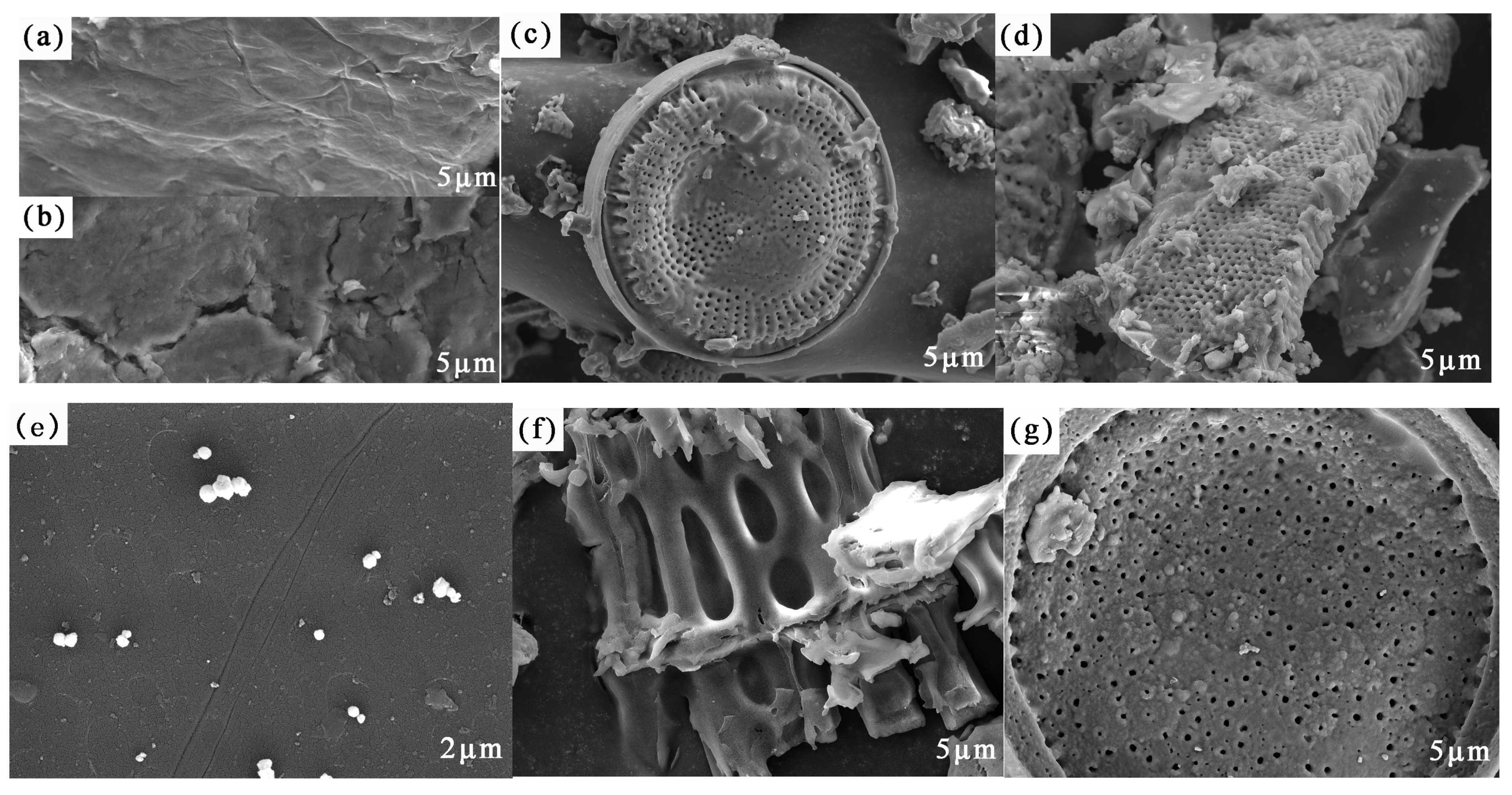
3.1. Material Characterization
As shown in
Figure 1, the surface of GO (a) prepared by the improved Hummers’ method was smooth, with an accumulated flaky structure and numerous wrinkles. Compared with the GO, the surface of GOH (b) was relatively rough, with numerous irregular wrinkles and cracks, which were possibly due to the activation of the epoxide and ester groups, and the conversion of hydroxyl groups (−OH) into carboxyl groups (–COOH) [
18,
19]. As observed in the SEM image (c) of the pretreated DE, DE was disc-shaped with a smooth surface and regular porosity [
20]. Many impurities were observed on the diatomite surface. Acidification pre-treatment was used to remove impurities and to better prepare adsorbents for integrating with the composite materials. Thus, the internal structure of CS (d) was solid and filamentous, with a relatively rough surface and porous structure. The SEM image of MCS (e) showed that the chitosan was grainy after magnetic modification, with agglomerated white round particles, and the diameter of the magnetic particles was about 30 nm [
21]. In the SEM image (f) of GOH/DCS, the interior of the modified adsorption material still showed the appearance of regular and large pores, thus increasing the specific surface area of the sheet structure. The increase in pore structure and the specific surface area provided a greater possibility for the adsorption of the rare earth La elements [
