1. Introduction
This paper is a part of ongoing research focused on an application of cold spray technology to restore worn-out areas of contact wires for electrified rail transport [
1,
2,
3]. Previously, the problem of restoring the local wear of contact wires was not solved. Previous works established technical requirements, optimal processing parameters, powder blend composition, and revealed properties of the obtained coating [
1,
2,
3]. In [
4], it is noted that a small number of works are devoted to the applied aspects of cold spraying. Moreover, in [
5], it is stated that cold gas dynamic spraying (CGDS) technology is a supplement to other known thermal sprays and so it is not a replacement either for any of them. Although there are reports on different successful applications of CGDS, the technology is not widespread due to insufficient bond strength between a substrate and a coating [
6,
7,
8,
9]. Particularly, several works attempted to apply Al-Sn coating on steel for bimetallic journal bearings [
10,
11,
12]. Having the experiment reproduced [
13,
14,
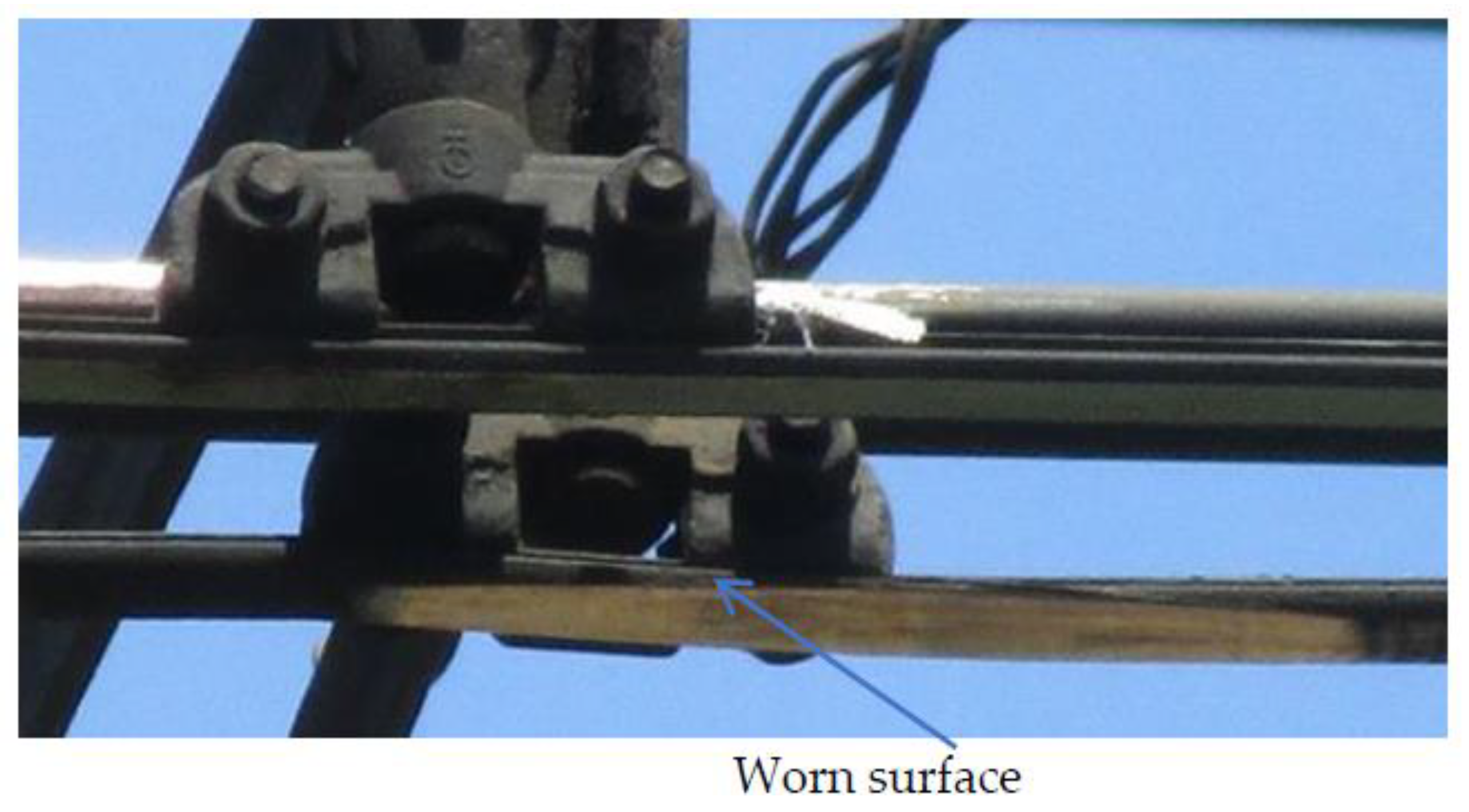
15], the authors of the current article revealed that the destruction of such Al-Sn coating occurred at the running-in stage which is caused by low adhesion and fatigue strength. Despite utilizing nitrogen or helium as working gas, the adhesion strength of the Al-Sn coatings is lower than that obtained by explosion welding or joint rolling. Thus, there is a major limitation for the CGDS technology for materials and applications demanding strong adhesion, especially in tribology, e.g., contact wires restoration. There are zones of severe wear on the contact wire up to 0.5 m long [
16,
17,
18,
19,
20,
21], indicating that these areas are worn out several times more intensely (
Figure 1). Today, repairing contact wire on high-speed lines is reduced to replacing whole segments at a length of 1.5 km once the limit wear of 0.5 m is detected.
Regular contact wire is a cold-drawn copper rod with a complex cross-section [
22] operated under-voltage and high tension of 100–300 MPa. To prevent recrystallization of the contact wire, the temperature during the lifecycle must be lower than 100 °C. Therefore, CGDS was taken as a primary technology since no recrystallization was observed in previous research [
2]. Earlier, satisfactory results were obtained during wire restoration proved by properties evaluated [
1,
2,
3,
23]. In [
1,
2,
3,
23], the results of a study of the mechanical and physical properties and functional characteristics of the coating depending on the composition of the charge and on the parameters of coating, as well as the effect of the coating process on the change in the mechanical properties and texture of the copper substrate, are presented. In [
23], it is shown that the addition of zinc to the powder mixture leads to a decrease in the electrical conductivity and adhesion of the coating to the copper substrate. In [
23], it is shown that the preliminary treatment of the surface of the copper substrate with coarse corundum leads to an increase in the adhesion strength of the coating to the copper substrate by several times. In this work, attempts to find correlations between microstructure and properties of the obtained coating were made. This makes it possible to study the mechanisms for ensuring the required properties of the coating, in particular, the strength of the adhesion of the coating to the substrate, and the specific electrical conductivity of the coating. In particular, the reasons for the influence of the pretreatment of the substrate surface and zinc additives on the adhesion strength and electrical conductivity of the coating.
