Influence of Double-Pulse Electrodeposition Parameters on the Performance of Nickel/Nanodiamond Composite Coatings
Abstract
:1. Introduction
2. Experimental Preparation
2.1. Substrate Selection and Pre-Treatment
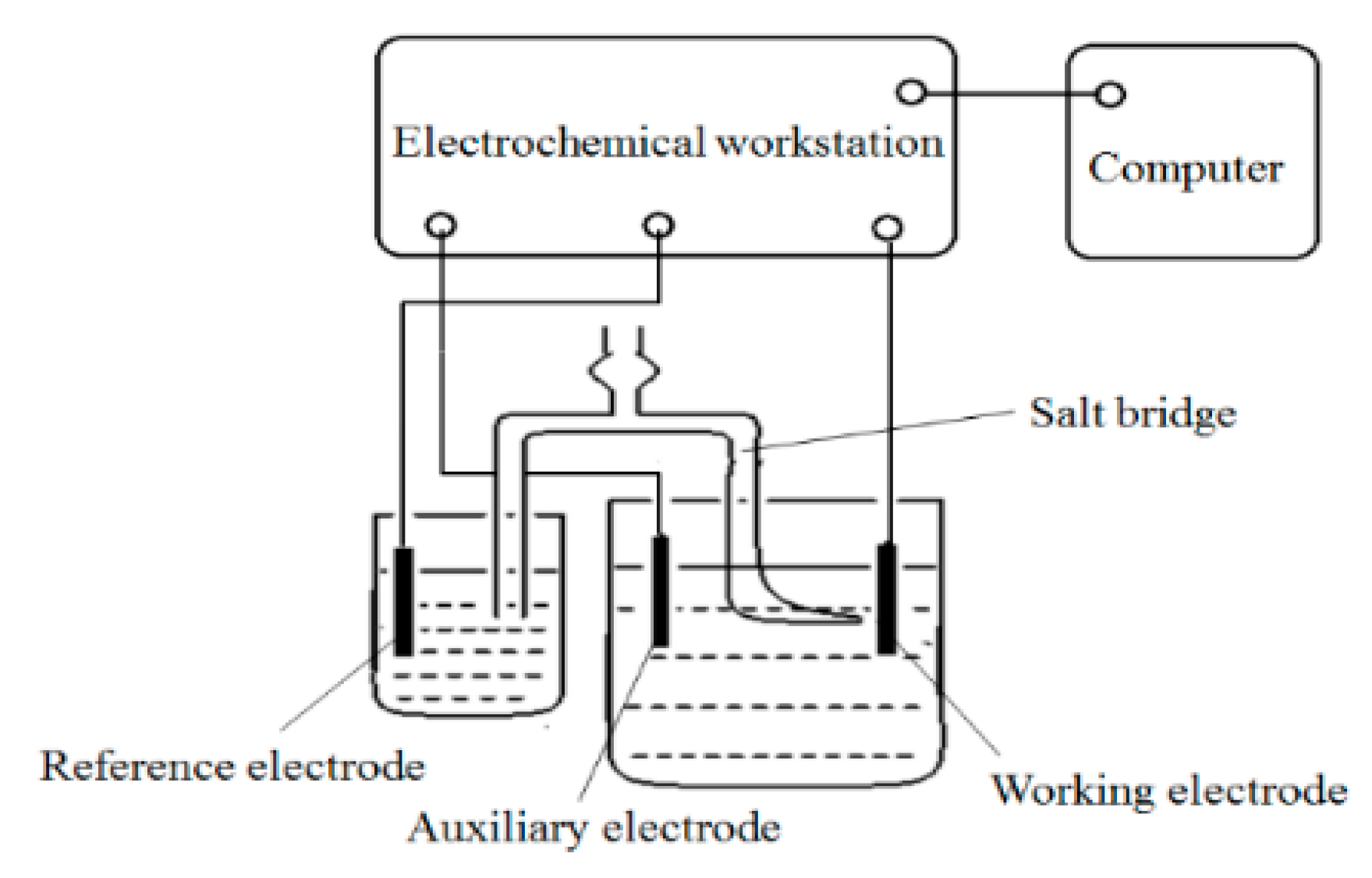
2.2. Analysis of the Polarisation Behaviour of Nanodiamond Particles in Plating Solutions
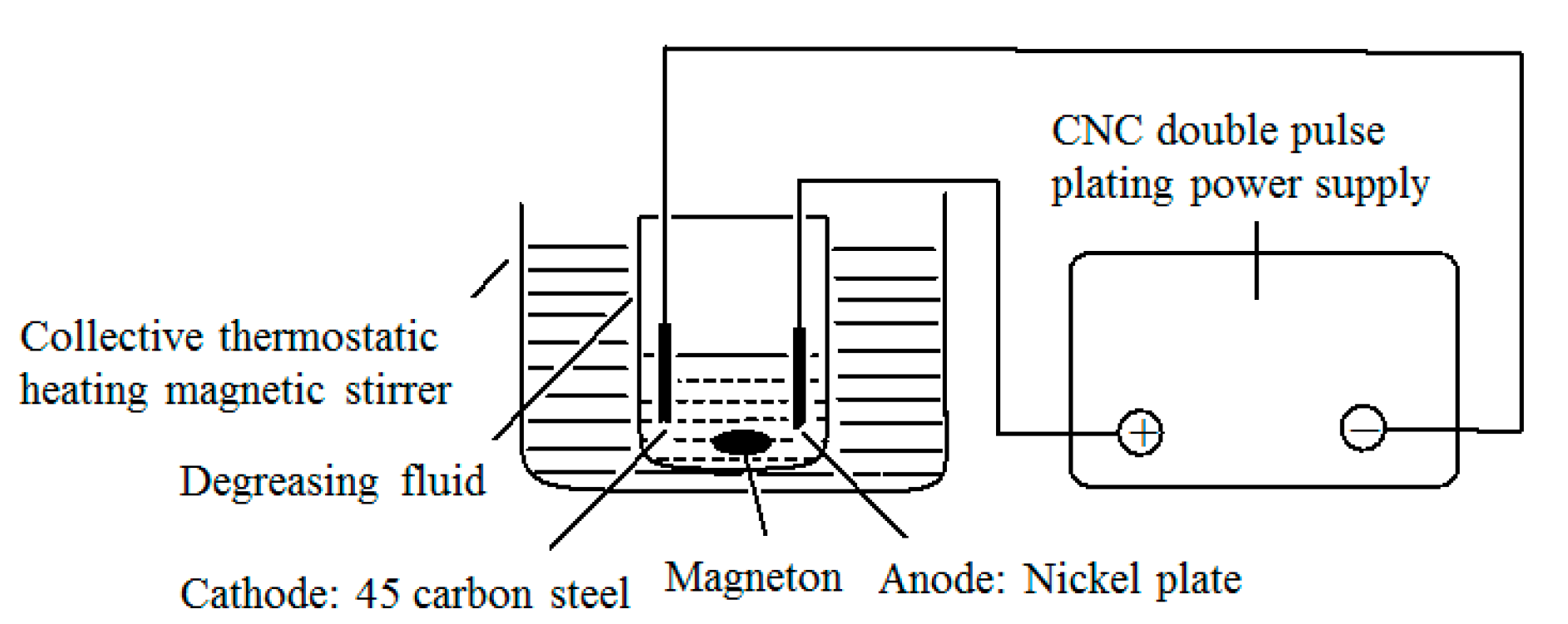
2.3. Selection of Parameters for Double-Pulse Electrodeposition
2.4. Components of the Compound Plating Solution
2.5. Testing of Plating Properties
3. Results and Analysis
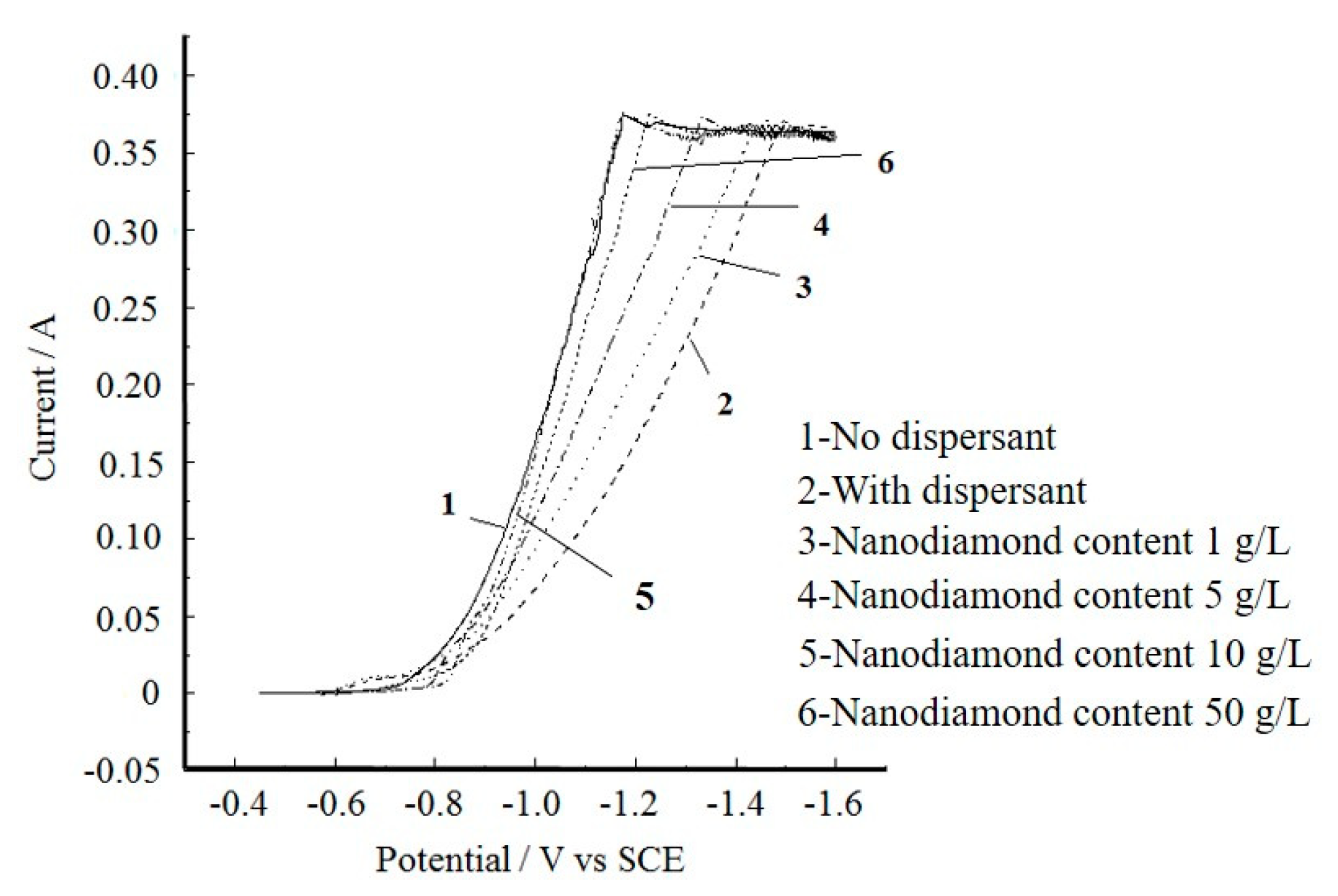
3.1. Analysis of the Cathodic Polarisation Behaviour of Plating Solutions Containing Nanodiamond Particles
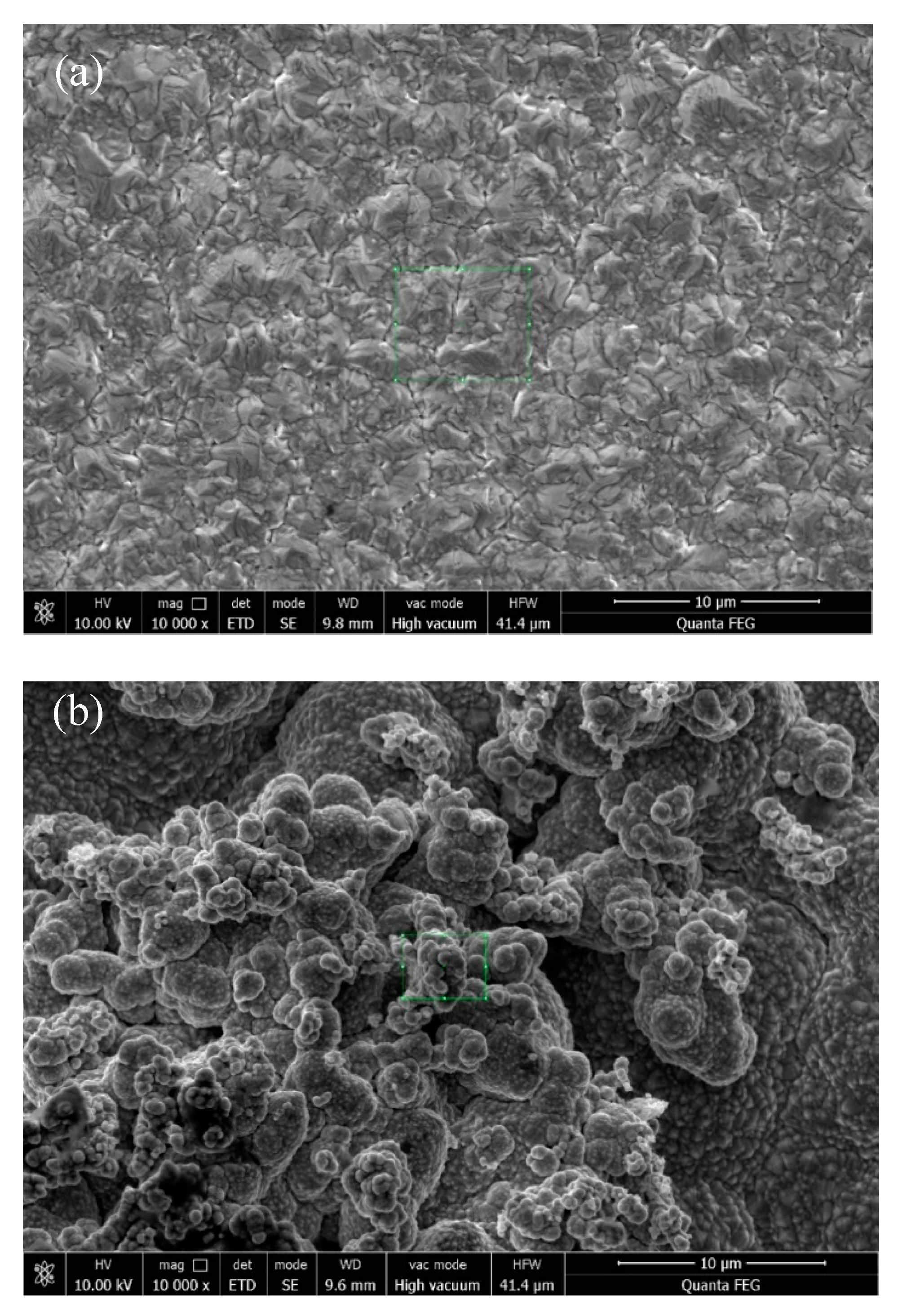
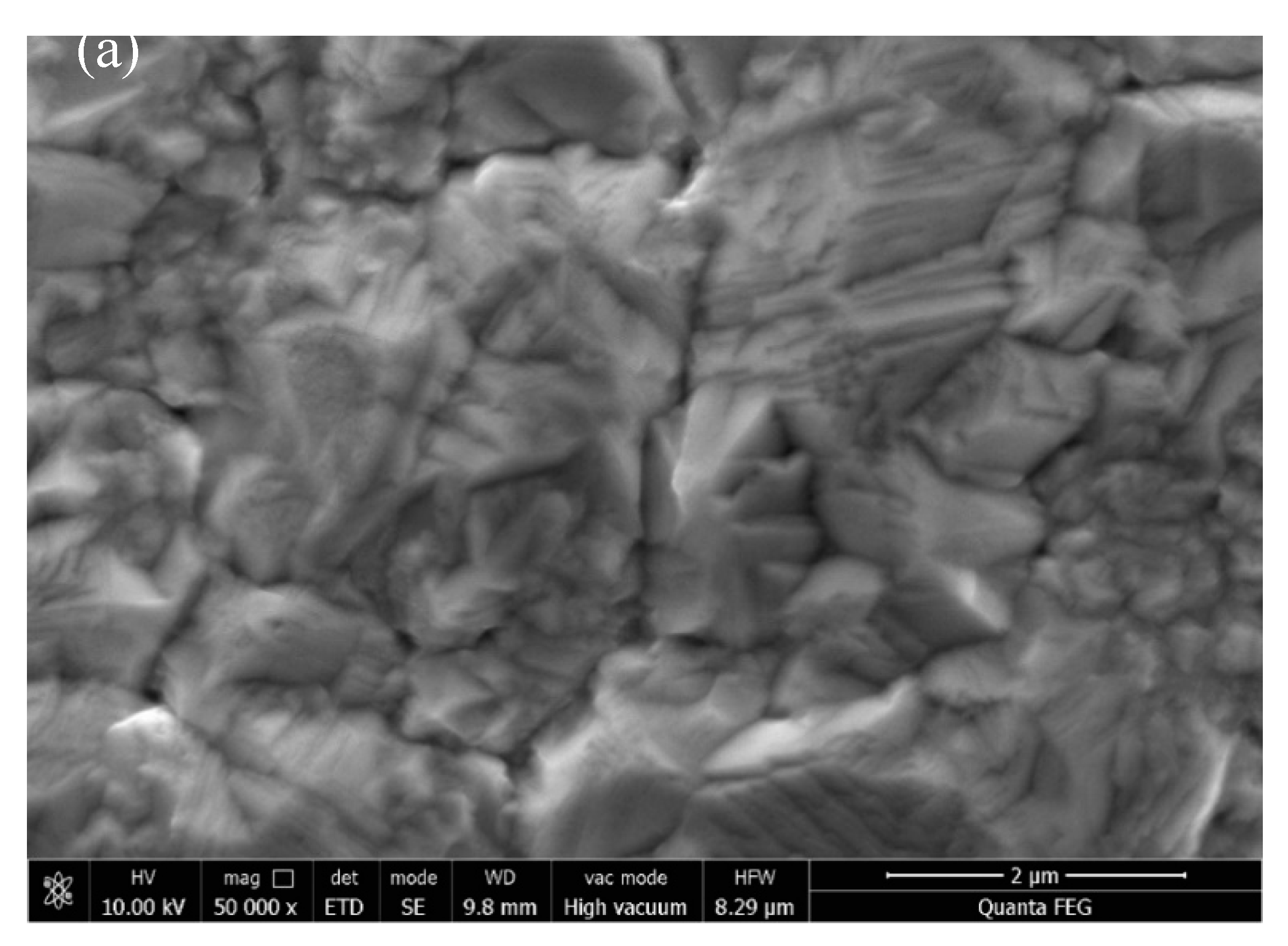
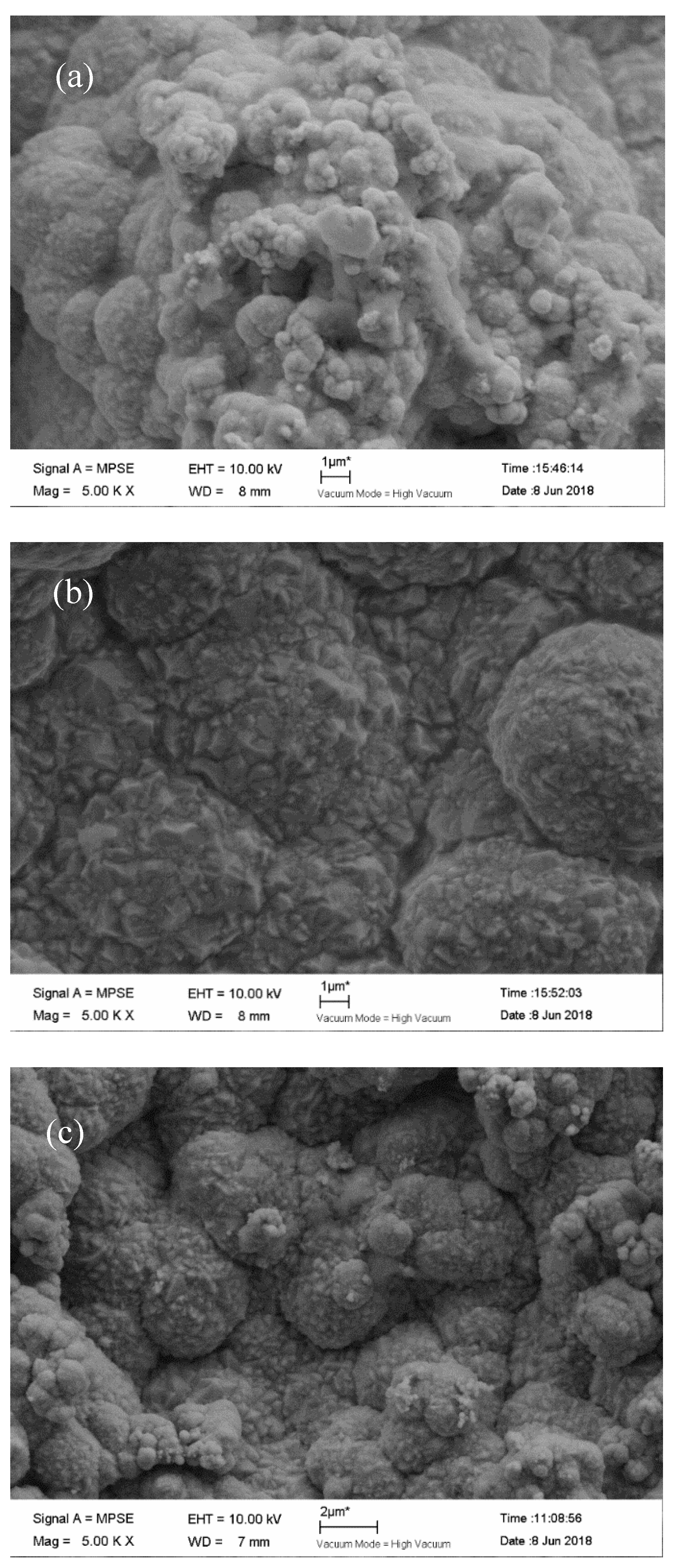
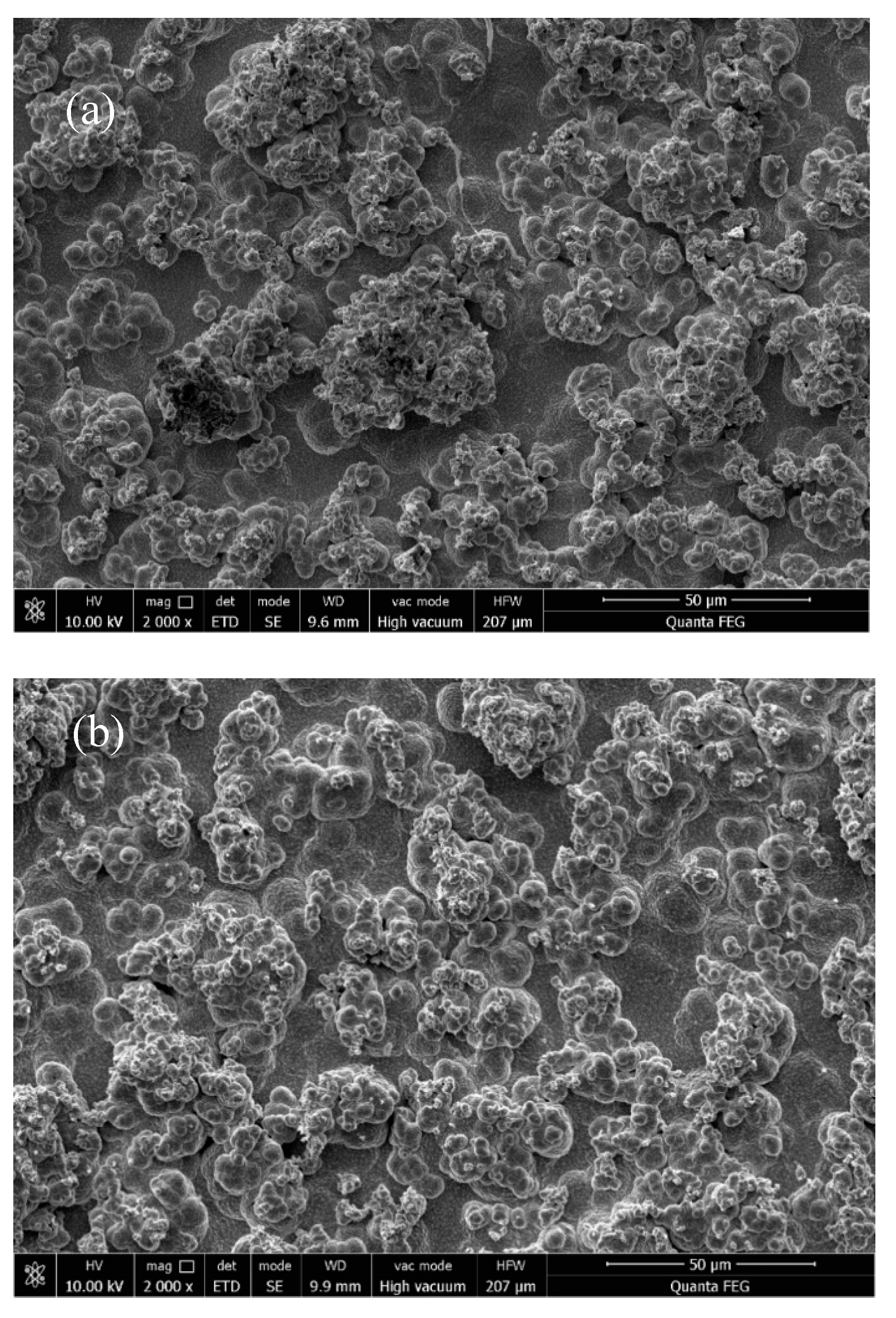
3.2. Effect of Different Deposition Methods on the Morphology of Composite Coatings
3.3. The Effect of Reverse Working Time on the Properties of Composite Plating
3.4. Effect of Reverse Duty Cycle on the Performance of Composite Coatings
3.5. Effect of Forward Duty Cycle on the Performance of Composite Plating
