Enhanced Electrochromic Properties of Nanostructured WO3 Film by Combination of Chemical and Physical Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Characterization
3. Results and Discussion
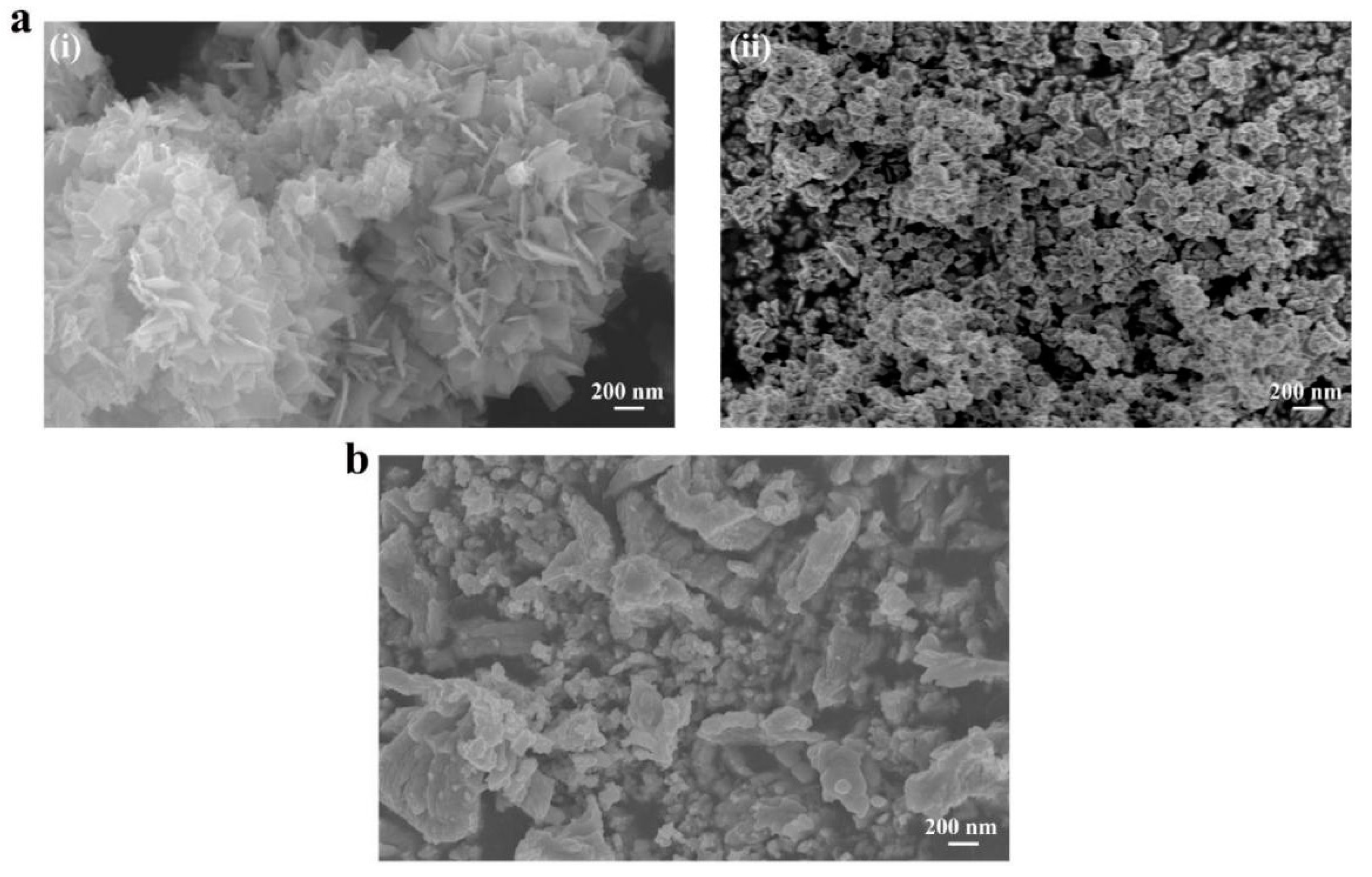
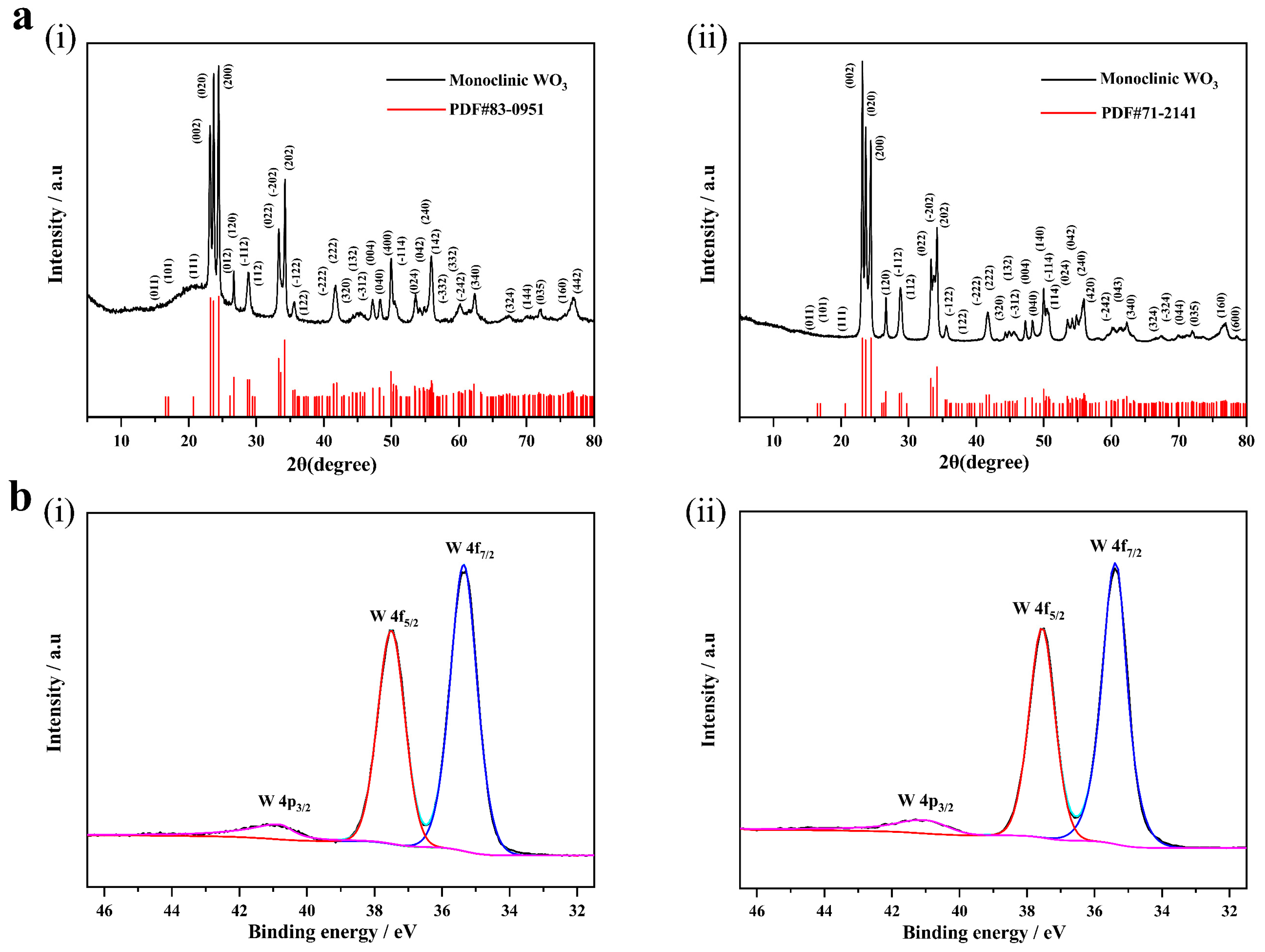
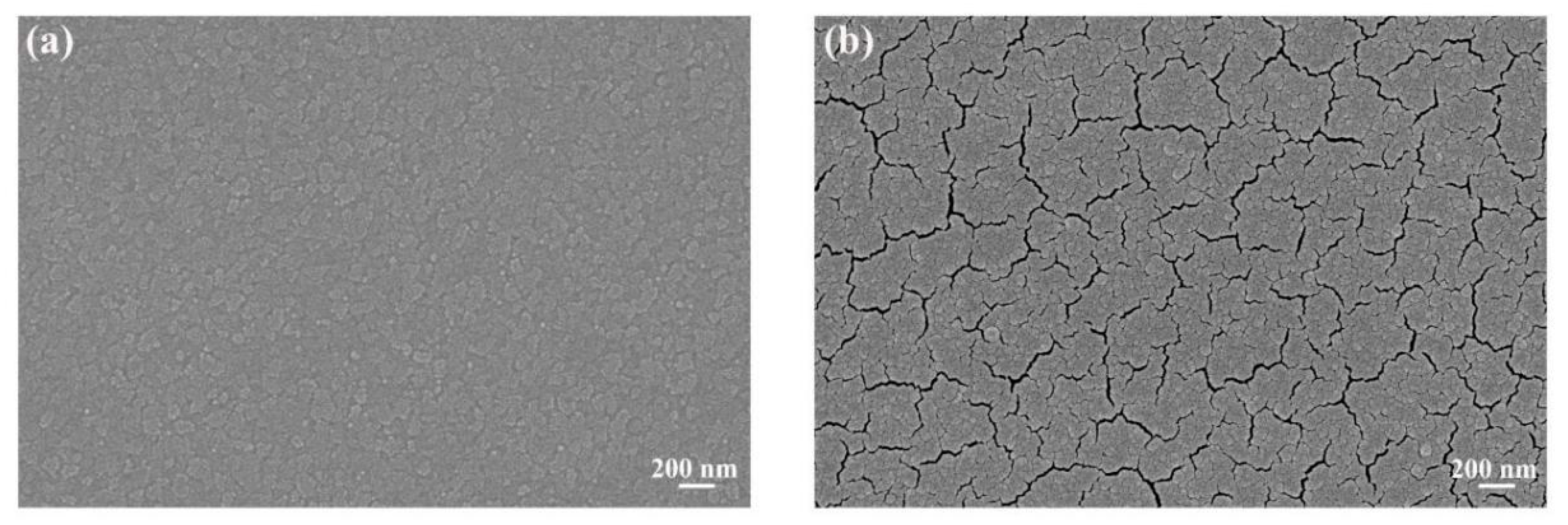
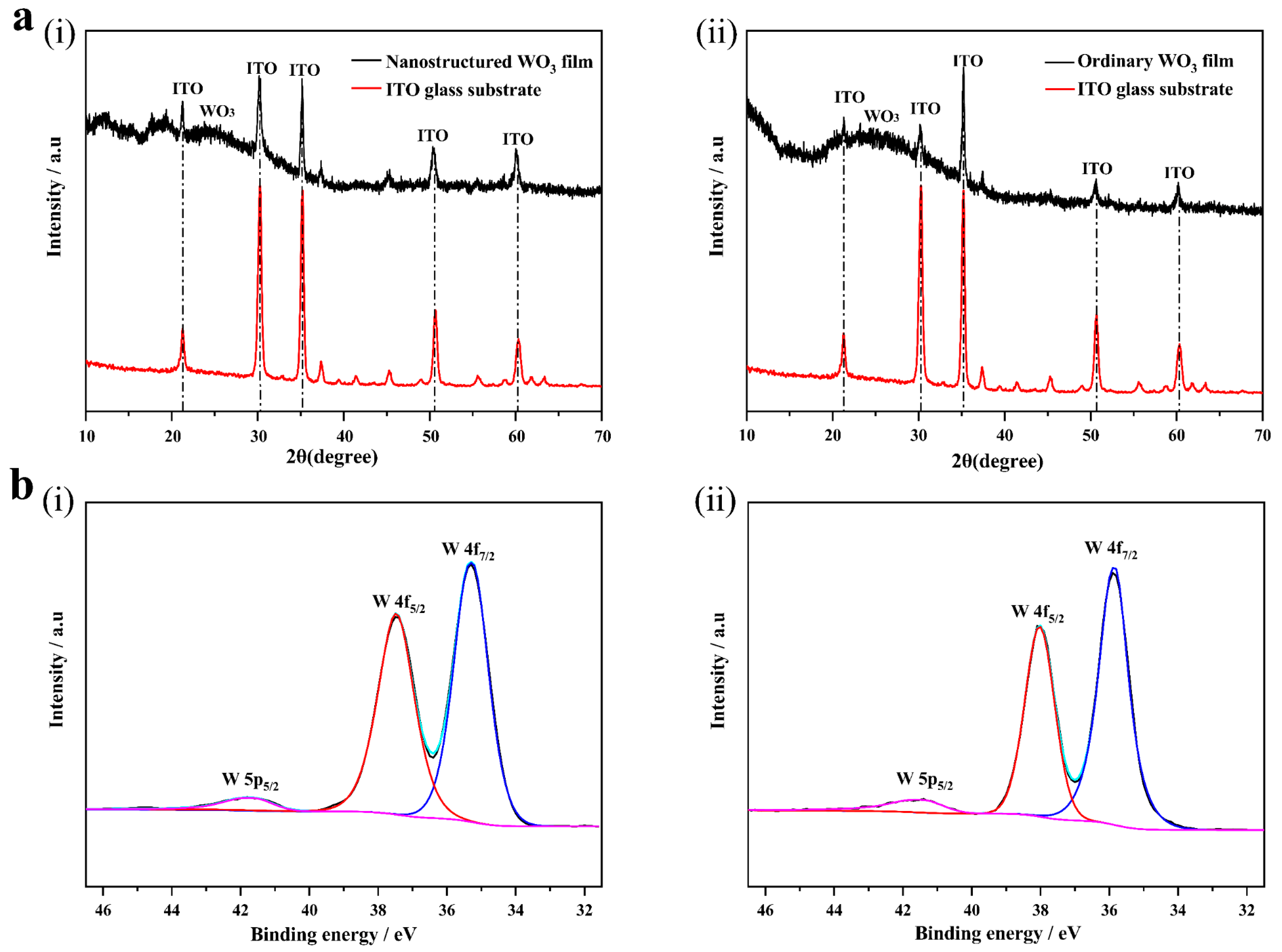
3.1. Morphology and Structure
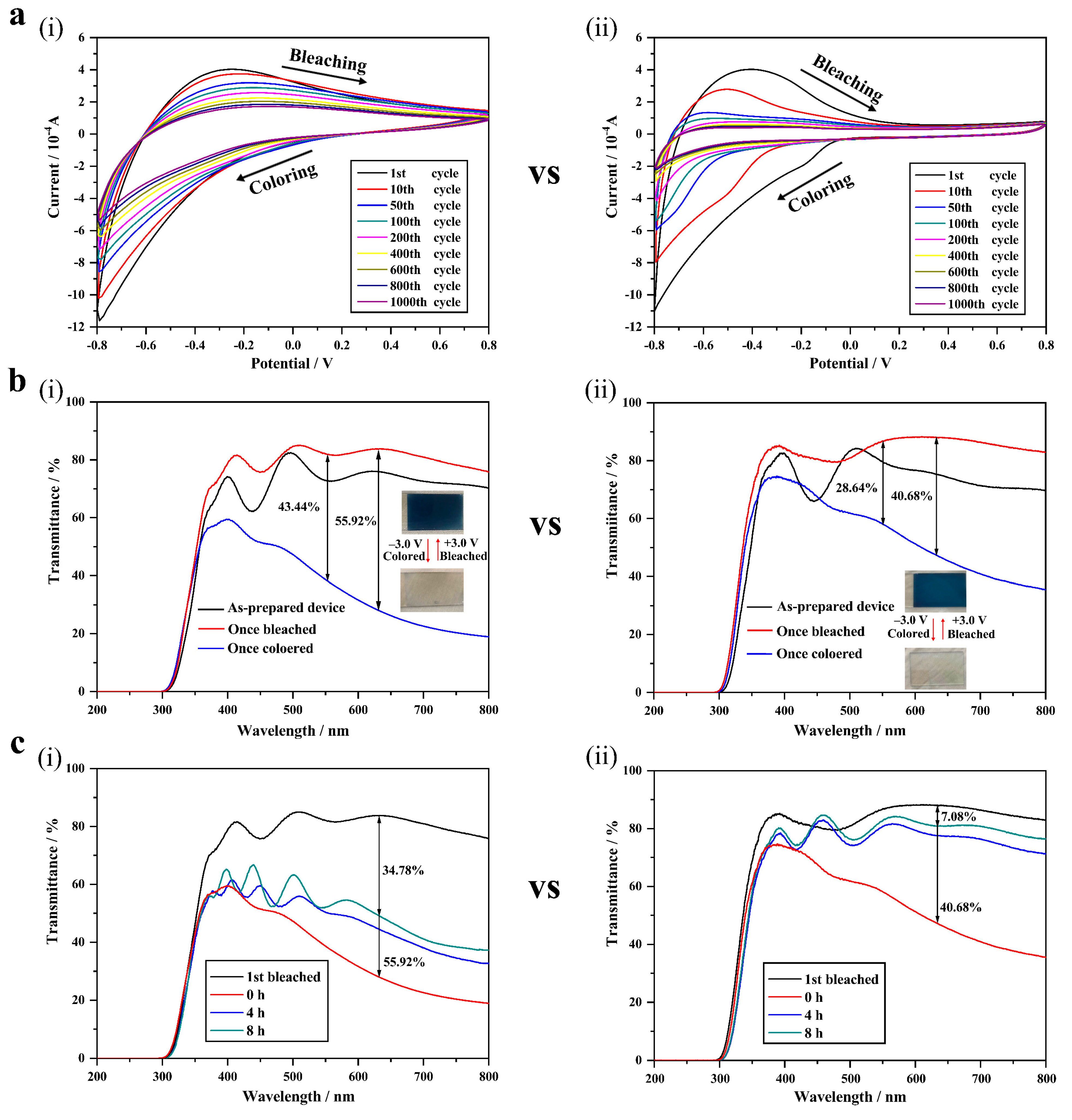
3.2. Electrochromic Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Evecan, D.; Zayim, E. Highly uniform electrochromic tungsten oxide thin films deposited by e-beam evaporation for energy saving systems. Curr. Appl. Phys. 2019, 2, 198–203. [Google Scholar] [CrossRef]
- Kumar, K.N.; Shaik, H.; Madhavi, V.; Sattar, S.A. On the bonding and electrochemical performance of sputter deposited WO3 thin films. IOP Conf. Ser. Mater. Sci. Eng. 2020, 872, 012147. [Google Scholar] [CrossRef]
- Shinde, P.A.; Lokhande, A.C.; Patil, A.M.; Lokhande, C.D. Facile synthesis of self-assembled WO3 nanorods for high-performance electrochemical capacitor. J. Alloys Compd. 2019, 770, 1130–1137. [Google Scholar] [CrossRef]
