Hydrophilic Surface Treatment of Carbon Powder Using CO2 Plasma Activated Gas
Abstract
:1. Introduction
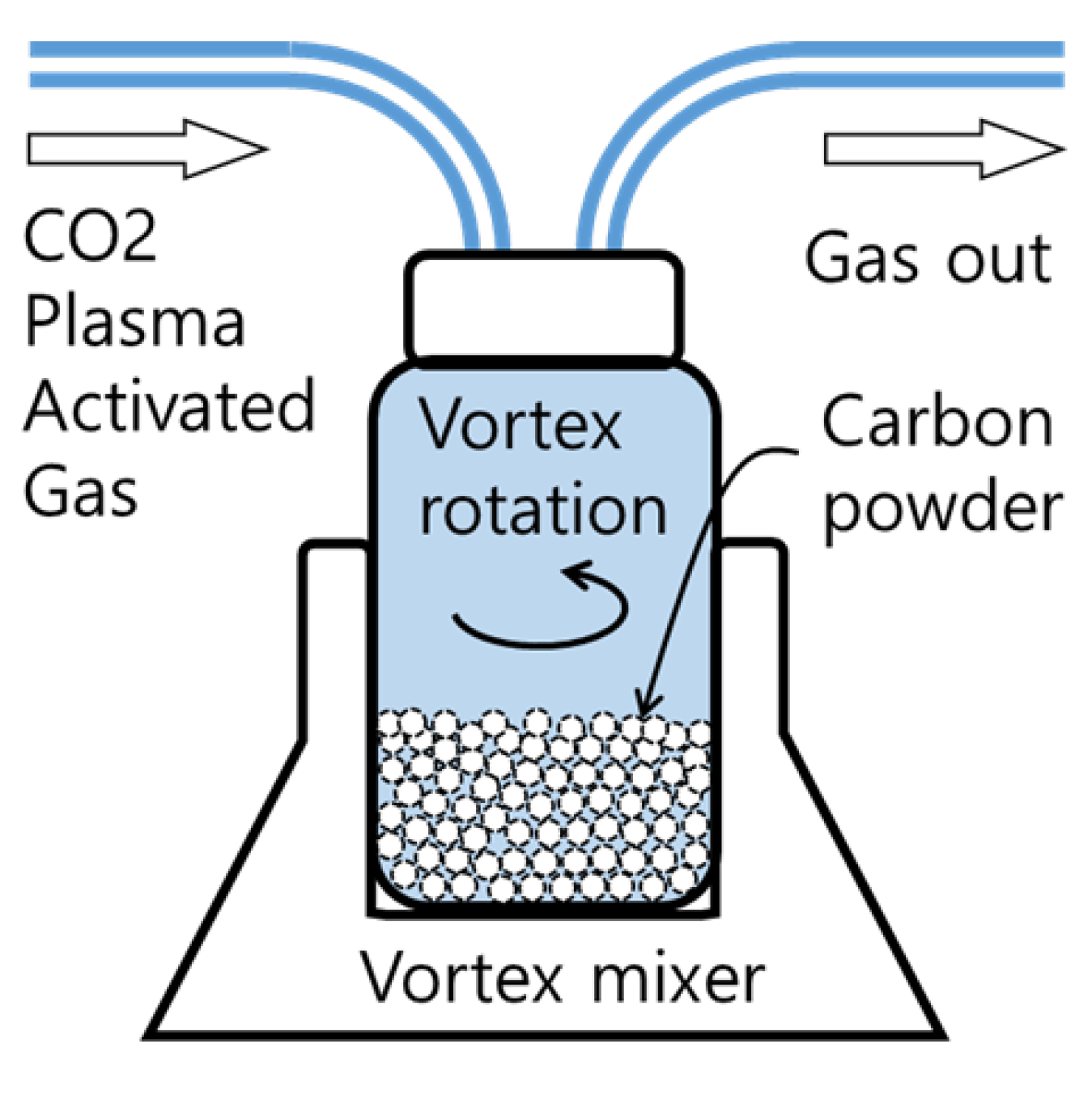
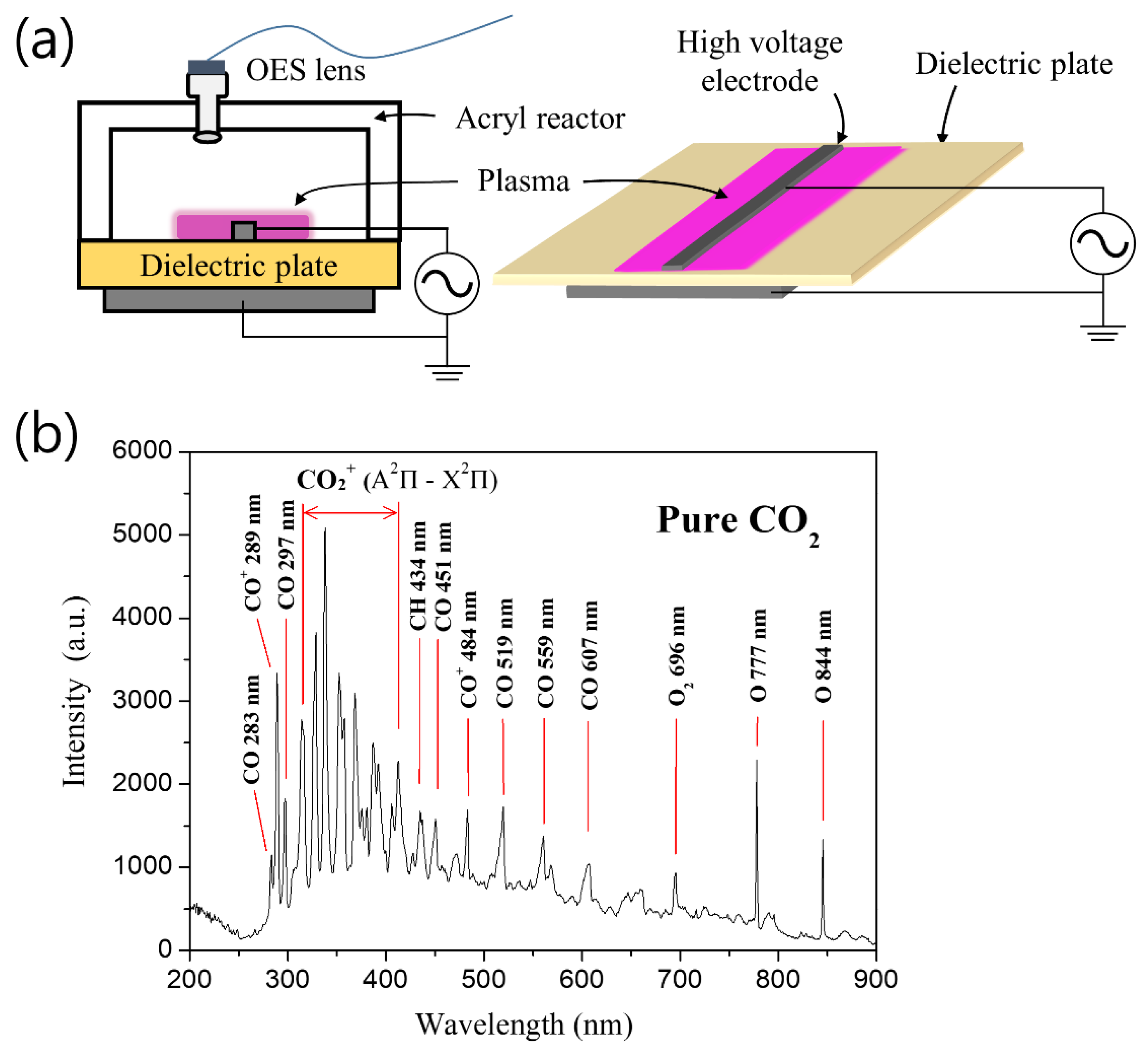
2. Materials and Methods
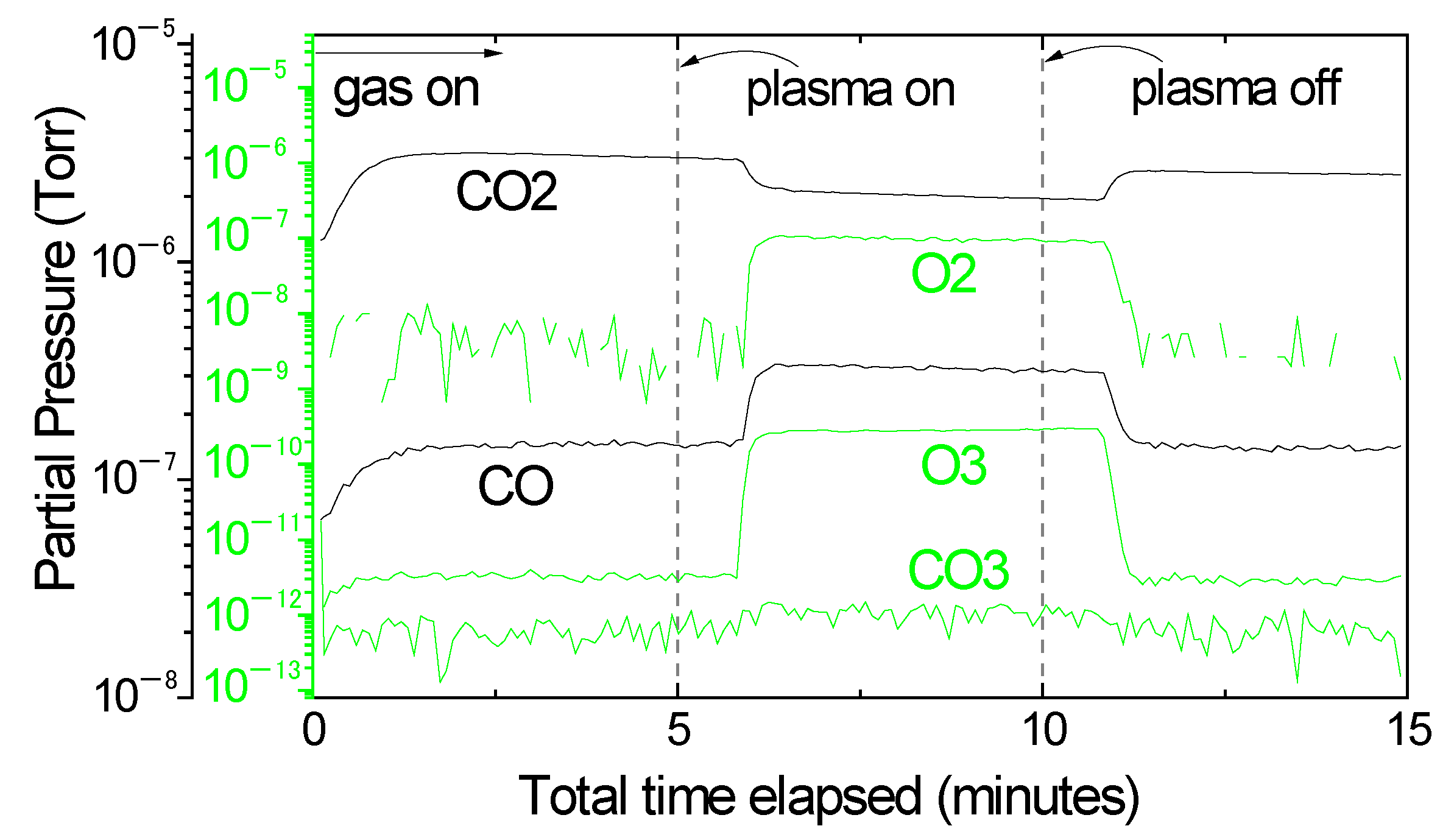
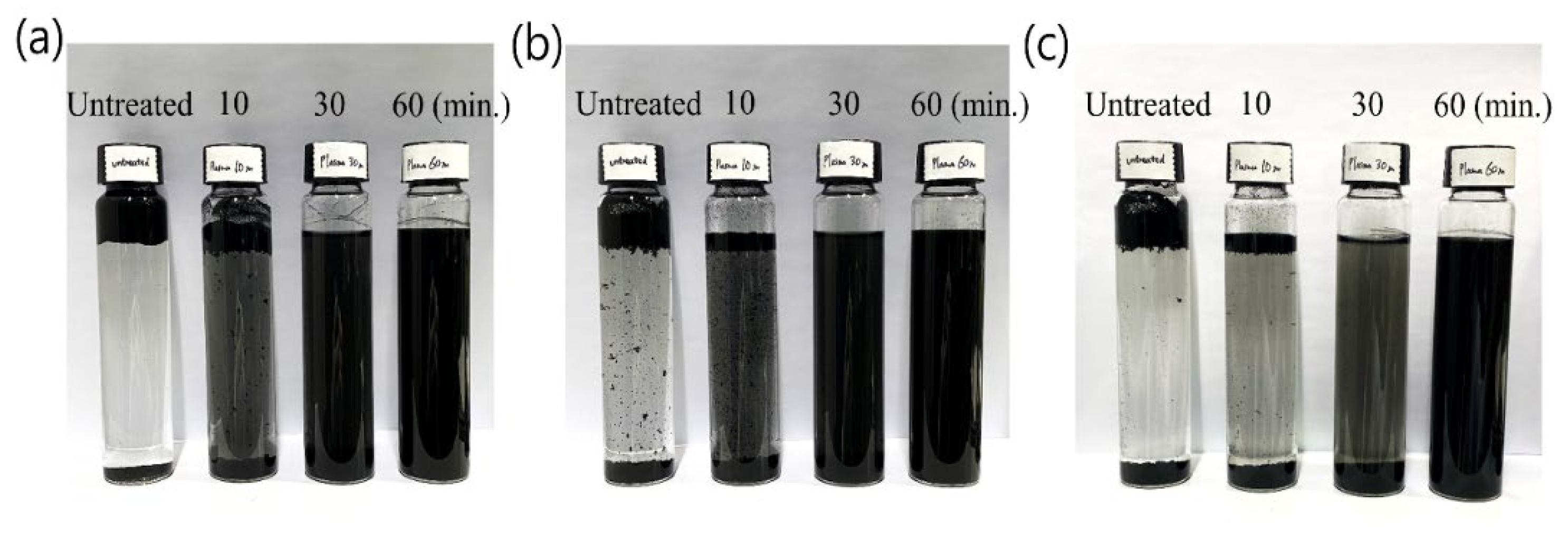
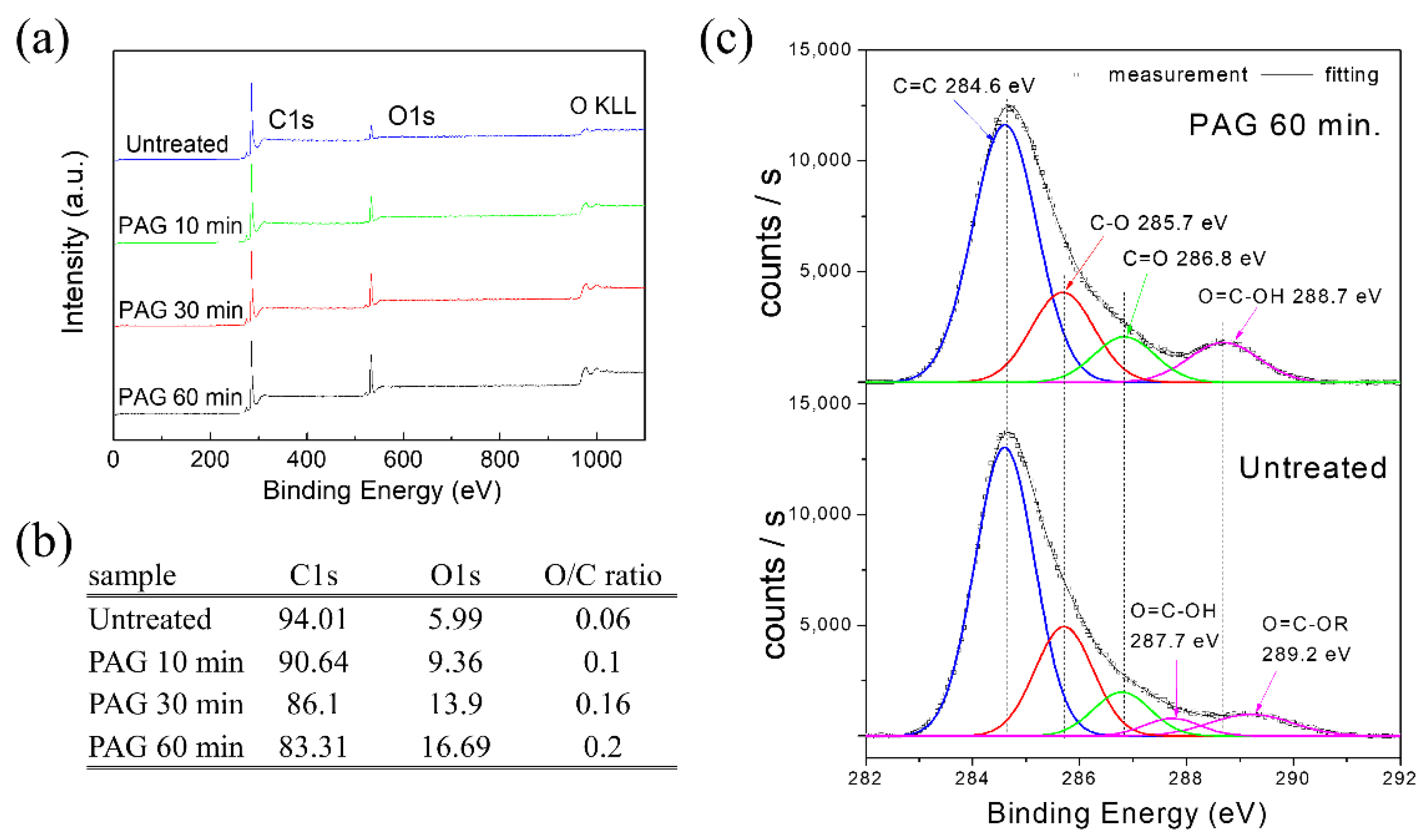
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, J.-S.; Liu, Z.; Yan, Z.; He, Y. A novel slurry blending method for a uniform dispersion of carbon nanotubes in nature rubber composites. Results Phys. 2019, 15, 102720. [Google Scholar] [CrossRef]
- Konda, K.; Moodakare, S.B.; Kumar, P.L.; Battabyal, M.; Seth, J.R.; Juvekar, V.A.; Gopalan, R. Comprehensive effort on electrode slurry preparation for better electrochemical performance of LiFePO4 battery. J. Power Sources 2020, 480, 228837. [Google Scholar] [CrossRef]
- Sis, H.; Birinci, M. Effect of nonionic and ionic surfactants on zeta potential and dispersion properties of carbon black powders. Colloids Surf. A Physicochem. Eng. Aspects 2009, 341, 60–67. [Google Scholar] [CrossRef]