22]. Furthermore, GOH/DMCS (g) had a fold on the porous disk structure, which differed from those on the DE surface, which was covered with a thick layer of folds, increasing the dispersity. This was attributed to the successful compounding of the carboxylated graphene oxide and modified materials. In addition, the apparent surface roughness of the intact DE structure, and the irregular adhesion of magnetic chitosan particles on the surface of the modified materials, confirmed the successful preparation of the magnetic chitosan/diatomite-modified carboxylated graphene oxide.
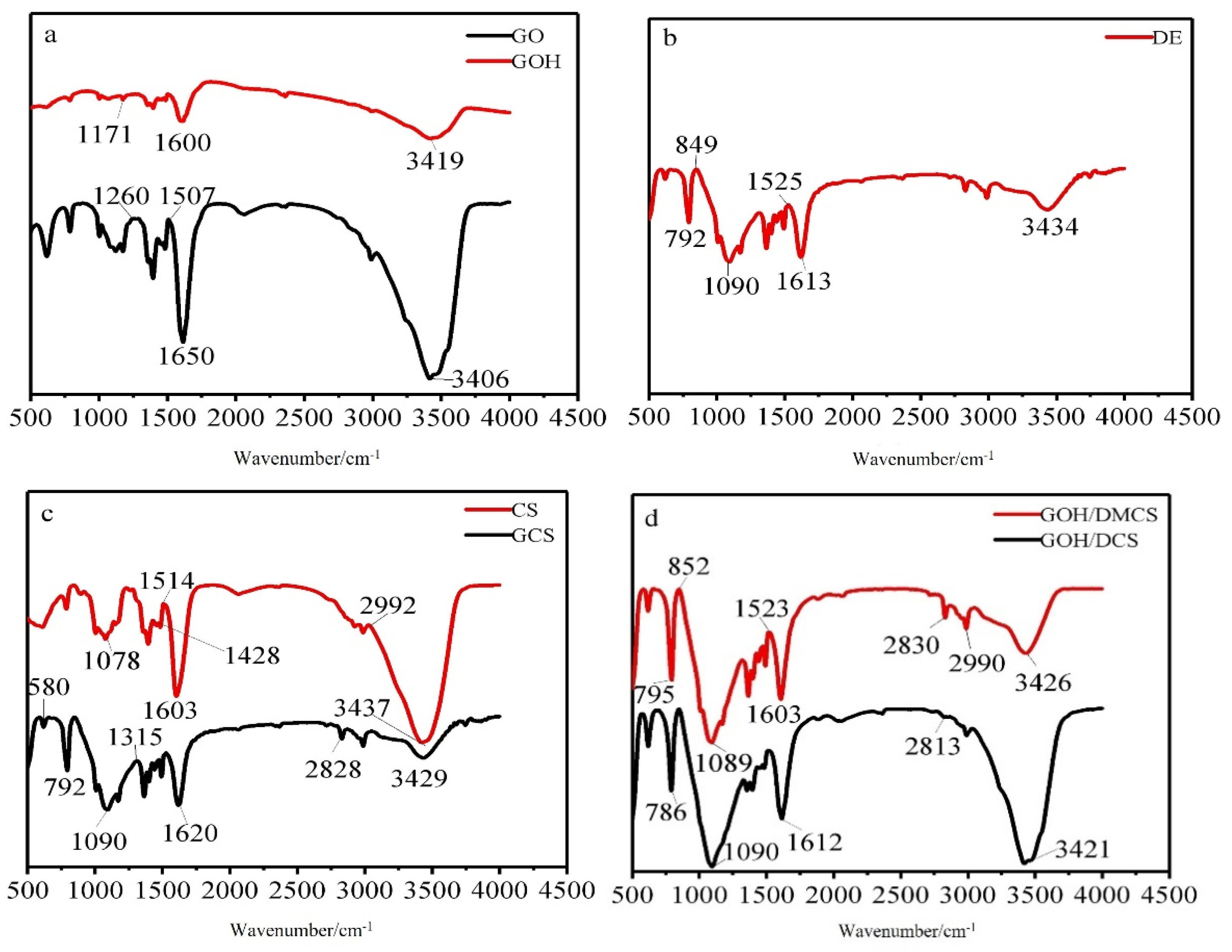
The infrared spectrograms of GO, GOH, DE, CS, GCS, GOH/DCS, and GOH/DMCS are shown in
Figure 2. As shown in the FTIR spectrogram for GO in
Figure 2a, the prominent peak at 3406 cm
−1 corresponds to the asymmetric stretching vibration peak of −OH, while the vibration at 1650 cm
−1 was the stretching vibration peak of C=O in the carboxyl group of GO, and C=O was grafted on the edge of GO. The peak at 1507 cm
−1 reflected the vibration absorption of the C=C skeleton and the characteristic bending band of the O-H bond in the undamaged aromatic region. The C−O stretching vibration band and C−O−C vibration absorption band appeared at 1260 cm
−1 [
23]. Many oxygen-containing functional groups appeared in the interlayer and edge of GO, and the above conclusions indicated that GO was successfully prepared by the modified Hummers’ method. For GOH, the FTIR spectrum shows characteristic bands for GO, and a characteristic peak at 1171 cm
−1 was attributed to the antisymmetric tensile vibration of C−O−C. Compared with GO, the characteristic bending of the O−H bond and the stretching vibration of C−O for the carboxyl group also appeared at 1600 cm
−1. The prominent stretching vibration of −OH in GO shifted from 3406 cm
