TiO2 Nanotubes Architectures for Solar Energy Conversion
Abstract
:1. Introduction
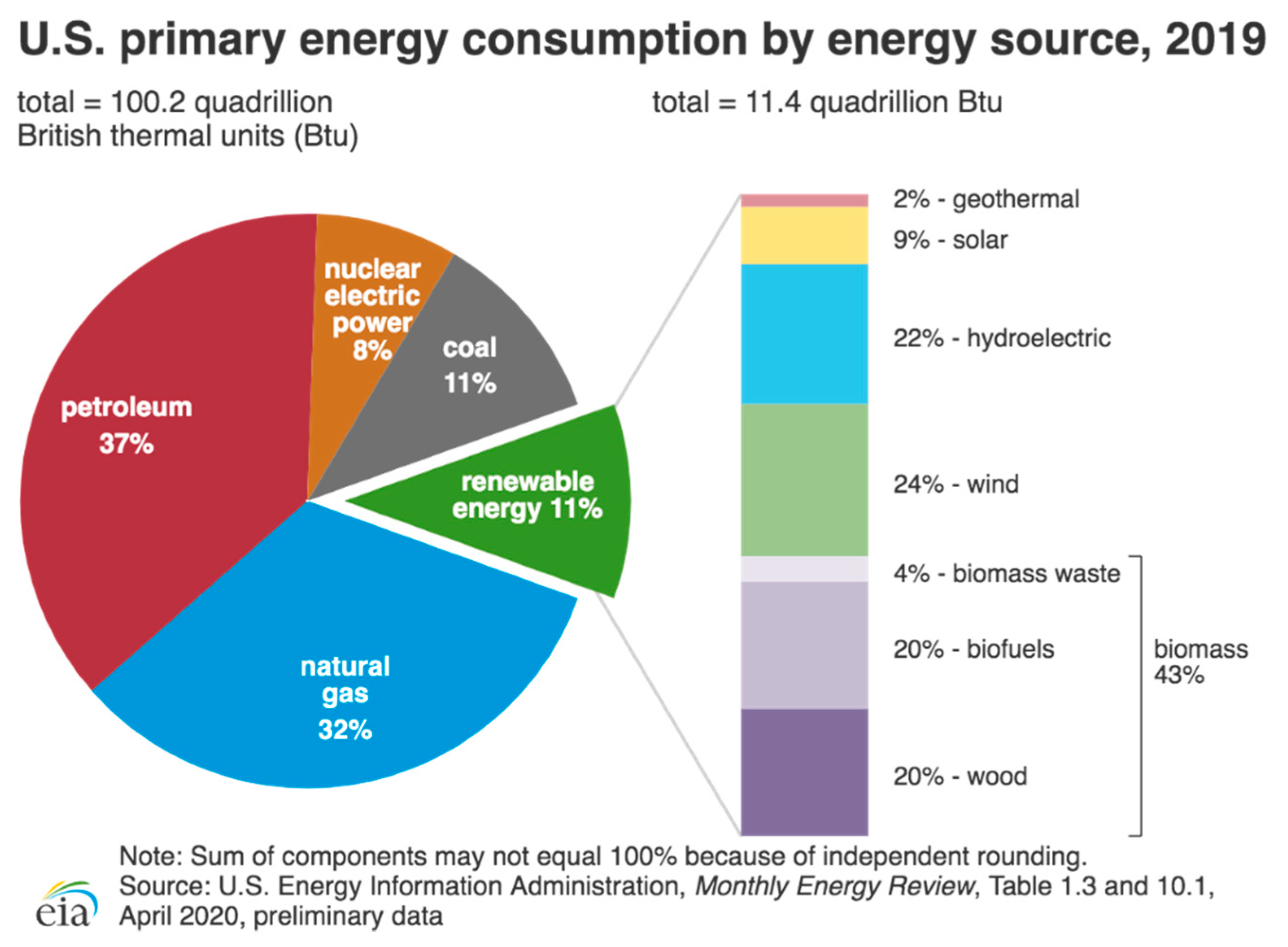
1.1. Water Splitting for Energy Conversion and Storage
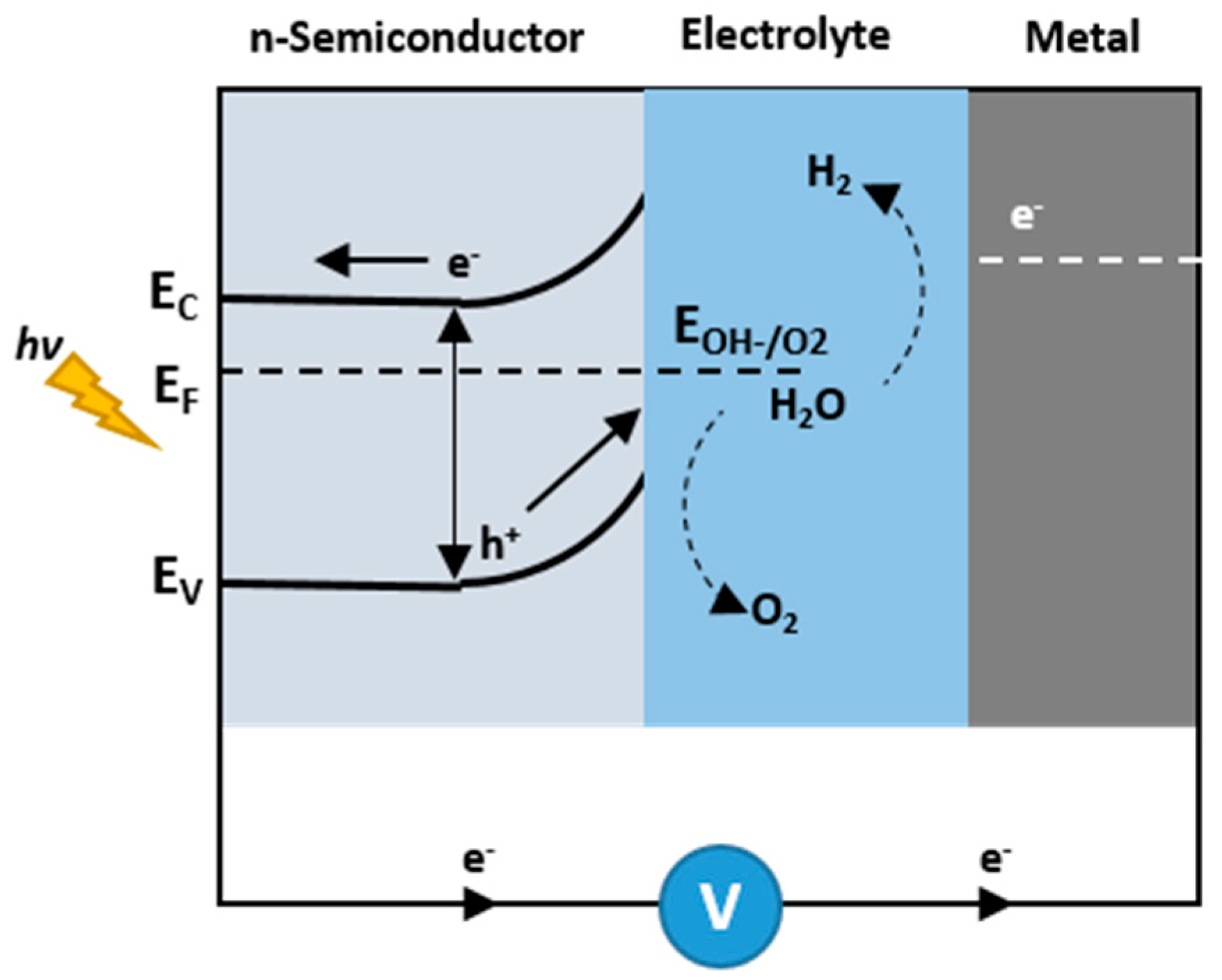
1.2. Photoelectrochemical Cells
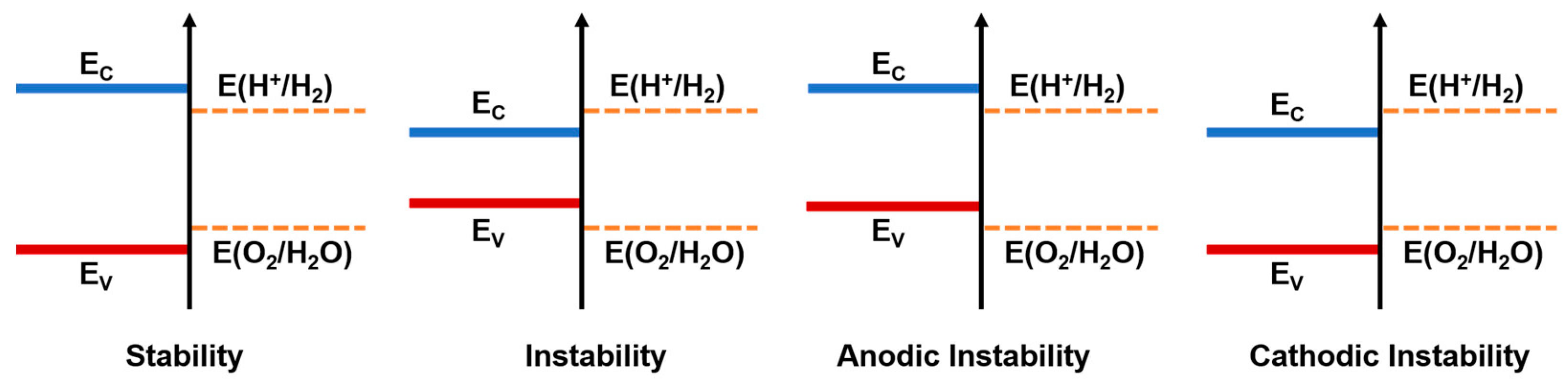
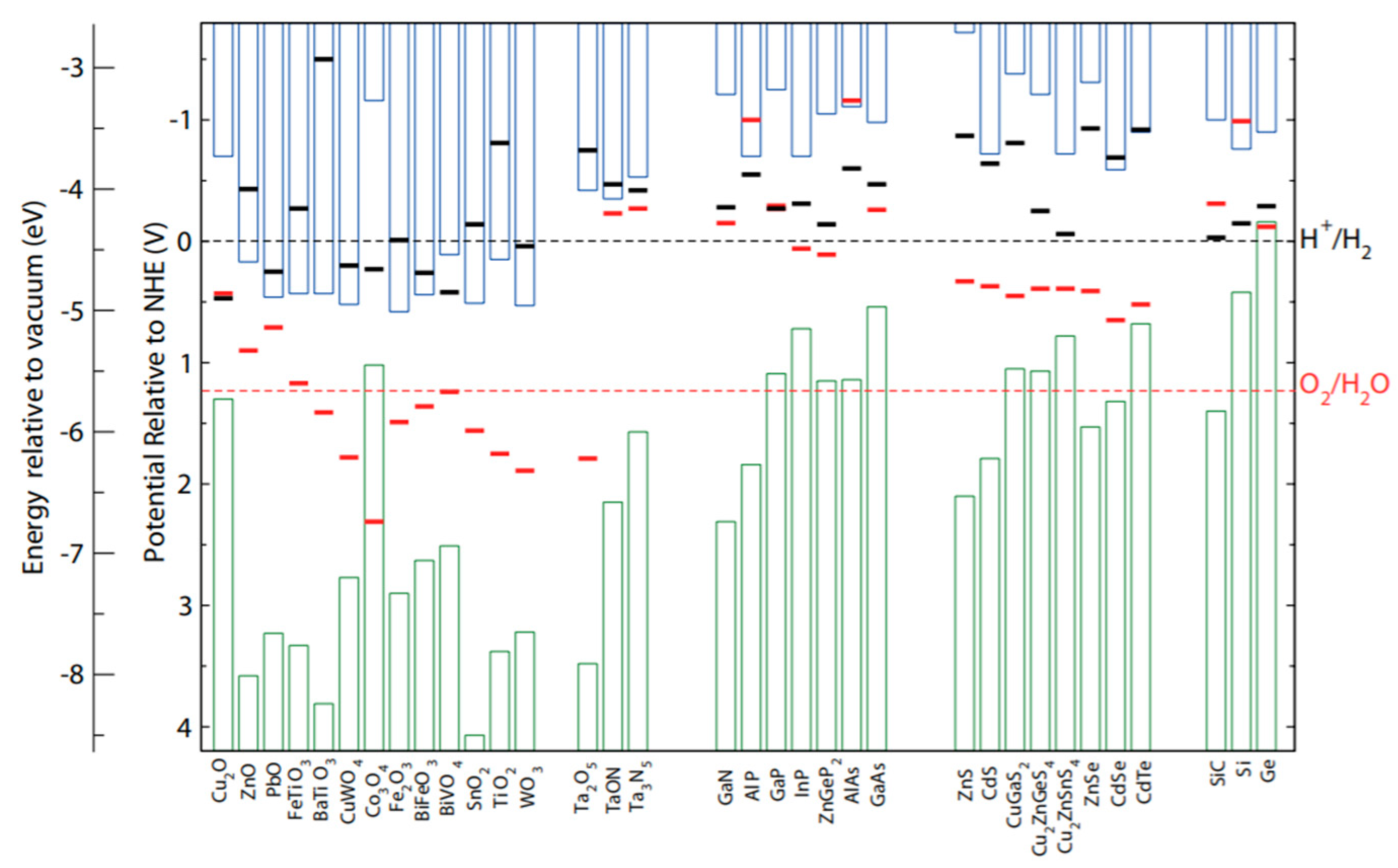
1.3. Semiconductors for Water Splitting
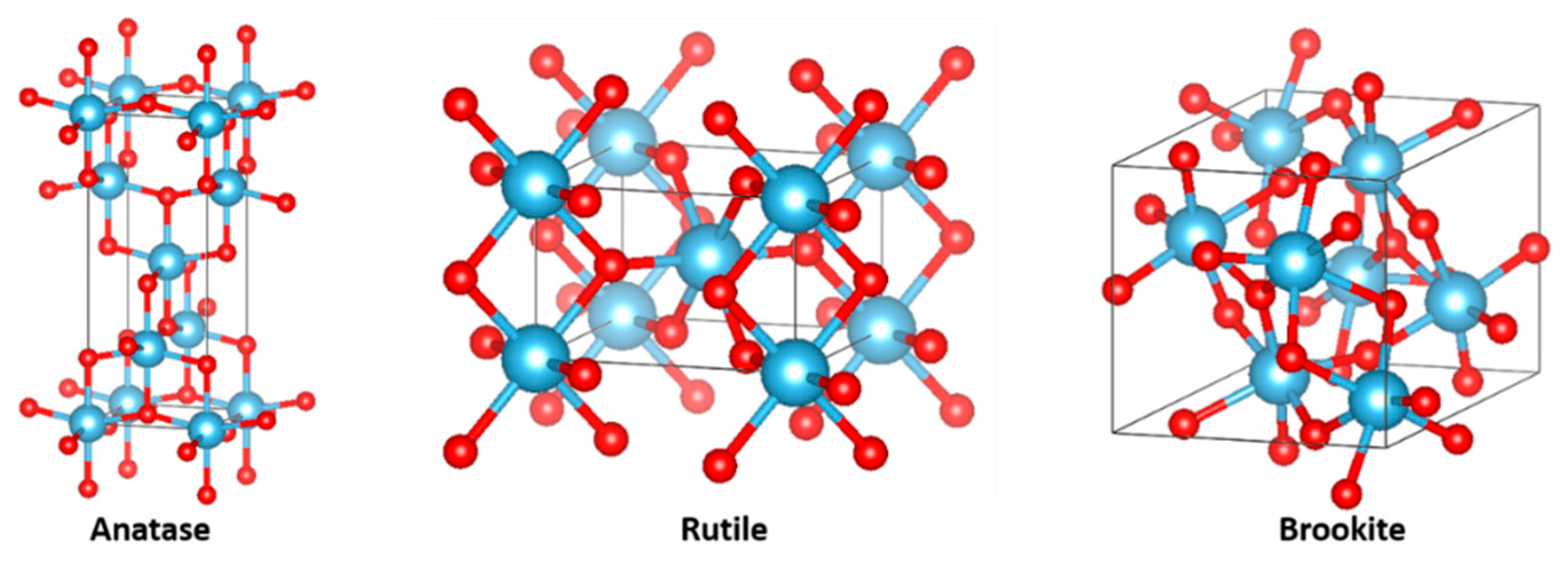
1.4. TiO2 as Photoanodes
2. Anodic TiO2 Nanotube Arrays (NTs)
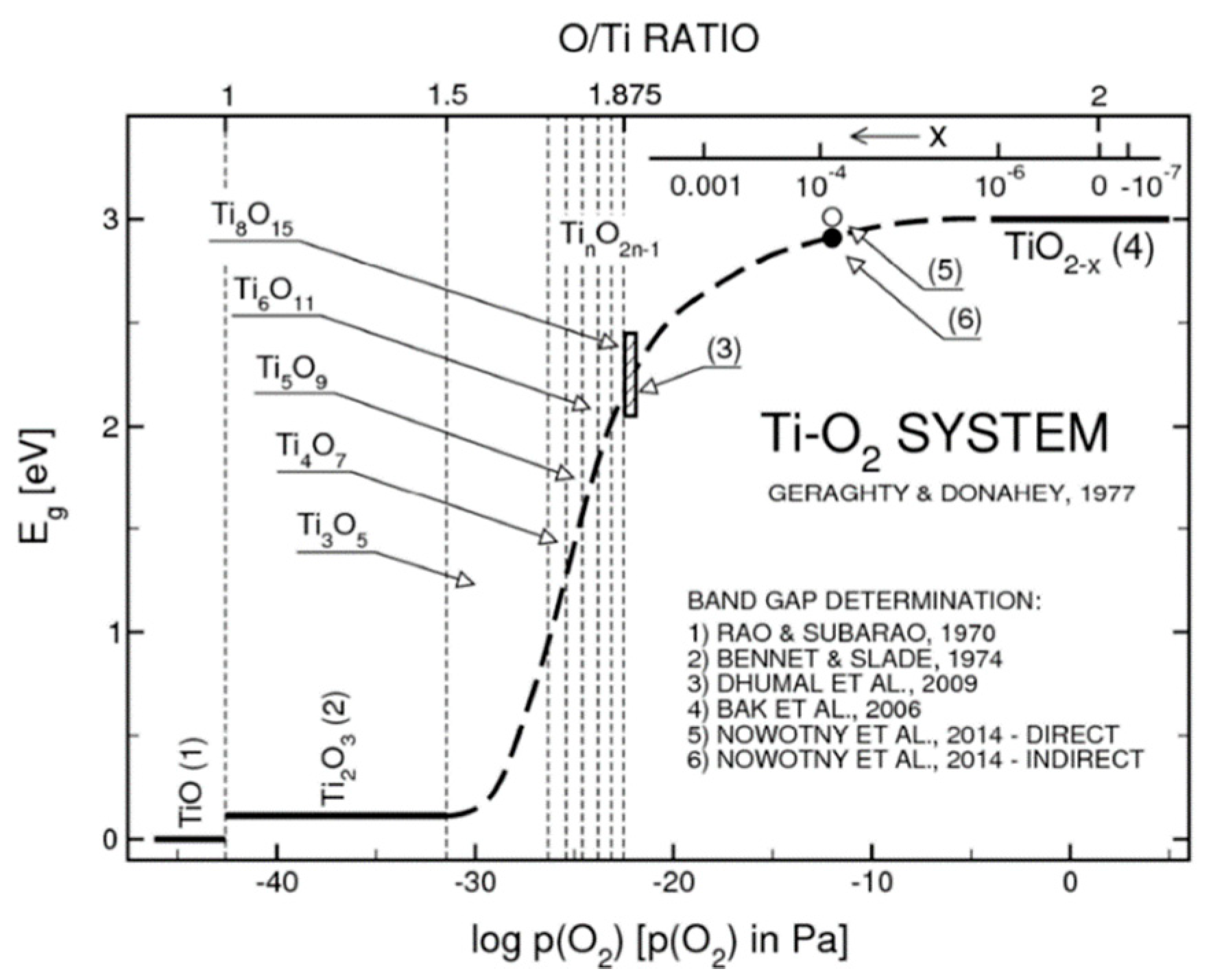
2.1. Defects in TiO2 and TiO2-x Sub-Stoichiometries
2.2. Water Oxidation at the Interface
2.3. Manipulating TiO2 Defects to Enhance Solar Conversion
3. Nanotubes and Their Modification
3.1. Nanotubes Formation and Modification
3.2. Characterization Methods
3.3. Evaluation for Water Oxidation Selectivity at Electrode/Electrolyte Interface
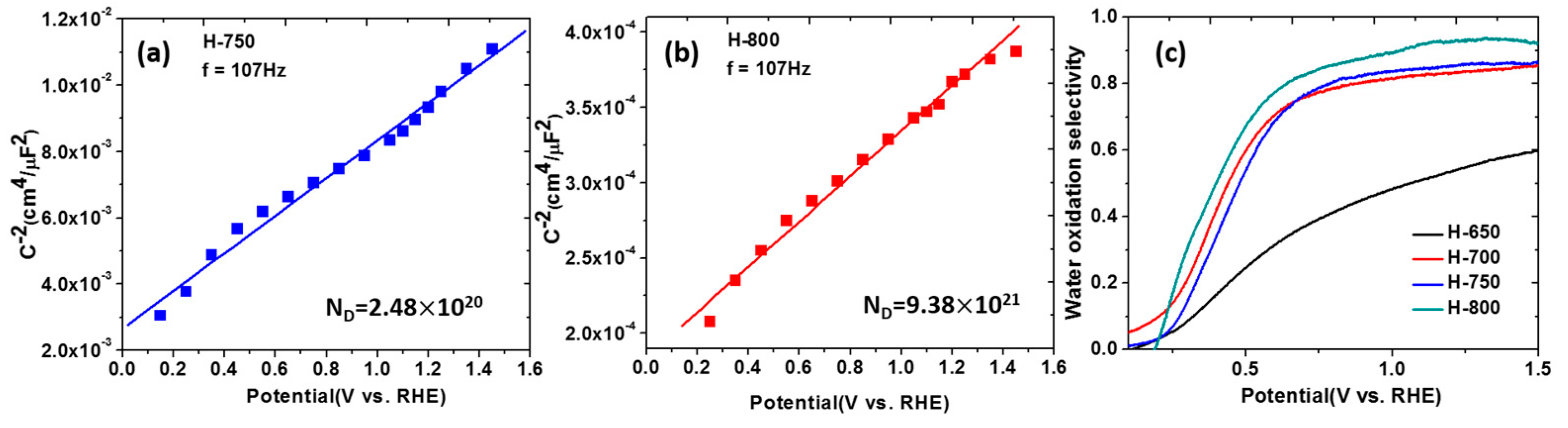
3.4. Determining the Density of Defects and Flat-Band Potential on TiO2 NTs
4. Some Examples of Rational TiO2 Modification
4.1. Intercalation, Sensitization and Catalysis
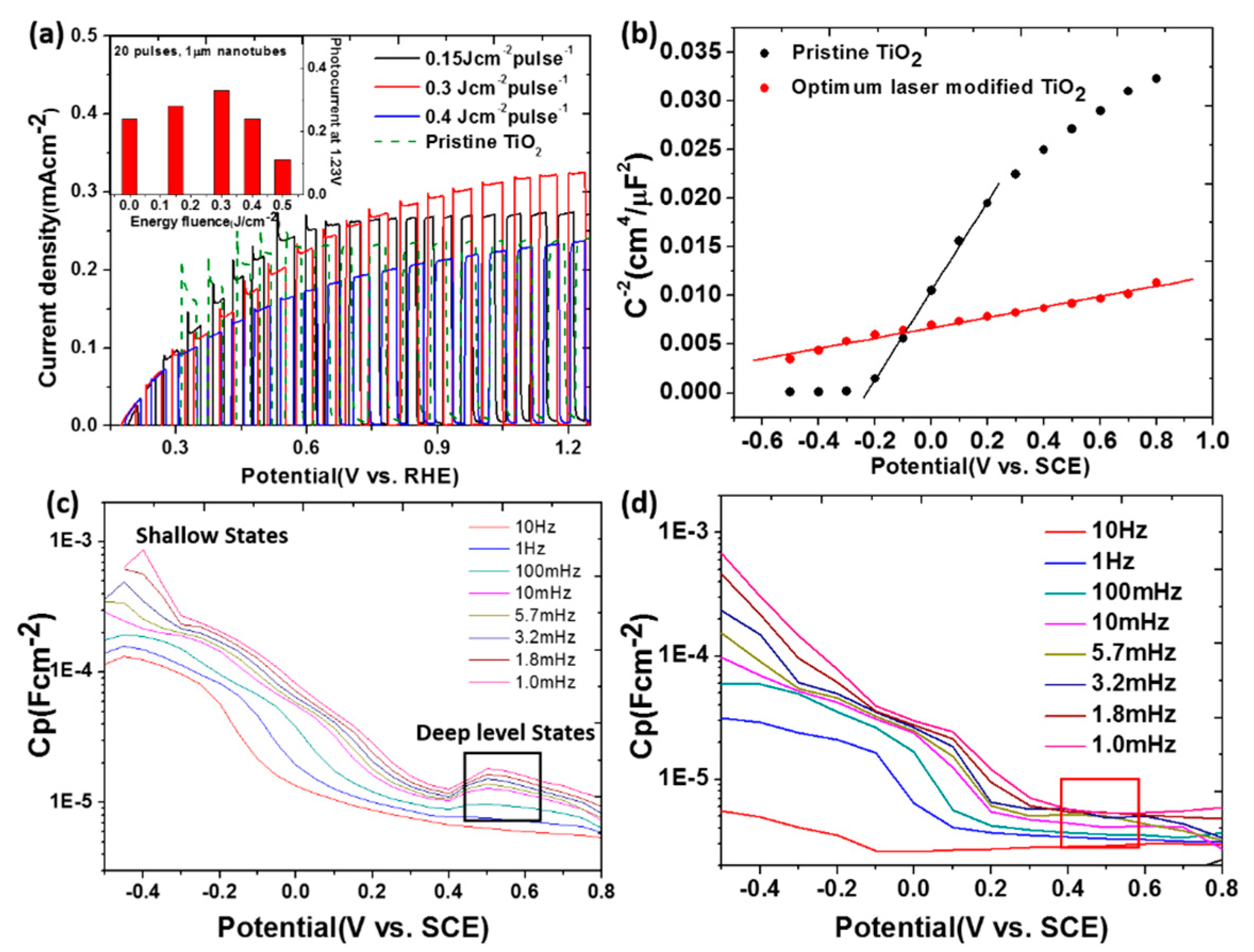
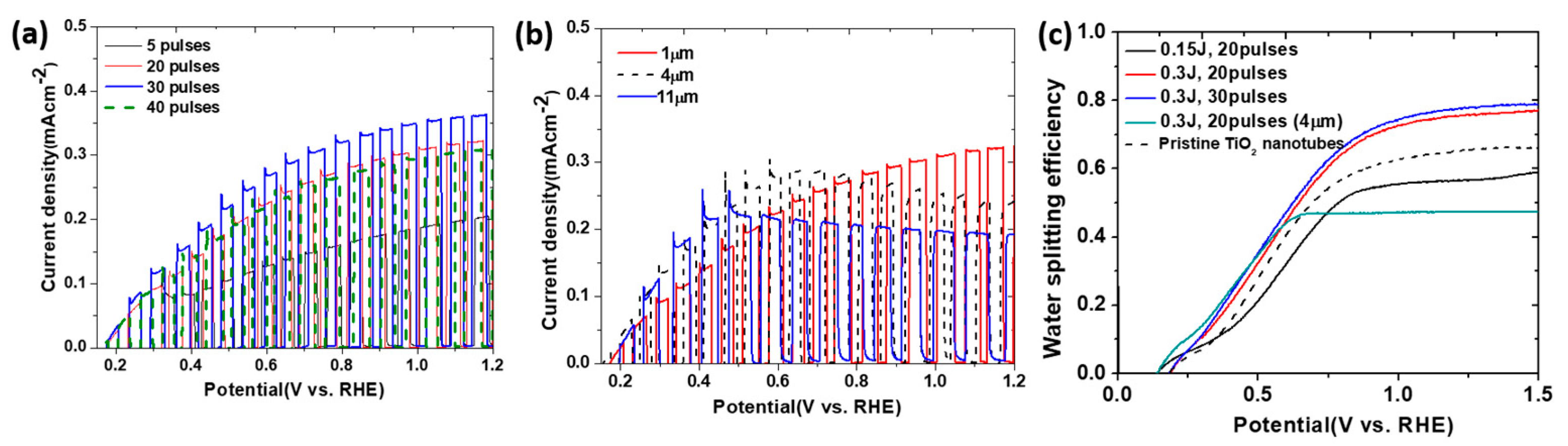
4.2. Laser Irradiation for Surface Modification
4.3. Phase Transformation of TiO2 for PEC
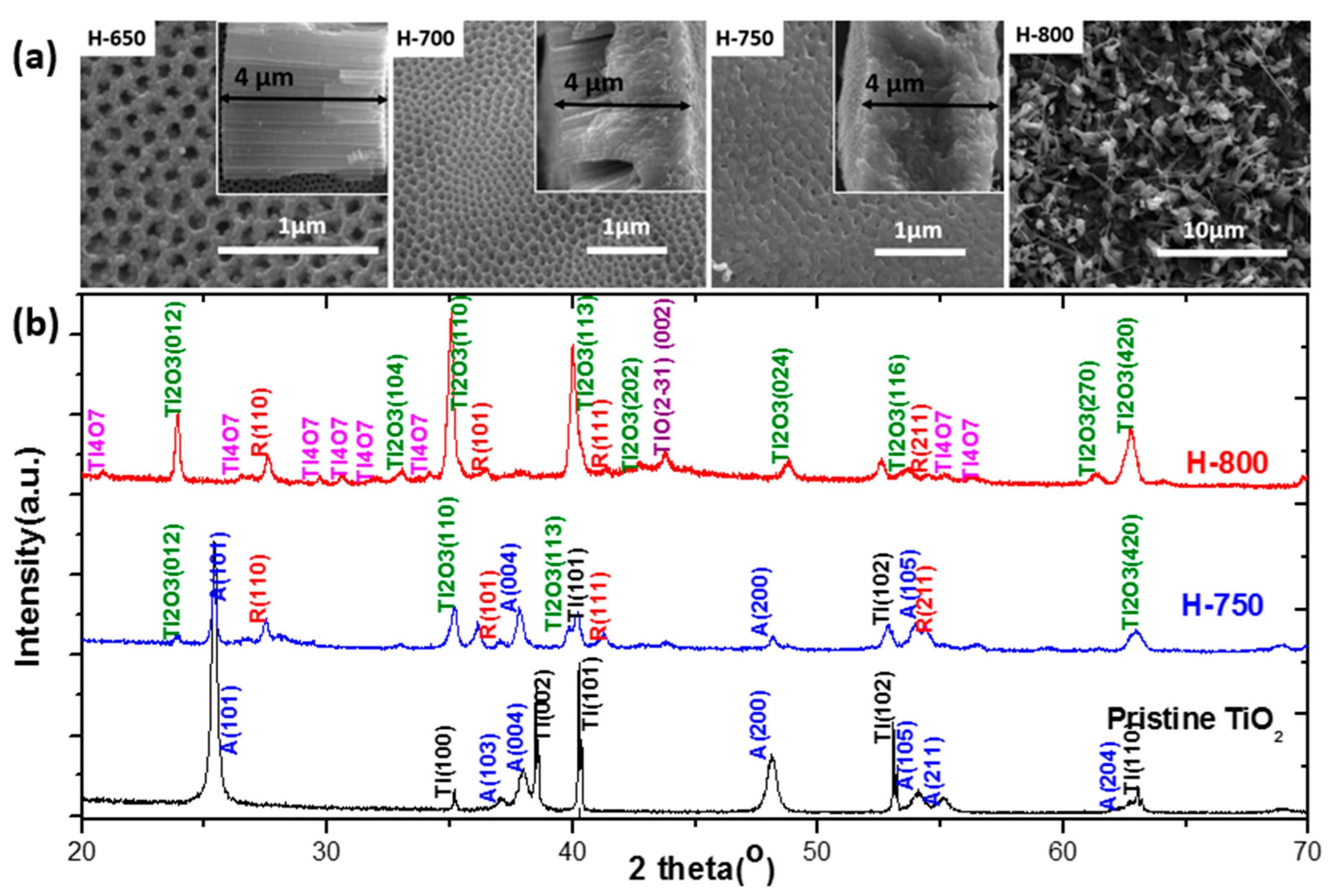
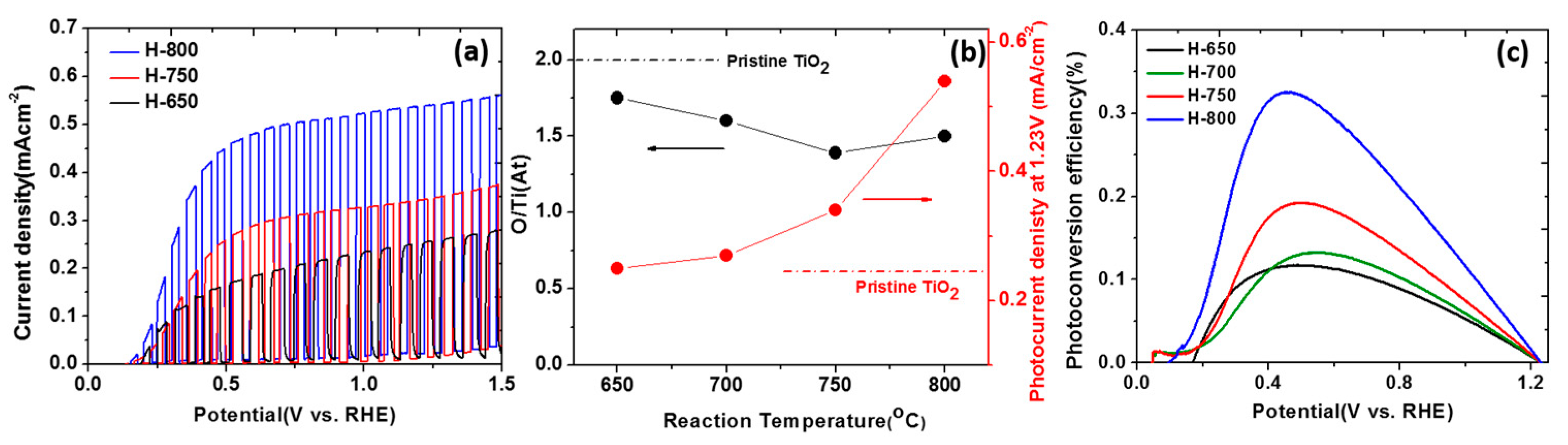
4.4. Introduction of Ti3+ and Oxygen Vacancies in TiO2 NTs
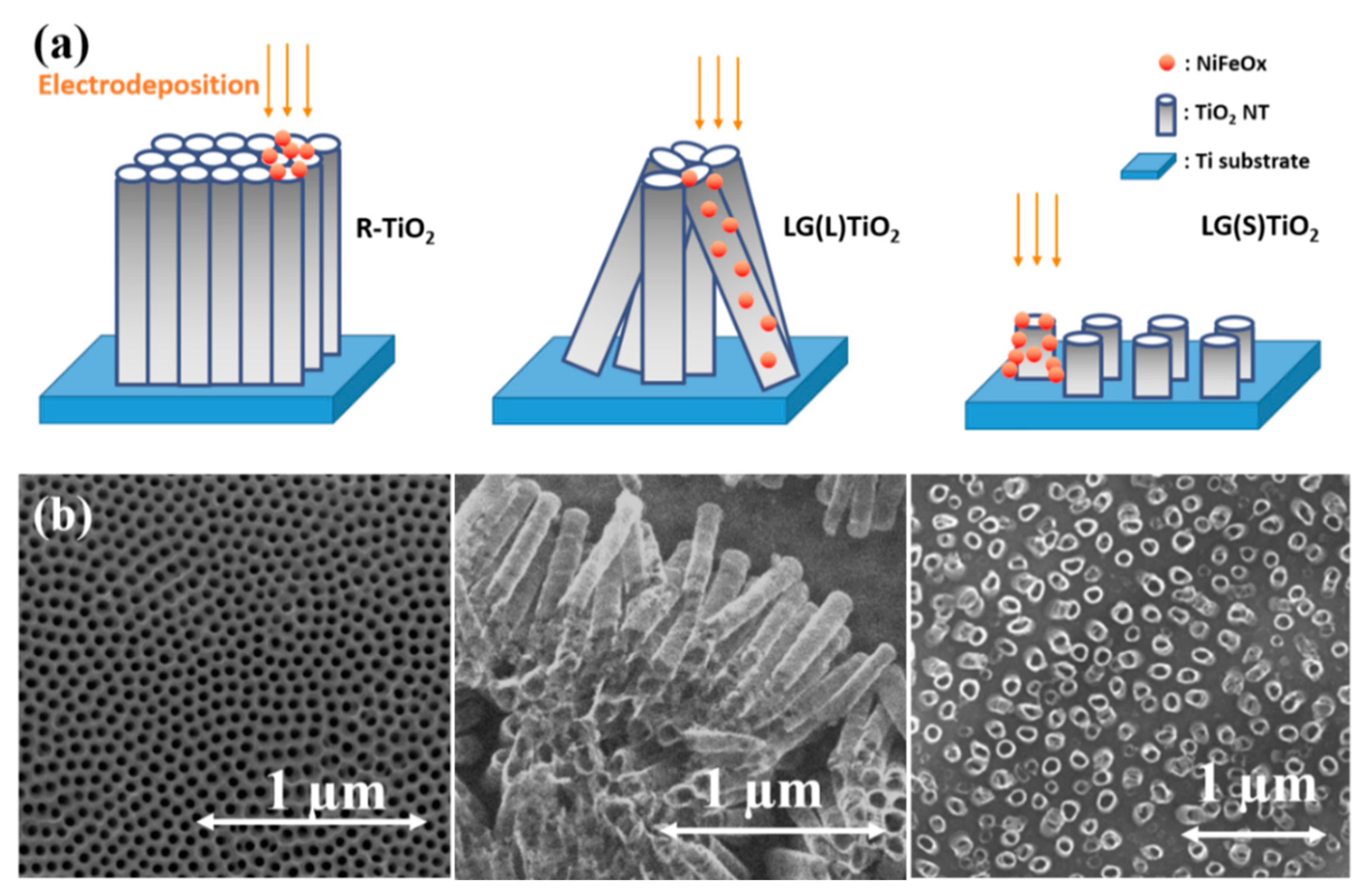
4.5. Deposition of Electrocatalysts on TiO2 NTs Surface
4.6. Black TiO2: Improved Performance
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balat, M.; Balat, H. Biogas as a renewable energy source—A review. Energy Sources Part A 2009, 31, 1280–1293. [Google Scholar] [CrossRef]
- Lewis, N.S.; Nocera, D.G. Powering the planet: Chemical challenges in solar energy utilization. Proc. Natl. Acad. Sci. USA 2006, 103, 15729–15735. [Google Scholar] [CrossRef] [Green Version]
- Eisenberg, R.; Gray, H.B.; Crabtree, G.W. Addressing the challenge of carbon-free energy. Proc. Natl. Acad. Sci. USA 2019, 117, 12543–12549. [Google Scholar] [CrossRef] [Green Version]
- Panwar, N.; Kaushik, S.; Kothari, S. Role of renewable energy sources in environmental protection: A review. Renew. Sustain. Energy Rev. 2011, 15, 1513–1524. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef]
- Gray, H.B. Powering the planet with solar fuel. Nat. Chem. 2009, 1, 7. [Google Scholar] [CrossRef] [PubMed]
- Holladay, J.; Hu, J.; King, D.; Wang, Y. An overview of hydrogen production technologies. Catal. Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- Nathan, S.L.; Crabtree, G.; Nozik, A.J.; Wasielewski, M.R.; Alivisatos, P. Basic Research Needs for Solar Energy Utilization: Report on the Basic Energy Sciences Workshop on Solar Energy Utilization; CIo Technology: Santa Barbara, CA, USA, 2005. [Google Scholar]
- Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Design of electrocatalysts for oxygen-and hydrogen-involving energy conversion reactions. Chem. Soc. Rev. 2015, 44, 2060–2086. [Google Scholar] [CrossRef]