- Li, H.-Y.; Chen, H.-Z.; Xu, W.-J.; Yuan, F.; Wang, J.-R.; Wang, M. Polymer-encapsulated hydrophilic carbon black nanoparticles free from aggregation. Colloids Surf. A Physicochem. Eng. Aspects 2005, 254, 173–178. [Google Scholar] [CrossRef]
- Rosen, M.J. Surfactants and Interfacial Phenomena, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1989; pp. 337–354. [Google Scholar]
- Arpagaus, C.; Oberbossel, G.; Rohr, P.R. Plasma treatment of polymer powders–from laboratory research to industrial application. Plasma Process. Polym. 2018, 15, 1800133. [Google Scholar] [CrossRef]
- Castilla, C.M.; García, M.A.F.; Joly, J.P.; Toledo, I.B.; Marín, F.C.; Utrilla, J.R. Activated carbon surface modifications by nitric acid, hydrogen peroxide, and ammonium peroxydisulfate treatments. Langmuir 1995, 11, 4386–4392. [Google Scholar] [CrossRef]
- Otake, Y.; Jenkins, R.G. Characterization of oxygen-containing surface complexes created on a microporous carbon by air and nitric acid treatment. Carbon 1993, 31, 109–121. [Google Scholar] [CrossRef]
- García, M.D.; Garzón, F.J.L.; Mendoza, M.P. Effect of some oxidation treatments on the textural characteristics and surface chemical nature of an activated carbon. J. Colloid Interface Sci. 2000, 222, 233–240. [Google Scholar] [CrossRef]
- Şahin, Ö.; Yardim, Y.; Baytar, O.; Saka, C. Enhanced electrochemical double-layer capacitive performance with CO2 plasma treatment on activated carbon prepared from pyrolysis of pistachio shells. Int. J. Hydrogen Energy 2020, 45, 8843–8852. [Google Scholar] [CrossRef]
- Kodama, S.; Habaki, H.; Sekiguchi, H.; Kawasaki, J. Surface modification of adsorbents by dielectric barrier discharge. Thin Solid Films 2002, 407, 151–155. [Google Scholar] [CrossRef]
- Lee, D.; Hong, S.-H.; Paek, K.-H.; Ju, W.-T. Adsorbability enhancement of activated carbon by dielectric barrier discharge plasma treatment. Surf. Coat. Technol. 2005, 200, 2277–2282. [Google Scholar] [CrossRef]
- García, A.B.; Alonso, A.M.; Leon, C.A.L.; Tascón, J.M.D. Modification of the surface properties of an activated carbon by oxygen plasma treatment. Fuel 1998, 77, 613–624. [Google Scholar] [CrossRef]
