Role of Stem Cells in Augmenting Dental Implant Osseointegration: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Data Extraction
2.5. Quality Assessment and Data Synthesis
2.6. Risk of Bias Assessment
3. Results
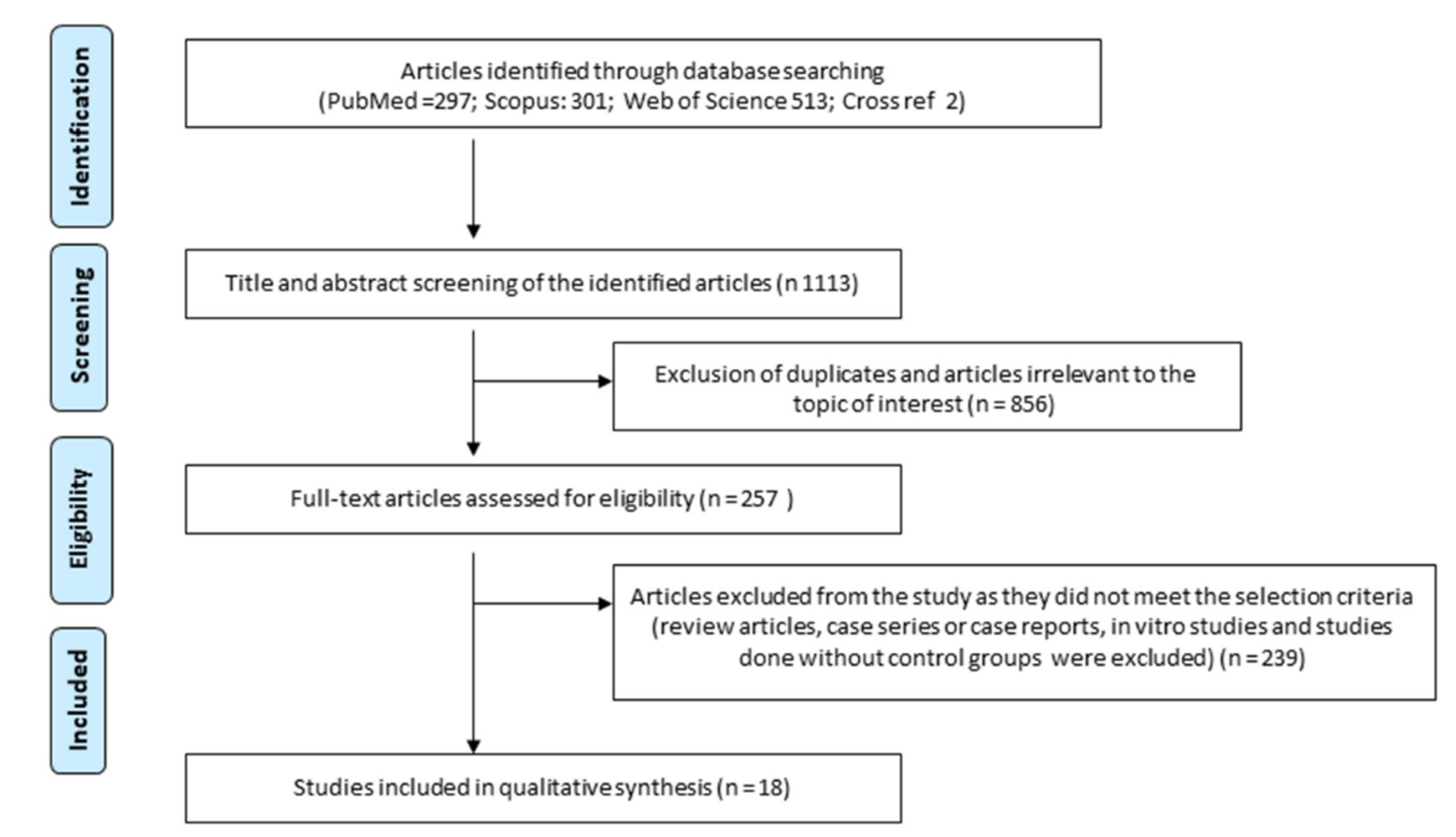
3.1. Selection of Articles
3.2. Characteristics of the Included Studies
3.3. Risk of Bias
3.4. Qualitative Analysis of the Effect of Stem Cells on Osseointegration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Parnia, F.; Yazdani, J.; Maleki Dizaj, S. Applications of Mesenchymal Stem Cells in Sinus Lift Augmentation as a Dental Implant Technology. Stem Cells Int. 2018, 2018, 3080139. [Google Scholar] [CrossRef] [PubMed]
- Setzer, F.C.; Kim, S. Comparison of long-term survival of implants and endodontically treated teeth. J. Dent. Res. 2014, 93, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Levin, L. Dealing with dental implant failures. J. Appl. Oral Sci. 2008, 16, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Ahmed, H.B.; Crespi, R.; Romanos, G.E. Role of primary stability for successful osseointegration of dental implants: Factors of influence and evaluation. Interv. Med. Appl. Sci. 2013, 5, 162–167. [Google Scholar] [CrossRef]
- Mittal, Y.; Jindal, G.; Garg, S. Bone manipulation procedures in dental implants. Indian J. Dent. 2016, 7, 86–94. [Google Scholar] [CrossRef]
- Chen, Z.; Bachhuka, A.; Wei, F.; Wang, X.; Liu, G.; Vasilev, K.; Xiao, Y. Nanotopography-based strategy for the precise manipulation of osteoimmunomodulation in bone regeneration. Nanoscale 2017, 9, 18129–18152. [Google Scholar] [CrossRef]
- Liu, W.; Li, J.; Cheng, M.; Wang, Q.; Yeung, K.W.K.; Chu, P.K.; Zhang, X. Zinc-Modified Sulfonated Polyetheretherketone Surface with Immunomodulatory Function for Guiding Cell Fate and Bone Regeneration. Adv. Sci. 2018, 5, 1800749. [Google Scholar] [CrossRef]
- Chen, L.; Wang, D.; Qiu, J.; Zhang, X.; Liu, X.; Qiao, Y.; Liu, X. Synergistic effects of immunoregulation and osteoinduction of ds-block elements on titanium surface. Bioact. Mater. 2021, 6, 191–207. [Google Scholar] [CrossRef]
- Chen, Z.; Bachhuka, A.; Han, S.; Wei, F.; Lu, S.; Visalakshan, R.M.; Vasilev, K.; Xiao, Y. Tuning Chemistry and Topography of Nanoengineered Surfaces to Manipulate Immune Response for Bone Regeneration Applications. ACS Nano 2017, 11, 4494–4506. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, A.; Ioannis, K.; Winter, K.; Schramm, A.; Wilde, F. Clinical results of autologous bone augmentation harvested from the mandibular ramus prior to implant placement. An analysis of 104 cases. GMS Interdiscip. Plast. Reconstr. Surg. DGPW 2016, 5, Doc21. [Google Scholar] [CrossRef]
- Demetriades, N.; Park, J.I.; Laskarides, C. Alternative bone expansion technique for implant placement in atrophic edentulous maxilla and mandible. J. Oral Implantol. 2011, 37, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Pistilli, R.; Felice, P.; Piatelli, M.; Nisii, A.; Barausse, C.; Esposito, M. Blocks of autogenous bone versus xenografts for the rehabilitation of atrophic jaws with dental implants: Preliminary data from a pilot randomised controlled trial. Eur. J. Oral Implantol. 2014, 7, 153–171. [Google Scholar] [PubMed]
- He, S.; Nakada, D.; Morrison, S.J. Mechanisms of stem cell self-renewal. Annu. Rev. Cell. Dev. Biol. 2009, 25, 377–406. [Google Scholar] [CrossRef] [PubMed]
