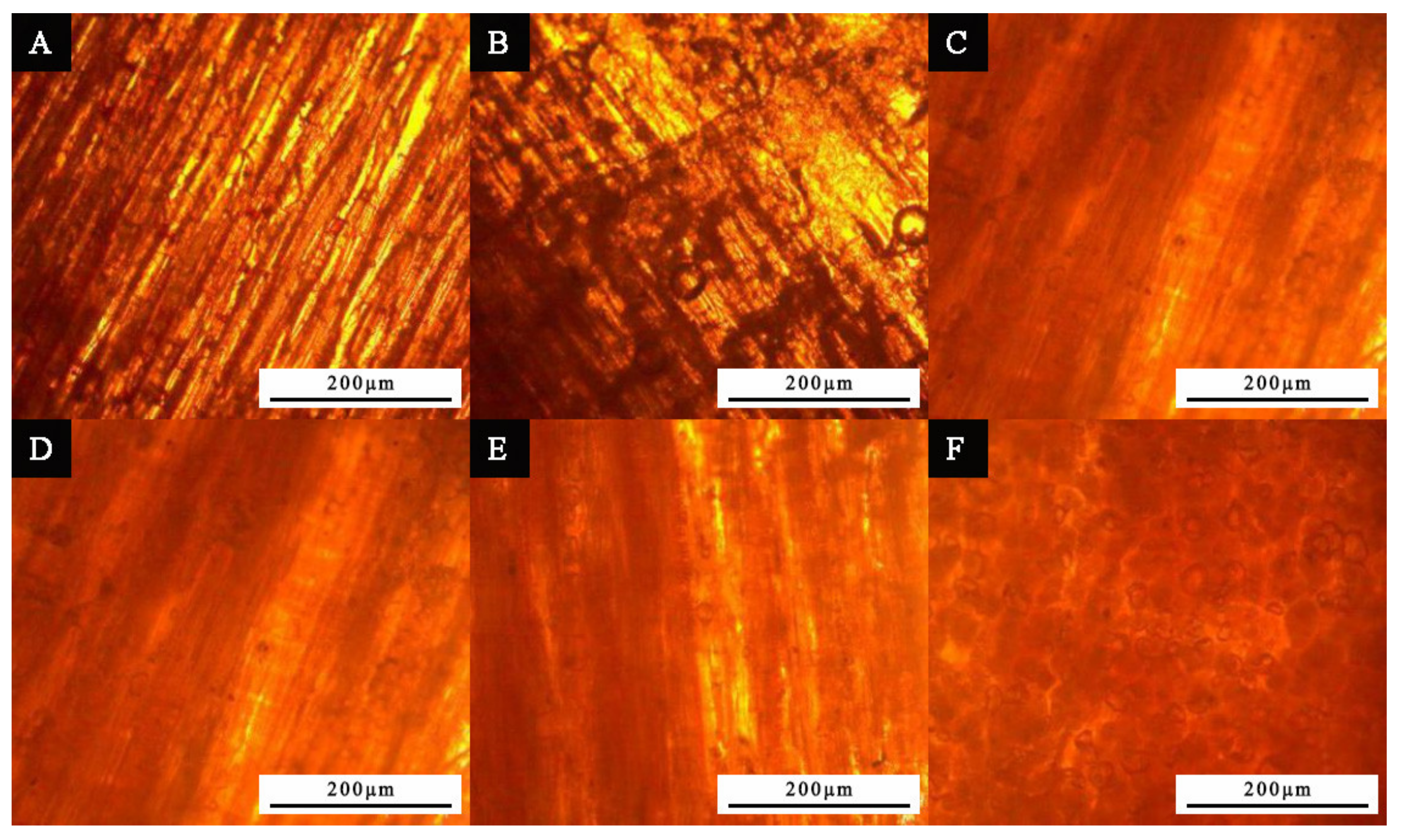
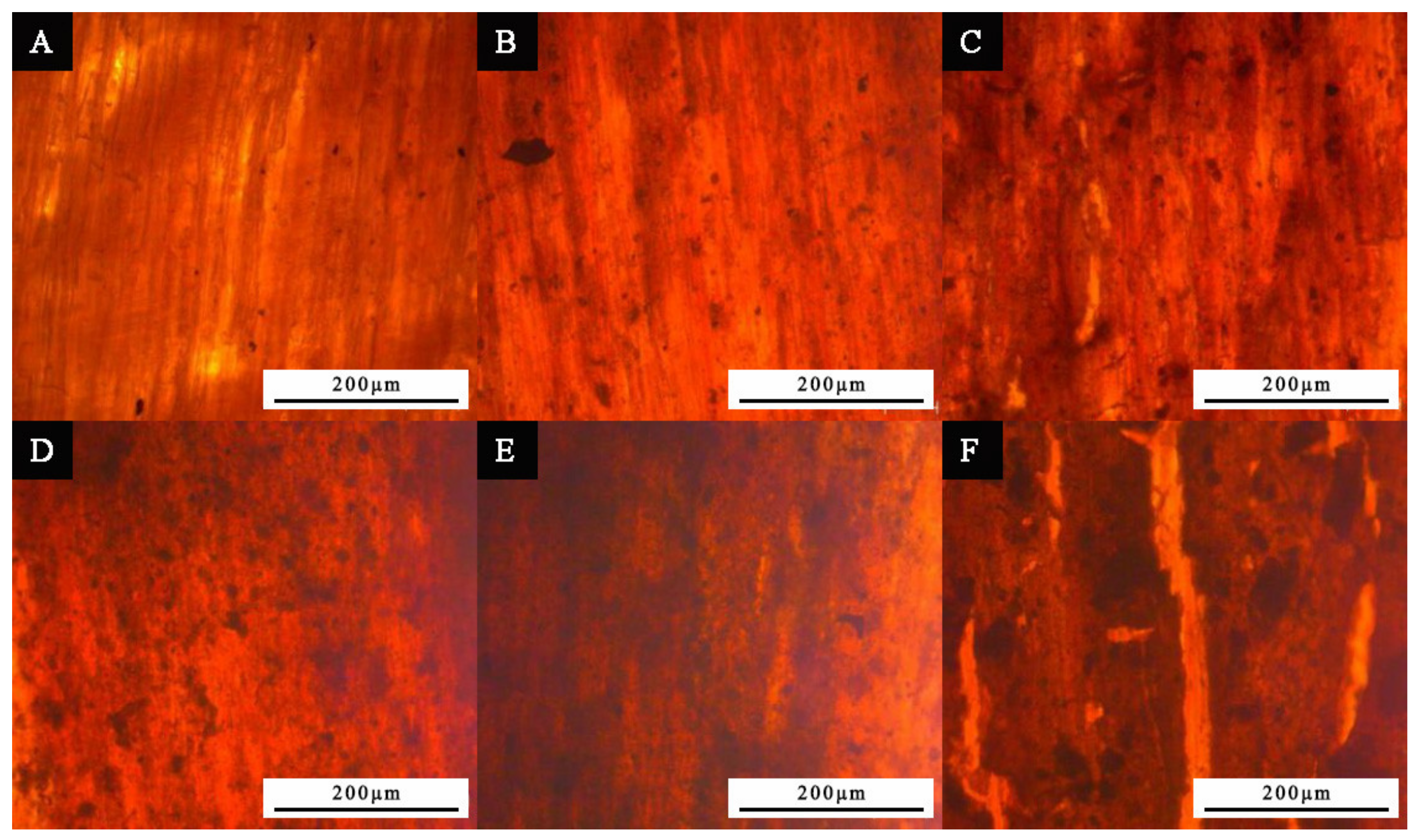
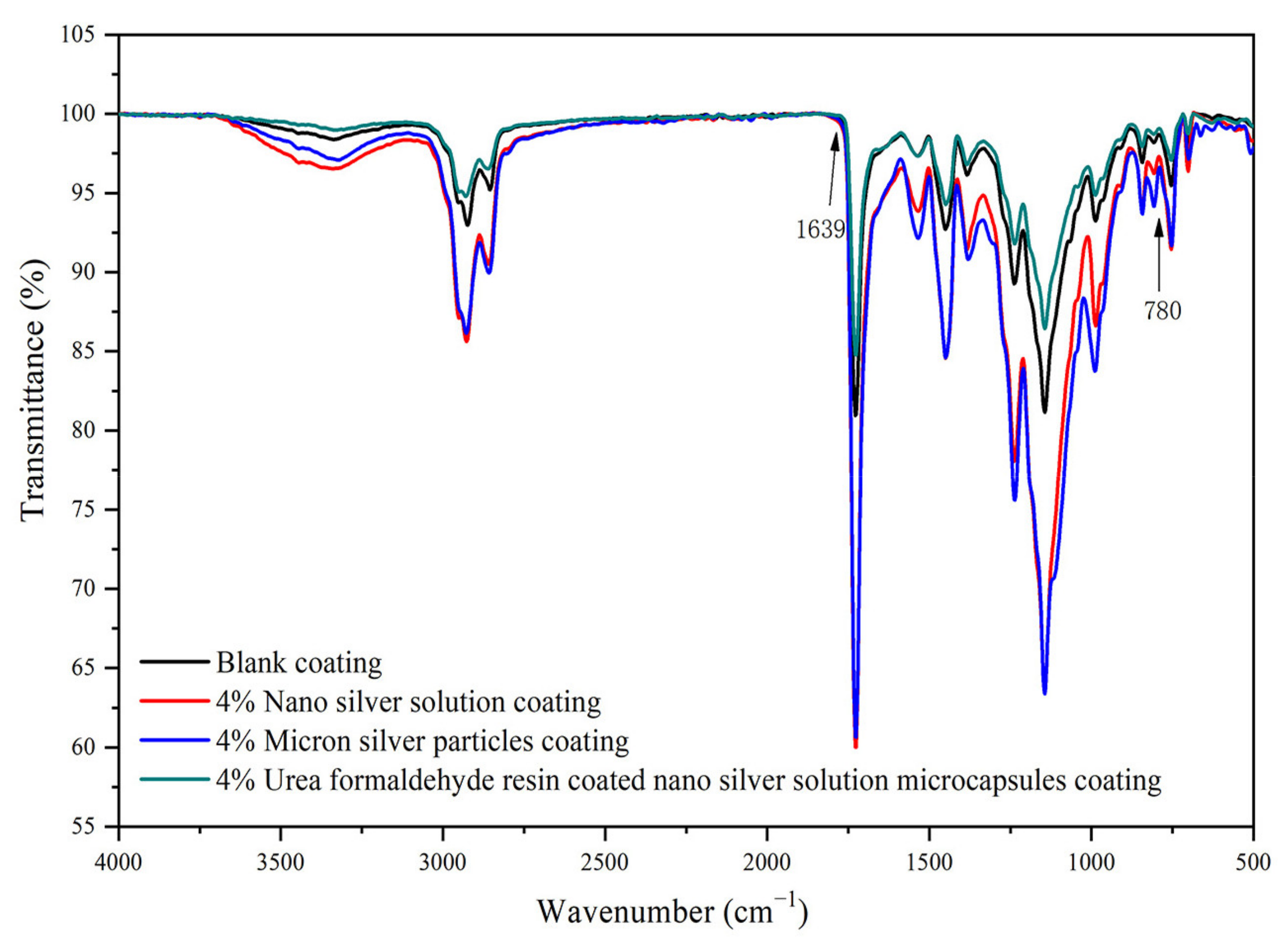
3.3. Microstructure and Composition Analysis of Paint Film
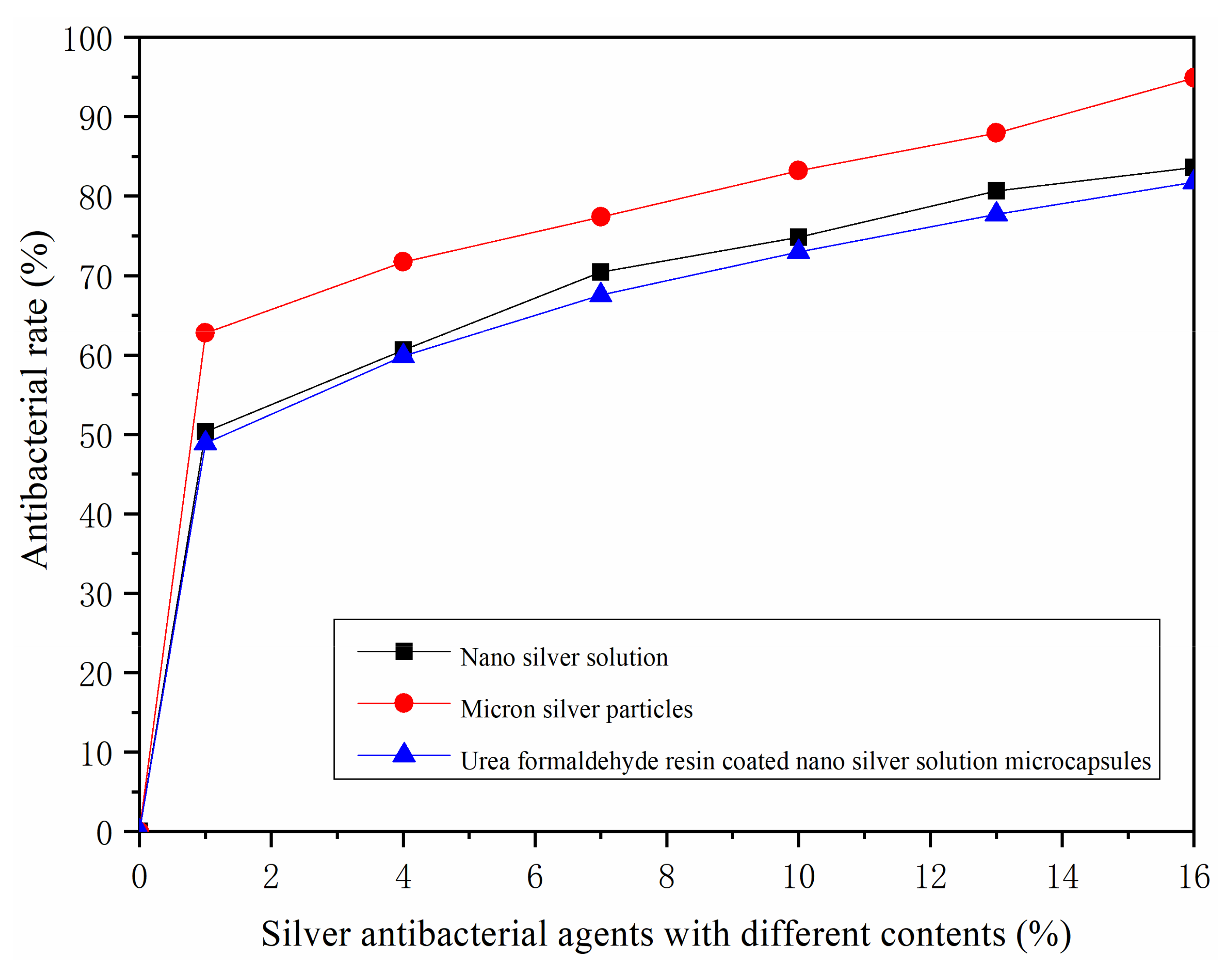
3.4. Analysis of Antibacterial Rate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duan, X.P.; Liu, S.M.; Huang, E.T.; Shen, X.Y.; Wang, Z.; Li, S.; Jin, C.D. Superhydrophobic and antibacterial wood enabled by polydopamine-assisted decoration of copper nanoparticles. Colloid. Surface. A. 2020, 602, 125145. [Google Scholar] [CrossRef]
- Xu, W.; Fang, X.Y.; Han, J.T.; Wu, Z.H.; Zhang, J.L. Effect of coating thickness on sound absorption property of four wood species commonly used for piano soundboards. Wood Fiber Sci. 2020, 52, 28–43. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.Q.; Gao, D.; Xu, W. Influence of the bottom color modification and material color modification process on the perfor-mance of modified Poplar. Coatings 2021, 11, 660. [Google Scholar] [CrossRef]
- Singh, A.P.; Kim, Y.S.; Chavan, R.R. Relationship of wood cell wall ultrastructure to bacterial degradation of wood. IAWA J. 2019, 40, 845–870. [Google Scholar] [CrossRef]
- Wu, S.S.; Tao, X.; Xu, W. Thermal conductivity of Poplar wood veneer impregnated with graphene/polyvinyl alcohol. Forests 2021, 12, 777. [Google Scholar] [CrossRef]