- Gong, M.; Dai, H. A mini review of NiFe-based materials as highly active oxygen evolution reaction electrocatalysts. Nano Res. 2015, 8, 23–39. [Google Scholar] [CrossRef] [Green Version]
- Tahir, M.B.; Pan, L.; Idrees, F.; Zhang, X.; Wang, L.; Zou, J.-J.; Wang, Z.L. Electrocatalytic oxygen evolution reaction for energy conversion and storage: A comprehensive review. Nano Energy 2017, 37, 136–157. [Google Scholar] [CrossRef]
- Grätzel, M. Photoelectrochemical cells. Nat. Cell Biol. 2001, 414, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Tsui, L.-K.; Zangari, G. Titania nanotubes by electrochemical anodization for solar energy conversion. J. Electrochem. Soc. 2014, 161, D3066–D3077. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.; Leung, D.Y.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nat. Cell Biol. 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, L.-W. Thermodynamic oxidation and reduction potentials of photocatalytic semiconductors in aqueous solution. Chem. Mater. 2012, 24, 3659–3666. [Google Scholar] [CrossRef] [Green Version]
- Lin, Z.; Orlov, A.; Lambert, A.R.M.; Payne, M.C. New insights into the origin of visible light photocatalytic activity of nitrogen-doped and oxygen-deficient anatase TiO2. J. Phys. Chem. B 2005, 109, 20948–20952. [Google Scholar] [CrossRef]
- Thompson, T.L.; Yates, J.T. Surface science studies of the photoactivation of TiO2 new photochemical processes. Chem. Rev. 2006, 106, 4428–4453. [Google Scholar] [CrossRef]
- He, Y.; Tilocca, A.; Dulub, O.; Selloni, A.; Diebold, U. Local ordering and electronic signatures of submonolayer water on anatase TiO2(101). Nat. Mater. 2009, 8, 585–589. [Google Scholar] [CrossRef]
- Maeda, K. Direct splitting of pure water into hydrogen and oxygen using rutile titania powder as a photocatalyst. Chem. Commun. 2013, 49, 8404–8406. [Google Scholar] [CrossRef]
- Murphy, A.; Barnes, P.; Randeniya, L.; Plumb, I.; Grey, I.; Horne, M.; Glasscock, J.A. Efficiency of solar water splitting using semiconductor electrodes. Int. J. Hydrogen Energy 2006, 31, 1999–2017. [Google Scholar] [CrossRef]
- Tsui, L.-K.; Saito, M.; Homma, T.; Zangari, G. Trap-state passivation of titania nanotubes by electrochemical doping for enhanced photoelectrochemical performance. J. Mater. Chem. A 2014, 3, 360–367. [Google Scholar] [CrossRef]
- Houser, J.E.; Hebert, K.R. The role of viscous flow of oxide in the growth of self-ordered porous anodic alumina films. Nat. Mater. 2009, 8, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Hebert, K.R.; Albu, S.P.; Paramasivam, I.; Schmuki, P. Morphological instability leading to formation of porous anodic oxide films. Nat. Mater. 2012, 11, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Varghese, O.K.; Paulose, M.; Shankar, K.; Mor, G.K.; Grimes, C.A. Water-photolysis properties of micron-length highly-ordered titania nanotube-arrays. J. Nanosci. Nanotechnol. 2005, 5, 1158–1165. [Google Scholar] [CrossRef]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 nanotubes: Synthesis and applications. Angew. Chem. Int. Ed. 2011, 50, 2904–2939. [Google Scholar] [CrossRef] [PubMed]
