Effect of Electromigration and Thermal Ageing on the Tin Whiskers’ Formation in Thin Sn–0.7Cu–0.05Ga Lead (Pb)-Free Solder Joints
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Dipping for Solder Coating
2.3. Whiskers Accelerated Test by Electromigration Testing and Electromigration with Thermal Ageing
2.4. Tin Whiskers Analysis and Characterization
3. Results
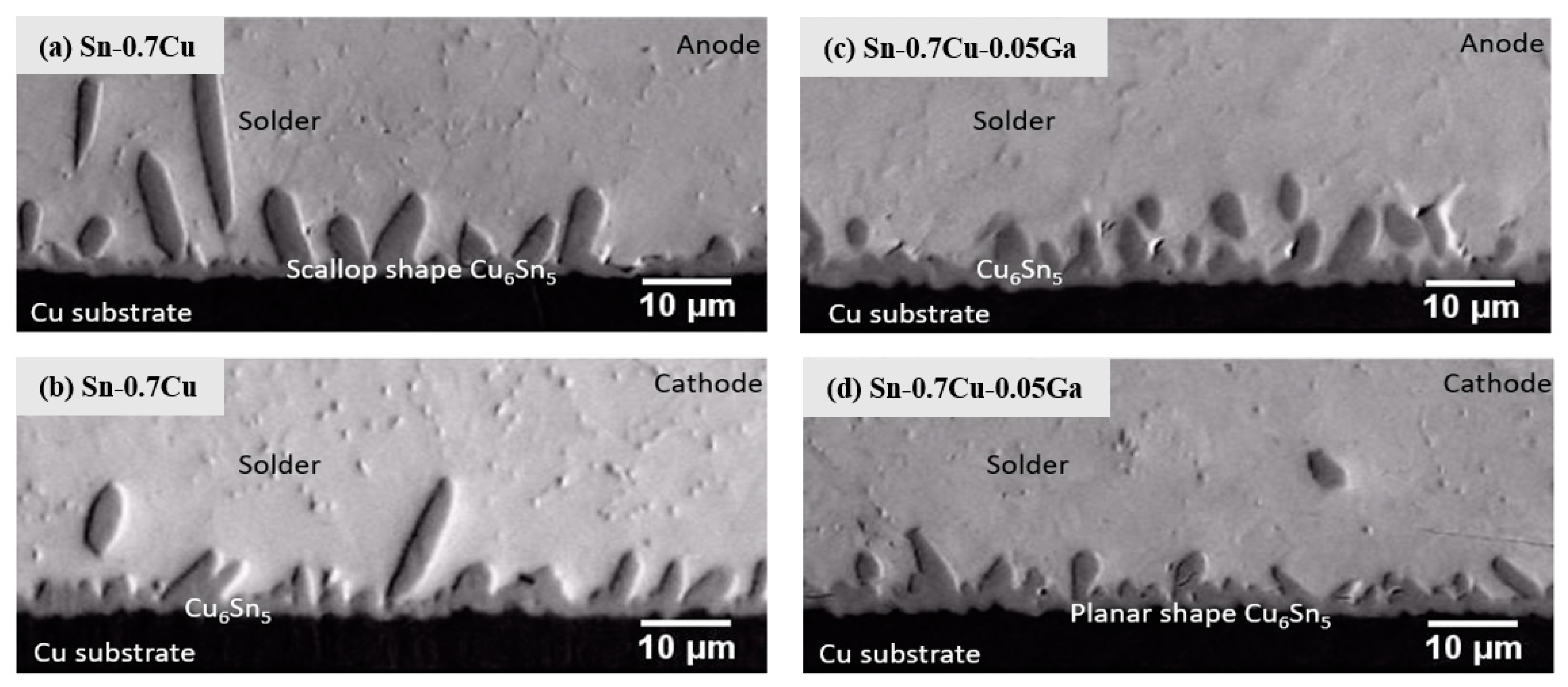
3.1. Microstructure Analysis
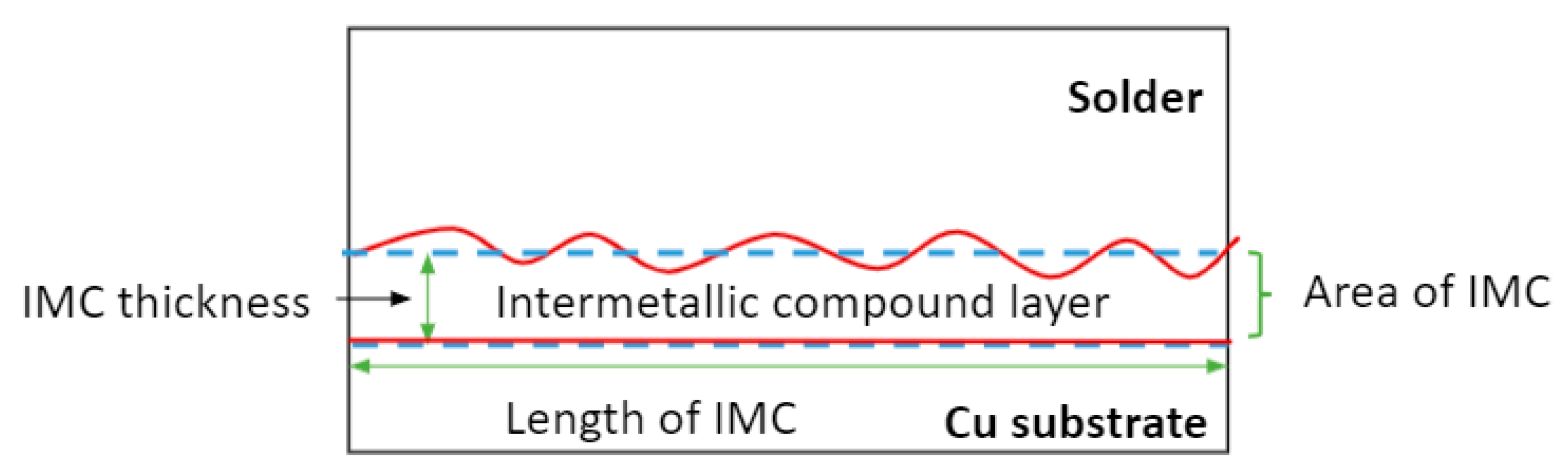
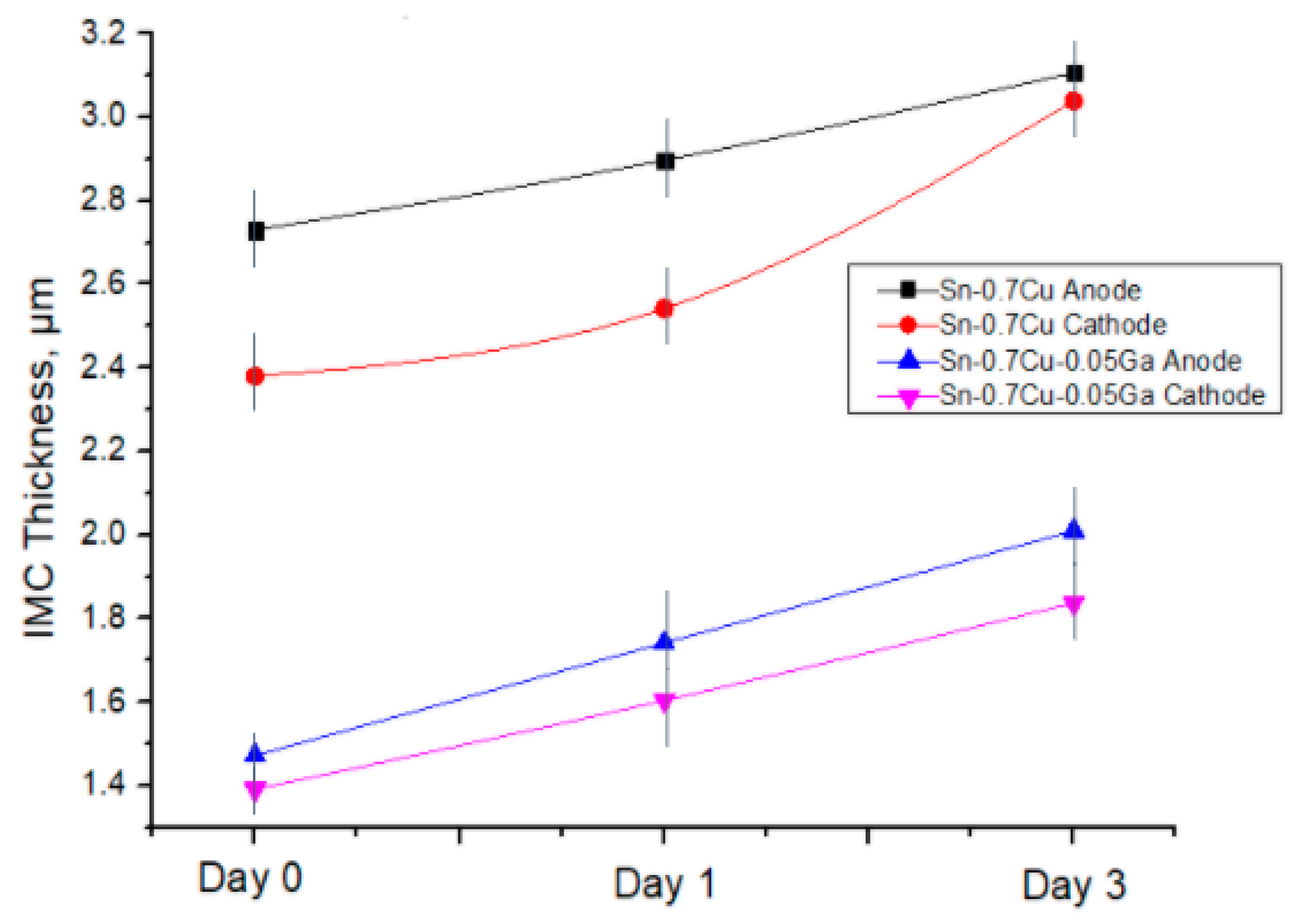
3.2. Interfacial Intermetallic Compound (IMC) during Electromigration
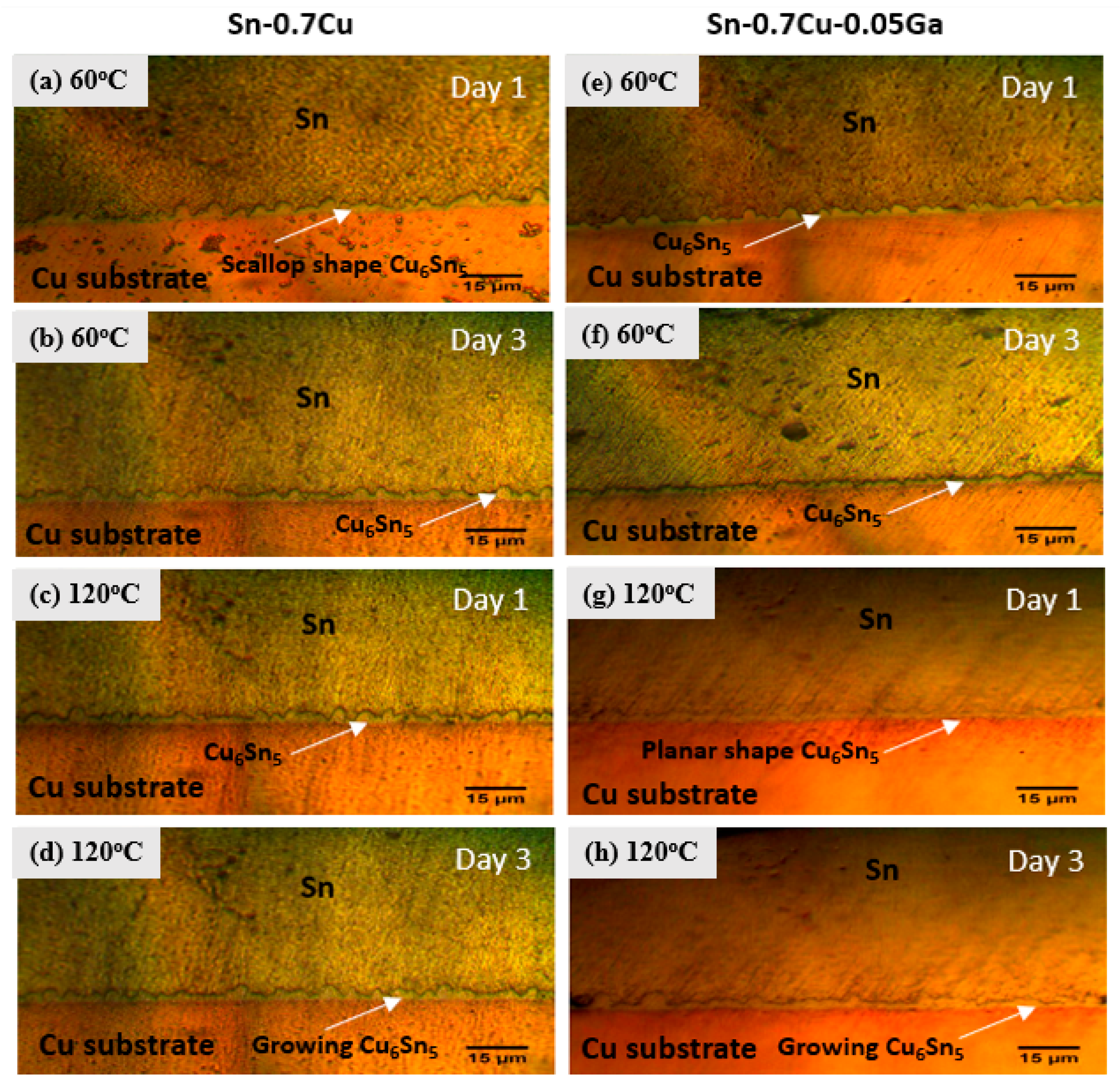
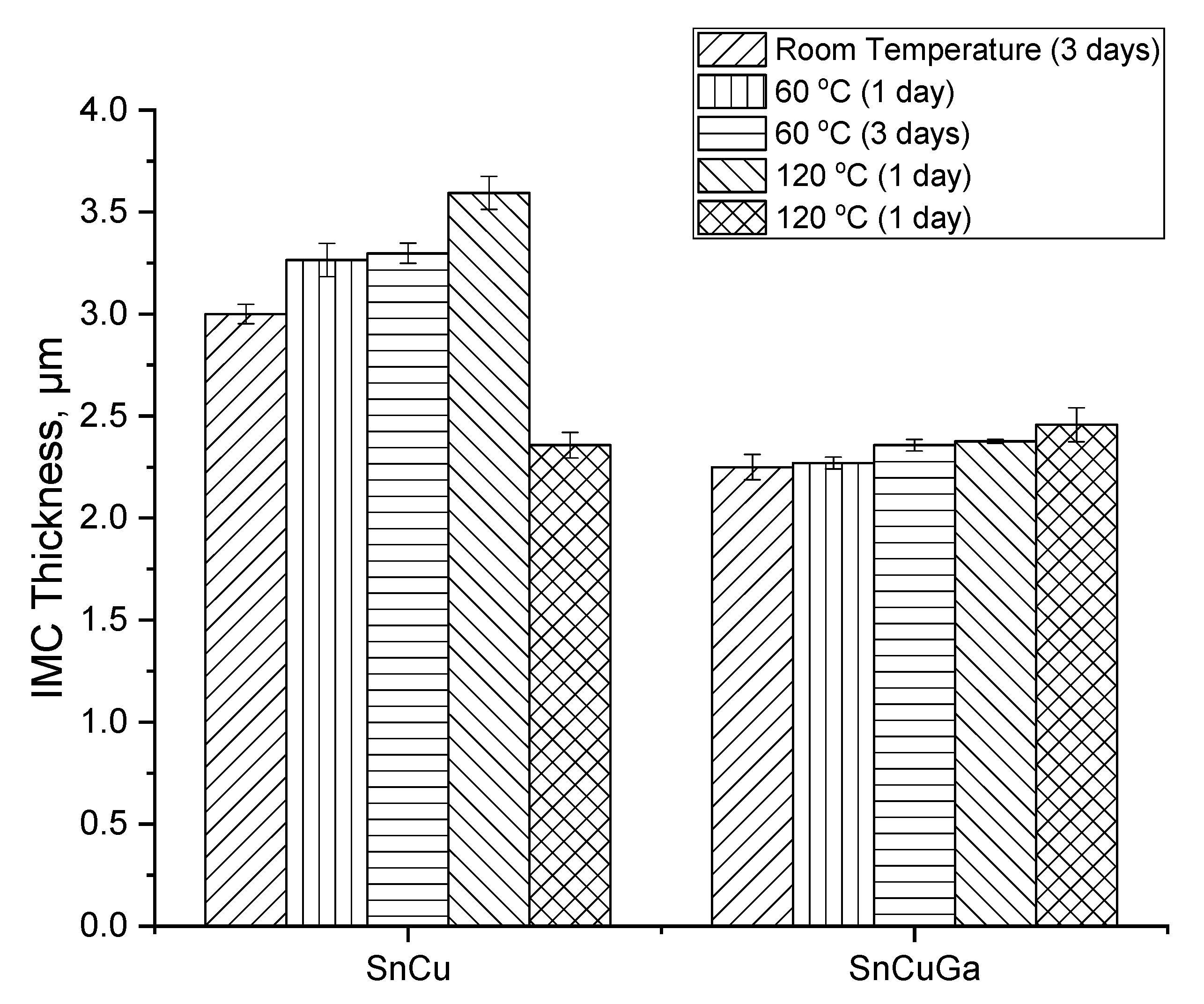