- Woodbury, D.; Schwarz, E.J.; Prockop, D.J.; Black, I.B. Adult rat and human bone marrow stromal cells differentiate into neurons. J. Neurosci. Res. 2000, 61, 364–370. [Google Scholar] [CrossRef]
- Lagasse, E.; Connors, H.; Al-Dhalimy, M.; Reitsma, M.; Dohse, M.; Osborne, L.; Wang, X.; Finegold, M.; Weissman, I.L.; Grompe, M. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat. Med. 2000, 6, 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.P.; Nawata, M.; Wakitani, S.; Kametani, K.; Ota, M.; Toda, A.; Konishi, I.; Ebara, S.; Nikaido, T. Human amniotic mesenchymal cells differentiate into chondrocytes. Cloning Stem Cells 2009, 11, 19–26. [Google Scholar] [CrossRef]
- Garg, P.; Mazur, M.M.; Buck, A.C.; Wandtke, M.E.; Liu, J.; Ebraheim, N.A. Prospective Review of Mesenchymal Stem Cells Differentiation into Osteoblasts. Orthop. Surg. 2017, 9, 13–19. [Google Scholar] [CrossRef]
- Birbrair, A. Stem Cell Microenvironments and Beyond. Adv. Exp. Med. Biol. 2017, 1041, 1–3. [Google Scholar] [CrossRef]
- Tuch, B.E. Stem cells—A clinical update. Aust. Fam. Physician 2006, 35, 719–721. [Google Scholar]
- Petit-Zeman, S. Regenerative medicine. Nat. Biotechnol. 2001, 19, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.; Decker, A.M.; Nibali, L.; Pilipchuk, S.P.; Berglundh, T.; Giannobile, W.V. Regenerative Medicine for Periodontal and Peri-implant Diseases. J. Dent. Res. 2016, 95, 255–266. [Google Scholar] [CrossRef]
- Yin, L.; Zhou, Z.-X.; Shen, M.; Chen, N.; Jiang, F.; Wang, S.-L. The Human Amniotic Mesenchymal Stem Cells (hAMSCs) Improve the Implant Osseointegration and Bone Regeneration in Maxillary Sinus Floor Elevation in Rabbits. Stem Cells Int. 2019, 2019, 9845497. [Google Scholar] [CrossRef]
- Bressan, E.; Botticelli, D.; Sivolella, S.; Bengazi, F.; Guazzo, R.; Sbricoli, L.; Ricci, S.; Ferroni, L.; Gardin, C.; Velez, J.U.; et al. Adipose-Derived Stem Cells as a Tool for Dental Implant Osseointegration: An Experimental Study in the Dog. Int. J. Mol. Cell. Med. 2015, 4, 197–208. [Google Scholar] [PubMed]
- Han, X.; Liu, H.; Wang, D.; Su, F.; Zhang, Y.; Zhou, W.; Li, S.; Yang, R. Alveolar bone regeneration around immediate implants using an injectable nHAC/CSH loaded with autogenic blood-acquired mesenchymal progenitor cells: An experimental study in the dog mandible. Clin. Implant. Dent. Relat. Res. 2013, 15, 390–401. [Google Scholar] [CrossRef]
- Jhin, M.-J.; Kim, K.-H.; Kim, S.-H.; Kim, Y.-S.; Kim, S.-T.; Koo, K.-T.; Kim, T.-I.; Seol, Y.-J.; Ku, Y.; Rhyu, I.-C. Ex vivo bone morphogenetic protein-2 gene delivery using bone marrow stem cells in rabbit maxillary sinus augmentation in conjunction with implant placement. J. Periodontol. 2013, 84, 985–994. [Google Scholar] [CrossRef]
- Zou, D.; Guo, L.; Lu, J.; Zhang, X.; Wei, J.; Liu, C.; Zhang, Z.; Jiang, X. Engineering of bone using porous calcium phosphate cement and bone marrow stromal cells for maxillary sinus augmentation with simultaneous implant placement in goats. Tissue Eng. Part A 2012, 18, 1464–1478. [Google Scholar] [CrossRef] [PubMed]
- Marei, M.K.; Saad, M.M.; El-Ashwah, A.M.; El-Backly, R.M.; Al-Khodary, M.A. Experimental formation of periodontal structure around titanium implants utilizing bone marrow mesenchymal stem cells: A pilot study. J. Oral Implantol. 2009, 35, 106–129. [Google Scholar] [CrossRef]
- Ito, K.; Yamada, Y.; Nakamura, S.; Ueda, M. Osteogenic potential of effective bone engineering using dental pulp stem cells, bone marrow stem cells, and periosteal cells for osseointegration of dental implants. Int. J. Oral Maxillofac. Implant. 2011, 26, 947–954. [Google Scholar]