- Zhen, Y.; Jelle, B.P.; Gao, T. Electrochromic properties of WO3 thin films: The role of film thickness. Anal. Sci. Adv. 2020, 1, 1–8. [Google Scholar] [CrossRef]
- Leitzke, D.W.; Cholant, C.M.; Landarin, D.M.; Lucio, C.S.; Krüger, L.U.; Gündel, A.; Flores, W.H.; Rodrigues, M.P.; Balboni, R.D.C.; Pawlicka, A.; et al. Electrochemical properties of WO3 sol-gel thin films on indium tin oxide/poly (ethylene terephthalate) substrate. Thin Solid Films 2019, 683, 8–15. [Google Scholar] [CrossRef]
- Bouzid, K.; Maindron, T.; Kanaan, H. Thin-film encapsulated white organic light top-emitting diodes using a WO3/Ag/WO3 cathode to enhance light out-coupling. J. Soc. Inf. Disp. 2016, 24, 563–568. [Google Scholar] [CrossRef]
- Zheng, G.; Wang, J.; Liu, H.; Murugadoss, V.; Zu, G.; Che, H.; Lai, C.; Li, H.; Ding, T.; Gao, Q.; et al. Tungsten oxide nanostructures and nanocomposites for photoelectrochemical water splitting. Nanoscale 2019, 11, 18968–18994. [Google Scholar] [CrossRef] [PubMed]
- Shaath, M.; Ansari, M.N.M.; Nordin, N.A.; Al-Furjan, M.S.H. Mechanical properties of tungsten tri-oxide (WO3) reinforced poly (lactic-acid) (PLA) nanocomposites. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1128, 012030. [Google Scholar] [CrossRef]