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Søresen, P.; Kill, S.; Johansen, D.K.; Weinell, C. Anticorrosive coating: A review. J. Coat. Technol. 2009, 6, 135–176. [Google Scholar] [CrossRef]
- Müler, K.; Bugnicourt, E.; Latorre, M.; Jorda, M.; Sanz, Y.E.; Lagaron, J.M. Review on the processing and properties of nanocoposites and nanocoating and their applications in the packing, automotive and solar energy fields. Nanomaterials 2017, 7, 74. [Google Scholar] [CrossRef] [Green Version]
- Mariello, M.; Guido, F.; Mastronardi, V.M.; Giannuzzi, R.; Algieri, L.; Qualteri, A.; Maffezzoli, A.; De Vittorio, M. Reliability of Protective Coatings for Flexible Piezoelectric Transducers in Aqueous Environments. Micromachines 2019, 10, 739. [Google Scholar] [CrossRef] [Green Version]
- Bao, W.; Deng, Z.; Zhang, S.; Ji, Z.; Zhang, H. Next-Generation Composite Coating System: Nanocoating. Front. Mater. 2019, 6, 72. [Google Scholar] [CrossRef]
- Safavi, M.S.; Rasooli, A. Ni-P-TiO2 nanocomposite coatings with uniformly dispersed Ni3Ti intermetallics: Effects of current density and post heat treatment. Surf. Coat. Technol. 2019, 372, 252–259. [Google Scholar] [CrossRef]
- Abdoli, M.; Rouhaghdam, A.S. Preparation and characterization of Ni–P/nanodiamond coatings: Effects of surfactants. Diam. Relat. Mater. 2012, 31, 30–37. [Google Scholar] [CrossRef]
