Use of Digital Image Correlation Method to Measure Bio-Tissue Deformation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Deformation and Displacement
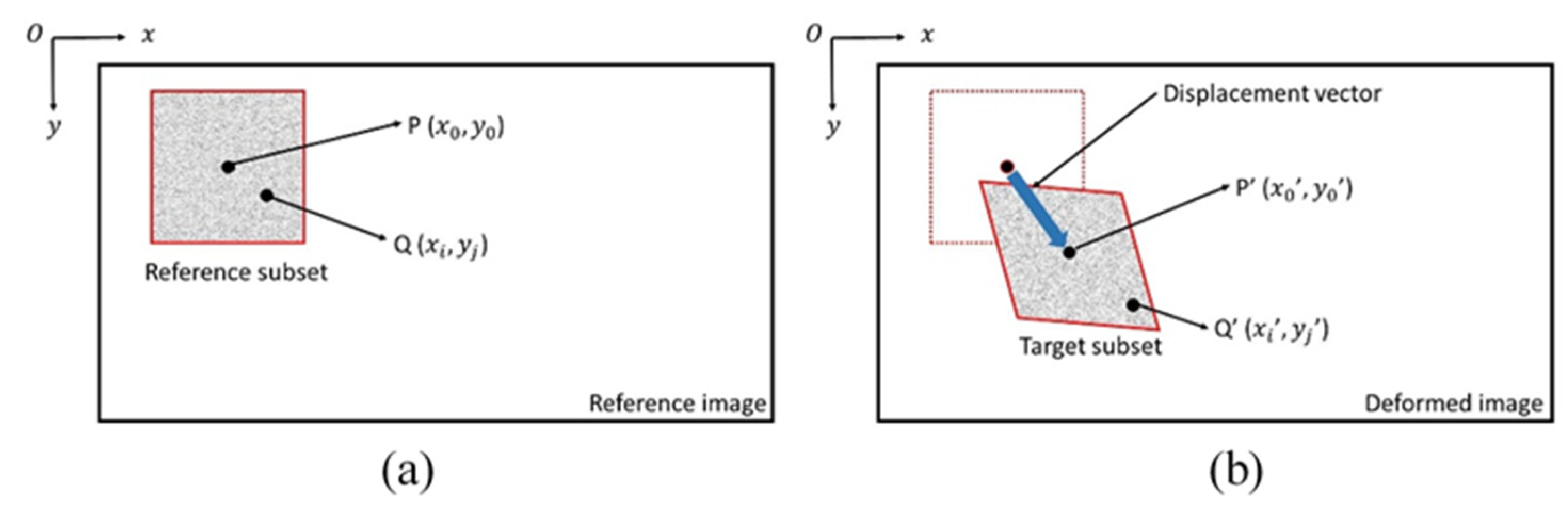
2.2. Digital Image Correlation Method