- Bi, Z.; Li, X.; Chen, Y.; Xu, X.; Zhang, S.; Zhu, Q. Bi-functional flexible electrodes based on tungsten trioxide/zinc oxide nanocomposites for electrochromic and energy storage applications. Electrochim. Acta 2017, 227, 61–68. [Google Scholar] [CrossRef]
- Upadhyay, K.K.; Altomare, M.; Eugénio, S.; Schmuki, P.; Silva, T.M.; Montemor, M.F. On the supercapacitive behaviour of anodic porous WO3-based negative electrodes. Electrochim. Acta 2017, 232, 192–201. [Google Scholar] [CrossRef] [Green Version]
- Spanu, D.; Recchia, S.; Schmuki, P.; Altomare, M. Thermal-oxidative growth of substoichiometric WO3–x nanowires at mild conditions. Phys. Status Solidi RRL 2020, 14, 2000235. [Google Scholar] [CrossRef]
- Mineo, G.; Ruffino, F.; Mirabella, S.; Bruno, E. Investigation of WO3 electrodeposition leading to nanostructured thin films. Nanomaterials 2020, 10, 1493. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Li, Q.; Jiang, D.; Wang, Q.; Feng, T. Microwave intercalation synthesis of WO3 nanoplates and their NO-sensing properties. J. Mater. Eng. Perform. 2015, 24, 274–279. [Google Scholar] [CrossRef]
