Theoretical Analysis of Si2H6 Adsorption on Hydrogenated Silicon Surfaces for Fast Deposition Using Intermediate Pressure SiH4 Capacitively Coupled Plasma
Abstract
:1. Introduction
2. Materials and Methods
2.1. DFT Calculations
2.2. Plasma Discharge Simulations
2.3. Experimental Details
3. Results
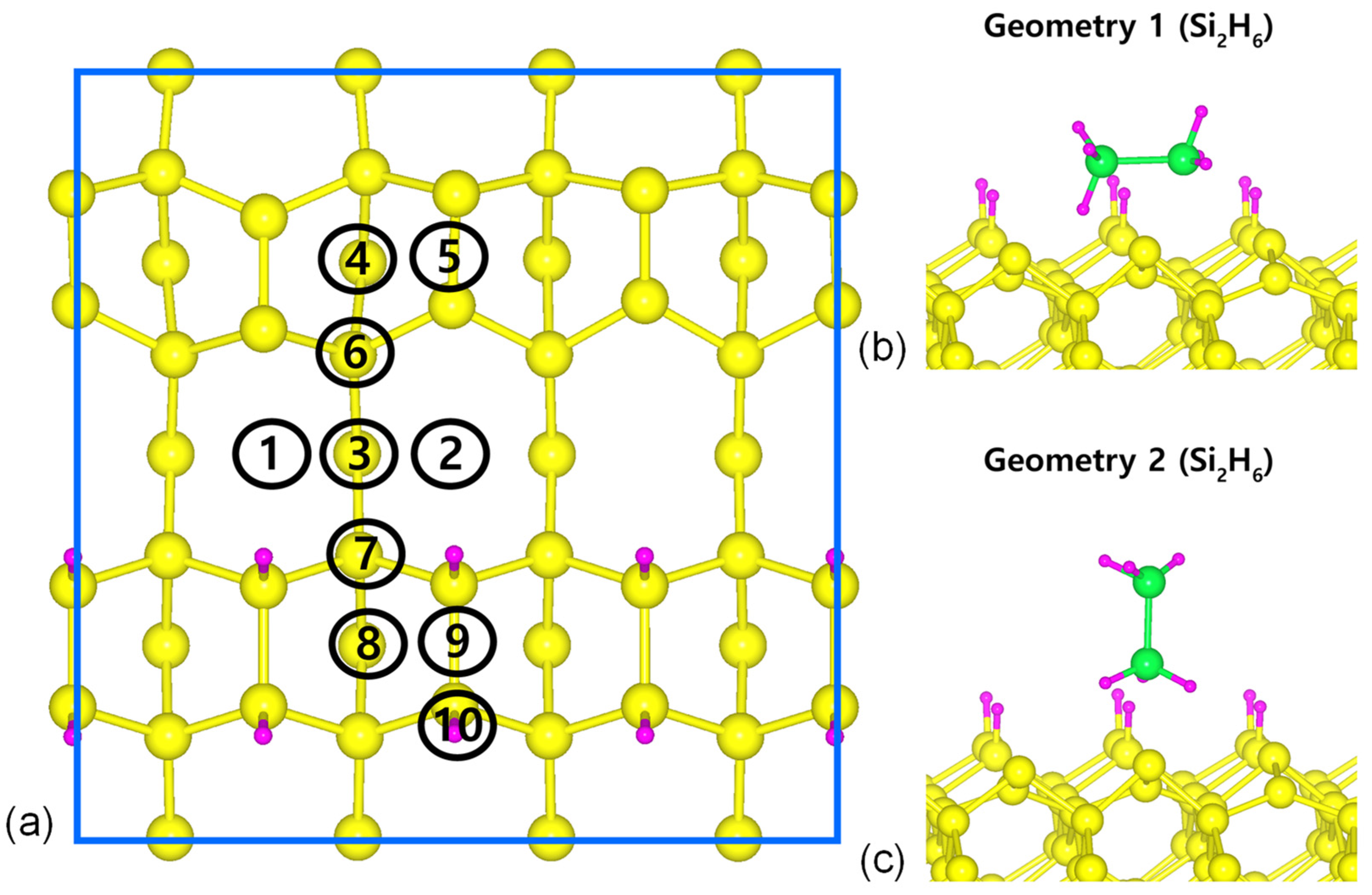
3.1. DFT Results: Si2H6 Adsorption
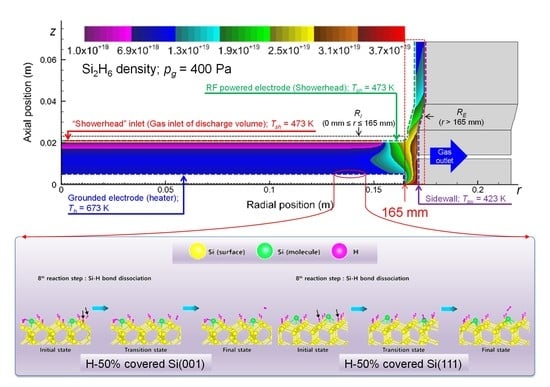
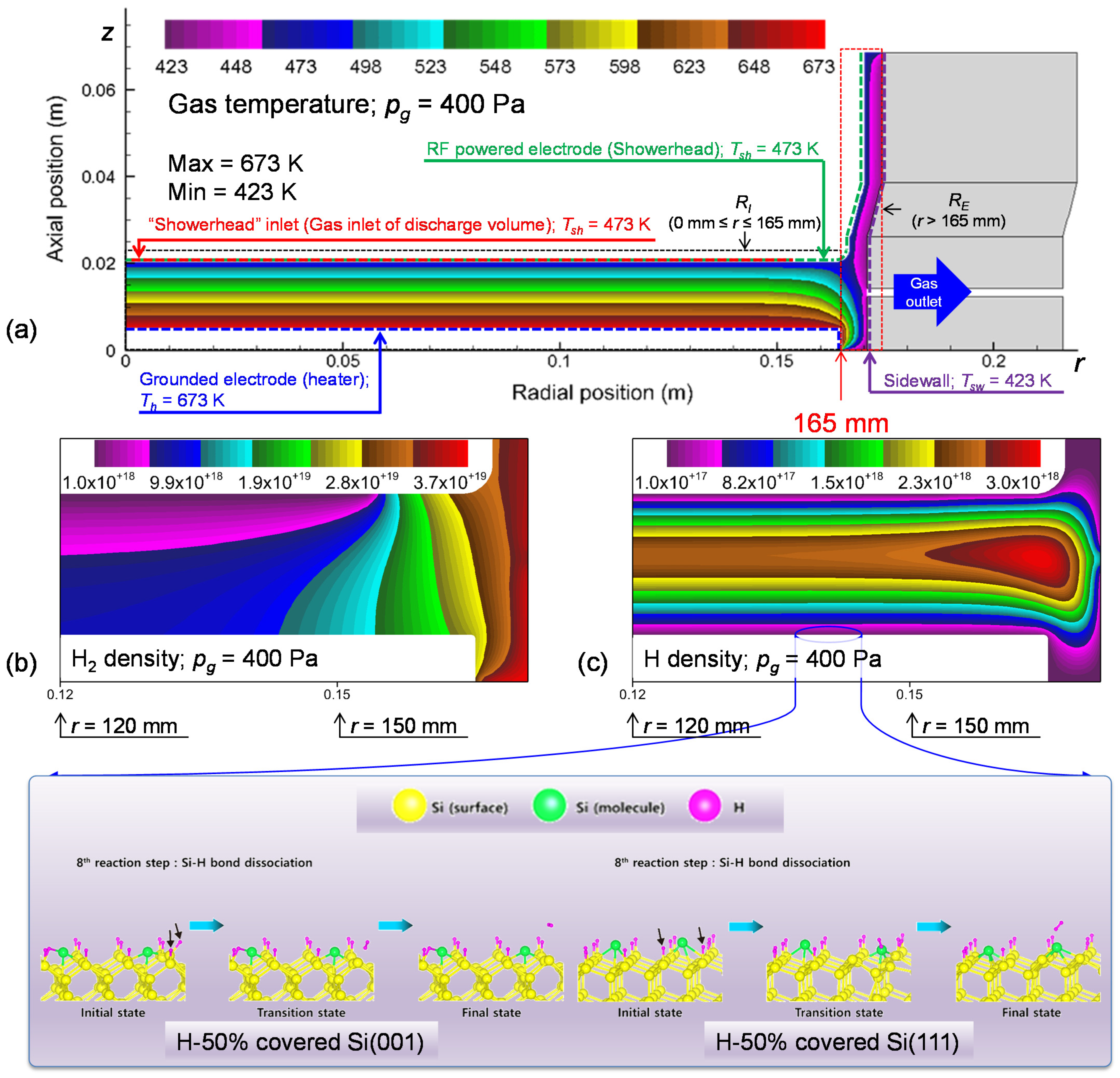
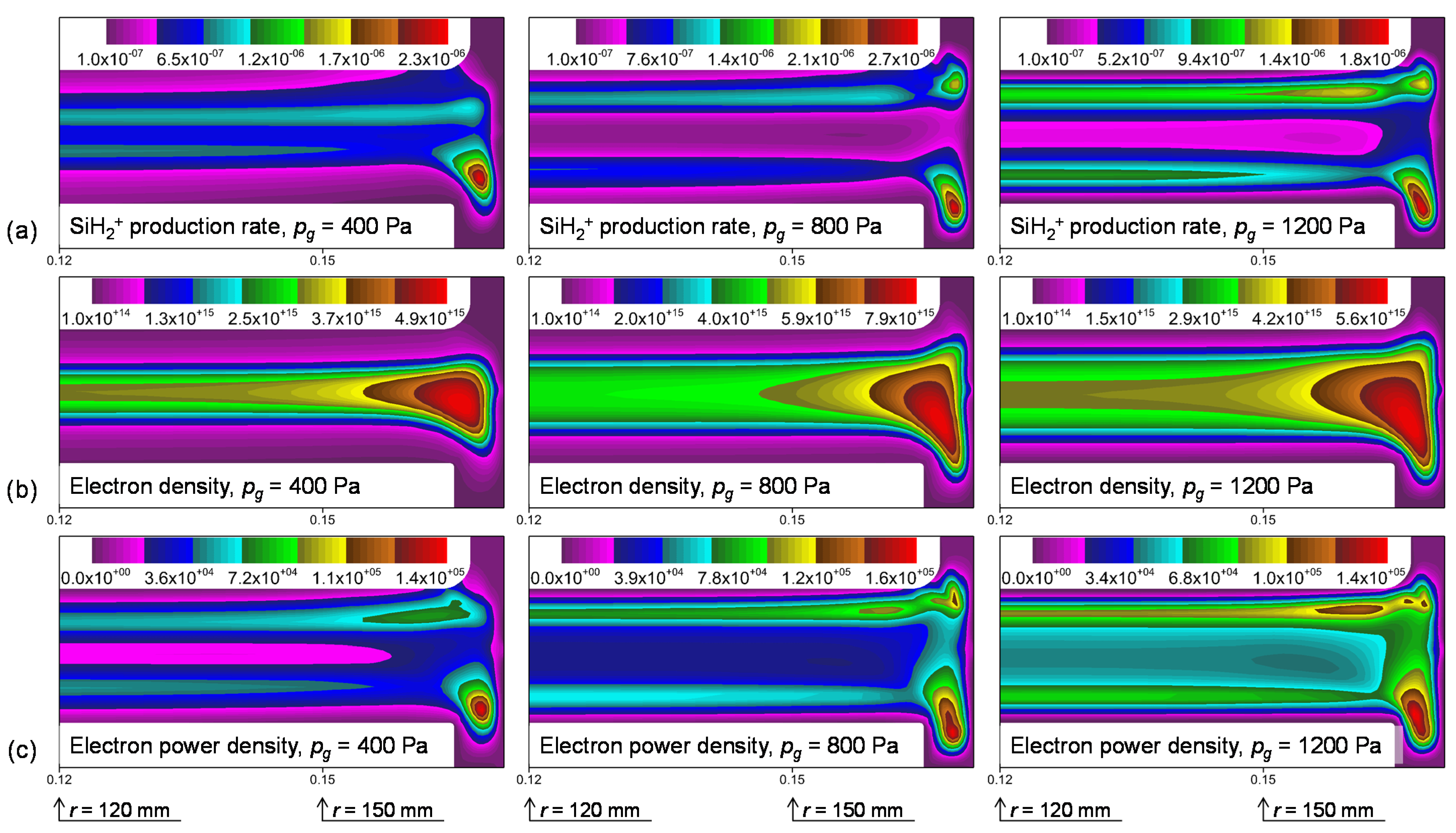
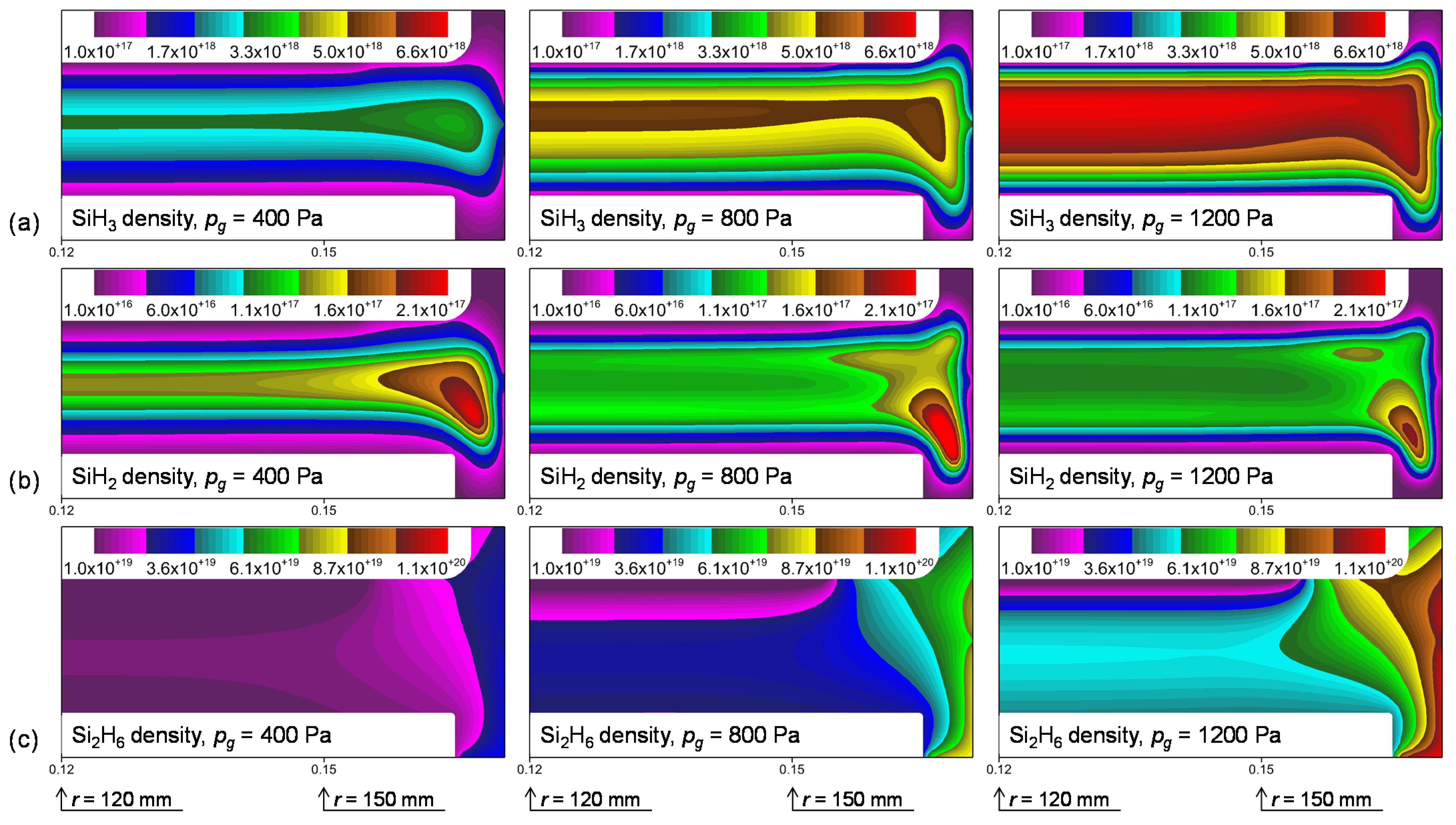
3.2. Plasma Modeling Results: SiH4/He Discharges
kr = 2.0 × 10−16 [1 − (1 + 0.0032 pg (Pa))−1] (m3·s−1)
kr = 1.5 × 10−16 (m3 s−1)
4. Discussion
4.1. Si2H6 Reactivity and Dependence on the Si Surface Orientation
4.2. Comparisons of Si2H6 Surface Reactivities
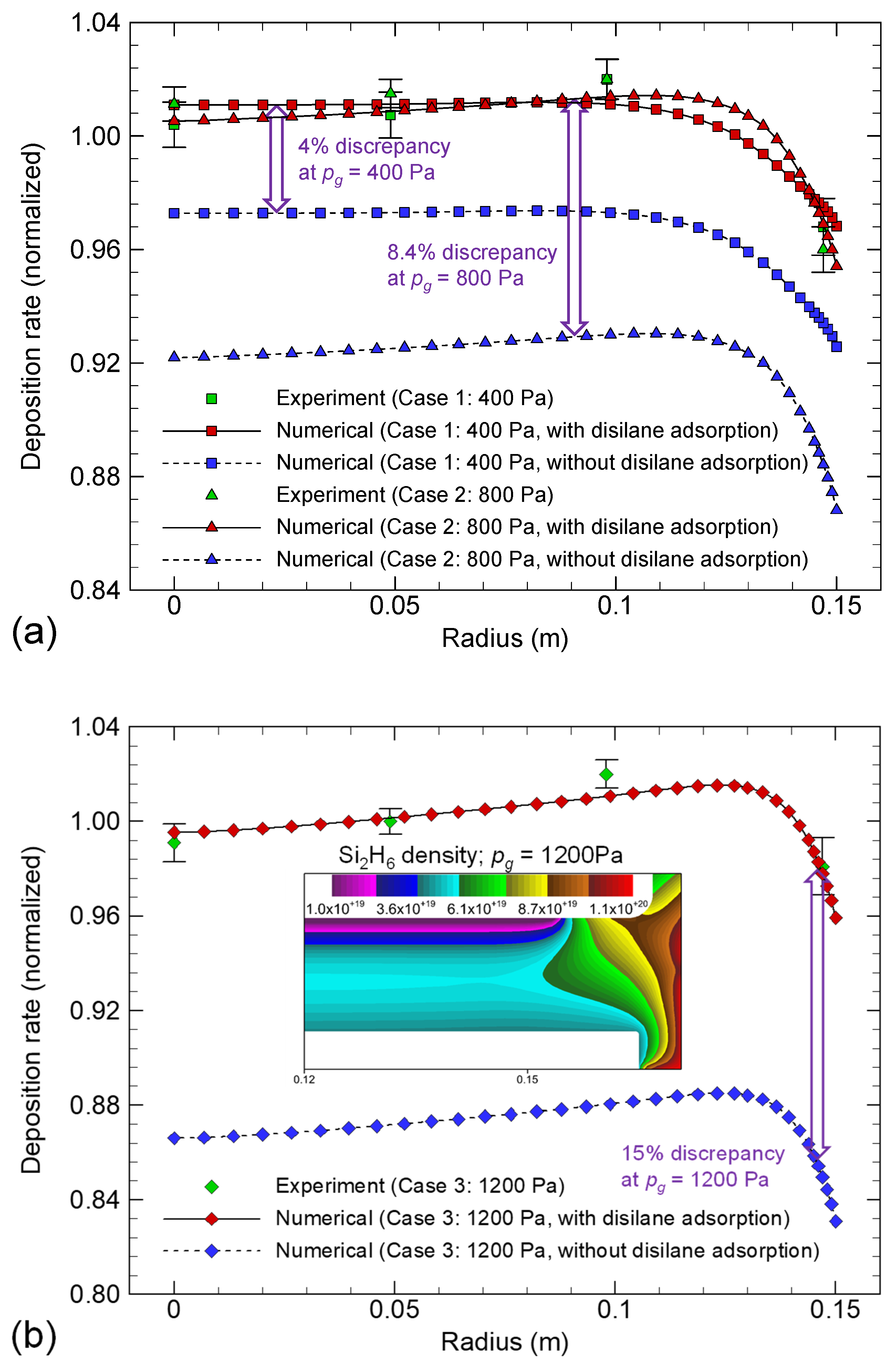
4.3. Deposition Rate Profiles of Si:H Films: Effects of Si2H6 Adsorption
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Adsorption Energy, Activation Energy and Reaction Energy
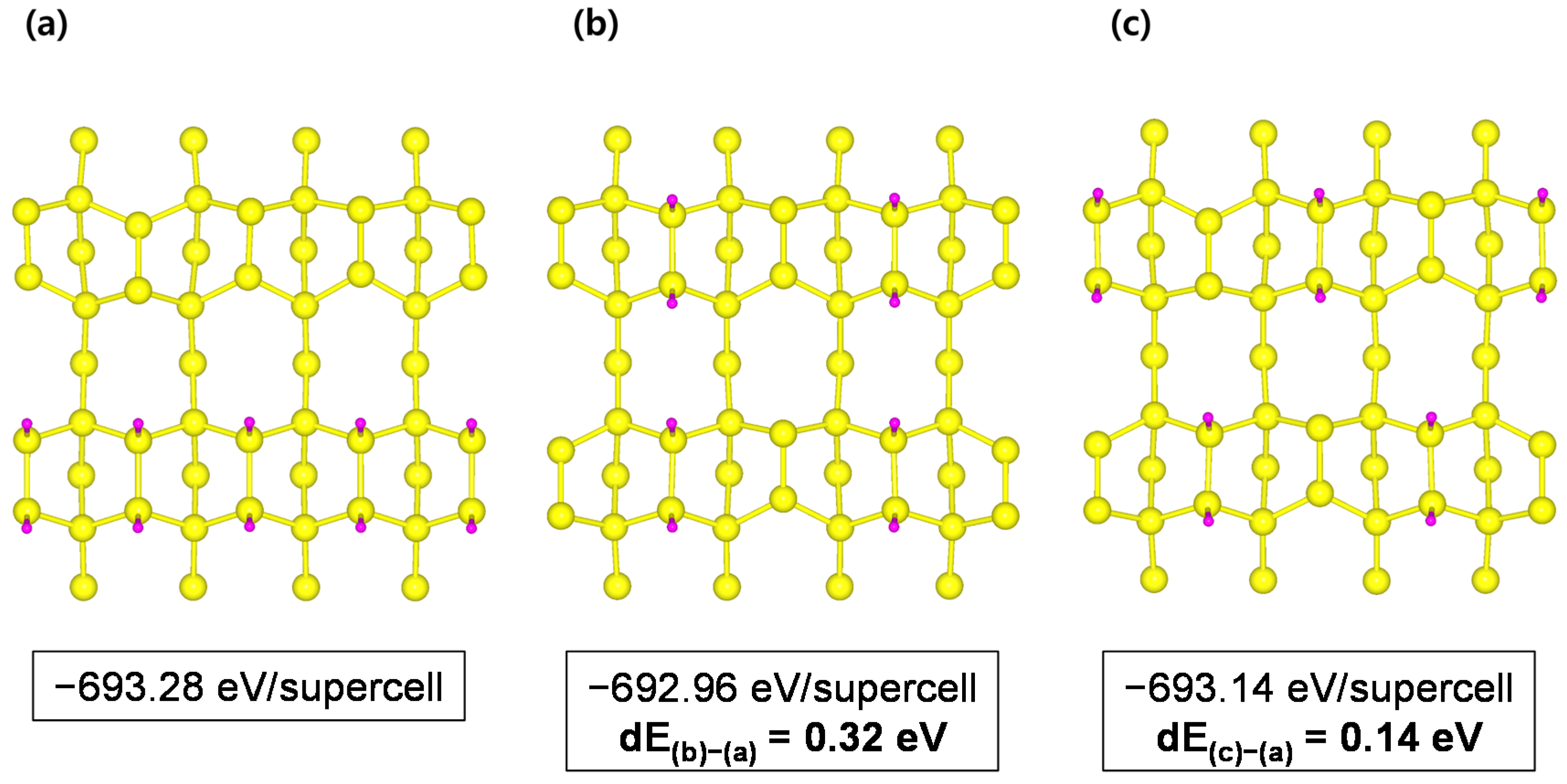
Appendix A.2. H-50% Covered Si(001) and H-50% Covered Si(111) Surfaces
References
- Maydan, D. The future of equipment development and semiconductor production. Mater. Sci. Eng. A 2001, 302, 1–5. [Google Scholar] [CrossRef]