- Zafar, F.; Ghosal, A.; Sharmin, E.; Chaturvedi, R.; Nishat, N. A review on cleaner production of polymeric and nanocomposite coatings based on waterborne polyurethane dispersions from seed oils. Prog. Org. Coat. 2019, 131, 259–275. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Gao, D.; Xu, W. Effect of sanding processes on the surface properties of modified Poplar coated by primer compared with Mahogany. Coatings 2020, 10, 856. [Google Scholar] [CrossRef]
- Jiang, G.F.; Li, X.F.; Che, Y.L.; Lv, Y.; Liu, F.; Wang, Y.Q.; Zhao, C.C.; Wang, X.J. Antibacterial and anticorrosive properties of CuZnO@RGO waterborne polyurethane coating in circulating cooling water. Environ. Sci. Pollut. Res. 2019, 26, 9027–9040. [Google Scholar] [CrossRef]
- Tang, S.H.; Zheng, J. Antibacterial activity of silver nanoparticles: structural effects. Adv. Healthc. Mater. 2018, 7, 1701503. [Google Scholar] [CrossRef]
- Bechtold, M.; Valerio, A.; de Souza, A.A.U.; de Oliveira, D.; Franco, C.V.; Serafim, R.; Souza, S.M.A.G.U. Synthesis and application of silver nanoparticles as biocidal agent in polyurethane coating. J. Coat. Technol. Res. 2020, 17, 613–620. [Google Scholar] [CrossRef]
- Auclair, J.; Turcotte, P.; Gagnon, C.; Peyrot, C.; Wilkinson, K.J.; Gagne, F. The influence of surface coatings of silver nanoparticles on the bioavailability and toxicity to elliptio complanata mussels. J. Nanomater. 2019, 2019, 7843025. [Google Scholar] [CrossRef]
- Cruz-Pacheco, A.F.; Munoz-Castiblanco, D.T.; Cuaspud, J.A.G.; Paredes-Madrid, L.; Vargas, C.A.P.; Zambrano, J.J.M.; Gomez, C.A.P. Coating of polyetheretherketone films with silver nanoparticles by a simple chemical reduction method and their antibacterial activity. Coatings 2019, 9, 91. [Google Scholar] [CrossRef] [Green Version]