2.3. Strain Field Calculation
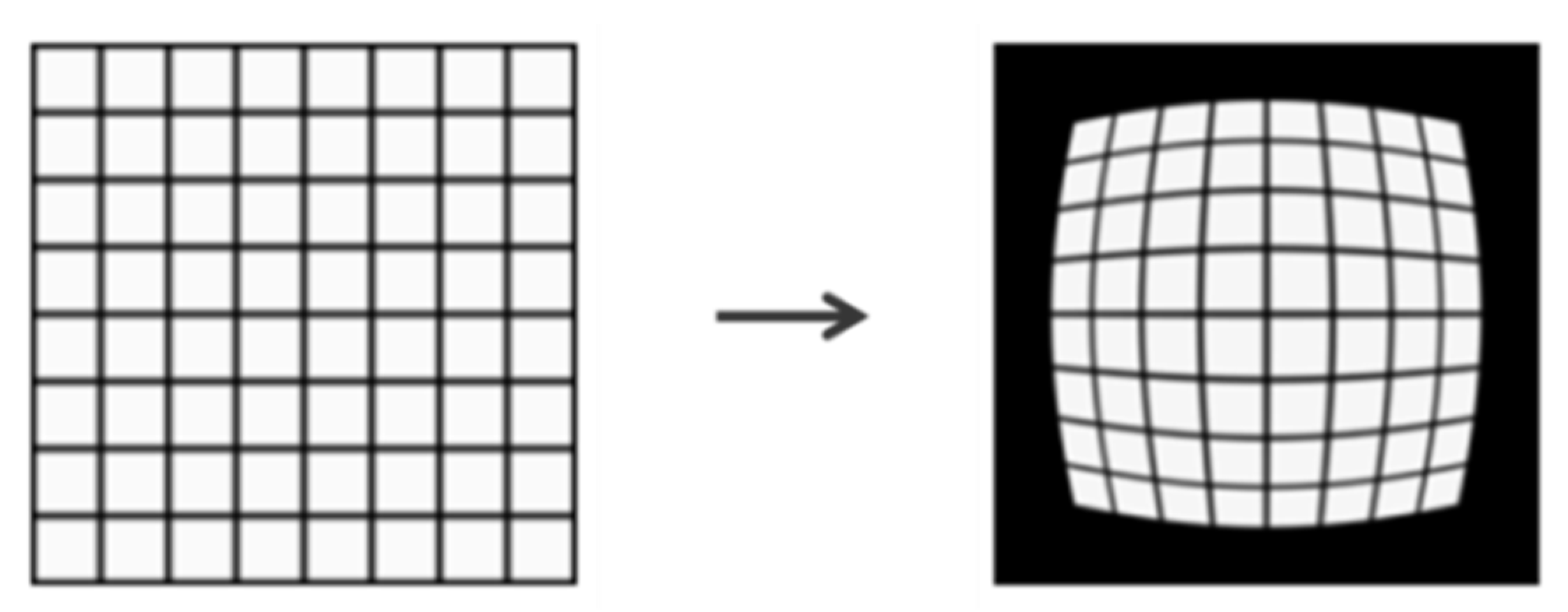
2.4. Image Distortion Correction
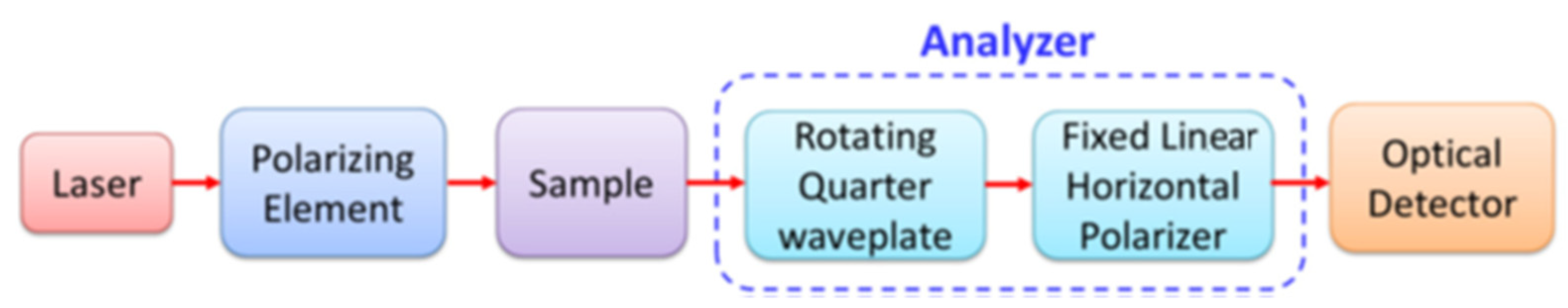