3.3. Interfacial Intermetallic Compound (IMC) under Electromigration and Thermal Ageing
3.4. Synchrotron Micro-XRF
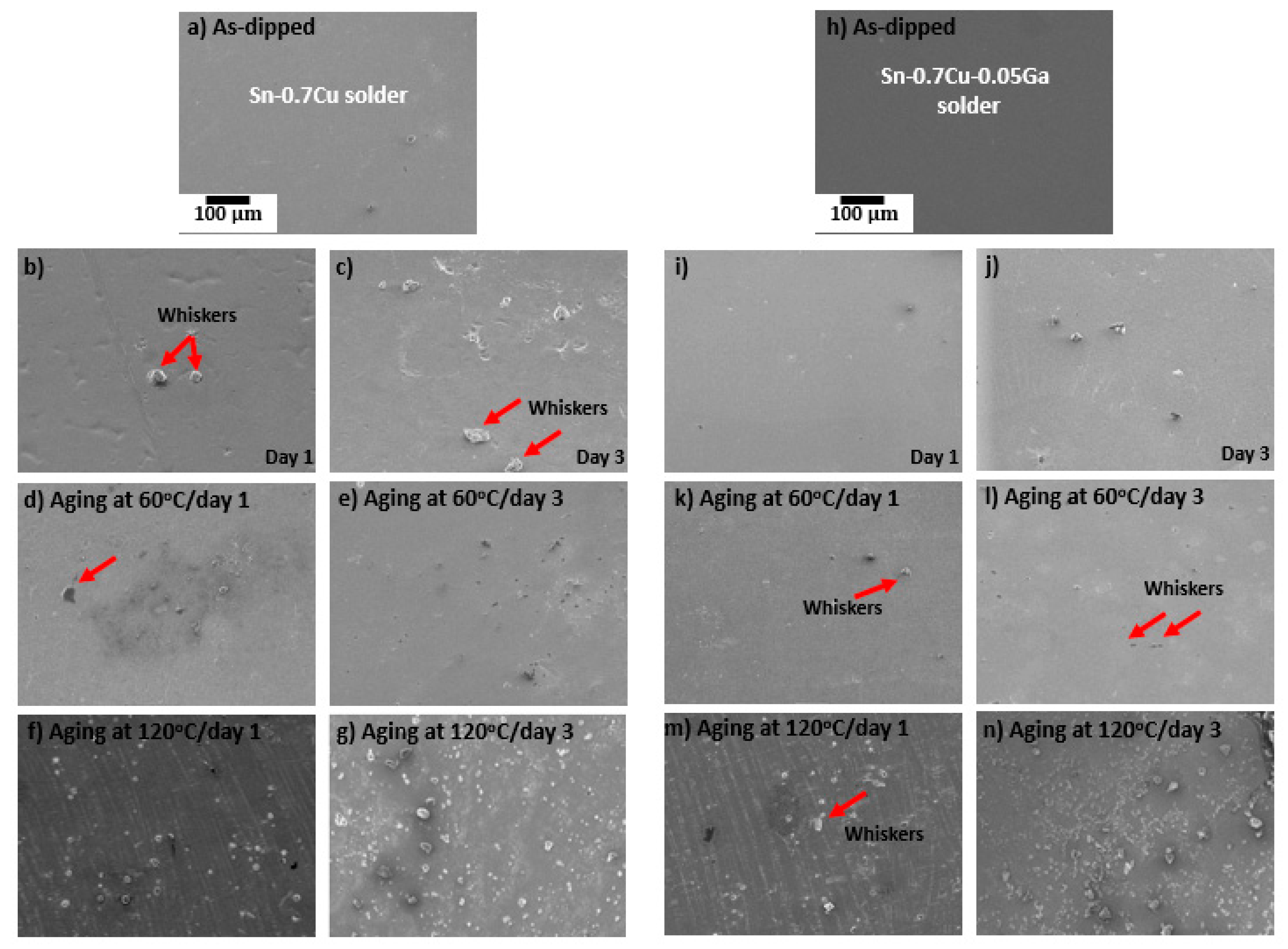
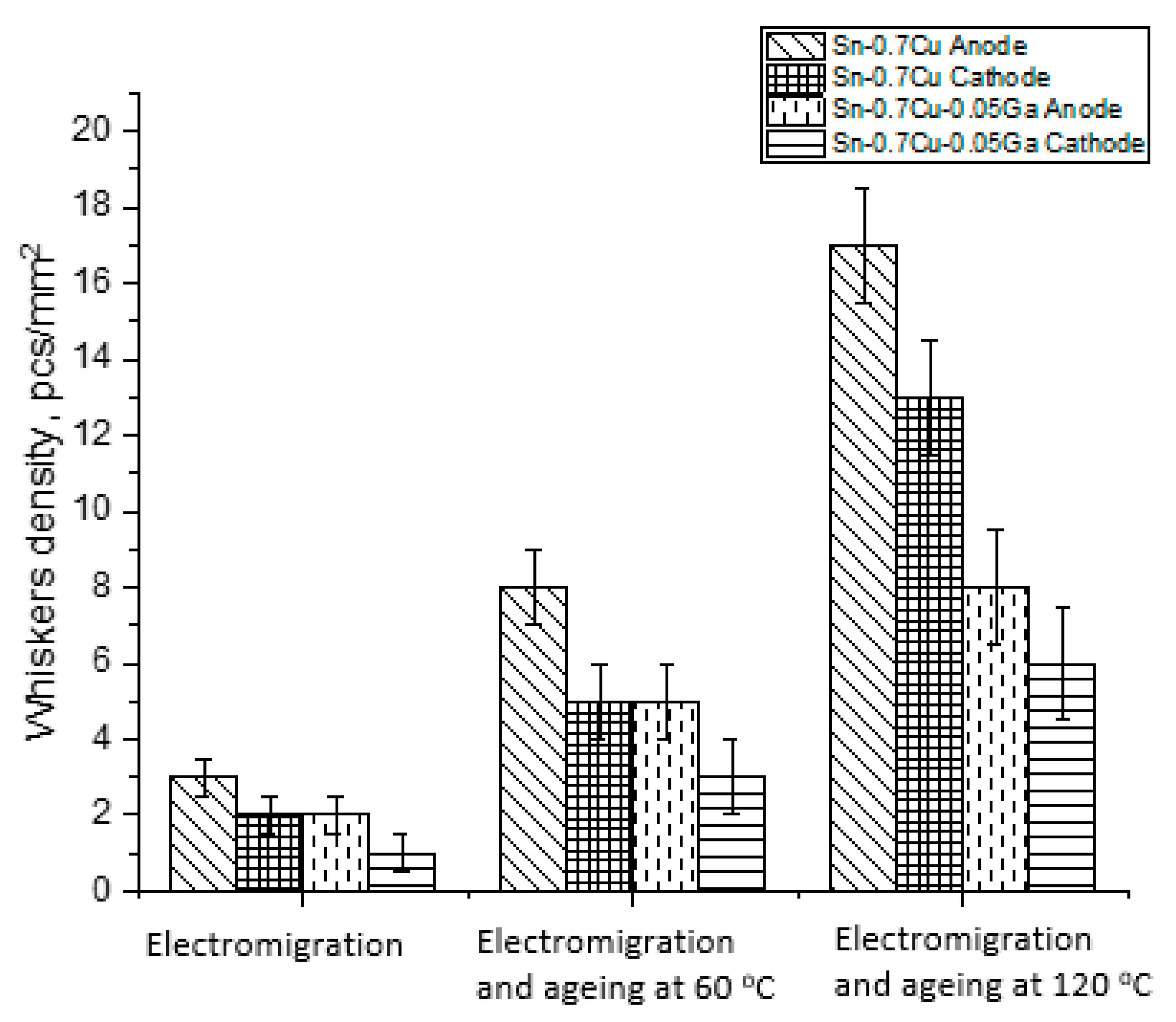
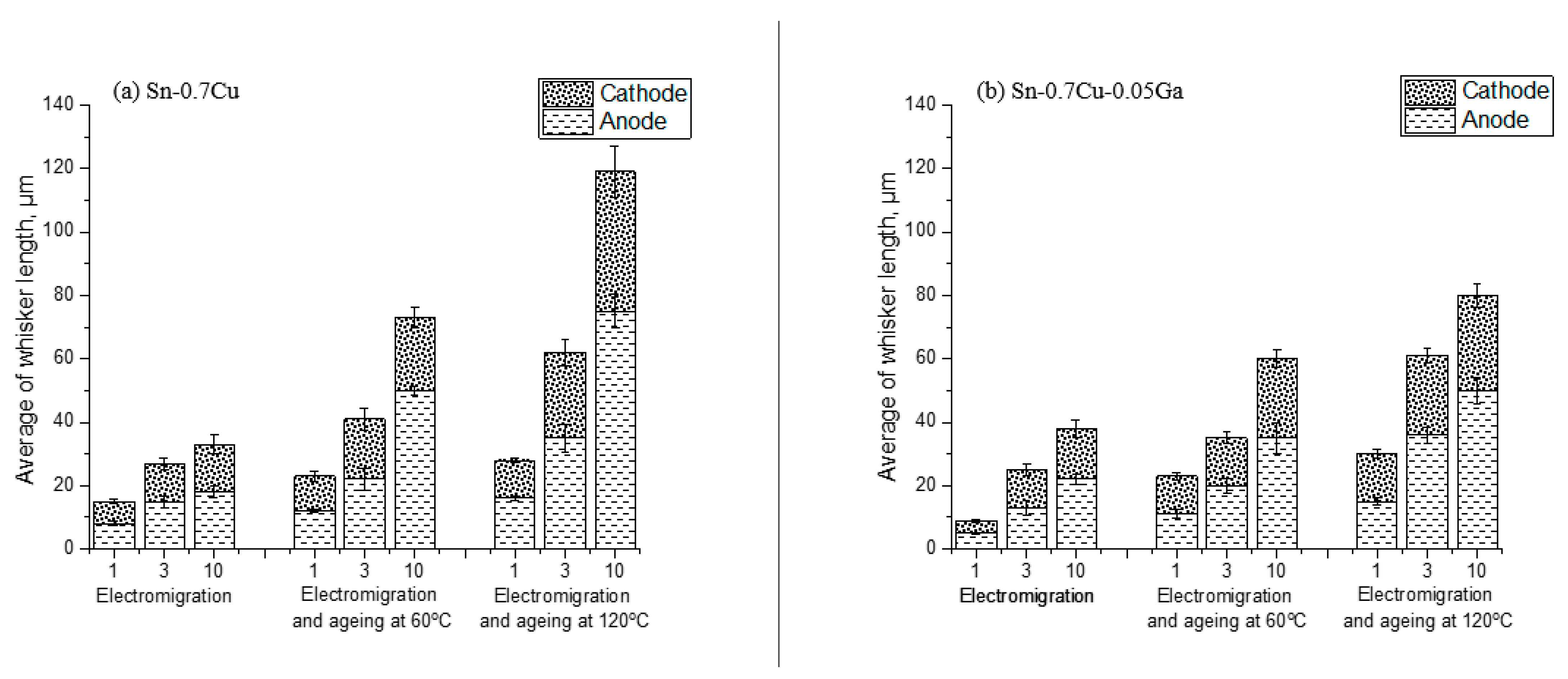
3.5. Properties of Tin Whiskers of the Solder
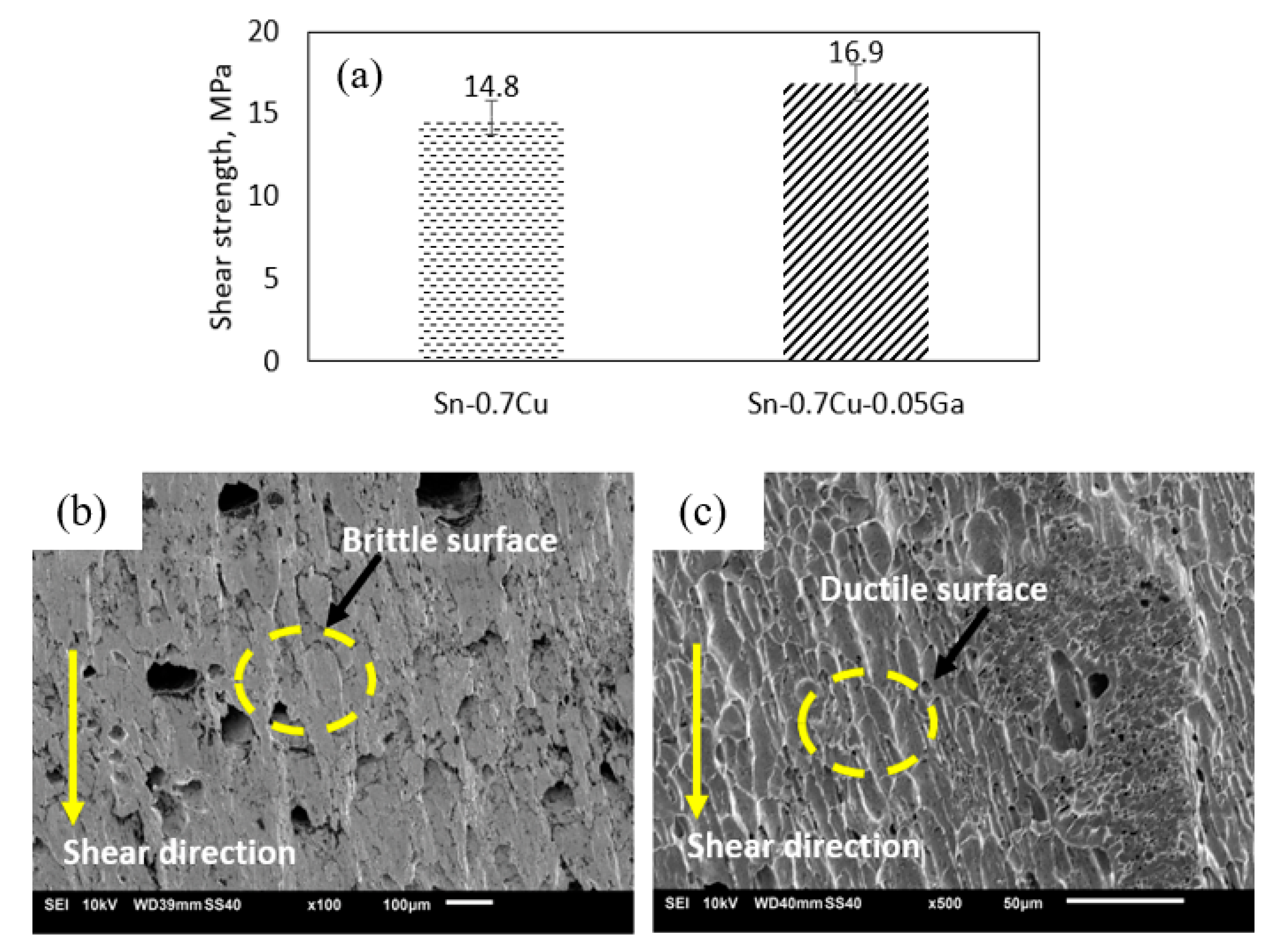
3.6. Mechanical Properties and Fracture Morphology
4. Conclusions
- The thickness of the IMC of Sn–0.7Cu and Sn–0.7Cu–0.05Ga increased from 0.6% to 4.2%, corresponding with the increasing number of ageing temperature and ageing time.
- Whiskers’ growth was reduced with the addition of 0.05 wt.% of Ga, which also acted as a solid-solution strengthening agent.
- The micro-XRF analysis showed the elemental distribution of Ga in Sn–0.7Cu–0.05Ga solder both under electromigration with and without thermal ageing. The distribution of Ga in the matrix decreases whiskers’ growth in the Sn–0.7Cu–0.05Ga solder joint.
- The shear strength of the Sn–0.7Cu solder (14.8 MPa) improved by 14% due to Ga addition (~16.9 MPa).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arnold, S.M. Repressing the Growth of Tin Whiskers. Plating 1966, 53, 96–99. Available online: https://hlinstruments.com/RoHS_articles/1966_Arnold_Repressing_Growth_of_Tin_Whiskers.pdf (accessed on 2 August 2021).