- Pieri, F.; Lucarelli, E.; Corinaldesi, G.; Iezzi, G.; Piattelli, A.; Giardino, R.; Bassi, M.; Donati, D.; Marchetti, C. Mesenchymal stem cells and platelet-rich plasma enhance bone formation in sinus grafting: A histomorphometric study in minipigs. J. Clin. Periodontol. 2008, 35, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.V.; Suaid, F.F.; Ruiz, K.G.S.; Rodrigues, T.L.; Carvalho, M.D.; Nociti, F.H.; Sallum, E.A.; Casati, M.Z. Effect of autologous bone marrow-derived cells associated with guided bone regeneration or not in the treatment of peri-implant defects. Int. J. Oral Maxillofac. Surg. 2012, 41, 121–127. [Google Scholar] [CrossRef]
- Wang, L.; Zou, D.; Zhang, S.; Zhao, J.; Pan, K.; Huang, Y. Repair of bone defects around dental implants with bone morphogenetic protein/fibroblast growth factor-loaded porous calcium phosphate cement: A pilot study in a canine model. Clin. Oral Implant. Res. 2011, 22, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Hao, P.-J.; Wang, Z.-G.; Xu, Q.-C.; Xu, S.; Li, Z.-R.; Yang, P.-S.; Liu, Z.H. Effect of umbilical cord mesenchymal stem cell in peri-implant bone defect after immediate implant: An experiment study in beagle dogs. Int. J. Clin. Exp. Med. 2014, 7, 4131–4138. [Google Scholar] [PubMed]
- Yun, J.-H.; Han, S.-H.; Choi, S.-H.; Lee, M.-H.; Lee, S.-J.; Song, S.U.; Oh, N. Effects of bone marrow-derived mesenchymal stem cells and platelet-rich plasma on bone regeneration for osseointegration of dental implants: Preliminary study in canine three-wall intrabony defects. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 1021–1030. [Google Scholar] [CrossRef]
- Yamada, Y.; Nakamura, S.; Ito, K.; Sugito, T.; Yoshimi, R.; Nagasaka, T.; Ueda, M. A Feasibility of Useful Cell-Based Therapy by Bone Regeneration with Deciduous Tooth Stem Cells, Dental Pulp Stem Cells, or Bone-Marrow-Derived Mesenchymal Stem Cells for Clinical Study Using Tissue Engineering Technology. Tissue Eng. Part A 2010, 16, 1891–1900. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Kim, K.-H.; Seo, B.-M.; Koo, K.-T.; Kim, T.-I.; Seol, Y.-J.; Ku, Y.; Rhyu, I.-C.; Chung, C.-P.; Lee, Y.-M. Alveolar bone regeneration by transplantation of periodontal ligament stem cells and bone marrow stem cells in a canine peri-implant defect model: A pilot study. J. Periodontol. 2009, 80, 1815–1823. [Google Scholar] [CrossRef]
- Ito, K.; Yamada, Y.; Naiki, T.; Ueda, M. Simultaneous implant placement and bone regeneration around dental implants using tissue-engineered bone with fibrin glue, mesenchymal stem cells and platelet-rich plasma. Clin. Oral Implants. Res. 2006, 17, 579–586. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, W.; Lv, K.; Yu, W.; Jiang, X.; Zhang, F. Peri-Implant Bone Regeneration Using rhPDGF-BB, BMSCs, and β-TCP in a Canine Model. Clin. Implant. Dent. Relat. Res. 2016, 18, 241–252. [Google Scholar] [CrossRef]
- Wang, F.; Zhou, Y.; Zhou, J.; Xu, M.; Zheng, W.; Huang, W.; Zhou, W.; Shen, Y.; Zhao, K.; Wu, Y.; et al. Comparison of Intraoral Bone Regeneration with Iliac and Alveolar BMSCs. J. Dent. Res. 2018, 97, 1229–1235. [Google Scholar] [CrossRef]
- Stramandinoli-Zanicotti, R.-T.; Sassi, L.-M.; Rebelatto, C.-L.-K.; Boldrine-Leite, L.M.; Brofman, P.-R.; Carvalho, A.-L. The effect of bone marrow-derived stem cells associated with platelet-rich plasma on the osseointegration of immediately placed implants. J. Clin. Exp. Dent. 2021, 13, e8–e13. [Google Scholar] [CrossRef]