2.5. Stokes Vector and Mueller Matrix Polarimetry
3. Experimental Procedure
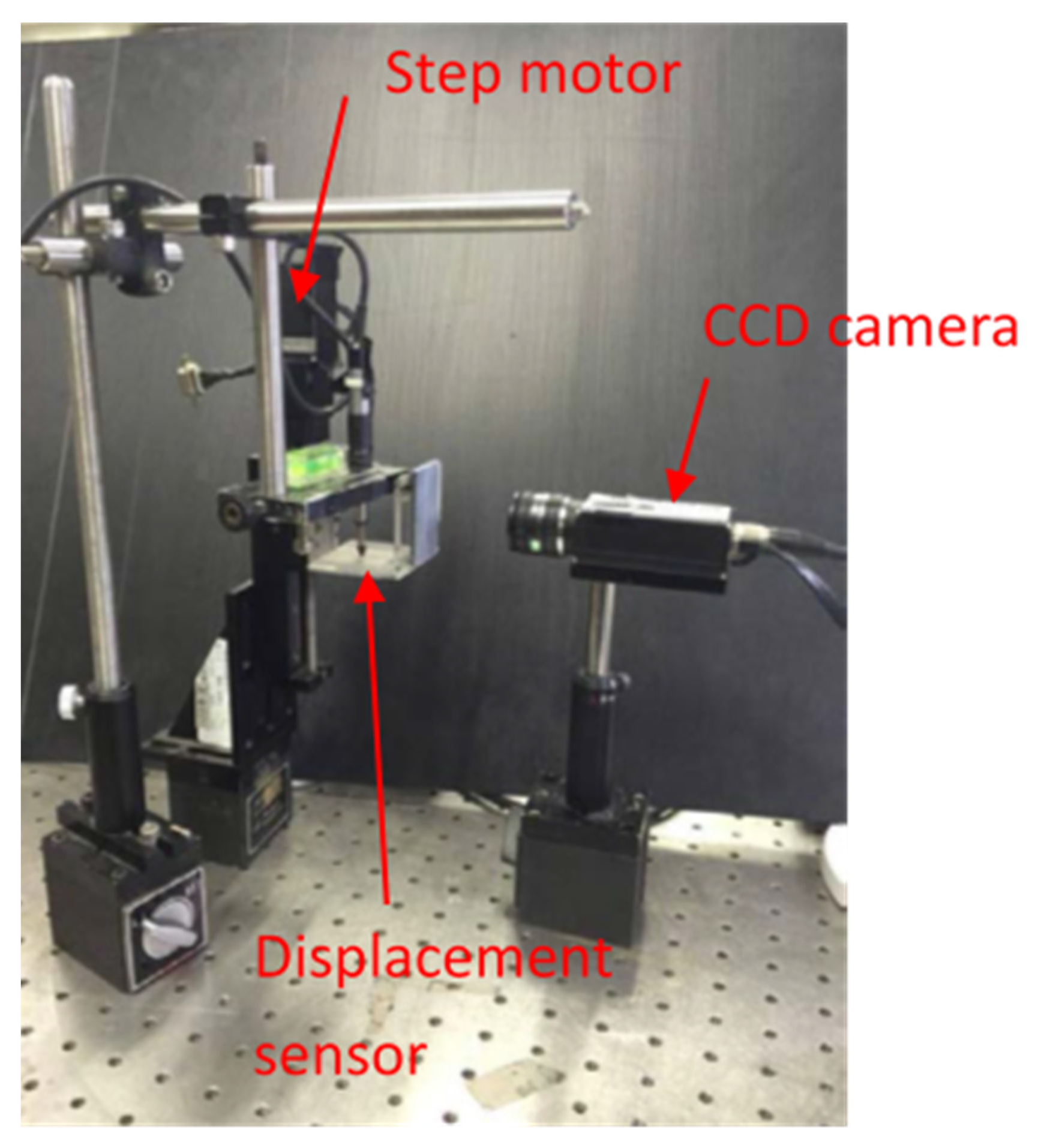