- Liang, J.; Dariavach, N.; Shangguan, D. Tin whisker nucleation and growth on Sn-Pb eutectic coating layer inside plated through holes with press-fit pins. IEEE Trans. Compon. Packag. Technol. 2008, 31, 152–158. [Google Scholar] [CrossRef]
- Wei, C.C.; Liu, P.C.; Chen, C. Electromigration-induced Pb and Sn whisker growth in SnPb solder stripes. J. Mater. Res. 2008, 23, 2017–2022. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Zhang, H.; Wu, P. Whisker growth in Sn and SnPb thin films under electromigration. Vacuum 2014, 107, 103–107. [Google Scholar] [CrossRef]
- Tu, K.; Zeng, K. Tin–lead (SnPb) solder reaction in flip chip technology. Mater. Sci. Eng. R Rep. 2001, 34, 1–58. [Google Scholar] [CrossRef]
- Ku, A.; Oetinscitan, O.; Saphores, J.-D.; Shapirod, A.; Schoenunp, J.M. Lead-free solders. Issues of toxicity, availability and impacts of extraction. In Proceedings of the 53rd Electronic Components and Technology Conference, New Orleans, LA, USA, 27–30 May 2003. [Google Scholar]
- Morris, J.W.; Goldstein, J.L.F.; Mei, Z. Microstructure and mechanical properties of Sn-In and Sn-Bi solders. JOM 1993, 45, 25–27. [Google Scholar] [CrossRef]
- Ramli, M.I.I.; Mohd Salleh, M.A.A.; Yasuda, H.; Chaiprapa, J.; Nogita, K. The effect of Bi on the microstructure, electrical, wettability and mechanical properties of Sn-0.7Cu-0.05Ni alloys for high strength soldering. Mater. Des. 2019, 186, 108281. [Google Scholar] [CrossRef]
- Xian, J.W.; Mohd Salleh, M.A.A.; Belyakov, S.A.; Su, T.C.; Zeng, G.; Nogita, K.; Gourlay, C.M. Influence of Ni on the refinement and twinning of primary Cu6Sn5 in Sn-0.7Cu-0.05Ni. Intermetallics 2018, 102, 34–45. [Google Scholar] [CrossRef]
- Cheng, S.; Huang, C.M.; Pecht, M. A review of lead-free solders for electronics applications. Microelectron. Reliab. 2017, 75, 77–95. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, L.; Liu, Z.-Q.; Xiong, M.-Y.; Sun, L. Structure and properties of Sn-Cu lead-free solders in electronics packaging. Sci. Technol. Adv. Mater. 2019, 20, 421–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Illés, B.; Krammer, O.; Hurtony, T.; Dušek, K.; Bušek, D.; Skwarek, A. Kinetics of Sn whisker growth from Sn thin-films on Cu substrate. J. Mater. Sci. Mater. Electron. 2020, 31, 16314–16323. [Google Scholar] [CrossRef]
- Chason, E.; Jadhav, N.; Pei, F.; Buchovecky, E.; Bower, A. Growth of whiskers from Sn surfaces: Driving forces and growth mechanisms. Prog. Surf. Sci. 2013, 88, 103–131. [Google Scholar] [CrossRef]
- Somidin, F.; Maeno, H.; Salleh, M.M.; Tran, X.Q.; McDonald, S.D.; Matsumura, S.; Nogita, K. Characterising the polymorphic phase transformation at a localised point on a Cu6Sn5 grain. Mater. Charact. 2018, 138, 113–119. [Google Scholar] [CrossRef]
- Hashim, A.N.; Salleh, M.A.A.M.; Mokhtar, N.Z.M.; Idris, S. Tin (Sn) whisker growth from electroplated Sn finished. IOP Conf. Ser. Mater. Sci. Eng. 2019, 701, 012005. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, Y.; Fan, C.; Abys, J.A. Driving force for the formation of Sn whiskers: Compressive stress-pathways for its generation and remedies for its elimination and minimization. IEEE Trans. Electron. Packag. Manuf. 2005, 28, 31–35. [Google Scholar]
- Horváth, B. Influence of copper diffusion on the shape of whiskers grown on bright tin layers. Microelectron. Reliab. 2013, 53, 1009–1020. [Google Scholar] [CrossRef]
- Hsu, T.-Y.; Chang, J.-Y.; Chang, H.-M.; Ouyang, F.-Y. Electromigration induced spontaneous Ag whisker growth in fine Ag-alloy bonding interconnects: Novel polarity effect. Mater. Lett. 2016, 182, 55–58. [Google Scholar] [CrossRef]
- Mokhtar, N.Z.M.; Salleh, M.A.A.M.; Hashim, N.M. Preliminary Study of Electrical Current Stressing on Tin Whisker Formation. IOP Conf. Ser. Mater. Sci. Eng. 2019, 551, 012096. [Google Scholar] [CrossRef]
- Jin, Z.; Shen, Y.-A.; He, S.; Zhou, S.; Chan, Y.C.; Nishikawa, H. Novel polarity effect on intermetallic compound thickness changes during electromigration in Cu/Sn-3.0Ag-0.5Cu/Cu solder joints. J. Appl. Phys. 2019, 126, 185109. [Google Scholar] [CrossRef]
- Liu, S.H.; Chen, C.; Liu, P.C.; Chou, T. Tin whisker growth driven by electrical currents. J. Appl. Phys. 2004, 95, 7742–7747. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, Y.; Osterman, M.; Pecht, M. The impact of electrical current, mechanical bending, and thermal annealing on tin whisker growth. Microelectron. Reliab. 2007, 47, 88–92. [Google Scholar] [CrossRef]
- Lin, S.K.; Liu, Y.C.; Chiu, S.J.; Liu, Y.T.; Lee, H.Y. The electromigration effect revisited: Non-uniform local tensile stress-driven diffusion. Sci. Rep. 2017, 7, 3082. [Google Scholar] [CrossRef]