- Sajjadnejad, M.; Omidvar, H.; Javanbakht, M.; Mozafari, A. Textural and structural evolution of pulse electrodeposited Ni/diamond nanocomposite coatings. J. Alloys Compd. 2017, 704, 809–817. [Google Scholar] [CrossRef]
- Ogihara, H.; Safuan, M.; Saji, T. Effect of electrodeposition conditions on hardness of Ni–B/diamond composite films. Surf. Coatings Technol. 2012, 212, 180–184. [Google Scholar] [CrossRef]
- Deng, S.-H.; Gong, Z.-Q.; Chen, W.-G. Research status and development of nano-material electrodeposition. Electroplat. Finish. 2001, 30, 35–39. (In Chinese) [Google Scholar]
- Li, L.; Yang, Y.W.; Huang, X.H.; Li, G.; Zhang, L. Pulsed electrodeposition of single-crystalline Bi2Te3 nanowire arrays. Nanotechnology 2006, 17, 1706–1712. [Google Scholar] [CrossRef]
- He, X.K.; Chen, B.Z.; Wu, L.Y.; Li, X.D.; He, Q.G. Process of pulse electrodeposition of nanocrystalline Ni-Cr alloy from trivalent chromium bath. Chin. J. Nonferrous Met. 2006, 16, 1281–1287. [Google Scholar]
- Xiang, G.-P.; Zhou, E.-B. Study of Ni-Co Alloy Pulse-Plating. Electroplat. Finish. 1994, 13, 18–22. [Google Scholar]
- Xie, J.-L.; Zhu, H.-F. Research of Pulse Plating Gold Process Technology. Microelectron. Comput. 1999, 22, 21–23. [Google Scholar]