2. Materials and Methods
The initial materials for the powder blend preparation were Cu, Zn, and Al
2O
3 provided by the JSC “Dimet” (Obninsk, Russia). Before the experiments, feedstock powders were studied using OCCHIO 500 nano granulomorphometer (Occhio, Liege, Belgium) according to [
24]. Copper powder particles were measured to be 10–60 µm (area diameter) with an average circularity factor of 0.483. Zinc particles were 20–60 µm with a circularity of 0.769. Alumina particles were from 10 to 80 µm with a shape factor of 0.686. The three powders were mixed in a planetary mixer Inversina 2 L (Bioengineering AG, Wald, Switzerland) and the following powder blends were used in the research: Cu, 60% Cu-40% Al
2O
3, 50% Cu-50% Al
2O
3, 70% Cu-30% Al
2O
3, 40% Cu-60% Al
2O
3, 50% Cu-40% Al
2O
3-10% Zn, 40% Cu-50% Al
2O
3-10% Zn, 40% Cu-40% Al
2O
3-20% Zn, 45% Cu-50% Al
2O
3-5% Zn. The powder blends were cold sprayed using a Dimet 405 setup (JSC “Dimet”, Obninsk, Russia). To obtain the uniform particle size distribution ranged 30–50 µm as recommended by the cold-spray machine manufacturer, the powders were sieved using a Retsch AS400 shaking sieve (RETSCH, Haan, Germany). In addition, coarse alumina powder with particles sized 100–150 µm was used for the substrate preprocessing to improve adhesion. For cold-spraying, air heated up to 500 °C was used.