- Diebold, U. The surface science of titanium dioxide. Surf. Sci. Rep. 2003, 48, 53–229. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Zuo, F.; Wang, L.; Wu, T.; Zhang, Z.; Borchardt, D.; Feng, P. Self-doped Ti3+ enhanced photocatalyst for hydrogen production under visible light. J. Am. Chem. Soc. 2010, 132, 11856–11857. [Google Scholar] [CrossRef] [PubMed]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Termin, A.; Hoffmann, M.R. The role of metal ion dopants in quantum-sized TiO2: Correlation between photoreactivity and charge carrier recombination dynamics. J. Phys. Chem. 1994, 98, 13669–13679. [Google Scholar] [CrossRef]
- Naldoni, A.; Allieta, M.; Santangelo, S.; Marelli, M.; Fabbri, F.; Cappelli, S.; Bianchi, C.; Psaro, R.; Santo, V.D. Effect of nature and location of defects on bandgap narrowing in black TiO2 nanoparticles. J. Am. Chem. Soc. 2012, 134, 7600–7603. [Google Scholar] [CrossRef] [PubMed]
- Nowotny, J.; Bak, T.; Alim, M. (High temperature materials division outstanding achievement award) semiconducting properties and defect disorder of titanium dioxide. ECS Trans. 2015, 64, 11–28. [Google Scholar] [CrossRef]
- Tsui, L.-K.; Homma, T.; Zangari, G. Photocurrent conversion in anodized TiO2 nanotube arrays: Effect of the water content in anodizing solutions. J. Phys. Chem. C 2013, 117, 6979–6989. [Google Scholar] [CrossRef]
- Kowalski, D.; Mallet, J.; Michel, J.; Molinari, M. Low electric field strength self-organization of anodic TiO2 nanotubes in diethylene glycol electrolyte. J. Mater. Chem. A 2015, 3, 6655–6661. [Google Scholar] [CrossRef]
- Hunger, T.; Lapicque, F.; Storck, A. Electrochemical oxidation of sulphite ions at graphite electrodes. J. Appl. Electrochem. 1991, 21, 588–596. [Google Scholar] [CrossRef]
- Kim, T.W.; Choi, K.-S. Nanoporous BiVO4 photoanodes with dual-layer oxygen evolution catalysts for solar water splitting. Science 2014, 343, 990–994. [Google Scholar] [CrossRef]
- Zhou, M.; Bao, J.; Bi, W.; Zeng, Y.; Zhu, R.; Tao, M.; Xie, Y. Efficient water splitting via a heteroepitaxial BiVO4 photoelectrode decorated with Co-Pi catalysts. ChemSusChem 2012, 5, 1420–1425. [Google Scholar] [CrossRef]
- Gelderman, K.; Lee, L.; Donne, S. Flat-band potential of a semiconductor: Using the mott–schottky equation. J. Chem. Educ. 2007, 84. [Google Scholar] [CrossRef]
- Yahia, S.A.A.; Hamadou, L.; Kadri, A.; Benbrahim, N.; Sutter, E.M.M. Effect of anodizing potential on the formation and EIS characteristics of TiO2 nanotube arrays. J. Electrochem. Soc. 2012, 159, K83–K92. [Google Scholar] [CrossRef]
- Hanzu, I.; Djenizian, T.; Knauth, P. Electrical and Point Defect Properties of TiO2 Nanotubes Fabricated by Electrochemical Anodization. J. Phys. Chem. C 2011, 115, 5989–5996. [Google Scholar] [CrossRef]