- Ciobanu, M.; Lepadatu, A.M.; Asaftei, S. Chemical and electrochemical studies of carbon black surface by treatment with ozone and nitrogen oxide. Mater. Today 2016, 3, 252–257. [Google Scholar] [CrossRef]
- Boudou, J.P.; Alonzo, A.M.; Tascon, J.M.D. Introduction of acidic groups at the surface of activated carbon by microwave-induced oxygen plasma at low pressure. Carbon 2000, 38, 1021–1029. [Google Scholar] [CrossRef]
- Torre, L.E.C.; Bottani, E.J.; Alonso, A.M.; Cuesta, A.; García, A.B.; Tascón, J.M.D. Effects of oxygen plasma treatment on the surface of graphitized carbon black. Carbon 1998, 36, 277–282. [Google Scholar] [CrossRef]
- Yoo, S.; Seok, D.C.; Lee, K.; Jung, Y.H. Enhancement of polymer powder wettability by using surface dielectric barrier discharge. IEEE Trans. Plasma Sci. 2019, 47, 1302–1308. [Google Scholar] [CrossRef]
- Sim, K.-B.; Baek, D.; Shin, J.H.; Shim, G.-S.; Jang, S.-W.; Kim, H.-J.; Hwang, J.-W.; Roh, J.U. Enhanced surface properties of carbon fiber reinforced plastic by epoxy modified primer with plasma for automotive applications. Polymers 2020, 12, 556. [Google Scholar] [CrossRef] [Green Version]