- Lok, C.N.; Ho, C.M.; Chen, R.; He, Q.Y.; Yu, W.Y.; Sun, H.Z.; Tam, P.K.H.; Chiu, J.F.; Che, C.M. Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J. Proteome Res. 2006, 5, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Lou, C.W.; Chen, A.P.; Lic, T.T.; Lin, J.H. Antimicrobial activity of UV-induced chitosan capped silver nanoparticles. Mater. Lett. 2014, 128, 248–252. [Google Scholar] [CrossRef]
- Derakhshi, M.; Ashkarran, A.A.; Bahari, A.; Bonakdar, S. Shape selective silver nanostructures decorated amine-functionalized graphene: A promising antibacterial platform. Colloids Surf. A. 2018, 545, 101–109. [Google Scholar] [CrossRef]
- Bhullar, S.K.; Ruzgar, D.G.; Fortunato, G.; Aneja, G.K.; Orhan, M.; Saber-Samandari, S.; Sadighi, M.; Ahadian, S.; Ramalingam, M.A. Facile method for controlled fabrication of hybrid silver nanoparticle-poly (epsilon-caprolactone) fibrous constructs with antimicrobial properties. J. Nanosci. Nanotechnol. 2019, 19, 6949–6955. [Google Scholar] [CrossRef] [Green Version]
- Le, T.T.; Nguyen, T.V.; Nguye, T.A.; Nguye, T.T.H.; Thai, H.; Tran, D.L.; Dinh, D.A.; Nguyen, T.M.; Lu, L.T. Thermal, mechanical and antibacterial properties of water-based acrylic polymer/SiO2-Ag nanocomposite coating. Mater. Chem. Phys. 2019, 232, 362–366. [Google Scholar] [CrossRef]
- Zhang, X.H.; Wang, W.; Yu, D. Synthesis of waterborne polyurethane-silver nanoparticle antibacterial coating for synthetic leather. J. Coat. Technol. Res. 2018, 15, 415–423. [Google Scholar] [CrossRef]