- Cai, G.; Tu, J.; Zhou, D.; Wang, X.; Gu, C. Growth of vertically aligned hierarchical WO3 nano-architecture arrays on transparent conducting substrates with outstanding electrochromic performance. Sol. Energy Mater. Sol. Cells 2014, 124, 103. [Google Scholar] [CrossRef]
- Li, Y.; Chen, D.; Caruso, R.A. Enhanced electrochromic performance of WO3 nanowire networks grown directly on fluorine-doped tin oxide substrates. J. Mater. Chem. C 2016, 4, 10500–10508. [Google Scholar] [CrossRef]
- Lu, C.H.; Hon, M.H.; Kuan, C.Y.; Leu, I.C. Preparation of WO3 nanorods by a hydrothermal method for electrochromic device. Jpn. J. Appl. Phys. 2014, 53, 06JG08. [Google Scholar] [CrossRef]
- Parthibavarman, M.; Karthik, M.; Sathishkumar, P.; Poonguzhali, R. Rapid synthesis of novel Cr-doped WO3 nanorods: An efficient electrochemical and photocatalytic performance. J. Iran. Chem. Soc. 2018, 15, 1419–1430. [Google Scholar] [CrossRef]
- Wang, M.; Chen, Y.; Gao, B.; Hao, L. Electrochromic properties of nanostructured WO3 thin films deposited by glancing-angle magnetron sputtering. Adv. Electron. Mater. 2019, 5, 1800713. [Google Scholar] [CrossRef]
- Quy, V.H.H.; Jo, I.R.; Kang, S.H.; Ahn, K.S. Amorphous-crystalline dual phase WO3 synthesized by pulsed-voltage electrodeposition and its application to electrochromic devices. J. Ind. Eng. Chem. 2021, 94, 264–271. [Google Scholar] [CrossRef]