−1, and a less obvious peak appeared at 3419 cm
−1 in the FTIR spectrum of GOH, indicating that the carboxyl groups in the alkaline environment replaced some hydroxyl groups in GO. Thus, the FTIR results confirmed the successful preparation of GOH. In the FTIR spectrum of DE
Figure 2b, the peak at 3434 cm
−1 was attributed to the stretching vibration of the free silanol group (Si−OH). In addition, the bands at 1613 cm
−1 and 1525 cm
−1 were related to the bending vibrations of water molecules (H−O−H) [
24]. The band at 1090 cm
−1 reflected the stretching of the siloxane (Si−O−Si) group, while 849 and 792 cm
−1 were associated with Si−O tension and SiO−H vibrations. The peak at 572 cm
−1 reflected the stretching vibration of Si−O−Si [
25,
26]. In the FTIR spectrum of CS (c), there were apparent −OH asymmetric stretching vibrations at 3437 cm
−1 and N-H stretching vibrations for the absorption peak on −NHCO−. A C−H stretching vibration appeared at 2992 cm
−1, and at 1603 cm
−1, the stretching vibration of the alcohol hydroxyl group C=O from -NHCO− occurred. The peak at 1514 cm
−1 was related to the N-H stretching vibration of −NH
2, and a C−H absorption peak appeared at 1428 cm
−1. The absorption peak at 1078 cm
−1 was attributed to the stretching vibration of C−O. [
27] The FTIR spectrum of MCS showed that the characteristic peak at 580 cm
−1 was Fe
3O
4, which indicated that the magnetic particles were successfully loaded. The hydroxyl peak intensity at 3437 cm
−1 on CS weakened and moved to the left to 3429 cm
−1, which was possibly caused by the interactions between nano-ferric oxide particles and chitosan functional groups during magnetization.