- Cheng, L.S.; Ren, S.B.; Lu, X.N. Application of eco-friendly waterborne polyurethane composite coating incorporated with nano cellulose crystalline and silver nano particles on wood antibacterial board. Polymers 2020, 12, 407. [Google Scholar] [CrossRef] [Green Version]
- Ichimaru, H.; Harada, A.; Yoshimoto, S.; Miyazawa, Y.; Mizoguchi, D.; Kyaw, K.; Ono, K.; Tsutsuki, H.; Sawa, T.; Niidome, T. Gold coating of silver nanoplates for enhanced dispersion stability and efficient antimicrobial activity against intracellular bacteria. Langmuir 2018, 34, 10413–10418. [Google Scholar] [CrossRef]
- Guo, L.Y.; Yuan, W.Y.; Lu, Z.S.; Li, C.M. Polymer/nanosilver composite coatings for antibacterial applications. Colloids Surf. A. 2013, 439, 69–83. [Google Scholar] [CrossRef]
- Yang, X.F.; Liu, J.; Wu, Y.F.; Liu, J.L.; Cheng, F.; Jiao, X.J.; Lai, G.Q. Fabrication of UV-curable solvent-free epoxy modified silicone resin coating with high transparency and low volume shrinkage. Prog. Org. Coat. 2019, 129, 96–100. [Google Scholar] [CrossRef]
- Maidaniuc, A.; Miculescu, M.; Voicu, S.I.; Ciocan, L.T.; Niculescu, M.; Corobea, M.C.; Rada, M.E.; Miculescu, F. Effect of micron sized silver particles concentration on the adhesion induced by sintering and antibacterial properties of hydroxyapatite microcomposites. J. Aahes. Sci. Technol. 2016, 30, 1829–1841. [Google Scholar] [CrossRef]
- Yan, X.X.; Zhao, W.T.; Qian, X.Y. Effect of water-based emulsion core microcapsules on aging resistance and self-repairing properties of water-based coatings on Linden. Appl. Sci. 2021, 11, 4662. [Google Scholar] [CrossRef]