- Yavas, O.; Richter, E.; Kluthe, C.; Sickmoeller, M. Wafer-edge yield engineering in leading-edge DRAM manufacturing. Semicond. Fabtech 2009, 39, 1–5. [Google Scholar]
- Kim, H.J. Numerical simulation of semiconductor fabrication system. In Proceedings of the International Conference on Microelectronics and Plasma Technology 2014, Gunsan Saemangeum Convention Center (GSCO), Gunsan, Korea, 8–11 July 2014. [Google Scholar]
- Smith, D.L. Thin-Film Deposition: Principles and Practice; McGraw-Hill: New York, NY, USA, 1995. [Google Scholar]
- Lieberman, M.A.; Lichtenberg, A.J. Principles of Plasma Discharges and Materials Processing; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Street, R.A. Technology and Applications of Amorphous Silicon; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2000. [Google Scholar]
- Nienhuis, G.J.; Goedheer, W.J.; Hamers, E.A.G.; Van Sark, W.G.J.H.M.; Bezemer, J. A self-consistent fluid model for radio-frequency discharges in SiH4-H2 compared to experiments. J. Appl. Phys. 1997, 82, 2060–2071. [Google Scholar] [CrossRef]
- Nienhuis, G.J.; Goedheer, W. Modelling of a large scale reactor for plasma deposition of silicon. Plasma Sources Sci. Technol. 1999, 8, 295–298. [Google Scholar] [CrossRef]
- Isomura, M.; Kondo, M.; Matsuda, A. Device-grade amorphous silicon prepared by high-pressure plasma. Jpn. J. Appl. Phys. 2002, 41, 1947. [Google Scholar] [CrossRef]