In the FTIR spectrum of GOH/DCS (d), the characteristic peaks of DE and CS appeared beside the characteristic peaks of the carboxyl group. The appearance of NH stretching and the characteristic peak of the amide group at 1612 cm
−1 indicated that chitosan was successfully grafted onto the GOH molecule [
28]. The typical band of the chitosan polymer (OH) appeared at 3410 cm
−1. In addition, 2813 cm
−1 corresponded to the C-H tensile absorption band. The modified infrared band was wider and stronger, which indicated that GOH/DCS carried more chitosan. The band at 1090 cm
−1 reflected the stretching of the siloxane (Si−O−Si) group in DE. Thus, an SiO−H vibration peak appeared at 786 cm
−1.
In summary, the GOH/DCS materials were successfully modified and prepared. In the FTIR spectrum of GOH/DMCS, a shift in the OH stretching vibration peak of the carboxyl group appeared at 3421 cm−1, which indicated that hydrogen bonding occurred between the hydroxyl groups in each material. The other peaks at 786, 1090, and 1612 cm−1 corresponded to the FTIR spectra of DE. Thus, the modification of the carboxylated graphene oxide/diatomite composite did not change the functional group structure of DE, and GOH covered the surface of DE through covalent bonding. Additionally, the carboxyl groups in the carboxylated graphene oxide and original graphene oxide were successfully compounded with GCS through covalent bonding. The electron micrographs also showed that the composite modified by GOH/DMCS retained the DE structure, which