- Orazem, M.E.; Tribollet, B. Electrochemical Impedance Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 2011; Volume 48, ISBN 111820994X. [Google Scholar]
- Tsui, L.-K.; Huang, J.; Sabat, M.; Zangari, G. Visible light sensitization of TiO2 nanotubes by bacteriochlorophyll-C dyes for photoelectrochemical solar cells. ACS Sustain. Chem. Eng. 2014, 2, 2097–2101. [Google Scholar] [CrossRef]
- Tsui, L.-K.; Zangari, G. The influence of morphology of electrodeposited Cu2O and Fe2O3 on the conversion efficiency of TiO2 nanotube photoelectrochemical solar cells. Electrochim. Acta 2013, 100, 220–225. [Google Scholar] [CrossRef]
- Da Silva, D.L.; Delatorre, R.G.; Pattanaik, G.; Zangari, G.; Figueiredo, W.; Blum, R.-P.; Niehus, H.; Pasa, A.A. Electrochemical synthesis of vanadium oxide nanofibers. J. Electrochem. Soc. 2008, 155, E14–E17. [Google Scholar] [CrossRef]
- Lu, G.; Evans, P.; Zangari, G. Electrocatalytic properties of Ni-based alloys toward hydrogen evolution reaction in acid media. J. Electrochem. Soc. 2003, 150, A551–A557. [Google Scholar] [CrossRef]
- Lu, G.; Zangari, G. Study of the electroless deposition process of Ni-P-based ternary alloys. J. Electrochem. Soc. 2003, 150, C777–C786. [Google Scholar] [CrossRef]
- Lu, G.; Zangari, G. Corrosion resistance of ternary Ni P based alloys in sulfuric acid solutions. Electrochim. Acta 2002, 47, 2969–2979. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Z.; Lin, T.; Yin, H.; Lu, X.; Wan, D.; Xu, T.; Zheng, C.; Lin, J.; Huang, F.; et al. Core-shell nanostructured “Black” rutile titania as excellent catalyst for hydrogen production enhanced by sulfur doping. J. Am. Chem. Soc. 2013, 135, 17831–17838. [Google Scholar] [CrossRef] [PubMed]
- Leshuk, T.; Parviz, R.; Everett, P.; Krishnakumar, H.; Varin, R.A.; Gu, F. Photocatalytic activity of hydrogenated TiO2. ACS Appl. Mater. Interfaces 2013, 5, 1892–1895. [Google Scholar] [CrossRef]
- Leshuk, T.; Linley, S.; Gu, F. Hydrogenation processing of TiO2 nanoparticles. Can. J. Chem. Eng. 2013, 91, 799–807. [Google Scholar] [CrossRef]
- Lu, H.; Zhao, B.; Pan, R.; Yao, J.; Qiu, J.; Luo, L.; Liu, Y. Safe and facile hydrogenation of commercial Degussa P25 at room temperature with enhanced photocatalytic activity. RSC Adv. 2014, 4, 1128–1132. [Google Scholar] [CrossRef]
- Oskam, G.; Hoffmann, P.M.; Schmidt, J.C.; Searson, P.C. Energetics and kinetics of surface states at n-type silicon surfaces in aqueous fluoride solutions. J. Phys. Chem. 1996, 100, 1801–1806. [Google Scholar] [CrossRef]
- Oskam, G.; Hoffmann, P.M.; Searson, P.C. In situ measurements of interface states at silicon surfaces in fluoride solutions. Phys. Rev. Lett. 1996, 76, 1521–1524. [Google Scholar] [CrossRef]