- Misawa, M.Y.O.; Huynh-Ba, G.; Villar, G.M.; Villar, C.C. Efficacy of stem cells on the healing of peri-implant defects: Systematic review of preclinical studies. Clin. Exp. Dent. Res. 2016, 2, 18–34. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 350, g7647. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; De Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Ou, K.-L.; Weng, C.-C.; Wu, C.-C.; Lin, Y.-H.; Chiang, H.-J.; Yang, T.-S.; James, W.; Yen, Y.; Cheng, H.-Y.; Sugiatno, E. Research of StemBios Cell Therapy on Dental Implants Containing Nanostructured Surfaces: Biomechanical Behaviors, Microstructural Characteristics, and Clinical Trial. Implant. Dent. 2016, 25, 63–73. [Google Scholar] [CrossRef]
- Weng, C.-C.; Ou, K.-L.; Wu, C.-Y.; Huang, Y.-H.; Wang, J.; Yen, Y.; Cheng, H.-Y.; Lin, Y.-H. Mechanism and Clinical Properties of StemBios Cell Therapy: Induction of Early Osseointegration in Novel Dental Implants. Int. J. Oral Maxillofac. Implant. 2017, 32, e47–e54. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Spencer, J.A.; Koh, B.I.; Kobayashi, T.; Fujisaki, J.; Clemens, T.L.; Lin, C.P.; Kronenberg, H.M.; Scadden, D.T. Endogenous Bone Marrow MSCs Are Dynamic, Fate-Restricted Participants in Bone Maintenance and Regeneration. Cell Stem Cell 2012, 10, 259–272. [Google Scholar] [CrossRef]
- Park, S.-Y.; Kim, K.-H.; Gwak, E.-H.; Rhee, S.-H.; Lee, J.-C.; Shin, S.-Y.; Koo, K.-T.; Lee, Y.-M.; Seol, Y.-J. Ex vivo bone morphogenetic protein 2 gene delivery using periodontal ligament stem cells for enhanced re-osseointegration in the regenerative treatment of peri-implantitis. J. Biomed. Mater. Res. Part A 2015, 103, 38–47. [Google Scholar] [CrossRef]
- Zou, D.; He, J.; Zhang, K.; Dai, J.; Zhang, W.; Wang, S.; Zhou, J.; Huang, Y.; Zhang, Z.; Jiang, X. The Bone-Forming Effects of HIF-1α-Transduced BMSCs Promote Osseointegration with Dental Implant in Canine Mandible. PLoS ONE 2012, 7, e32355. [Google Scholar] [CrossRef][Green Version]
- Barfeie, A.; Wilson, J.; Rees, J. Implant surface characteristics and their effect on osseointegration. Br. Dent. J. 2015, 218, E9. [Google Scholar] [CrossRef]
- Kantarci, A.; Hasturk, H.; Van Dyke, T.E. Animal models for periodontal regeneration and peri-implant responses. Periodontology 2000, 68, 66–82. [Google Scholar] [CrossRef] [PubMed]
- Haney, J.M.; Zimmerman, G.J.; Wikesjö, U.M. Periodontal repair in dogs: Evaluation of the natural disease model. J. Clin. Periodontol. 1995, 22, 208–213. [Google Scholar] [CrossRef]
- Zablotsky, M.; Meffert, R.; Caudill, R.; Evans, G. Histological and clinical comparisons of guided tissue regeneration on dehisced hydroxylapatite-coated and titanium endosseous implant surfaces: A pilot study. Int. J. Oral Maxillofac. Implant. 1991, 6, 294–303. [Google Scholar]
- Misch, C.E. Density of bone: Effect on treatment plans, surgical approach, healing, and progressive boen loading. Int. J. Oral Implantol. 1990, 6, 23–31. [Google Scholar] [PubMed]
| S. No. | Reference Number | Origin of Stem Cells | Animal Model Used | Type and Size of Implants Used | Differentiation/Characterization/Application of Stem Cells | Site of Implant | Type of Defect | Total Period of Observation | Results and Conclusion |
|---|---|---|---|---|---|---|---|---|---|