Microstructural studies of the cold sprayed coatings were conducted using both the optical microscope Neophot 21 Carl Zeiss (Carl-Zeiss, Jena, Germany) and scanning electron microscope (SEM, TESCAN ORSAY HOLDING, Brno, Czech Republic) Tescan Vega 3, equipped with an energy-dispersive analysis (EDX) module X-Act (Oxford Instruments, Abington, UK).
To measure the microhardness of the cold sprayed coatings, microhardness Vickers tester Qness Q10 (QATM, Haan, Germany) was used with a 10 g load applied for 10 s. The standard procedure recommended by the manufacturer to determine minimum load involves a number of tests at different loads starting from the lowest to find a critical one, starting from which hardness values become stable. In this study, the minimum load was found to be 10 g resulting in clear indentation far enough from the edges. Indentation time 10 s was chosen to prevent any elastic deformation of the material.
3. Results and Discussion
Previously, while spraying Cu-Al
2O
3-Zn on copper, it was found that preprocessing the substrate with coarse alumina powder (>100 µm) improves the adhesion of the coating by 3–5 times [
23]. However, adding zinc decreases the bond strength by 1.3 times and electroconductivity by 1.4 times owing to microstructural changes [
23]. These features are summarized in
Table 1.
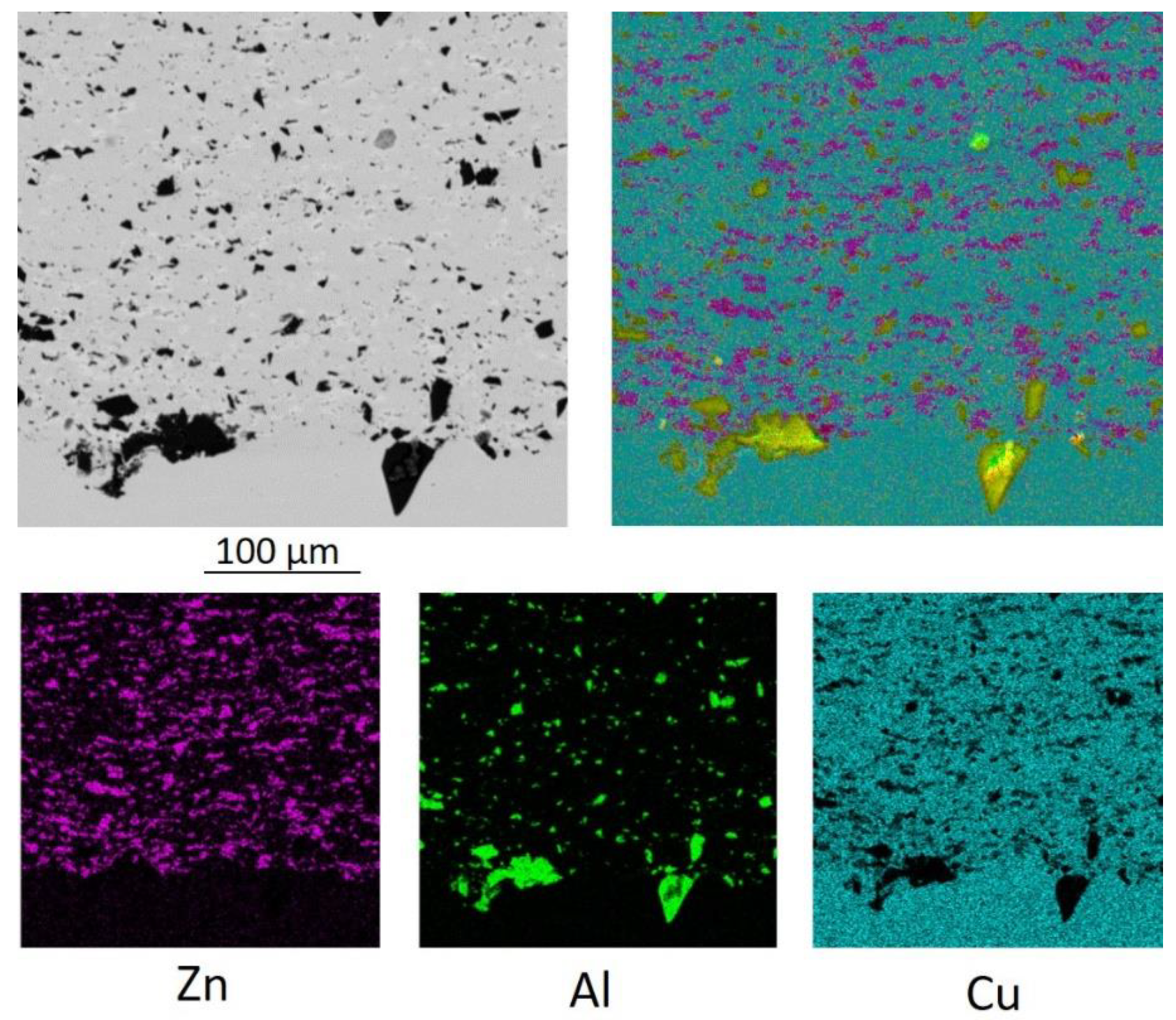
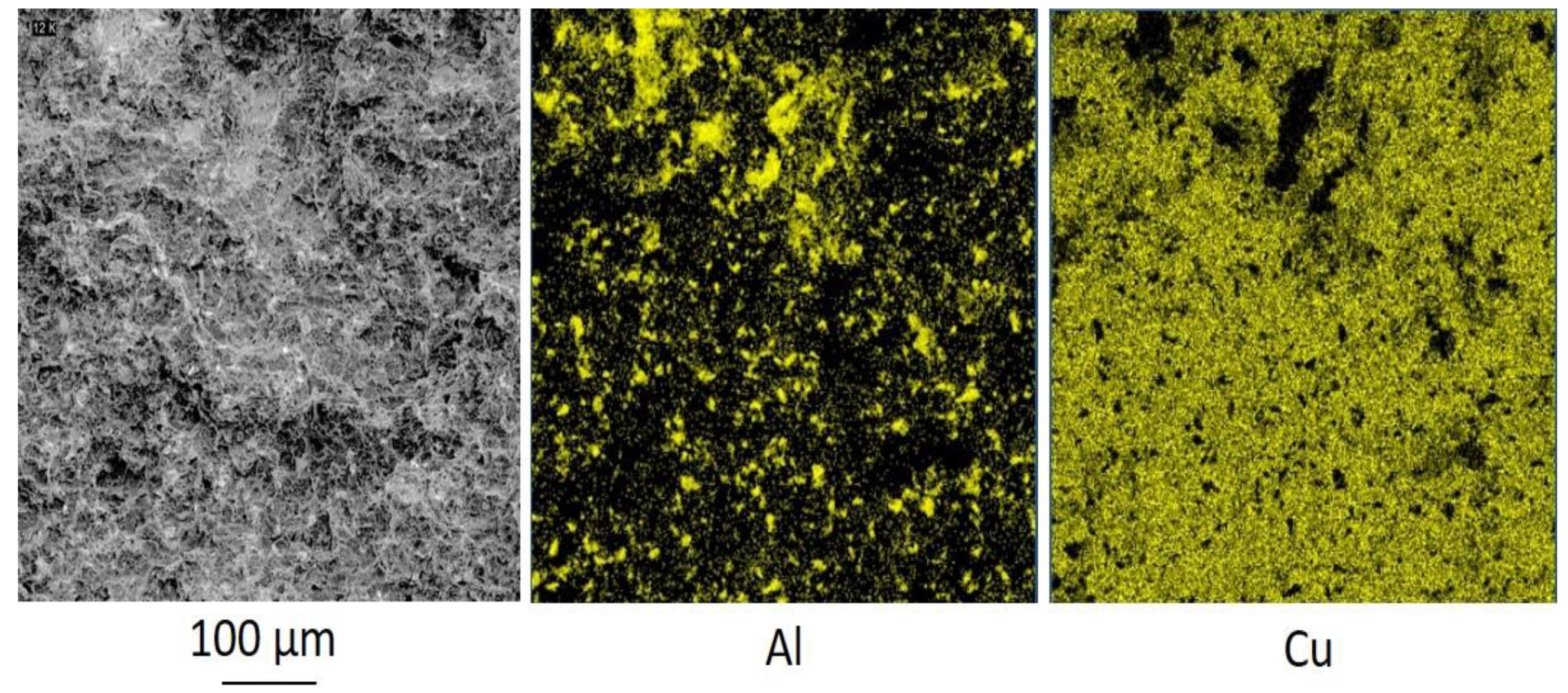
Figure 2 shows, that zinc is located along the boundaries of copper particles in almost continuous layers. Therefore, it contributes to both cohesion and adhesion strength [