- Hilliaro, S.; Baldinozzi, G.; Friedrich, D.; Kressman, S.; Strub, K.; Artero, V.; Laberty-Robert, C. Mesoporous thin film WO3 photoanode for photoelectrochemical water splitting: A sol-gel dip coating approach. Sustain. Energy Fuels 2017, 1, 145–153. [Google Scholar] [CrossRef] [Green Version]
- Kadam, A.V.; Bhosale, N.Y.; Patil, S.B.; Mali, S.S.; Hong, C.K. Fabrication of an electrochromic device by using WO3 thin films synthesized using facile single-step hydrothermal process. Thin Solid Films 2019, 673, 86–93. [Google Scholar] [CrossRef]
- Semenova, A.; Eruzin, A.; Bezrukov, P.; Sychov, M.; Mjakin, S.; Lukashova, T.; Sudarb, N. Improvement of parameters and conditions for magnetron sputtering of WO3 layers to enhance their electrochromic performances. Mater. Today Proc. 2020, 30, 606–610. [Google Scholar] [CrossRef]
- Madhuri, K.V.; Babu, M.B. Studies on Electron Beam Evaporated WO3 Thin Films. Mater. Today Proc. 2016, 3, 84–89. [Google Scholar] [CrossRef]
- Tang, X.; Chen, G.; Liao, H.; Li, Z.; Zhang, J.; Luo, J. Unveiling mechanical degradation for a monolithic electrochromic device: Glass/ITO/WO3/LiClO4 (PEO)/TiO2/ITO/glass. Electrochim. Acta 2019, 329, 135182. [Google Scholar] [CrossRef]