was consistent with the obtained FTIR results. Therefore, the excellent properties of DE, such as numerous pores, large specific surface area, and strong adsorption capacity, were retained when the composite was successfully prepared.
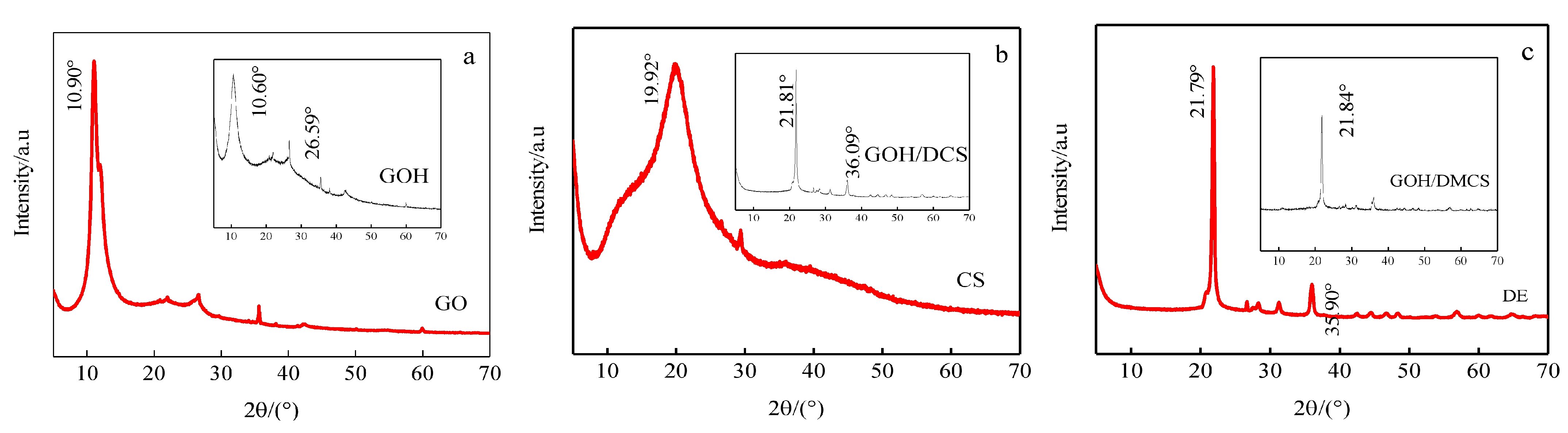
The crystal structure changes in each sample were analyzed by XRD, and the XRD analysis results of GO, GOH, CS, GOH/DCS, DE, and GOH/DMCS are shown in
Figure 3. The diffraction peak of the (d001) crystal plane appeared in the GO (a), which was prepared by the improved Hummers’ method at 2θ = 10.9, according to Bragg formula (2dsinθ = nλ), with an interlayer spacing of 0.81 nm. In the XRD results for GOH, the diffraction peak appeared at 2θ = 10.6, and the interlayer spacing was 0.83 nm, which indicated that the diffraction peak of the same crystal plane shifted to a low angle. The calculated results showed that the introduction of chloroacetic acid increased the interlayer spacing of GOH, and a dense layered graphene oxide structure was layered into the disordered sheets of GOH. In addition, carboxylation treatment increased the number of -COOH groups at the edge and interlayers of GO. In the XRD results of CS, (b) the characteristic peak of the amorphous chitosan structure appeared at 2θ = 19.92, and the diffraction peak of the GOH/DCS composite decreased to 2θ = 21.81, indicating that the CS crystal phase content declined. Then, the diffraction peak of GOH appeared again, and the diatomite diffraction peak appeared at 2θ = 