| 1. | [24] | Human amniotic mesenchymal stem cells | Twelve New Zealand white rabbits | Mini implant 1.5 mm × 5 mm (Bioconcept Co., Ltd., China) | Insertion of hAMSC-gel (AMSCs re-suspended in fibrin solution) into the maxillary sinus before implant placement and only fibrin in the control group | Maxillary sinus | Maxillary sinus floor elevation | 4 and 12 weeks | Bone volume, bone volume/tissue volume, bone-to-implant contact ratio, and vessel-like structures were better in the Bio OSS hAMSC group in comparison with other groups. ALP was higher in hAMSC and hAMSC/BioOSS group |
| 2. | [25] | Adipose-derived stem cells derived from dog’s Bichat bulla | Six beagle dogs | 10 × 3.3 mm Premium TM, Sweden and Martina | HA-based scaffolds previously seeded with ADSCs | Mandibular premolars and the first molars | Peri-implant–bone defect | 1 month | ADSCs increased bone regeneration new vessels, osteoblasts, and new bone matrix, absence of inflammation |
| 3. | [26] | UCMSCs (Lifeline Cell Technology, FC0020) | Eight male beagle dogs | SuperLine implants (Dentium Biomaterial Co., Ltd., Korea), 3.6 × 8 mm | Injection of UCMSCs with PRF into the peri-implant bone defect | Second, third, and fourth mandibular premolars | Peri-implant– bone defect | 2, 4 and 8 weeks | A significantly higher percentage of new bone formation in the case group in comparison with the control |
| 4. | [27] | Human clonal bone marrow mesenchymal stem cells | Four male adult mongrel dogs | GSII, Osstem, Korea 4 × 8.5 mm | Placement of cells with graft material randomly placed at the mesial bone defect area | Mandibular first molars and premolars | Peri-implant– bone defect | 6 and 12 weeks | Highest level of bone density and bone–implant contact in HA, stem cells, and PRP group (no statistical significance) |
| 5. | [28] | Canine BMMSCs (cBMMSCs), canine DPSCs (cDPSCs), puppy DTSCs (pDTSCs), | Adult hybrid dogs, sample size not mentioned | 3.7 × 7 mm HA-coated JMM implants (POI = Finatite, Japan MedicalMaterials Corporation, Osaka, Japan) | Injection of the PRP, cBMMSCs PRP, cDPSCs PRP, and pDTSCs PRP admixtures into the bone defect before implant placement | 1st molar, 1st, 2nd and 3rd and third premolars | Peri-implant– bone defect | 16 weeks | Well-formed mature bone and neovascularization in all the three groups in comparison with control. Bone implant contact was highest in pDTSCs = PRP group > cDPSCs = PRP group, cBMMSCs = PRP group. PRP group. Control (statistically significant) |
| 6. | [29] | Autologous periodontal ligament stem cells (PDLSCs) and bone marrow SCs (BMSCs) | Four adults, male beagle dogs | 3.3 × 10 mm implant (brand not mentioned) | Placement of the graft material onto the defect after implant placement | Bilateral all mandibular premolars and first molars | Peri-implant–bone defect | 8 and 16 weeks | Highest new bone formation in BMSC group > PDLSC > control group |
| 7. | [30] | Dog mesenchymal stem cells (dMSCs) from bone marrow | Twelve adult hybrid dogs | 3.75 × 7 mm Branemark implants | Simultaneous placement of implant and graft material | First molar, premolars, and the second and third premolars | Peri-implant– bone defect | 2, 4 and 8 weeks | Natural margin bone level in dMSCs/PRP/fibrin and dMSCs/fibrin with no exposure if implant thread in comparison with only fibrin and control group. Bone implant contact dMSCs/PRP/fibrin > dMSCs/fibrin > fibrin > control |