- Walther, F.; Davydovskaya, P.; Zürcher, S.; Kaiser, M.; Herberg, H.; Gigler, A.M.; Stark, R.W.J. Stability of the hydrophilic behavior of oxygen plasma activated SU-8. Micromech. Microeng. 2007, 17, 524–531. [Google Scholar] [CrossRef] [Green Version]
- Seok, D.C.; Jeong, H.Y.; Jung, Y.H.; Lho, T. Optimizing factors on high concentration of ozone production with dielectric barrier discharge. Ozone Sci. Eng. 2015, 37, 1–6. [Google Scholar] [CrossRef]
- Reyes, P.G.; Gómez, A.; Vergara, J.; Martínez, H.; Torres, C. Plasma diagnostics of glow discharges in mixtures of CO2 with noble gases. C. Rev. Mex. Fis. 2017, 63, 363–371. [Google Scholar]
- Liu, Y.; Rehman, F.; Zimmerman, W.B. Reaction engineering of carbon monoxide generation by treatment with atmospheric pressure, low power CO2 DBD plasma. Fuel 2017, 209, 117–126. [Google Scholar] [CrossRef]
- Khan, M.I.; Rehman, N.U.; Khan, S.; Ullah, N.; Masood, A.; Ullah, A. Spectroscopic study of CO2 and CO2-N2 mixture plasma using dielectric barrier discharge. AIP Adv. 2019, 9, 085015. [Google Scholar] [CrossRef] [Green Version]