1]. Moreover, alumina particles are also found between the copper ones, but compared to zinc, do not surround copper in the same conformal way due to lack of plasticity. As a result, to get through the wire, an electrical current passes through zinc particles as well. The electrical conductivity of zinc is 3.5 times less than the electrical conductivity of copper. Therefore, the addition of zinc leads to a decrease in the electrical conductivity of the coating. Thus, the microstructure is a key factor for elucidating the emerged problem of changing properties of the coating.
For zinc-containing powder systems, satisfying adhesion is observed only when a zinc-rich layer appears at the interface with the substrate [
1]. Therefore, the adhesion mechanism of the copper-zinc coating mostly involves zinc. This study raises the possibility that for sprayed powders with zinc, the adhesion of the sprayed layer to the substrate occurs as a result of the adhesion of zinc particles to the copper substrate. The sprayed copper particles adhere to the zinc particles on the substrate surface.
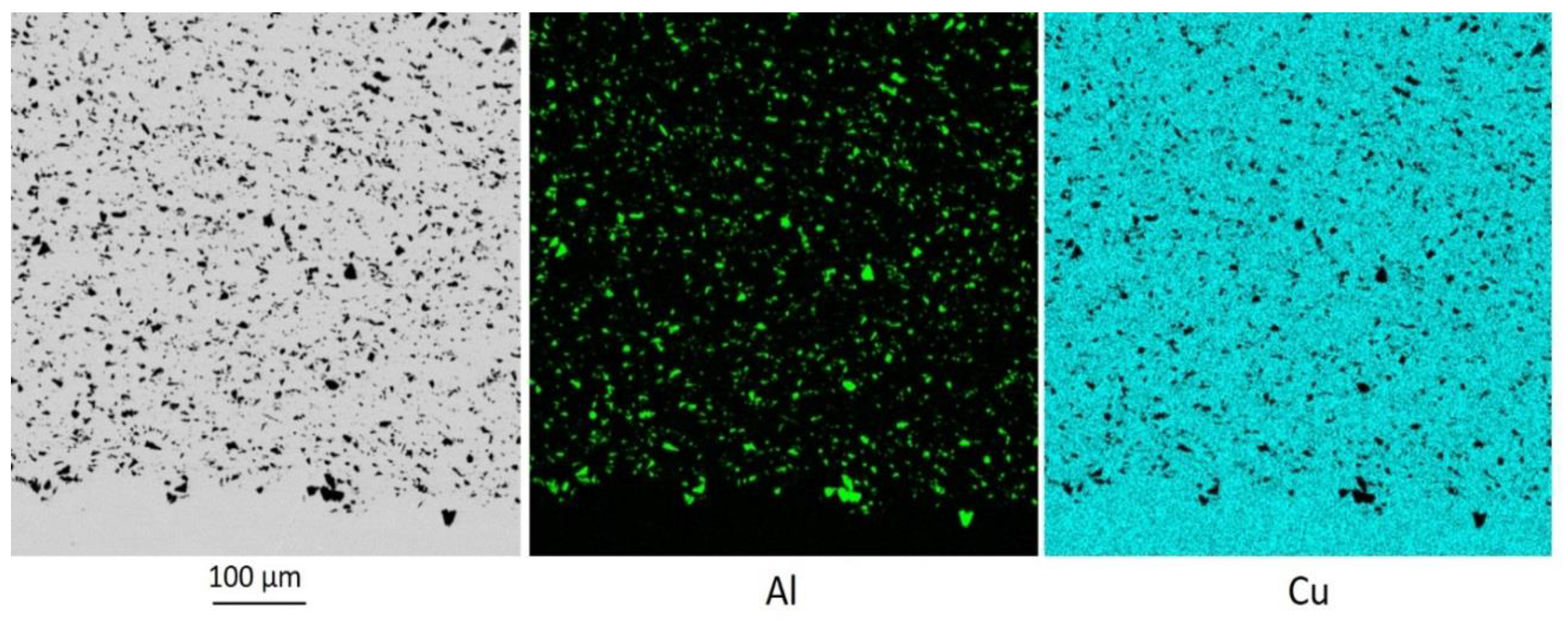
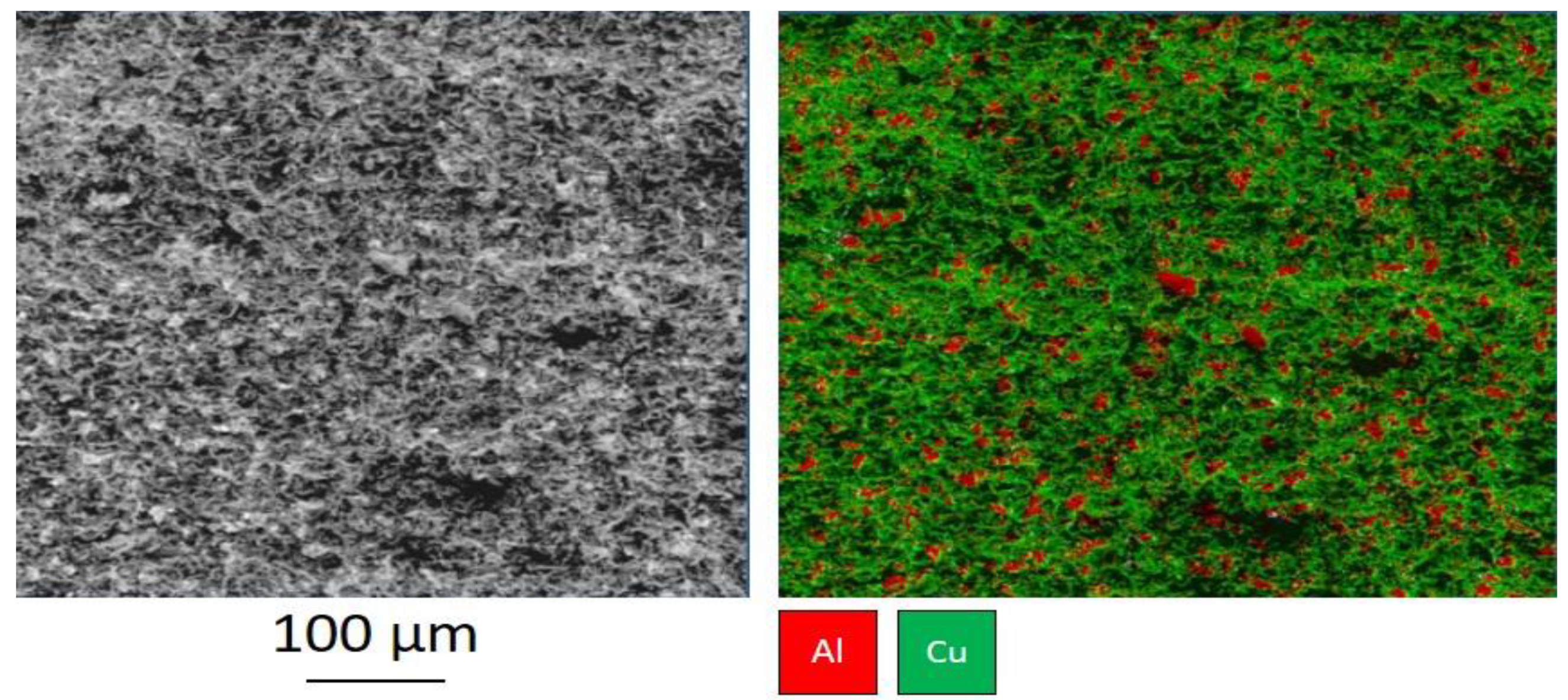
Cold spraying the Cu 60%-Al
2O
3 powder blend without preliminary surface treatment with coarse Al
2O
3 particles, the boundary between the layer and the substrate is a wavy surface with a wavelength of about 100 μm (
Figure 3). This shape is caused by a local introduction of the group of the Al
2O
3 particles into the substrate.
Figure 4 shows the group of Al
2O
3 particles on top of the wave.