- Liu, L.; Yu, P.Y.; Chen, X.; Mao, S.; Shen, D.Z. Hydrogenation and disorder in engineered black TiO2. Phys. Rev. Lett. 2013, 111, 065505. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Melia, M.A.; Tsui, L.-K.; Fitz-Gerald, J.M.; Zangari, G. Laser-induced surface modification at anatase TiO2 nanotube array photoanodes for photoelectrochemical water oxidation. J. Phys. Chem. C 2017, 121, 17121–17128. [Google Scholar] [CrossRef]
- van de Lagemaat, J.; Park, N.-G.; Frank, A.J. Influence of electrical potential distribution, charge transport, and recombination on the photopotential and photocurrent conversion efficiency of dye-sensitized nanocrystalline TiO2 solar cells: A study by electrical impedance and optical modulation techniques. J. Phys. Chem. B 2000, 104, 2044–2052. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Huang, F. Black titanium dioxide (TiO2) nanomaterials. Chem. Soc. Rev. 2015, 44, 1861–1885. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, H.; Ling, Y.; Tang, Y.; Yang, X.; Fitzmorris, R.C.; Wang, C.; Zhang, J.Z.; Li, Y. Hydrogen-treated TiO2 nanowire arrays for photoelectrochemical water splitting. Nano Lett. 2011, 11, 3026–3033. [Google Scholar] [CrossRef]
- Chen, B.; Hou, J.; Lu, K. Formation mechanism of TiO2 nanotubes and their applications in photoelectrochemical water splitting and supercapacitors. Langmuir 2013, 29, 5911–5919. [Google Scholar] [CrossRef]
- Zhu, W.-D.; Wang, C.-W.; Chen, J.-B.; Li, D.-S.; Zhou, F.; Zhang, H.-L. Enhanced field emission from hydrogenated TiO2nanotube arrays. Nanotechnology 2012, 23, 455204. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2010, 46, 855–874. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Lin, Q.; Ahmed, R.; Hoglund, E.R.; Zangari, G. Synthesis of TiO2-based nanocomposites by anodizing and hydrogen annealing for efficient photoelectrochemical water oxidation. J. Power Sour. 2019, 410, 59–68. [Google Scholar] [CrossRef]
- Albu, S.; Tsuchiya, H.; Fujimoto, S.; Schmuki, P. TiO2 nanotubes—Annealing effects on detailed morphology and structure. Eur. J. Inorg. Chem. 2010, 2010, 4351–4356. [Google Scholar] [CrossRef]
- Amano, F.; Nakata, M.; Yamamoto, A.; Tanaka, T. Effect of Ti3+ ions and conduction band electrons on photocatalytic and photoelectrochemical activity of rutile titania for water oxidation. J. Phys. Chem. C 2016, 120, 6467–6474. [Google Scholar] [CrossRef]
- Tsui, L.-K.; Xu, Y.; Dawidowski, D.; Cafiso, D.; Zangari, G. Efficient water oxidation kinetics and enhanced electron transport in Li-doped TiO2 nanotube photoanodes. J. Mater. Chem. A 2016, 4, 19070–19077. [Google Scholar] [CrossRef]
- Beranek, R.; Tsuchiya, H.; Sugishima, T.; Macak, J.; Taveira, L.V.; Fujimoto, S.; Kisch, H.; Schmuki, P. Enhancement and limits of the photoelectrochemical response from anodic TiO2 nanotubes. Appl. Phys. Lett. 2005, 87, 243114. [Google Scholar] [CrossRef]