- Abdel-Fattah, E.; Sugai, H. Combined effects of gas pressure and exciting frequency on electron energy distribution functions in hydrogen capacitively coupled plasmas. Phys. Plasmas 2013, 20, 023501. [Google Scholar] [CrossRef]
- Rauscher, H. The interaction of silanes with silicon single crystal surfaces: Microscopic processes and structures. Surf. Sci. Rep. 2001, 42, 207–328. [Google Scholar] [CrossRef]
- Lin, D.-S.; Hirschorn, E.S.; Chiang, T.-C.; Tsu, R.; Lubben, D.; Greene, J.E. Scanning-tunneling-microscopy studies of disilane adsorption and pyrolytic growth on Si(100)-(2 × 1). Phys. Rev. B 1992, 45, 3494–3498. [Google Scholar] [CrossRef]
- Price, R.W.; Tok, E.S.; Zhang, J. Probing the silane, disilane and germane adsorption kinetics on the silicon (001) surface. J. Cryst. Growth 2000, 209, 306–310. [Google Scholar] [CrossRef]
- Park, H.; Yoon, E.; Lee, G.-D.; Kim, H.J. Analysis of surface adsorption kinetics of SiH4 and Si2H6 for deposition of a hydrogenated silicon thin film using intermediate pressure SiH4 plasmas. Appl. Surf. Sci. 2019, 496, 143728. [Google Scholar] [CrossRef]
- Lin, D.-S.; Miller, T.; Chiang, T.-C. Adsorption and thermal reactions of disilane and the growth of Si films on Ge(100)-(2 × 1). Phys. Rev. B 1993, 47, 6543–6554. [Google Scholar] [CrossRef]