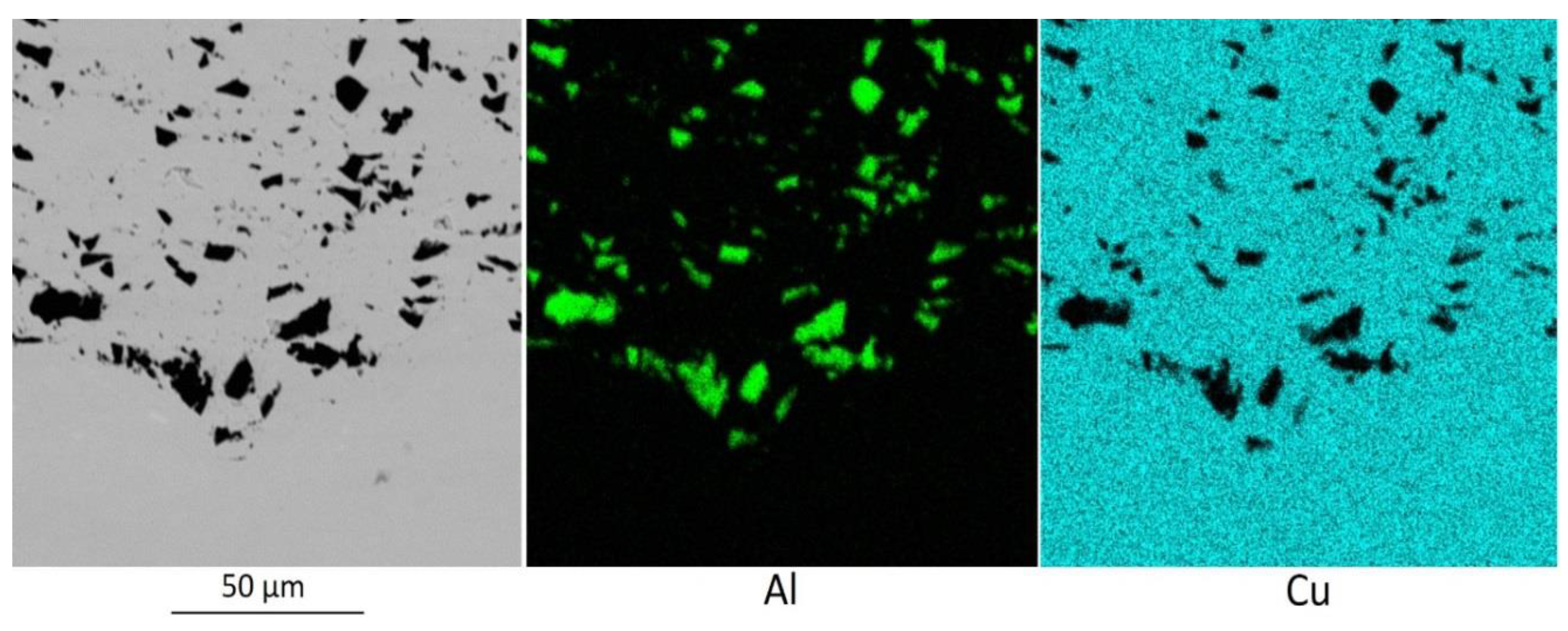
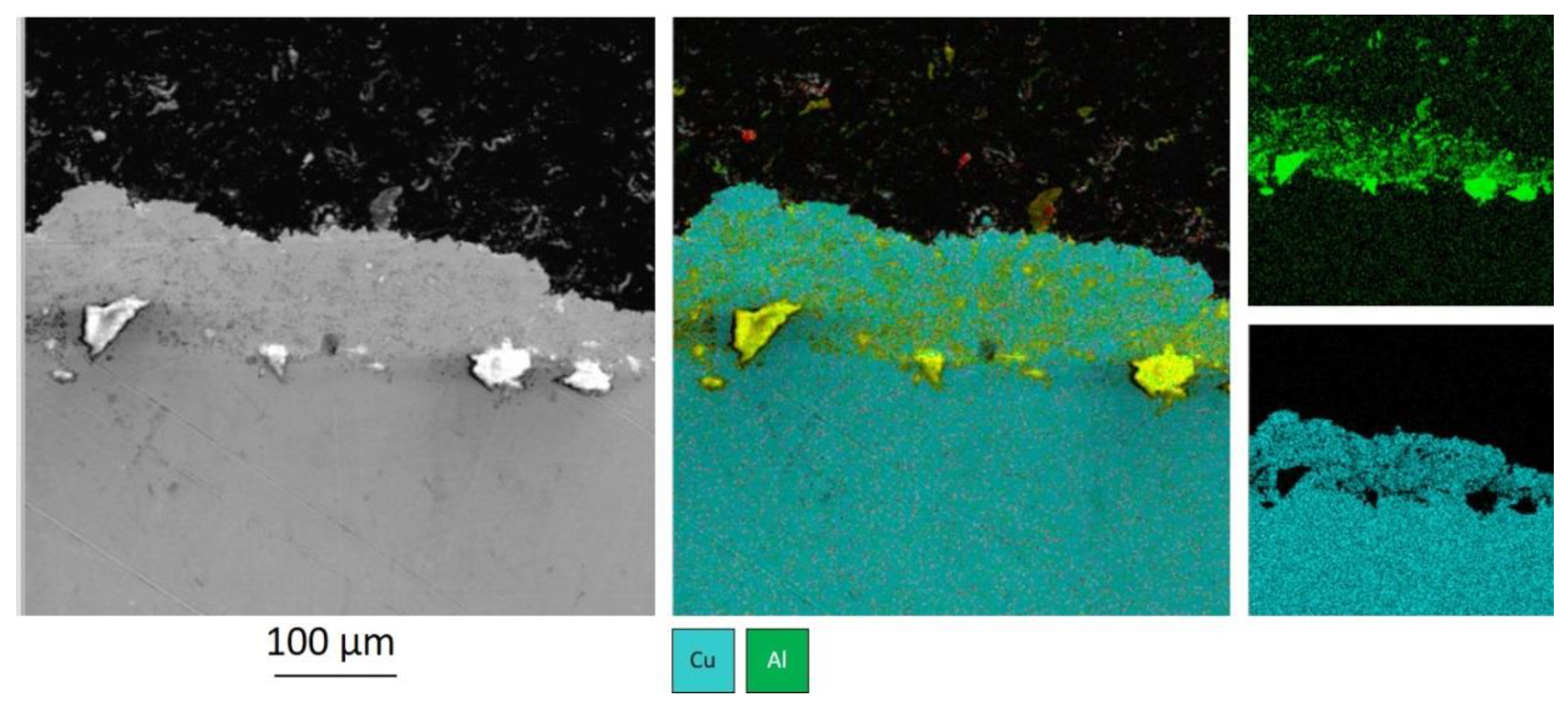
Substrate preprocessing ensures a surface with periodically embedded coarse Al
2O
3 particles.
Figure 5 shows the boundary between the substrate and the coating, sprayed after pretreatment of the substrate surface with coarse aluminum particles. The coating was sprayed with a powder mixture Cu-60% Al
2O
3. The embedding depth was measured to be up to 30 μm and the distance between the closest alumina particles—from 20 to 300 μm. After coating, the interface between the coarse alumina particles consists mainly of copper and a small amount of fine cold sprayed Al
2O
3 particles. A similar structure is formed when zinc is added to the powder mixture.