| 8. | [31] | Autologous bone marrow mesenchymal stem cells | Six male adult labrador dogs | 3.75 × 10 mm implants (pure titanium, Cibei Medical Devices Co., Ltd. Zhejiang, Shanghai, China) | Placement of graft material following implant placement | Bilateral first, second, third, and fourth mandibular premolar teeth | Peri-implant– bone defects | 3, 6, 9 weeks | Osseointegration highest in rhPDGF-BB/BMSCs/β-TCP constructs > rhPDGF-BB/β-TCP constructs > BMSCs/βTCP constructs > TCP particles alone. No significant differences in bone–implant contact although rhPDGF-BB/BMSCs/β-TCP constructs had the highest value |
| 9. | [32] | Dog iliac bone marrow mesenchymal stem cells (I-BMSCs) and alveolar bone marrow mesenchymal stem cells (Al-BMSCs) | Four labrador dogs | 4.1 × 10.0 mm Beijing Leiden Biomaterial implant | Placement of graft material following implant placement | Mandibular premolar region | Peri-implant– bone defects | 12 weeks | Greater new bone formation and high bone–implant contact in Al-BMSC and I-BMSC group in comparison with the other groups and no significant difference between Al-BMSC and I-BMSC groups |
| 10. | [33] | Autologous bone marrow mesenchymal stem cells from the iliac crest | Four Brazilian male adult miniature pigs | 3.5 × 11 mm (ConeMor- se; Neodent, Curitiba, Brazil) | Placement of graft with cells before implant placement | Bilateral third and fourth mandibular premolar region | Peri-implant–bone defect | 90 days | Although statistically insignificant lesser implant loss rate (ILR), greater bone–implant contact (BIC), and bone density within the threads (BDWT) in the test group in comparison with the control |
| 11. | [34] | Dog hematopoietic mesenchymal progenitor cells (dBMPC) | Four adult male mongrel dogs | 3 × 10 mm Ti-24Nb-4Zr7.9Sn (T2448)1 | Placement of implants followed by graft in the same procedure | Bilateral second, third, and fourth mandibular premolars | Peri-implant–bone defect | 12 weeks | More bone formation in dBMPC + nHAC/CSH g than other groups. Significantly high bone–implant contact and bone density in dBMPC + nHAC/CSH g > nHAC/CSH > control |
| 12. | [35] | Autologous bone marrow stem cells from the iliac crest | 27 mature New Zealand rabbits | 1.4 × 6 mm implant | Placement of graft and implant in the same procedure | Maxillary sinus | Maxillary sinus augmentation | 2, 4 and 8 weeks | At 2 and 4 weeks, greater new bone formation and bone–implant contact in the BMP-2 transduced BMSC group in comparison with other 2 groups and at 8 weeks no significant difference between all the three groups although BMP-2 BMSC > non-transduced BMSC > control |
| 13. | [36] | Autologous bone marrow stem cells from the iliac crest | Nine healthy female goats | 3.3 × 12 ITI-SLA; Strauman AG | Simultaneous placement of implant and graft | The maxillary second and third premolar | Maxillary sinus floor elevation | 12 weeks/3 months | Bone formation and bone–implant contact highest in BMSCs/CPC > autogenous bone group > CPC alone group (statistically significant) |
| 14. | [37] | Goat bone marrow-derived mesenchymal stem cells from femur | Five goats | Titanium fixture not mentioned | Placement of implant and graft material simultaneously | Mandibular canine | Peri-implant–bone formation | 10 days and 4 weeks | More bone formation and PDL tissue regeneration in the case group in comparison with control. |