- Shivagan, D.D.; Shirage, P.M.; Pawar, S.H. Studies on the fabrication of Ag/Hg1Ba2Ca1Cu2O6 + &/CdSeheterostructuresusingthe pulse electrodepositiontechniqu. Semicond. Sci. Technol. 2004, 19, 323–330. [Google Scholar]
- Yin, Y.-T. Research and application of pulse power supply in local high-speed silver plating process. Microelectron. Technol. 2001, 29, 58–59. [Google Scholar]
- Yang, F.Z.; Xu, S.; Yao, S.; Chen, B.; Zheng, X.; Zhong, X.; Zhou, S. A Study on the Elect rodeposition of Palladium and it’s Nucleation. Electrochemistry 1997, 3, 103–108. [Google Scholar]
- Xu, J.-X.; Jiang, L.-H.; Liu, D.-Z.; Chen, S.-Y.; Feng, W.-S. Effect of Average Electric Current Density on Deposition Rate and Appearance and Structure of Pulse Electrodeposited Fe Coating. J. Mater. Prot. 2006, 39, 13–15. [Google Scholar]
- Xiang, G. Theory and Application of Pulse Electroplating; Tianjin Science and Technology Press: Tianjin, China, 1989; pp. 13–14. [Google Scholar]
- Liu, M.; Zhao, Y.; Meng, Y.; Li, F.-H.; Gong, Y.-I.; Feng, L. Morphology and Mechanicl Properties of Ni/Nano-Diamond Composite Coatings Prepared at Different Current Density in Different Nano-Diamond Concentrations. J. Mater. Prot. 2016, 1, 1–6. [Google Scholar]