- Yan, X.X.; Xu, G.Y. Effect of surface modification of Cu with Ag by ball-milling on the corrosion resistance of low infrared emissivity coating. Mater. Sci. Eng. B Adv. 2010, 166, 152–157. [Google Scholar] [CrossRef]
- Yan, X.X.; Wang, L.; Qian, X.Y. Effect of microcapsules with different core-wall ratios on properties of waterborne primer coating for European Linden. Coatings 2020, 10, 826. [Google Scholar] [CrossRef]
- HG/T 3950-2007 Antibacterial Coatings; National Development and Reform Commission of the People’s Republic of China: Beijing, China, 2007; pp. 1–9. (In Chinese)
- Cheng, Q.L.; Guo, X.W.; Hao, X.J.; Shi, Z.S.; Zhu, S.; Cui, Z.C. Fabrication of robust antibacterial coatings based on an organic-inorganic hybrid system. ACS Appl. Mater. Inter. 2019, 11, 42607–42615. [Google Scholar] [CrossRef]
- Galus, S.; Mikus, M.; Ciurzyńska, A.; Domian, E.; Kowalska, J.; Marzec, A.; Kowalska, H. The effect of whey protein-based edible coatings incorporated with lemon and lemongrass essential oils on the quality attributes of fresh-cut pears during storage. Coatings 2021, 11, 745. [Google Scholar] [CrossRef]
- Yan, X.X.; Peng, W.W.; Qian, X.Y. Effect of water-based acrylic acid microcapsules on the properties of paint film for furniture surface. Appl. Sci. 2021, 11, 7586. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.F. Morphology and crystallinity of urea-formaldehyde resin adhesives with different molar ratios. Polymers 2021, 13, 673. [Google Scholar] [CrossRef]