- Oberbossel, G.; Probst, C.; Giampietro, V.R.; Rohr, P.R. Plasma afterglow treatment of polymer powders: Process parameters, wettability improvement, and aging effects. Plasma Process Polym. 2017, 14, 1600144. [Google Scholar] [CrossRef]
- Washburn, E.W. The dynamics of capillary flow. Phys. Rev. 1921, 17, 273. [Google Scholar] [CrossRef]
- Thakker, M.; Karde, V.; Shah, D.O.; Shukla, P.; Ghoroi, C. Wettability measurement apparatus for porous material using the modified washburn method. Meas. Sci. Technol. 2013, 24, 125902. [Google Scholar] [CrossRef]
- Meng, X.; Ning, P.; Xu, G.; Cao, H. Removal of coke powder from coking wastewater by extraction technology. Sep. Purif. Technol. 2017, 175, 506–511. [Google Scholar] [CrossRef]
- Liu, L.; Shen, Z.; Zhang, X.; Ma, H. Highly conductive graphene/carbon black screen printing inks for flexible electronics. J. Colloid Interface Sci. 2021, 582, 12–21. [Google Scholar] [CrossRef]
- Liao, Y.; Zhang, R.; Wang, H.; Ye, S.; Zhou, Y.; Ma, T.; Zhu, J.; Pfefferle, L.D.; Qian, J. Highly conductive carbon-based aqueous inks toward electroluminescent devices, printed capacitive sensors and flexible wearable electronics. RSC Adv. 2019, 9, 15184. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Cheng, X.; Chong, Y.; Yuan, H.; Huang, J.-Q.; Zhang, Q. Advanced electrode processing of lithium ion batteries: A review of powder technology in battery fabrication. Particuology 2021, 57, 56–71. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, S.; Seok, D.; Jung, Y.; Lee, K. Hydrophilic Surface Treatment of Carbon Powder Using CO2 Plasma Activated Gas. Coatings 2021, 11, 925. https://doi.org/10.3390/coatings11080925
Yoo S, Seok D, Jung Y, Lee K. Hydrophilic Surface Treatment of Carbon Powder Using CO2 Plasma Activated Gas. Coatings. 2021; 11(8):925. https://doi.org/10.3390/coatings11080925
Chicago/Turabian StyleYoo, Seungryul, Dongchan Seok, Yongho Jung, and Kiyong Lee. 2021. "Hydrophilic Surface Treatment of Carbon Powder Using CO2 Plasma Activated Gas" Coatings 11, no. 8: 925. https://doi.org/10.3390/coatings11080925
APA StyleYoo, S., Seok, D., Jung, Y., & Lee, K. (2021). Hydrophilic Surface Treatment of Carbon Powder Using CO2 Plasma Activated Gas. Coatings, 11(8), 925. https://doi.org/10.3390/coatings11080925