- Brezesinski, T.; Rohlfing, D.F.; Sallard, S.; Antonietti, M.; Smarsly, B.M. Highly crystalline WO3 thin films with ordered 3D mesoporosity and improved electro-chromic performance. Small 2010, 2, 1203–1211. [Google Scholar] [CrossRef]
- Tang, X.; Huang, J.; Liao, H.; Chen, G.; Mo, Z.; Ma, D.; Zhan, R.; Li, Y.; Luo, J. Growth of W18O49/WOx/W dendritic nanostructures by one-step thermal evaporation and their high-performance photocatalytic activities in methyl orange degradation. CrystEngComm 2019, 21, 5905–5914. [Google Scholar] [CrossRef]
- Chu, J.; Lan, J.; Lu, D.; Ma, J.; Wang, X.; Wu, B.; Gong, M.; Zhang, R.; Xiong, S. Facile fabrication of WO3 crystalline nanoplate on FTO glass and their application in electrochromism. Micro Nano Lett. 2016, 11, 749–752. [Google Scholar] [CrossRef]



| Electrochromic Properties | Nanostructured WO3 Film | Ordinary WO3 Film |
|---|---|---|
| response time (coloring time/bleaching time) | 8.40 s/1.39 s | 9.27 s/3.82 s |
| cycling stability (ion capacity decreasing rate) | 53% | 81% |
| optical modulation rates (∆T550nm and ∆T633nm) | 43.44%/55.92% | 28.64%/40.68% |