- Liu, M.; Liu, H.; Wang, D.; Chu, Y.; Li, F.; Liu, S. Influence of Electrodeposition Parameters on Properties of Ni-Nanometer Diamond Composite Coating. J. Mater. Prot. 2018, 1, 62–66. [Google Scholar]
- Liu, M.; Wang, D.; Wang, H.; Shi, Y.; Liu, B.; Li, F.; Gong, Y.; Zhang, W. Study on Optimization Technology to Strengthen Ni-Based Composite Coating Electroplate Containing Nanodiamond. Materials 2019, 12, 1654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Liu, H.; Wang, D.; Liu, B.; Shi, Y.; Li, F.; Gong, Y.; Li, L.; Li, L.; Zhang, W. Effect of Nanodiamond Concentration and the Current Density of the Elec-trolyte on the Texture and Mechanical Properties of Ni/Nanodiamond Composite Coatings Produced by Electrodeposition. Materials 2019, 11, 1105. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Liu, M.; Feng, L.; Meng, Y.; Li, F.-H.; Chen, Z. Optimization of Technology for Electro-deposition of Nickel Coating on Q235A Steel Substrate. J. Mater. Prot. 2014, 47, 33–36. [Google Scholar]
- Wang, Y.; Yuan, X.-T.; Yu, H.-Y.; Sun, D.-B.; Li, H.-Q. Influence of pulse parameters on the micro-structure and microhardness of nickel electrodeposits. Mater. Sci. Technol. 2010, 18, 90–95. [Google Scholar]
- Zhang, Z.; Liu, H.; Meng, Q.; Wang, X.; Li, Y.; Yang, H. Effect of Duty Cycle on Ni-Cr-Mn Alloy Coating Prepared by Pulse Electroplating. Hydrometall. China 2017, 36, 115–118. [Google Scholar]