- Bard, A.J.; Bocarsly, A.B.; Fan, F.R.F.; Walton, E.G.; Wrighton, M.S. The concept of Fermi level pinning at semiconductor/liquid junctions. Consequences for energy conversion efficiency and selection of useful solution redox couples in solar devices. J. Am. Chem. Soc. 1980, 102, 3671–3677. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, N.; Schmuki, P. Ar+-ion bombardment of TiO2 nanotubes creates co-catalytic effect for photocatalytic open circuit hydrogen evolution. Electrochem. Commun. 2014, 49, 60–64. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Häublein, V.; Liu, N.; Nguyen, N.T.; Zolnhofer, E.M.; Tsuchiya, H.; Killian, M.S.; Meyer, K.; Frey, L.; Schmuki, P. TiO2 nanotubes: Nitrogen-ion implantation at low dose provides noble-metal-free photocatalytic H2-Evolution activity. Angew. Chem. Int. Ed. 2016, 55, 3763–3767. [Google Scholar] [CrossRef] [Green Version]
- Cronemeyer, D.C. Infrared absorption of reduced rutile TiO2 single crystals. Phys. Rev. 1959, 113, 1222–1226. [Google Scholar] [CrossRef]
- Wang, Z.; Wen, B.; Hao, Q.; Liu, L.-M.; Zhou, C.; Mao, X.; Lang, X.; Yin, W.-J.; Dai, D.; Selloni, A.; et al. Localized excitation of Ti3+ ions in the photoabsorption and photocatalytic activity of reduced rutile TiO2. J. Am. Chem. Soc. 2015, 137, 9146–9152. [Google Scholar] [CrossRef]
- Ihara, T.; Miyoshi, M.; Iriyama, Y.; Matsumoto, O.; Sugihara, S. Visible-light-active titanium oxide photocatalyst realized by an oxygen-deficient structure and by nitrogen doping. Appl. Catal. B Environ. 2003, 42, 403–409. [Google Scholar] [CrossRef]
- Di Valentin, C.; Pacchioni, G.; Selloni, A.; Livraghi, S.; Giamello, E. Characterization of paramagnetic species in N-doped TiO2 powders by EPR spectroscopy and DFT calculations. J. Phys. Chem. B 2005, 109, 11414–11419. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lou, Y.; Samia, A.C.S.; Burda, C.; Gole, J.L. Formation of oxynitride as the photocatalytic enhancing site in nitrogen-doped titania nanocatalysts: Comparison to a commercial nanopowder. Adv. Funct. Mater. 2005, 15, 41–49. [Google Scholar] [CrossRef]
- Emeline, A.; Sheremetyeva, N.V.; Khomchenko, N.V.; Ryabchuk, V.; Serpone, N. Photoinduced formation of defects and nitrogen stabilization of color centers in N-doped titanium dioxide. J. Phys. Chem. C 2007, 111, 11456–11462. [Google Scholar] [CrossRef]
- Batzill, M.; Morales, E.H.; Diebold, U. Influence of nitrogen doping on the defect formation and surface properties of TiO2 rutile and anatase. Phys. Rev. Lett. 2006, 96, 026103. [Google Scholar] [CrossRef] [PubMed]
- Serpone, N. Is the band gap of pristine TiO2 narrowed by anion- and cation-doping of titanium dioxide in second-generation photocatalysts? J. Phys. Chem. B 2006, 110, 24287–24293. [Google Scholar] [CrossRef]
- Hoang, S.; Berglund, S.; Hahn, N.T.; Bard, A.J.; Mullins, C.B. Enhancing visible light photo-oxidation of water with TiO2 nanowire arrays via cotreatment with H2 and NH3: Synergistic effects between Ti3+ and N. J. Am. Chem. Soc. 2012, 134, 3659–3662. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, X.; Lin, B.; Gao, B. Origin of the visible-light photoactivity of NH3-treated TiO2: Effect of nitrogen doping and oxygen vacancies. Appl. Surf. Sci. 2013, 264, 845–852. [Google Scholar] [CrossRef]