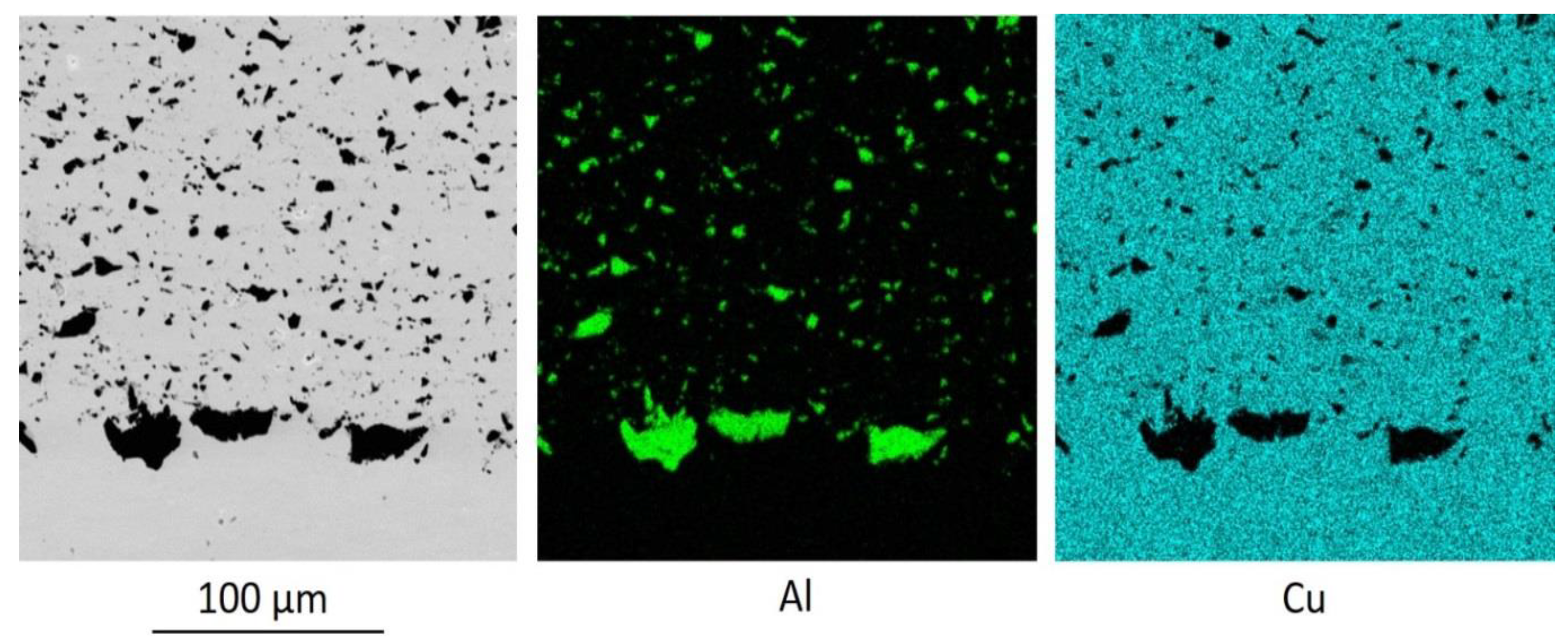
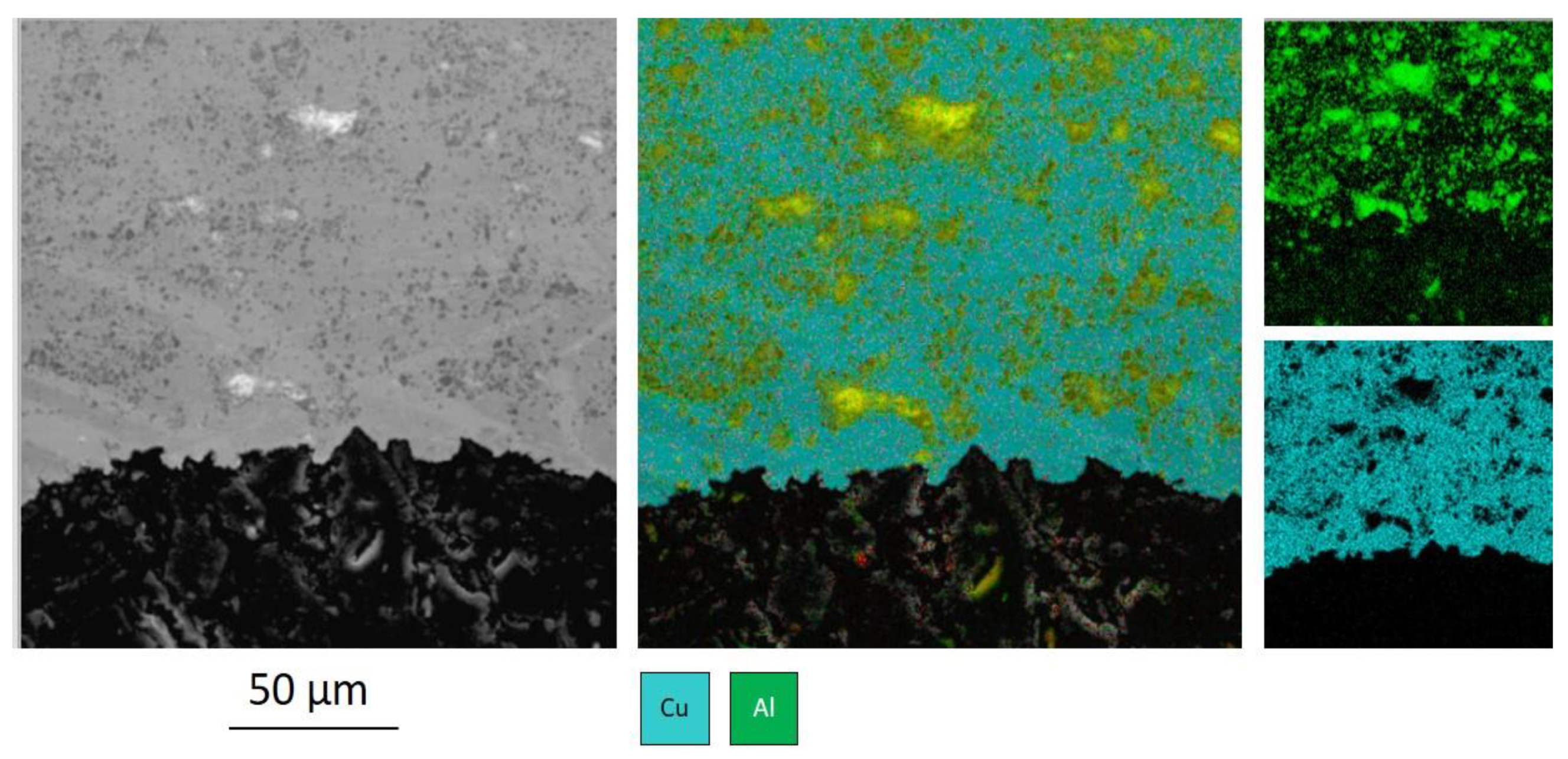
Figure 6 shows the microstructure of the interface between the substrate and the coating with zinc. The coating was applied with a powder mixture Cu-60% Al
2O
3-10% Zn after preliminary treatment of the copper substrate with coarse aluminum particles.
The boundary shown in
Figure 5 differs from the boundary shown in
Figure 4 in that coarse aluminum particles are introduced into the substrate with preliminary treatment (
Figure 5), and groups of relatively small aluminum particles from the sprayed powder mixture are introduced into the substrate without preliminary treatment (
Figure 4). Moreover, the aluminum particles from the sprayed powder mixture (
Figure 4) are significantly smaller than the coarse aluminum particles (
Figure 5) for preliminary treatment of the substrate surface.