36.09. The analysis results showed that GOH was related to DE and CS. In DE(c) and GOH/DMCS, the diffraction peaks were very similar, indicating that silicon dioxide accounted for a large proportion of DE. The diffraction peak of magnetic ferric oxide simultaneously appeared, verifying the successful preparation of the magnetic graphene oxide-based composites.
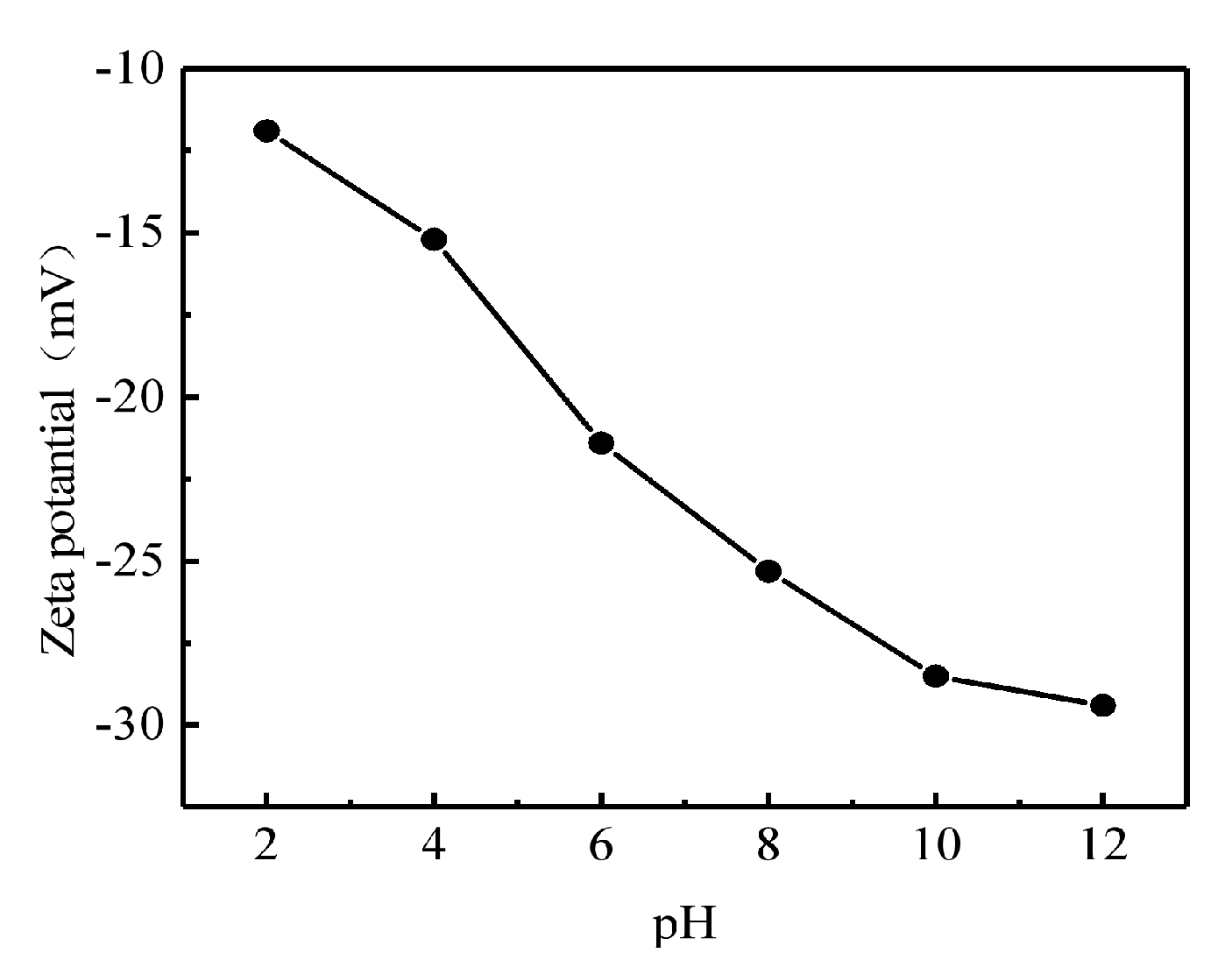
In this work, the zeta potential of GOH/DMCS in the water phase was measured using a Zetasizer Nano S90 potential analyzer from the Malvern company, in a 2–12 pH environment. The zeta potential results of GOH/DMCS are shown in
Figure 4. The figure shows that the electronegativity of the GOH/DMCS surface gradually increased from acidic to alkaline with changes in pH, from −11.9 mV at pH = 2 to −30.4 mV at pH = 12. In addition, compared to a potential value of −9.5 mV when the pH of graphene oxide was 2, the electronegativity of the GOH/DMCS surface was enhanced by the oxygen-containing functional groups, which were ionized as they were exposed to electricity in the aqueous solution.
As shown from the negative zeta potential results under acid and alkaline conditions, the entire surface of the material became negatively charged, which was beneficial for the adsorption of positively charged rare earth La3+ elements. The negative charge density on the GOH/DMCS surface also increased with increased pH, and the sol stability of the composite material also increased. This did not cause aggregation when in solution form.