- Guo, F.; Xu, G.; He, H.; Zhao, M.; Sun, J.; Wang, C.H. Effect of Electromigration and Isothermal Aging on the Formation of Metal Whiskers and Hillocks in Eutectic Sn-Bi Solder Joints and Reaction Films. J. Electron. Mater. 2009, 38, 2647. [Google Scholar] [CrossRef]
- Zhang, Q.K.; Long, W.M.; Yu, X.Q.; Pei, Y.Y.; Qiao, P.X. Effects of Ga addition on microstructure and properties of Sn–Ag–Cu/Cu solder joints. J. Alloy. Compd. 2015, 622, 973–978. [Google Scholar] [CrossRef]
- Ramli, M.I.I.; Mohd Salleh, M.A.A.; Mohd Sobri, F.A.; Narayanan, P.; Sweatman, K.; Nogita, K. Relationship between free solder thickness to the solderability of Sn–0.7Cu–0.05Ni solder coating during soldering. J. Mater. Sci. Mater. Electron. 2019, 30, 3669–3677. [Google Scholar]
- Somidin, F.; Salleh, M.A.A.; Ahmad, K.R. Intermetallic compound formation on solder alloy/cu-substrate interface using lead-free Sn-0.7Cu/recycled-aluminum composite solder. J. Adv. Mater. Res. 2013, 620, 105–111. [Google Scholar] [CrossRef]
- Yu, H.; Xin, Y.; Wang, M.; Liu, Q. Hall-Petch relationship in Mg alloys: A review. J. Mater. Sci. Technol. 2018, 34, 248–256. [Google Scholar] [CrossRef]
- Chen, K.I.; Cheng, S.C.; Cheng, C.H.; Wu, S.; Jiang, Y.-L.; Cheng, T.-C. The Effects of Gallium Additions on Microstructures and Thermal and Mechanical Properties of Sn-9Zn Solder Alloys. Adv. Mater. Sci. Eng. 2014, 2014, 606814. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.T.; Hsu, H.H.; Wu, A.T. Electromigration-induced back stress in critical solder length for three-dimensional integrated circuits. J. Appl. Phys. 2014, 115, 034904. [Google Scholar] [CrossRef]
- Lin, Y.W.; Lai, Y.S.; Lin, Y.L.; Tu, C.T.; Kao, C.R. Tin Whisker Growth Induced by High Electron Current Density. J. Electron. Mater. 2008, 37, 17–22. [Google Scholar] [CrossRef]
- Wang, F.; Liu, L.; Li, D.; Wu, M. Electromigration behaviors in Sn-58Bi solder joints under different current densities and temperatures. J. Mater. Sci. Mater. Electron. 2018, 29, 21157–21169. [Google Scholar] [CrossRef]
- Abdelhadi, O.M.; Ladani, L. IMC growth of Sn-3.5Ag/Cu system: Combined chemical reaction and diffusion mechanisms. J. Alloy. Compd. 2012, 537, 87–99. [Google Scholar] [CrossRef]
- Yu, C.-F.; Chan, C.-M.; Hsieh, K.-C. The effect of tin grain structure on whisker growth. Microelectron. Reliab. 2010, 50, 1146–1151. [Google Scholar] [CrossRef]
- Li, M.Y.; Yang, H.F.; Zhang, Z.H.; Gu, J.H.; Yang, S.H. Fast formation and growth of high-density Sn whiskers in Mg/Sn-based solder/Mg joints by ultrasonic-assisted soldering: Phenomena, mechanism and prevention. Sci. Rep. 2016, 6, 27522. [Google Scholar] [CrossRef] [Green Version]
- Jo, J.-L.; Nagao, S.; Hamasaki, K.; Tsujimoto, M.; Sugahara, T.; Suganuma, K. Mitigation of Sn Whisker Growth by Small Bi Additions. J. Electron. Mater. 2013, 43, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Sarobol, P.; Pedigo, A.; Koppes, J.; Wang, Y.; Chen, W.; Su, P.; Handwerker, C. Whisker Formation and Stress Relaxation in Tin Thin Films. Microsc. Microanal. 2011, 17, 1846–1847. [Google Scholar] [CrossRef] [Green Version]





| Test Type | Period | Temperature |
|---|---|---|
| Electromigration | 1 day, 3 days | 30 °C |
| Electromigration and ageing | 1, 3, and 10 days | 60 °C, 120 °C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokhtar, N.Z.M.; Salleh, M.A.A.M.; Sandu, A.V.; Ramli, M.M.; Chaiprapa, J.; Vizureanu, P.; Ramli, M.I.I. Effect of Electromigration and Thermal Ageing on the Tin Whiskers’ Formation in Thin Sn–0.7Cu–0.05Ga Lead (Pb)-Free Solder Joints. Coatings 2021, 11, 935. https://doi.org/10.3390/coatings11080935
Mokhtar NZM, Salleh MAAM, Sandu AV, Ramli MM, Chaiprapa J, Vizureanu P, Ramli MII. Effect of Electromigration and Thermal Ageing on the Tin Whiskers’ Formation in Thin Sn–0.7Cu–0.05Ga Lead (Pb)-Free Solder Joints. Coatings. 2021; 11(8):935. https://doi.org/10.3390/coatings11080935
Chicago/Turabian StyleMokhtar, Noor Zaimah Mohd, Mohd Arif Anuar Mohd Salleh, Andrei Victor Sandu, Muhammad Mahyiddin Ramli, Jitrin Chaiprapa, Petrica Vizureanu, and Mohd Izrul Izwan Ramli. 2021. "Effect of Electromigration and Thermal Ageing on the Tin Whiskers’ Formation in Thin Sn–0.7Cu–0.05Ga Lead (Pb)-Free Solder Joints" Coatings 11, no. 8: 935. https://doi.org/10.3390/coatings11080935
APA StyleMokhtar, N. Z. M., Salleh, M. A. A. M., Sandu, A. V., Ramli, M. M., Chaiprapa, J., Vizureanu, P., & Ramli, M. I. I. (2021). Effect of Electromigration and Thermal Ageing on the Tin Whiskers’ Formation in Thin Sn–0.7Cu–0.05Ga Lead (Pb)-Free Solder Joints. Coatings, 11(8), 935. https://doi.org/10.3390/coatings11080935