| 15. | [38] | Dog dental pulp stem cells (dDPSC), dog bone marrow stem cells (dBMSC), and dog periosteal cells (dPC) | 3 dogs | 3.7 × 8 mm (POI·EX(FINATITE) Japan Medical Materials | Placement of graft with or without cells and placement of implants 8 weeks after graft placement | Mandibular all premolars and first molar | Peri-implant–bone defect | 8 weeks after implant placement | Bone implant contact highest in dDPSC/PRP > dBMSC/PRP > dPC/PRP > control |
| 16. | [39] | Bone marrow mesenchymal cells from the iliac origin | 8 adult minipigs | 3.8 × 1 mm implantXiVE; Dentsply-Friadent | Placement of graft followed by implant simultaneously | Maxillary sinus region | Maxillary sinus augmentation | 12 weeks (3 months) | Significant increase in bone formation and high BIC in the test group (with MSC and PRP) |
| 17. | [39] | Bone marrow mesenchymal cells from the iliac origin | Eight beagle dogs | 4 × 8.5 mm (Biomet-3iTM do Brasil LTDA, São Paulo, SP, Brazil) | Placement of implant followed by graft material in the same procedure | 3rd and 4th mandibular premolar | Peri-implant–bone defect | 12 weeks (months) | Statistically significant higher bone fill in BMSC and BMSC-guided bone regeneration with control. No significant difference in bone fill in BMSC and BMSC + guided none regeneration. Statistically significant new bone area, bone-to-implant contact, new bone height, and new bone weight in BMSC-guided bone regeneration in comparison with control |
| 18. | [41] | Autologous bone marrow-derived mesenchymal stem cells | Five beagle dogs | 3.75 × 10 mm Brånemarks dental implant (Nobel Biocare, Göteborg, Sweden | Placement of implant followed by graft in the same procedure | Not clear | Peri-implant–bone defect | 12 weeks | Statistically significant mineral apposition in BMP + FGF + BMSCs + CPC > BMP + BMSCs + CPC > FGF + BMSCs + CPC > BMSC + CPC > control |
| S. No. | Author/Year | Selection Bias 1. Was the Allocation Sequence Adequately Generated and Applied? | Selection Bias 2. Were the Groups Similar at Baseline or Were They Adjusted for Confounders in the Analysis? | Selection Bias 3. Was the Allocation Adequately Concealed? | Performance Bias 4. Were the Animals Randomly Housed during the Experiment? | Performance Bias 5. Were the Caregivers and/or Investigators Blinded from Knowledge of Which Intervention Each Animal Received during the Experiment? | Detection Bias 6. Were Animals Selected at Random for Outcome Assessment? | Detection Bias 7. Was the Outcome Assessor Blinded? | Attrition Bias 8. Were Incomplete Outcome Data Adequately Addressed? | Reporting Bias 9. Are Reports of the Study Free of Selective Outcome Reporting? | Other Bias 10. Was the Study Apparently Free of Other Problems That Could Result in a High Risk of Bias? | Overall Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Yin/2019/China [24] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes | Yes | Moderate |
| 2. | Bressa.2015/Italy [25] | Unclear | Yes | Unclear | Not applicable (split-mouth design) | Not applicable (split-mouth design) | Unclear | Unclear | Yes | Yes | Yes | Moderate |
| 3. | Hao et al./2014/China [26] | Unclear | Yes | Unclear | Not applicable (split-mouth design) | Not applicable (split-mouth design) | Unclear | Unclear | Yes | Yes | Yes | Moderate |
| 4. | Yun/2019/Koreav [27] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes | High |
| 5. | Yamada et al./2010/Japan [28] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Unclear | High |
| 6. | Kim et al./2009/Korea [29] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes | High |