The boundary shown in
Figure 6 differs from the boundary shown in
Figure 5 by the presence of zinc between the coarse aluminum particles.
These findings have important implications for developing a concept for adhesion mechanisms. Juvenile surface and plastic deformation are mandatory for seizure and adhesion [
25]. In the powder blends used, the yield strength of copper surpasses that of zinc by 2 times [
26]. Consequently, zinc particles on impact deform more easily compared to copper particles. Thus, the probability of seizure of zinc particles with the copper substrate will be greater compared to the probability of seizure of copper particles. Simply put, when spraying powder mixtures with zinc, zinc particles are the first to cover the copper substrate, and then the copper particles are bound to the zinc-rich layer [
1]. Copper particles bond to each other through the zinc layer (
Figure 2), which, therefore, defines the strength of the whole coating. The formation of a zinc-rich layer [
1] described above suggests a low probability of setting copper particles to a copper substrate. This may be due to insufficient plastic deformation of the copper particles upon impact contributing to low adhesion in copper-copper systems [
23].
When a mixture of copper particles with Al
2O
3 particles is deposited, the latter are incorporated into the copper substrate (
Figure 4). As a result, the substrate is deformed. Cold deformation by the embedded particles leads to a local strengthening of the copper substrate, increasing its hardness. The distance between particles is about 100 microns (
Figure 4). It corresponds to a hardened area from one single alumina particle or a group of them. This reasoning is also valid for the preprocessed surface with embedded large Al
2O
3 particles (
Figure 5). The existence of hardened zones is confirmed by measuring the microhardness of the substrate after the deposited layer is detached from it. The microhardness of the substrate areas between the embedded Al
2O
3 particles (hardened zones) was 125–145 HV, while the other areas (non-hardened zones) were only 86–101 HV, giving an increase in hardness by 24%–69%. The substrate shown in
Figure 4 hardens less. The microhardness of the substrate in the hardening zones between the embedded particles is 115–125 HV. This is due to the fact that the size of the aluminum particles in the powder mixture for spraying is significantly smaller than the size of the aluminum particles for the pretreatment of the substrate. Accordingly, smaller particles deform and harden the substrate less when embedded than coarse particles do.
Copper particles hitting solid Al2O3 particles protruding from the substrate undergo severe deformation which continues as following fine Al2O3 particles reach the surface. Intense deformation of copper particles leads to the destruction of the oxide layer and the formation of juvenile surfaces, friction, and hardening. The microhardness of copper of the sprayed layer detached from the substrate was 145 HV, while the microhardness of the sprayed layer in the area of accumulation of fine Al2O3 particles was 180 HV (the microhardness of the sprayed copper layer without Al2O3 was about 80 HV). An increase in the hardness of copper in the sprayed layer is associated not only with its deformation and hardening during deposition as a result of collisions with aluminum oxide particles. The hardening of copper in the sprayed layer occurs as a result of dispersed hardening by particles of aluminum oxide. This is confirmed by the fact that the microhardness of copper near the accumulation of aluminum oxide particles is higher than in other places. The sprayed layer containing zinc is hardened to a lesser extent because the hardness and strength of zinc are significantly less than the strength and hardness of. copper The microhardness of such a layer usually does not exceed 120 HV. It should be noted that the strength and yield strength of the layer increases with hardness.
Figure 7 and
Figure 8 show microstructures and maps of the distribution of elements of a substrate without pretreatment after tearing off the coating sprayed with a mixture Cu-60% Al
2O
3 and microstructure of the separated coating sprayed with a mixture of Cu-60% Al
2O
3 on a substrate without pretreatment, respectively. The coating was sprayed with a Cu-60% Al
2O
3 mixture without preprocessing of the substrate. The substrate area presented in
Figure 7 contains almost no coating residues except for a few Al
2O
3 particles embedded in the substrate. This suggests that the coatings are torn off at the interface with the substrate, on which embedded Al
2O
3 particles can remain.
Figure 8 shows a section of a detached coating where alumina particles are observed and do not exceed the separation surface. Therefore, alumina particles embedded in a copper substrate remain there. These particles are located uniformly (
Figure 9). Thus, detaching mechanisms occur on the copper interface, while alumina particles remain on the substrate surface. Due to insufficient copper hardening adhesion strength of the coating is only 16.7 MPa.
Based on the above microstructures and microhardness measurements, the adhesion of the coating when the powder mixture is sprayed onto the substrate without pretreatment can be represented in the following way. First, there is an introduction of groups of aluminum particles into the copper substrate (
Figure 4). The penetration depth does not exceed 15 microns. Prior to this, the sprayed particles bounced off the substrate, or individual copper particles, after slight deformation upon impact on the substrate, seizure onto it. After the introduction of groups of aluminum particles into the substrate (
Figure 4), the copper particles after collision with them are deformed to a greater extent than upon collision with the copper substrate. As a result of the increased deformation, the copper particles seizure with the copper substrate with a higher strength compared to the adhesion strength of the copper particles to the substrate after collision only with the substrate. The results of measuring the microhardness show that the surface of the substrate is hardened to a lesser extent than the sprayed layer (115–125 HV and 145 HV, respectively). Therefore, on average, the adhesion strength of the coating to the substrate will be lower than the strength of the coating itself.
Due to insufficient copper hardening and weak retention of embedded aluminum particles by the substrate, adhesion strength of the coating is only 16.7 MPa.
Figure 10 shows an area of the preprocessed substrate with Cu-60% Al
2O
3 coating residues after tearing off coating from the substrate. The embedded coarse alumina particles are clearly observed as well as a part of the sprayed coating after tearing off the coating from the substrate. Together with the embedded particles, a part of the coating with a thickness of about 100 μm remained on the substrate.
Figure 11 demonstrates the corresponding detached part of the coating. Detaching the coating causes destruction propagating along the coating, not the interface as anticipated. There are no coarse Al
2O
3 particles in the detached layer observed (
Figure 11).
From
Figure 10 and
Figure 11, it follows that the destruction of the coating occurred over the area of the coating copper zones depleted in fine aluminum particles. In the zone with a low content of dispersed particles of Al
2O
3, copper is less hardened, therefore, its strength is lower than in areas with a high content of dispersed particles. The results of measuring the microhardness show that the surface of the substrate is hardened as well as the average sprayed layer (microhardness 125–145 and 145, respectively). Consequently, these zones have approximately the same ultimate strength. Therefore, in this case, destruction occurs at a weak point. Weak points of the coating are places that are depleted in fine aluminum particles. The microhardness in such places can be more than 80 HV and less than 145 HV. Therefore, these locations are less hardened than other coating locations and the surface of the substrate. This is due to the lower disperse hardening of copper due to the small number of dispersed particles. Strength in this coating reaches 62.49 MPa (
Table 1).