- Irie, H.; Watanabe, Y.; Hashimoto, K. Nitrogen-concentration dependence on photocatalytic activity of TiO2-x N x powders. J. Phys. Chem. B 2003, 107, 5483–5486. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Yang, F.; Yang, X. Excellent antimicrobial properties of mesoporous anatase TiO2 and Ag/TiO2 composite films. Microporous Mesoporous Mater. 2008, 114, 431–439. [Google Scholar] [CrossRef]
- Saha, N.C.; Tompkins, H.G. Titanium nitride oxidation chemistry: An X-ray photoelectron spectroscopy study. J. Appl. Phys. 1992, 72, 3072–3079. [Google Scholar] [CrossRef]
- Yates, H.; Nolan, M.; Sheel, D.; Pemble, M. The role of nitrogen doping on the development of visible light-induced photocatalytic activity in thin TiO2 films grown on glass by chemical vapour deposition. J. Photochem. Photobiol. A Chem. 2006, 179, 213–223. [Google Scholar] [CrossRef]
- Shard, A.G. Detection limits in XPS for more than 6000 binary systems using Al and Mg Kα X-rays. Surf. Interface Anal. 2014, 46, 175–185. [Google Scholar] [CrossRef]
- Anpo, M.; Che, M.; Fubini, B.; Garrone, E.; Giamello, E.; Paganini, M.C. Generation of superoxide ions at oxide surfaces. Top. Catal. 1999, 8, 189–198. [Google Scholar] [CrossRef]
- Kumar, C.P.; Gopal, N.O.; Wang, T.C.; Wong, A.M.-S.; Ke, S.C. EPR Investigation of TiO2Nanoparticles with Temperature-Dependent Properties. J. Phys. Chem. B 2006, 110, 5223–5229. [Google Scholar] [CrossRef] [PubMed]
- Di Valentin, C.; Finazzi, E.; Pacchioni, G.; Selloni, A.; Livraghi, S.; Paganini, M.C.; Giamello, E. N-doped TiO2: Theory and experiment. Chem. Phys. 2007, 339, 44–56. [Google Scholar] [CrossRef]
- Livraghi, S.; Paganini, M.C.; Giamello, E.; Selloni, A.; Di Valentin, C.; Pacchioni, G. Origin of photoactivity of nitrogen-doped titanium dioxide under visible light. J. Am. Chem. Soc. 2006, 128, 15666–15671. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ahmed, R.; Klein, D.; Cap, S.; Freedy, K.; McDonnell, S.; Zangari, G. Improving photo-oxidation activity of water by introducing Ti3+ in self-ordered TiO2 nanotube arrays treated with Ar/NH3. J. Power Sources 2019, 414, 242–249. [Google Scholar] [CrossRef]
- Liu, C.; Dasgupta, N.P.; Yang, P. Semiconductor nanowires for artificial photosynthesis. Chem. Mater. 2013, 26, 415–422. [Google Scholar] [CrossRef]
- Siuzdak, K.; Szkoda, M.; Sawczak, M.; Lisowska-Oleksiak, A.; Karczewski, J.; Ryl, J. Enhanced photoelectrochemical and photocatalytic performance of iodine-doped titania nanotube arrays. RSC Adv. 2015, 5, 50379–50391. [Google Scholar] [CrossRef]
- Berger, T.; Lana-Villarreal, T.; Monllor-Satoca, D.; Gómez, R. Charge transfer reductive doping of nanostructured TiO2 thin films as a way to improve their photoelectrocatalytic performance. Electrochem. Commun. 2006, 8, 1713–1718. [Google Scholar] [CrossRef]