- Niu, J.F.; Tang, G.; Tang, J.Y.; Yang, J.L.; Zhou, Z.Y.; Gao, Y.H.; Chen, X.; Tian, Y.Y.; Li, Y.; Li, J.Q.; et al. Functionalized silver nanocapsules with improved antibacterial activity using silica shells modified with quaternary ammonium polyethyleneimine as a bacterial cell-targeting agent. J. Agric. Food Chem. 2021, 69, 6485–6494. [Google Scholar] [CrossRef] [PubMed]
- Panicker, S.; Ahmady, I.M.; Han, C.; Chehimi, M.; Mohamed, A.A. On demand release of ionic silver from gold-silver alloy nanoparticles: fundamental antibacterial mechanisms study. Mater. Today Chem. 2020, 16, 100237. [Google Scholar] [CrossRef]

| Material | Molecular Formula | MW (g/mol) | CAS No. | Concentration | Producer |
|---|---|---|---|---|---|
| silver nitrate solid powder | AgNO3 | 169.87 | 7761-88-8 | 99.9% | Nanjing Chemical Reagent Co., Ltd., Nanjing, China |
| concentrated ammonia | NH3∙H2O | 35.00 | 1336-21-6 | 25% | Tianjin Fuchen Chemical Reagent Factory, Tianjin, China |
| nano-silver solution | - | - | - | 100 ppm | Luoyang Oulun Environmental Protection Technology Co., Ltd., Luoyang, China |
| formaldehyde solution | CH2O | 30.03 | 50-00-0 | 37.0% | Tianjin University Mao Chemical Reagent Factory, Tianjin, China |
| urea | CH4N2O | 60.06 | 57-13-6 | 99.9% | Tianjin Beichen Fangzheng Chemical Reagent Factory, Tianjin, China |
| triethanolamine | C6H15NO3 | 149.19 | 102-71-6 | 99.9% | Xilong Science Co., Ltd., Wuxi, China |
| absolute ethanol | C2H6O | 46.07 | 64-17-5 | 99.9% | Wuxi Yasheng Chemical Co., Ltd., Wuxi, China |
| sodium dodecyl benzene sulfonate | C18H29NaO3S | 348.48 | 25155-30-0 | 99.9% | Wuxi Yatai United Chemical Co., Ltd., Wuxi, China |