| memory effect (transmittance increasing rate) | 21.14% | 33.68% |
| adhesion (value of adhesion factor) | 0.99702 | 0.57148 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Li, Z.; He, W.; Chen, H.; Tang, X.; Chen, Y.; Chen, Y. Enhanced Electrochromic Properties of Nanostructured WO3 Film by Combination of Chemical and Physical Methods. Coatings 2021, 11, 959. https://doi.org/10.3390/coatings11080959
Li X, Li Z, He W, Chen H, Tang X, Chen Y, Chen Y. Enhanced Electrochromic Properties of Nanostructured WO3 Film by Combination of Chemical and Physical Methods. Coatings. 2021; 11(8):959. https://doi.org/10.3390/coatings11080959
Chicago/Turabian StyleLi, Xiaoni, Zhijie Li, Wanting He, Haolin Chen, Xiufeng Tang, Yeqing Chen, and Yu Chen. 2021. "Enhanced Electrochromic Properties of Nanostructured WO3 Film by Combination of Chemical and Physical Methods" Coatings 11, no. 8: 959. https://doi.org/10.3390/coatings11080959
APA StyleLi, X., Li, Z., He, W., Chen, H., Tang, X., Chen, Y., & Chen, Y. (2021). Enhanced Electrochromic Properties of Nanostructured WO3 Film by Combination of Chemical and Physical Methods. Coatings, 11(8), 959. https://doi.org/10.3390/coatings11080959