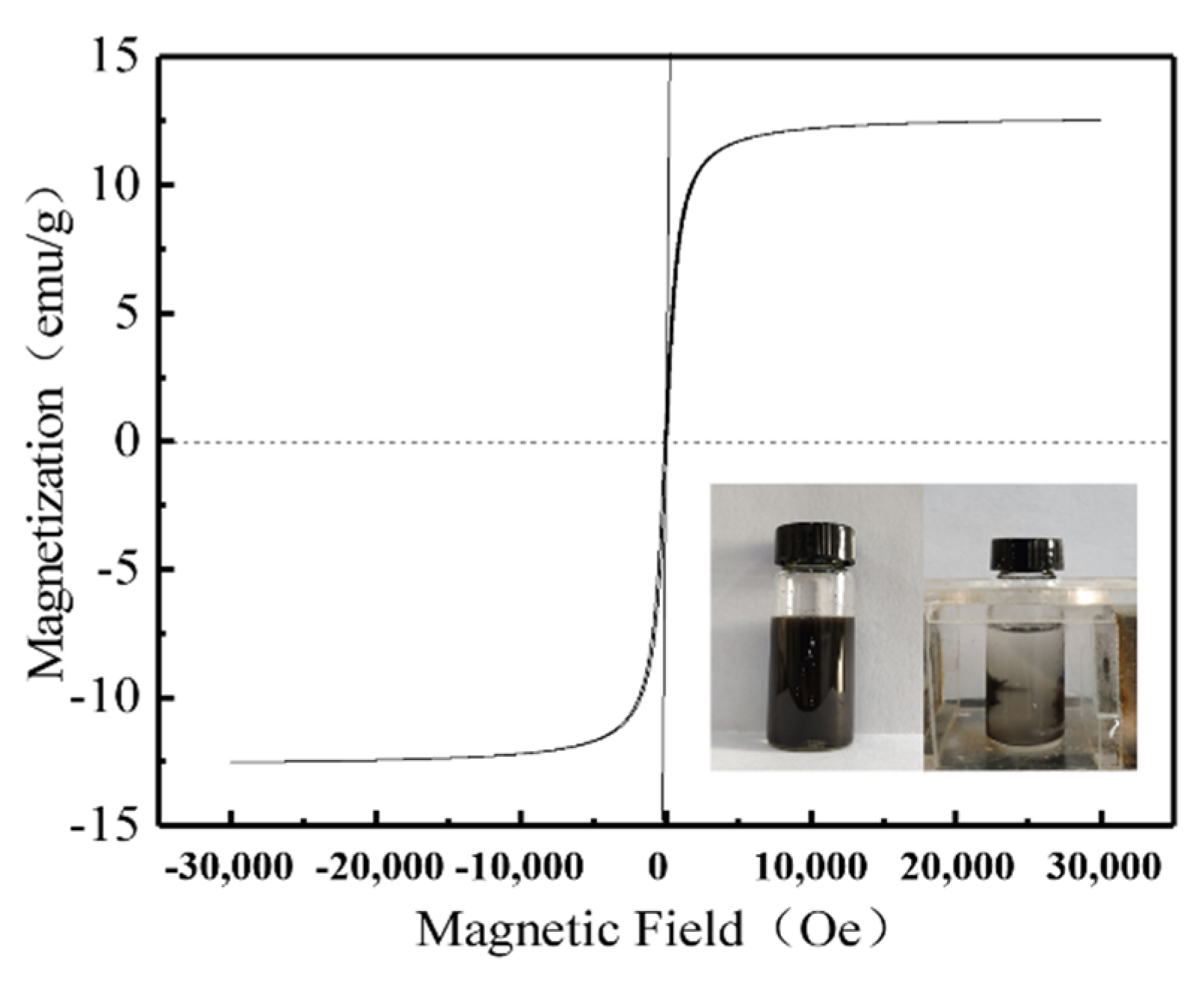
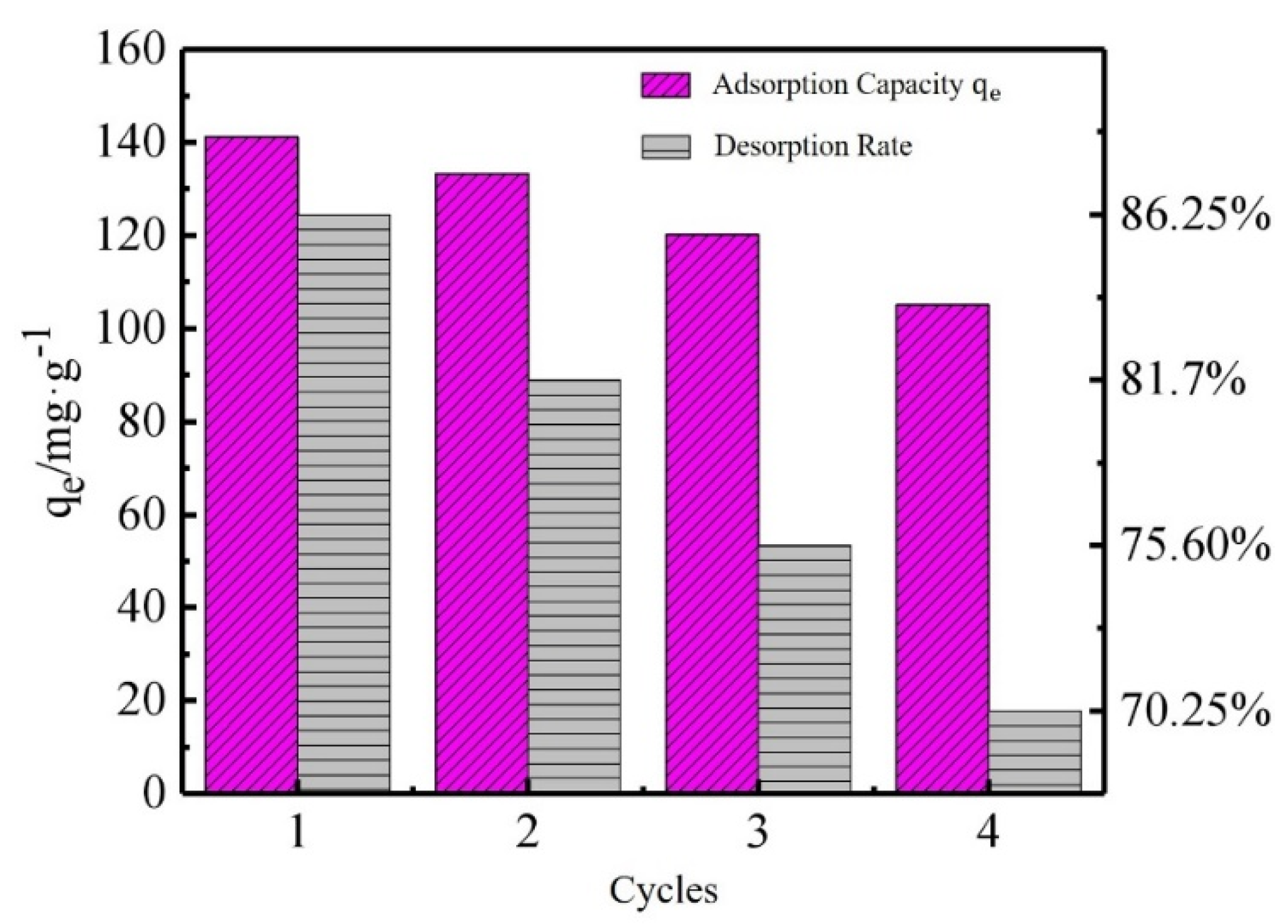
As observed in the hysteresis curve (
Figure 5), the hysteresis loop of the GOH/DMCS composite was S-shaped with an asymmetrical origin, and there was no obvious hysteresis loop. The maximum magnetization of the GOH/DMCS composite was 13.1 emu/g, which was lower than the magnetization of Fe
3O
4 by 52.70 emu/g [
29]. This was possibly due to the combination of GOH, DE, and CS non-magnetic materials, and the nanostructure of Fe
3O
4 during the preparation of the modified material, which weakened the magnetization of the composite material. However, the experimental diagram also showed that the ultrasonically dispersed uniform GOH/DMCS composite water solvent could be effectively separated in the aqueous solution in less than one minute, under an applied magnetic field. Thus, the above characterization results indicated that the GOH/DMCS composite was successfully prepared and could be used as an adsorbent that can be quickly separated and recovered.