| waterborne acrylic resin | - | - | 9003-01-4 | - | Dulux Paint Co., Ltd., Shanghai, China |
| citric acid monohydrate | C6H10O8 | 210.14 | 5949-29-1 | 99.9 | Kunshan Southeast Chemical Materials Co., Ltd., Kunshan, China |
| Tilia europaea | - | - | - | - | Shanghai Puhui industry and Trade Co., Ltd., Shanghai, China |
| nutrient broth | - | - | - | - | Guangdong huankai Microbial Technology Co., Ltd. Guangzhou, China |
| eluent | NaCl | 58.4428 | 7647-14-5 | 0.85% | Sichuan Kelun Pharmaceutical Co., Ltd., Chengdu, China |
| staphylococcus aureus AS1.89 | - | - | - | - | Beijing Baocang Biotechnology Co., Ltd., Beijing, China |
| nutrient agar | - | - | - | - | Hangzhou microbial Reagent Co., Ltd., Hangzhou, China |
| Percentage (%) | Mass of Primer (g) | Mass of Nano-Silver Solution and Other Antibacterial Agent (g) |
|---|---|---|
| 0 | 3.00 | 0 |
| 1.0 | 2.97 | 0.03 |
| 4.0 | 2.88 | 0.12 |
| 7.0 | 2.79 | 0.21 |
| 10.0 | 2.70 | 0.30 |
| 13.0 | 2.61 | 0.39 |
| 16.0 | 2.52 | 0.48 |
| Microcapsule Content (%) | Color Difference | Gloss (%) | Adhesion (Level) | Impact Resistance (kg·cm) |
|---|---|---|---|---|
| 0 | 0.73 | 30.5 | 1 | 5.0 |
| 1.0 | 1.92 | 29.1 | 2 | 7.0 |
| 4.0 | 2.45 | 8.0 | 2 | 7.0 |
| 7.0 | 2.78 | 3.6 | 3 | 8.0 |
| 10.0 | 2.89 | 2.7 | 3 | 8.0 |
| 13.0 | 3.64 | 2.6 | 4 | 9.0 |
| 16.0 | 4.22 | 2.5 | 4 | 9.0 |
| Nano-Silver Solution Content (%) | Color Difference | Gloss (%) | Adhesion (Level) | Impact Resistance (kg·cm) |
|---|---|---|---|---|
| 0 | 0.73 | 30.5 | 1 | 5.0 |
| 1.0 | 1.27 | 29.7 | 1 | 6.0 |