- Bhuiyan, E.H.; Moreno, S.; Wang, C.; Minary-Jolandan, M. Interconnect Fabrication by Electroless Plating on 3D-Printed Electroplated Patterns. ACS Appl. Mater. Interfaces 2021, 13, 19271–19281. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Bhuiyan, E.H.; Moreno, S.; Minary-Jolandan, M. Direct-Write Printing Copper–Nickel (Cu/Ni) Alloy with Controlled Composition from a Single Electrolyte Using Co-Electrodeposition. ACS Appl. Mater. Interfaces 2020, 12, 18683–18691. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Xu, L.; Wang, C.; Li, S.; Wu, D.; Shi, Y.; Liu, F.; Xue, X. Electrochemical Behavior and Electrodeposition of Sn Coating from Choline Chloride–Urea Deep Eutectic Solvents. Coatings 2020, 10, 1154. [Google Scholar] [CrossRef]






| Sample Number | Average Current/A | Working Time/ms | Duty Cycle | |||
|---|---|---|---|---|---|---|
| Positive Direction | Reverse Direction | Positive Direction | Reverse Direction | Positive Direction | Reverse Direction | |
| 1 | 0.14 | 0.01 | 100 | 10 | 0.2 | 0.1 |
| 2 | 0.14 | 0.01 | 100 | 10 | 0.2 | 0.1 |
| 3 | 0.14 | 0.01 | 100 | 13 | 0.2 | 0.1 |
| 4 | 0.14 | 0.02 | 100 | 20 | 0.2 | 0.1 |
| 5 | 0.14 | 0.02 | 100 | 10 | 0.2 | 0.2 |