- Goldsmith, Z.; Harshan, A.K.; Gerken, J.B.; Vörös, M.; Galli, G.; Stahl, S.S.; Hammes-Schiffer, S. Characterization of NiFe oxyhydroxide electrocatalysts by integrated electronic structure calculations and spectroelectrochemistry. Proc. Natl. Acad. Sci. USA 2017, 114, 3050–3055. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Ahmed, R.; Lin, Q.; Zangari, G. (Photo) electrochemical water oxidation at anodic TiO2 nanotubes modified by electrodeposited NiFe oxy-hydroxides catalysts. Electrochim. Acta 2019, 308, 91–98. [Google Scholar] [CrossRef]
- Liu, N.; Schneider, C.; Freitag, D.; Hartmann, M.; Venkatesan, U.; Müller, J.; Spiecker, E.; Schmuki, P. Black TiO2 nanotubes: Cocatalyst-free open-circuit hydrogen generation. Nano Lett. 2014, 14, 3309–3313. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, Z.; Li, W.; Cao, M. Distinctly improved photocurrent and stability in TiO2 nanotube arrays by ladder band structure. J. Phys. Chem. C 2017, 121, 20605–20612. [Google Scholar] [CrossRef]
- Wang, Q.; Watanabe, M.; Edalati, K. Visible-light photocurrent in nanostructured high-pressure TiO2-II (columbite) phase. J. Phys. Chem. C 2020, 124, 13930–13935. [Google Scholar] [CrossRef]
- Singh, S.V.; Kumar, M.P.; Anantharaj, S.; Mukherjee, B.; Kundu, S.; Pal, B.N. Direct evidence of an efficient plasmon-induced hot-electron transfer at an in situ grown Ag/TiO2 interface for highly enhanced solar H2 generation. ACS Appl. Energy Mater. 2020, 3, 1821–1830. [Google Scholar] [CrossRef]
- Luo, H.; Dimitrov, S.D.; Daboczi, M.; Kim, J.-S.; Guo, Q.; Fang, Y.; Stoeckel, M.-A.; Samorì, P.; Fenwick, O.; Sobrido, A.B.J.; et al. Nitrogen-doped carbon Dots/TiO2 nanoparticle composites for photoelectrochemical water oxidation. ACS Appl. Nano Mater. 2020, 3, 3371–3381. [Google Scholar] [CrossRef]
- Subramanyam, P.; Meena, B.; Suryakala, D.; Subrahmanyam, C. TiO2 photoanodes sensitized with Bi2Se3 nanoflowers for visible–near-infrared photoelectrochemical water splitting. ACS Appl. Nano Mater. 2021, 4, 739–745. [Google Scholar] [CrossRef]
- Chen, Y.-X.; Gombac, V.; Montini, T.; Lavacchi, A.; Filippi, J.; Miller, H.A.; Fornasiero, P.; Vizza, F. An increase in hydrogen production from light and ethanol using a dual scale porosity photocatalyst. Green Chem. 2018, 20, 2299–2307. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Zangari, G. TiO2 Nanotubes Architectures for Solar Energy Conversion. Coatings 2021, 11, 931. https://doi.org/10.3390/coatings11080931
Xu Y, Zangari G. TiO2 Nanotubes Architectures for Solar Energy Conversion. Coatings. 2021; 11(8):931. https://doi.org/10.3390/coatings11080931
Chicago/Turabian StyleXu, Yin, and Giovanni Zangari. 2021. "TiO2 Nanotubes Architectures for Solar Energy Conversion" Coatings 11, no. 8: 931. https://doi.org/10.3390/coatings11080931
APA StyleXu, Y., & Zangari, G. (2021). TiO2 Nanotubes Architectures for Solar Energy Conversion. Coatings, 11(8), 931. https://doi.org/10.3390/coatings11080931