| 7. | Ito et al./2005/Japan [30] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes | High |
| 8. | Xu et al./2015/China [31] | Unclear | Yes | Unclear | Not applicable as each animal received one construct from each of the groups | Not applicable | Unclear | Unclear | Yes | Yes | Yes | High |
| 9. | Wang et al./China/2018 [32] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes | High |
| 10. | Zanicottiet al/2021/Brazil [33] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes | High |
| 11. | Han et al./China/2011 [34] | Unclear | Yes | Unclear | Not applicable as each animal received one construct from each of the groups | Not applicable | Unclear | Yes | Yes | Yes | Yes | Moderate |
| 12. | Jhin et al./2012/South Korea [35] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes | High |
| 13. | Zhou et al./2012/China [36] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes | High |
| 14. | Marei et al./2009/Egypt [37] | No | Yes | Unclear | Not applicable split-mouth design | Not applicable split-mouth design | Unclear | Unclear | Yes | Yes | Yes | High |
| 15. | Ito et a;/2011/Japan [38] | Unclear | Yes | Unclear | Not applicable | Not applicable | Unclear | Unclear | Yes | Yes | Yes | High |
| 16. | Pieri/2008/Italy [39] | Unclear | Yes | Unclear | Not applicable (split-mouth design) | Not applicable (split-mouth design) | Unclear | Unclear | Yes | Yes | Yes | High |
| 17. | Ribeiro/2012/Brazil [40] | Yes | Yes | unclear | Not applicable (split-mouth design) | Not applicable (split-mouth design) | Unclear | Yes | Yes | Yes | Yes | Low |
| 18. | Wang et al./2011/China [41] | Unclear | Yes | Unclear | Not applicable (split-mouth design) | Not applicable (split-mouth design) | Unclear | Unclear | Yes | Yes | Yes | High |
| Number of Studies | Type of Scaffold Used |
|---|---|
| 4 | Platelet-rich plasma (PRP) |
| 3 | Tricalcium phosphate (TCP) |
| 2 | Hydroxyapatite |
| 1 | Bio OSS graft, platelet-rich fibrin (PRF), deproteinized bovine bone mineral, PRP and fluorohydroxyapatite (FH), BMP-2 with bFGF and CPC, guided bone regeneration, PLG scaffold, nHAC/CSH, and calcium phosphate cement |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sayed, M.E.; Mugri, M.H.; Almasri, M.A.; Al-Ahmari, M.M.; Bhandi, S.; Madapusi, T.B.; Varadarajan, S.; Raj, A.T.; Reda, R.; Testarelli, L.; et al. Role of Stem Cells in Augmenting Dental Implant Osseointegration: A Systematic Review. Coatings 2021, 11, 1035. https://doi.org/10.3390/coatings11091035
Sayed ME, Mugri MH, Almasri MA, Al-Ahmari MM, Bhandi S, Madapusi TB, Varadarajan S, Raj AT, Reda R, Testarelli L, et al. Role of Stem Cells in Augmenting Dental Implant Osseointegration: A Systematic Review. Coatings. 2021; 11(9):1035. https://doi.org/10.3390/coatings11091035
Chicago/Turabian StyleSayed, Mohammed E., Maryam H. Mugri, Mazen A. Almasri, Manea Musa Al-Ahmari, Shilpa Bhandi, Thodur Balaji Madapusi, Saranya Varadarajan, A. Thirumal Raj, Rodolfo Reda, Luca Testarelli, and et al. 2021. "Role of Stem Cells in Augmenting Dental Implant Osseointegration: A Systematic Review" Coatings 11, no. 9: 1035. https://doi.org/10.3390/coatings11091035
APA StyleSayed, M. E., Mugri, M. H., Almasri, M. A., Al-Ahmari, M. M., Bhandi, S., Madapusi, T. B., Varadarajan, S., Raj, A. T., Reda, R., Testarelli, L., & Patil, S. (2021). Role of Stem Cells in Augmenting Dental Implant Osseointegration: A Systematic Review. Coatings, 11(9), 1035. https://doi.org/10.3390/coatings11091035