- Xia, L.-Q.; Jones, M.E.; Maity, N.; Engstrom, J.R. Dissociation and pyrolysis of Si2H6 on Si surfaces: The influence of surface structure and adlayer composition. J. Chem. Phys. 1995, 103, 1691–1701. [Google Scholar] [CrossRef]
- Suemitsu, M.; Nakazawa, H.; Morita, T.; Miyamoto, N. Observation of hydrogen-coverage- and temperature-dependent adsorption kinetics of disilane on Si(100) during Si gas-source molecular beam epitaxy. Jpn. J. Appl. Phys. 1997, 36, L625–L628. [Google Scholar] [CrossRef]
- Niwano, M.; Shinohara, M.; Neo, Y.; Yokoo, K. Si2H6 adsorption and hydrogen desorption on Si(100) investigated by infrared spectroscopy. Appl. Surf. Sci. 2000, 162, 111–115. [Google Scholar] [CrossRef]
- Buss, R.J.; Ho, P.; Breiland, W.G.; Coltrin, M.E. Reactive sticking coefficients for silane and disilane on polycrystalline silicon. J. Appl. Phys. 1988, 63, 2808–2819. [Google Scholar] [CrossRef]
- Bramblett, T.R.; Lu, Q.; Karasawa, T.; Hasan, M.-A.; Jo, S.K.; Greene, J.E. Si(001)2 × 1 gas-source molecular-beam epitaxy from Si2H6: Growth kinetics and boron doping. J. Appl. Phys. 1994, 76, 1884–1888. [Google Scholar] [CrossRef]
- Hu, X.F.; Xu, Z.; Lim, D.; Downer, M.C.; Parkinson, P.S.; Gong, B.; Hess, G.; Ekerdt, J.G. In situ optical second-harmonic-generation monitoring of disilane adsorption and hydrogen desorption during epitaxial growth on Si(001). Appl. Phys. Lett. 1997, 71, 1376–1378. [Google Scholar] [CrossRef]