In order to elucidate mechanisms of forming and destruction, sprayed coating separation surfaces were studied.
Figure 12 shows an image of a portion of the substrate surface, from which a coating sprayed with a composition of 40% Cu-60% Al
2O
3 after preliminary processing was torn off. There is a cellular structure of the separation zone and scaly-shaped particles, which characterizes plastic fracture while no regular presence of coarse alumina was found comparing to areas shown in
Figure 5,
Figure 6 and
Figure 10. It suggests that adhesion of the coating is higher than cohesion strength, determining destruction manner. The separation surface studied on the substrate is similar to that on the coating.
The adhesion of the coating to the pretreated substrate is the same as to the substrate without pretreatment. The difference is that on the surface of the pre-treated substrate there are already ready-made aluminum particles embedded in the substrate. Their sizes are much larger than the sizes of particles incorporated into the substrate without pretreatment. Therefore, the degree of deformation of copper particles upon collision with embedded particles, in this case, was significantly higher than the degree of deformation of copper particles upon collision with small embedded particles of aluminum into the substrate without pretreatment. Therefore, the seizure of the copper particles to the substrate, in this case, led to a higher adhesion strength compared to the substrate without pretreatment.
Large alumina particles from the preprocessing stage are almost absent. The observed ones were found on the coating side of the separation surface (
Figure 13). No copper was found on the alumina particle surface. Consequently, copper does not seizure to corundum, and destruction occurs along the copper particles. These findings suggest that copper particles deform more when deposited onto the preprocessed substrate which is due to large, embedded alumina particles. It results in stronger adhesion of the coating. Consequently, the adhesion strength of the coating to the substrate may be lower in some places than the strength of the coating itself. Apparently, this is a rare and not the typical case for a substrate with preliminary treatment.
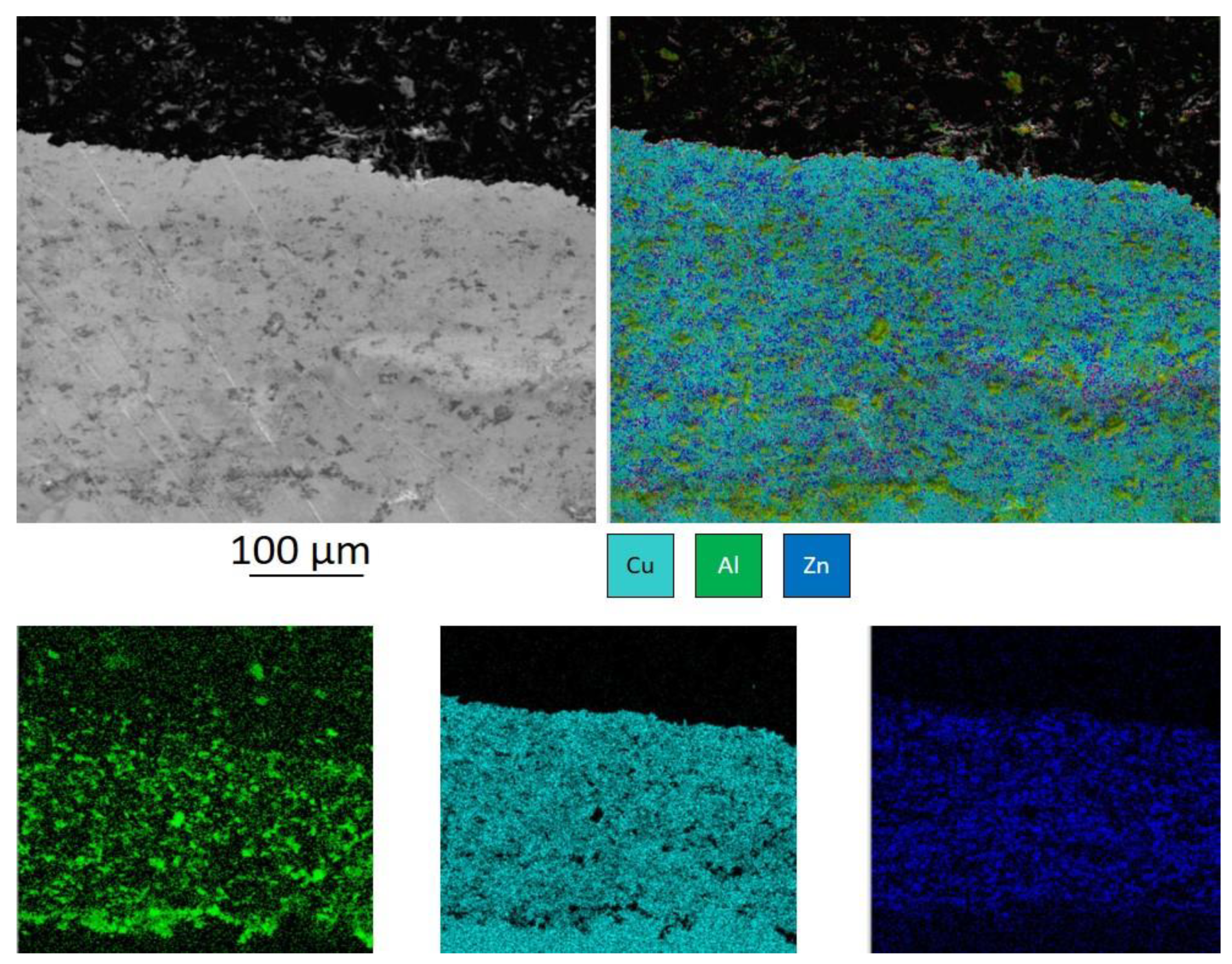
In contrast, separation surfaces of the 40% Cu 50% Al
2O
3-10% Zn coating demonstrate that most copper is coated with zinc (
Figure 2). At the same time, alumina particles were found to have no zinc on their surface indicating zero bonding of zinc and Al
2O
3. The less cellular structure indicates less deformation of copper which is reasonable since zinc deforms first and provides a bond.
Figure 14 shows an area of the substrate with the Cu-60% Al
2O
3-10% Zn coating residues up to 100 μm width after tearing off the coating from the substrate with preliminary treatment. Due to preprocessing coarse embedded alumina particles are observed. The destruction of the coating occurred inside the coating in a zone depleted of dispersed fine particles of Al
2O
3 similar to the coating without zinc. Since zinc strength is lower than that of hardened copper, the cohesion of zinc-containing coating is lower and is 41.55 MPa.