| 6 | 0.14 | 0.03 | 100 | 13 | 0.2 | 0.2 |
| 7 | 0.14 | 0.04 | 100 | 20 | 0.2 | 0.2 |
| 8 | 0.14 | 0.03 | 100 | 10 | 0.2 | 0.3 |
| 9 | 0.14 | 0.04 | 100 | 13 | 0.2 | 0.3 |
| 10 | 0.14 | 0.06 | 100 | 20 | 0.2 | 0.3 |
| 11 | 0.14 | 0.02 | 100 | 10 | 0.1 | 0.1 |
| 12 | 0.14 | 0.03 | 100 | 13 | 0.1 | 0.1 |
| 13 | 0.14 | 0.04 | 100 | 20 | 0.1 | 0.1 |
| 14 | 0.14 | 0.04 | 100 | 10 | 0.1 | 0.2 |
| 15 | 0.14 | 0.05 | 100 | 13 | 0.1 | 0.2 |
| 16 | 0.14 | 0.08 | 100 | 20 | 0.1 | 0.2 |
| 17 | 0.14 | 0.06 | 100 | 10 | 0.1 | 0.3 |
| 18 | 0.14 | 0.08 | 100 | 13 | 0.1 | 0.3 |
| 19 | 0.14 | 0.12 | 100 | 20 | 0.1 | 0.3 |
| 20 | 0.14 | 0.01 | 100 | 10 | 0.3 | 0.1 |
| 21 | 0.14 | 0.02 | 100 | 13 | 0.3 | 0.1 |
| 22 | 0.14 | 0.01 | 100 | 20 | 0.3 | 0.1 |
| 23 | 0.14 | 0.01 | 100 | 10 | 0.3 | 0.2 |
| 24 | 0.14 | 0.02 | 100 | 13 | 0.3 | 0.2 |
| 25 | 0.14 | 0.03 | 100 | 20 | 0.3 | 0.2 |
| 26 | 0.14 | 0.02 | 100 | 10 | 0.3 | 0.3 |
| 27 | 0.14 | 0.03 | 100 | 13 | 0.3 | 0.3 |
| 28 | 0.14 | 0.04 | 100 | 20 | 0.3 | 0.3 |
| Reverse Working Time/ms | Thickness/mm | Hardness/kgf·mm−2 | Roughness/μm |
|---|---|---|---|
| 10 | 0.2589 | 269 | 1.509 |
| 13 | 0.3374 | 285 | 1.791 |
| 20 | 0.1944 | 291 | 1.423 |
| Reverse Duty Cycle | Thickness/mm | Hardness/kgf·mm−2 | Roughness/μm |