- Le, T.N.-M.; Raghunath, P.; Huynh, L.K.; Lin, M.C. A computational study on the adsorption configurations and reactions of SiHx(x = 1-4) on clean and H-covered Si(100) surfaces. Appl. Surf. Sci. 2016, 387, 546–556. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Kittel, C. Introduction to Solid State Physics, 8th ed.; Wiley: New York, NY, USA, 2004. [Google Scholar]
- Kim, H.J.; Lee, H.J. 2D fluid model analysis for the effect of 3D gas flow on a capacitively coupled plasma deposition reactor. Plasma Sources Sci. Technol. 2016, 25, 035006. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, H.J. Numerical analysis of the effect of electrode spacing on deposition rate profiles in a capacitively coupled plasma reactor. Plasma Sources Sci. Technol. 2016, 25, 065006. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, H.J. Analysis of intermediate pressure SiH4/He capacitively coupled plasma for deposition of an amorphous hydrogenated silicon film in consideration of thermal diffusion effects. Plasma Sources Sci. Technol. 2017, 26, 085003. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, H.J. Uniformity control of the deposition rate profile of a-Si:H film by gas velocity and temperature distributions in a capacitively coupled plasma reactor. J. Appl. Phys. 2018, 123, 113302. [Google Scholar] [CrossRef]