| 4.0 | 1.37 | 26.4 | 2 | 7.0 |
| 7.0 | 1.57 | 20.8 | 3 | 7.0 |
| 10.0 | 1.60 | 17.5 | 3 | 8.0 |
| 13.0 | 2.09 | 14.7 | 3 | 8.0 |
| 16.0 | 2.11 | 11.2 | 3 | 8.0 |
| Micron Silver Particle Content (%) | Color Difference | Gloss (%) | Adhesion (Level) | Impact Resistance (kg·cm) |
|---|---|---|---|---|
| 0 | 0.73 | 30.5 | 1 | 5.0 |
| 1.0 | 3.64 | 14.1 | 1 | 7.0 |
| 4.0 | 4.08 | 7.2 | 2 | 10.0 |
| 7.0 | 5.32 | 4.4 | 2 | 11.0 |
| 10.0 | 5.92 | 2.8 | 3 | 12.0 |
| 13.0 | 7.09 | 1.6 | 3 | 13.0 |
| 16.0 | 8.91 | 1.2 | 3 | 14.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; Pan, P. Preparation of Silver Antibacterial Agents with Different Forms and Their Effects on the Properties of Water-Based Primer on Tilia europaea Surface. Coatings 2021, 11, 1066. https://doi.org/10.3390/coatings11091066
Yan X, Pan P. Preparation of Silver Antibacterial Agents with Different Forms and Their Effects on the Properties of Water-Based Primer on Tilia europaea Surface. Coatings. 2021; 11(9):1066. https://doi.org/10.3390/coatings11091066
Chicago/Turabian StyleYan, Xiaoxing, and Pan Pan. 2021. "Preparation of Silver Antibacterial Agents with Different Forms and Their Effects on the Properties of Water-Based Primer on Tilia europaea Surface" Coatings 11, no. 9: 1066. https://doi.org/10.3390/coatings11091066
APA StyleYan, X., & Pan, P. (2021). Preparation of Silver Antibacterial Agents with Different Forms and Their Effects on the Properties of Water-Based Primer on Tilia europaea Surface. Coatings, 11(9), 1066. https://doi.org/10.3390/coatings11091066