|---|---|---|---|
| 0.1 | 0.3018 | 279 | 1.942 |
| 0.2 | 0.1944 | 291 | 1.423 |
| 0.3 | 0.118 | 275 | 0.85 |
| Forward Duty Cycle | Thickness/mm | Hardness/kgf·mm−2 | Roughness/μm |
|---|---|---|---|
| 0.1 | 0.1064 | 444 | 1.075 |
| 0.2 | 0.1565 | 297 | 1.865 |
| 0.3 | 0.0394 | 569 | 1.755 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Liu, M.; Zhu, Y.; Li, F. Influence of Double-Pulse Electrodeposition Parameters on the Performance of Nickel/Nanodiamond Composite Coatings. Coatings 2021, 11, 1068. https://doi.org/10.3390/coatings11091068
Wang D, Liu M, Zhu Y, Li F. Influence of Double-Pulse Electrodeposition Parameters on the Performance of Nickel/Nanodiamond Composite Coatings. Coatings. 2021; 11(9):1068. https://doi.org/10.3390/coatings11091068
Chicago/Turabian StyleWang, Dongai, Meihua Liu, Yuanmin Zhu, and Feihui Li. 2021. "Influence of Double-Pulse Electrodeposition Parameters on the Performance of Nickel/Nanodiamond Composite Coatings" Coatings 11, no. 9: 1068. https://doi.org/10.3390/coatings11091068
APA StyleWang, D., Liu, M., Zhu, Y., & Li, F. (2021). Influence of Double-Pulse Electrodeposition Parameters on the Performance of Nickel/Nanodiamond Composite Coatings. Coatings, 11(9), 1068. https://doi.org/10.3390/coatings11091068