- Kim, H.J. Effect of electrode heating on the distribution of the ion production rate in a capacitively coupled plasma deposition reactor in consideration of thermal decomposition. Vacuum 2021, 189, 110264. [Google Scholar] [CrossRef]
- ESI Group. CFD-ACE+ 2020.0 Manual; ESI US R&D Inc.: Huntsville, AL, USA, 2020. [Google Scholar]
- Perrin, J.; Leroy, O.; Bordage, M.C. Cross-sections, rate constants and transport coefficients in silane plasma chemistry. Contrib. Plasma Phys. 1996, 36, 3–49. [Google Scholar] [CrossRef]
- Perrin, J.; Shiratani, M.; Kae-Nune, P.; Videlot, H.; Jolly, J.; Guillon, J. Surface reaction probabilities and kinetics of H, SiH3, Si2H5, CH3, and C2H5 during deposition of a-Si:H and a-C:H from H2, SiH4, and CH4 discharges. J. Vac. Sci. Technol. A. 1998, 16, 278. [Google Scholar] [CrossRef]
- Babaeva, N.Y.; Kushner, M.J. Penetration of plasma into the wafer-focus ring gap in capacitively coupled plasmas. J. Appl. Phys. 2007, 101, 113307. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.; Rauf, S.; Collins, K. Gas heating mechanisms in capacitively coupled plasmas. Plasma Sources Sci. Technol. 2012, 21, 055012. [Google Scholar] [CrossRef]
- Denpoh, K.; Moroz, P.; Kato, T.; Matsukuma, M. Multiscale plasma and feature profile simulations of plasma-enhanced chemical vapor deposition and atomic layer deposition processes for titanium thin film fabrication. Jpn. J. Appl. Phys. 2020, 59, SHHB02. [Google Scholar] [CrossRef]
- Bird, R.B.; Stewart, W.E.; Lightfoot, E.N. Transport Phenomena; John Wiley & Sons, Inc.: New York, NY, USA, 2007. [Google Scholar]
- Shi, J.; Tok, E.S.; Kang, H.C. The dissociative adsorption of silane and disilane on Si(100)-(2 × 1). J. Chem. Phys. 2007, 127, 164713. [Google Scholar] [CrossRef] [PubMed]
- Paulus, B.; Rościszewski, K.; Stoll, H.; Birkenheuer, U. Ab initio incremental correlation treatment with non-orthogonal localized orbitals. Phys. Chem. Chem. Phys. 2003, 5, 5523–5529. [Google Scholar] [CrossRef]
- Sobolewski, M.A. Power coupling and utilization efficiencies of silicon-depositing plasmas in mixtures of H2, SiH4, Si2H6, and Si3H8. J. Vac. Sci. Technol. A 2014, 32, 041307. [Google Scholar] [CrossRef]
- Leroy, O.; Gousset, G.; Alves, L.L.; Perrin, J.; Jolly, J. Two-dimensional modelling of SiH4-H2 radio-frequency discharges for a-Si:H deposition. Plasma Sources Sci. Technol. 1998, 7, 348. [Google Scholar] [CrossRef]


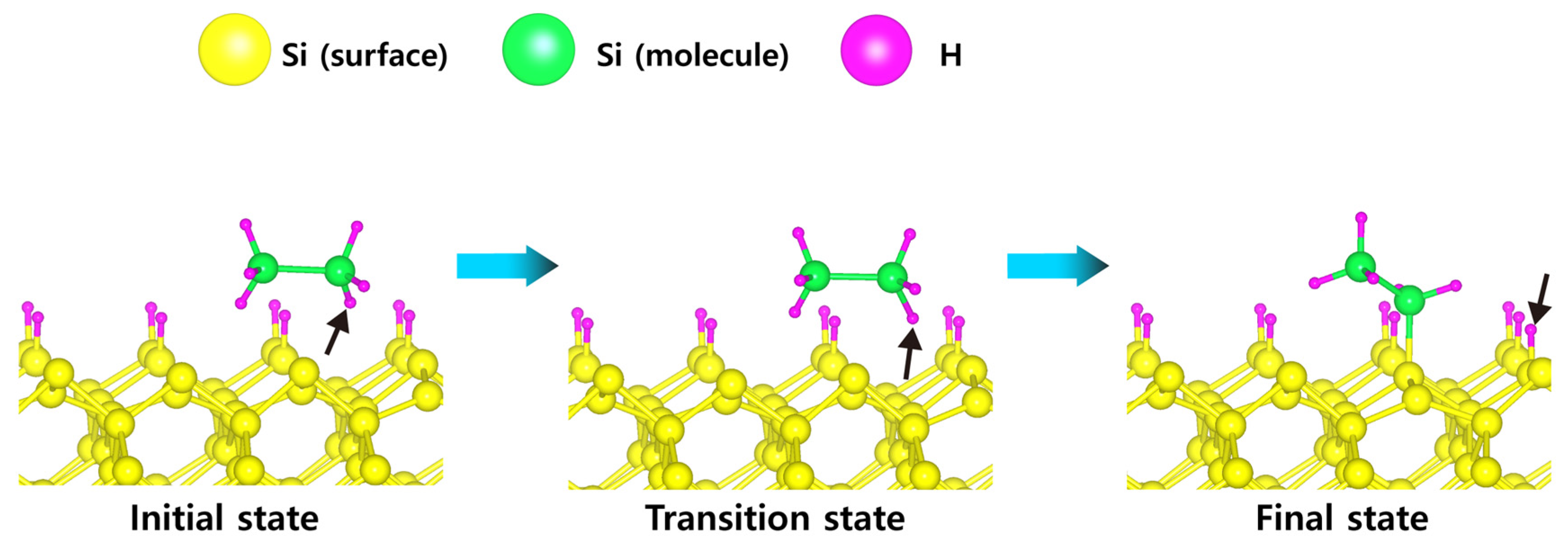
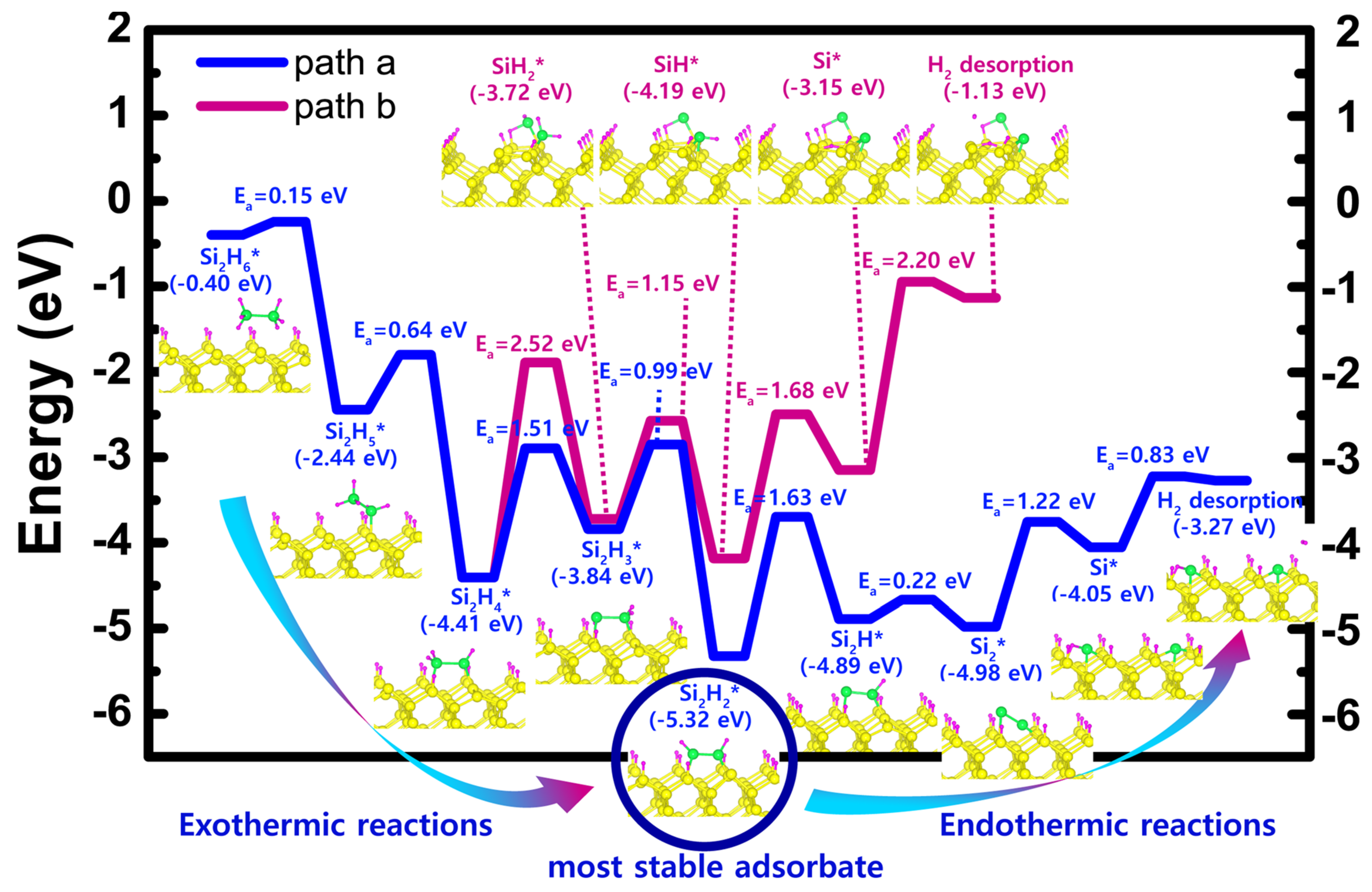
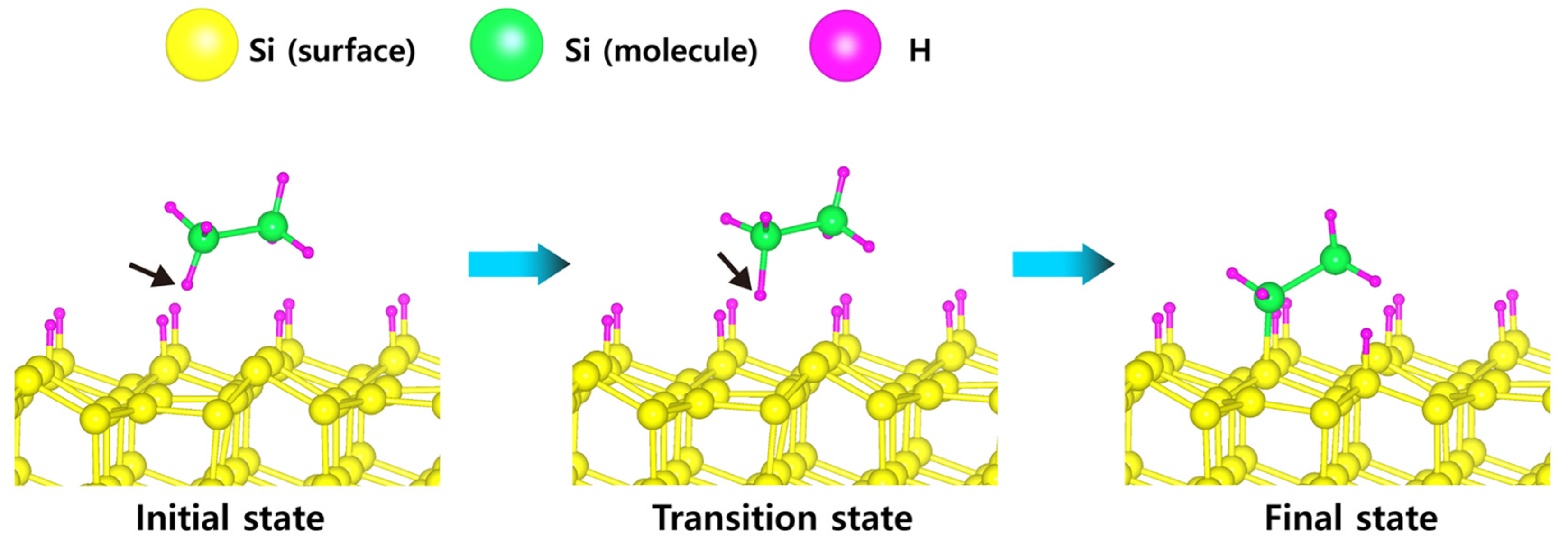
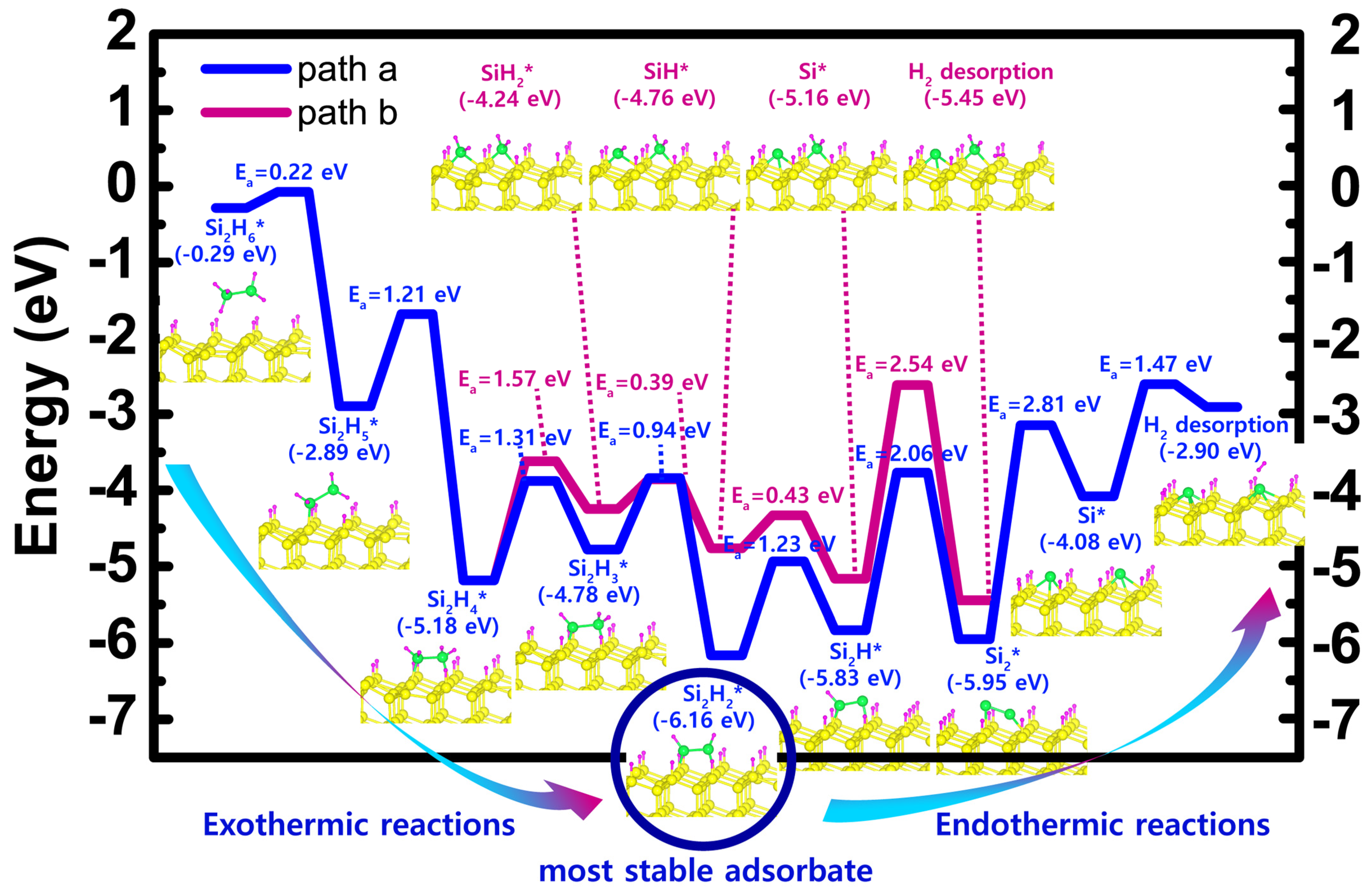
| Scheme | Einitial adsorbate | Emost stable adsorbate | Erxn | Ea, min | Ea, max | Path |
|---|---|---|---|---|---|---|
| H-50% covered Si(001) | −0.40 (Si2H6) | −5.32 (Si2H2) | −4.92 | 0.15 | 1.51 | a |
| H-50% covered Si(111) | −0.22 (Si2H6) | −6.16 (Si2H2) | −5.87 | 0.22 | 1.31 | a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.; Kim, H.J. Theoretical Analysis of Si2H6 Adsorption on Hydrogenated Silicon Surfaces for Fast Deposition Using Intermediate Pressure SiH4 Capacitively Coupled Plasma. Coatings 2021, 11, 1041. https://doi.org/10.3390/coatings11091041
Park H, Kim HJ. Theoretical Analysis of Si2H6 Adsorption on Hydrogenated Silicon Surfaces for Fast Deposition Using Intermediate Pressure SiH4 Capacitively Coupled Plasma. Coatings. 2021; 11(9):1041. https://doi.org/10.3390/coatings11091041
Chicago/Turabian StylePark, Hwanyeol, and Ho Jun Kim. 2021. "Theoretical Analysis of Si2H6 Adsorption on Hydrogenated Silicon Surfaces for Fast Deposition Using Intermediate Pressure SiH4 Capacitively Coupled Plasma" Coatings 11, no. 9: 1041. https://doi.org/10.3390/coatings11091041
APA StylePark, H., & Kim, H. J. (2021). Theoretical Analysis of Si2H6 Adsorption on Hydrogenated Silicon Surfaces for Fast Deposition Using Intermediate Pressure SiH4 Capacitively Coupled Plasma. Coatings, 11(9), 1041. https://doi.org/10.3390/coatings11091041