3.1. Image Measurement System
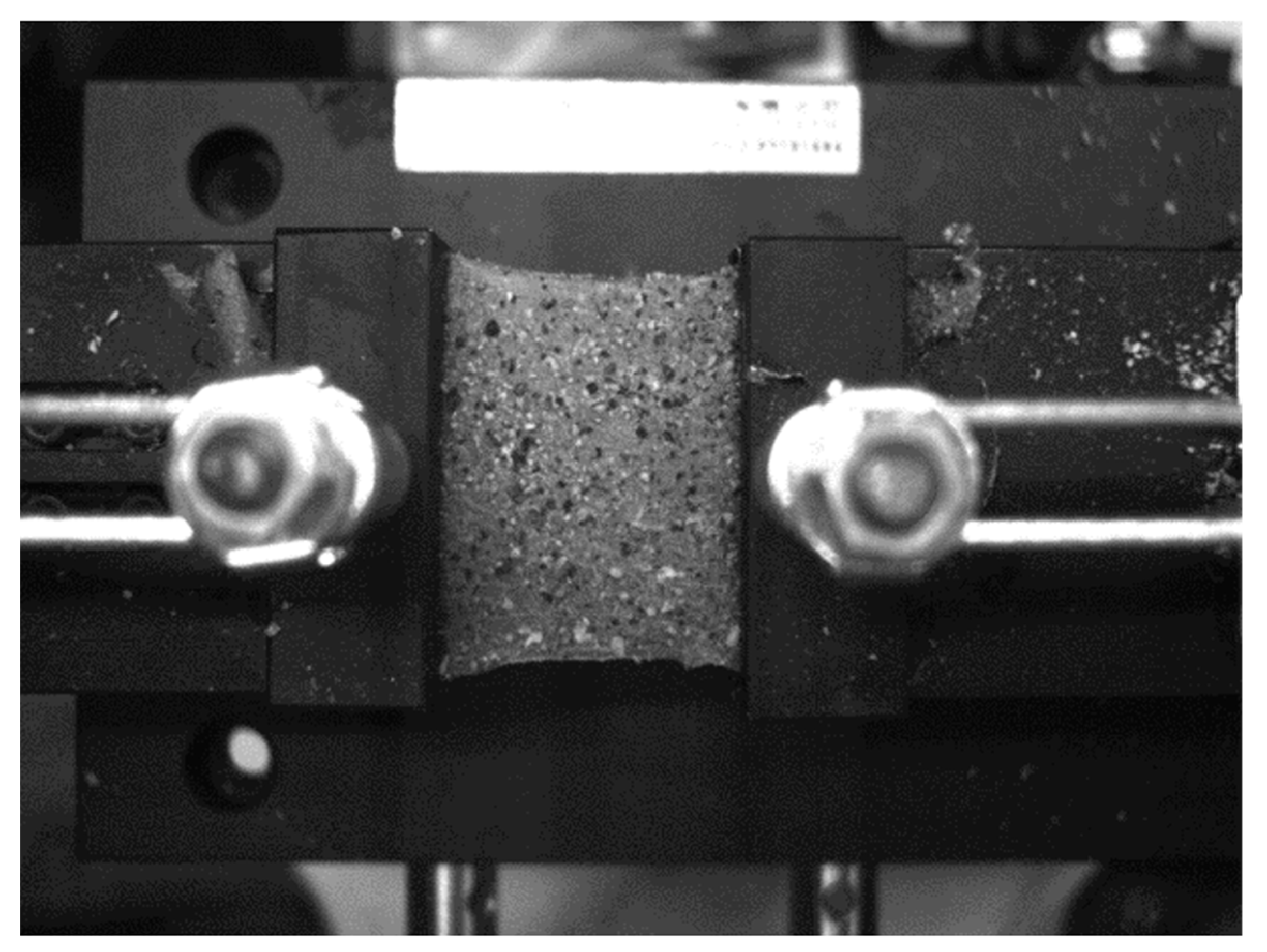
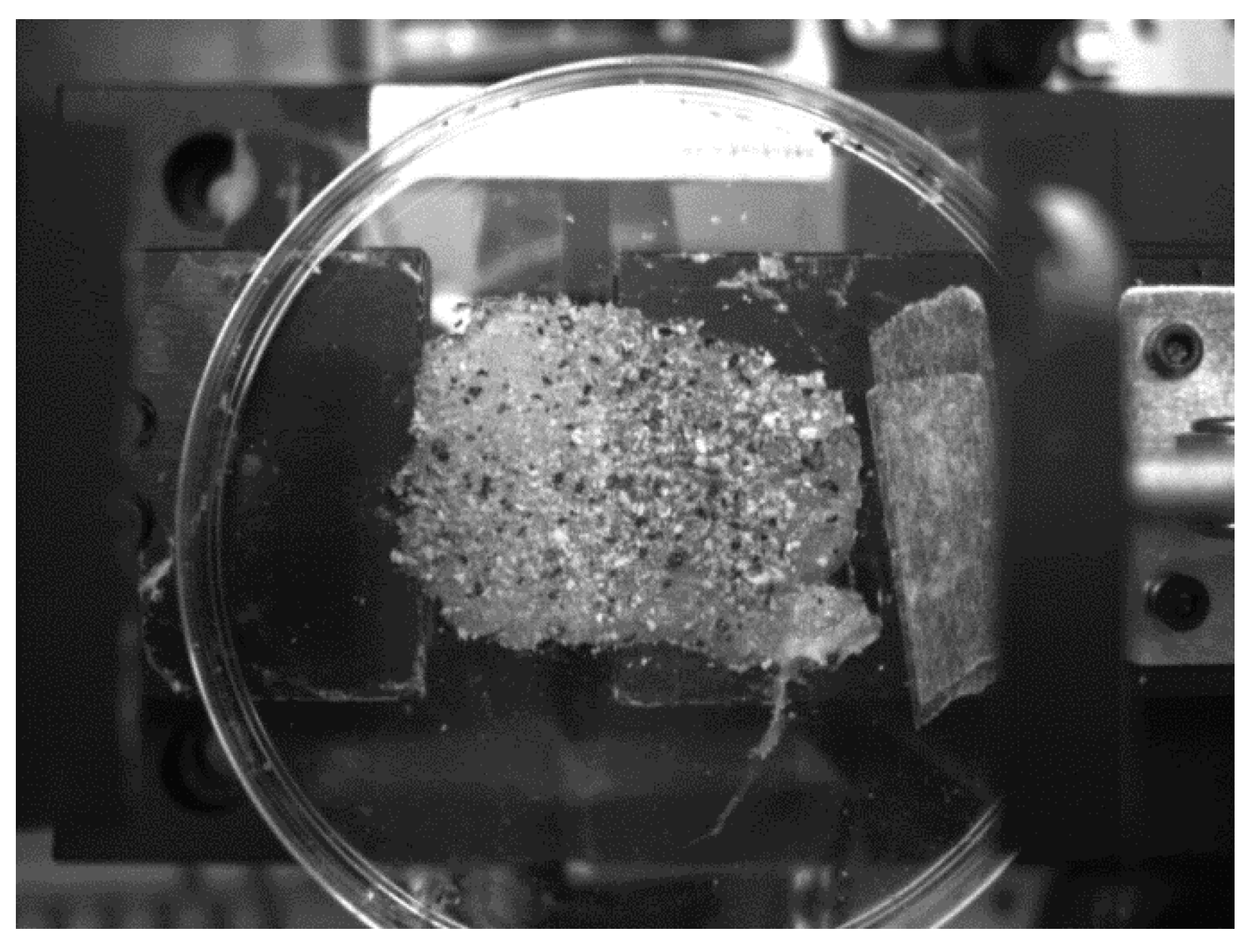
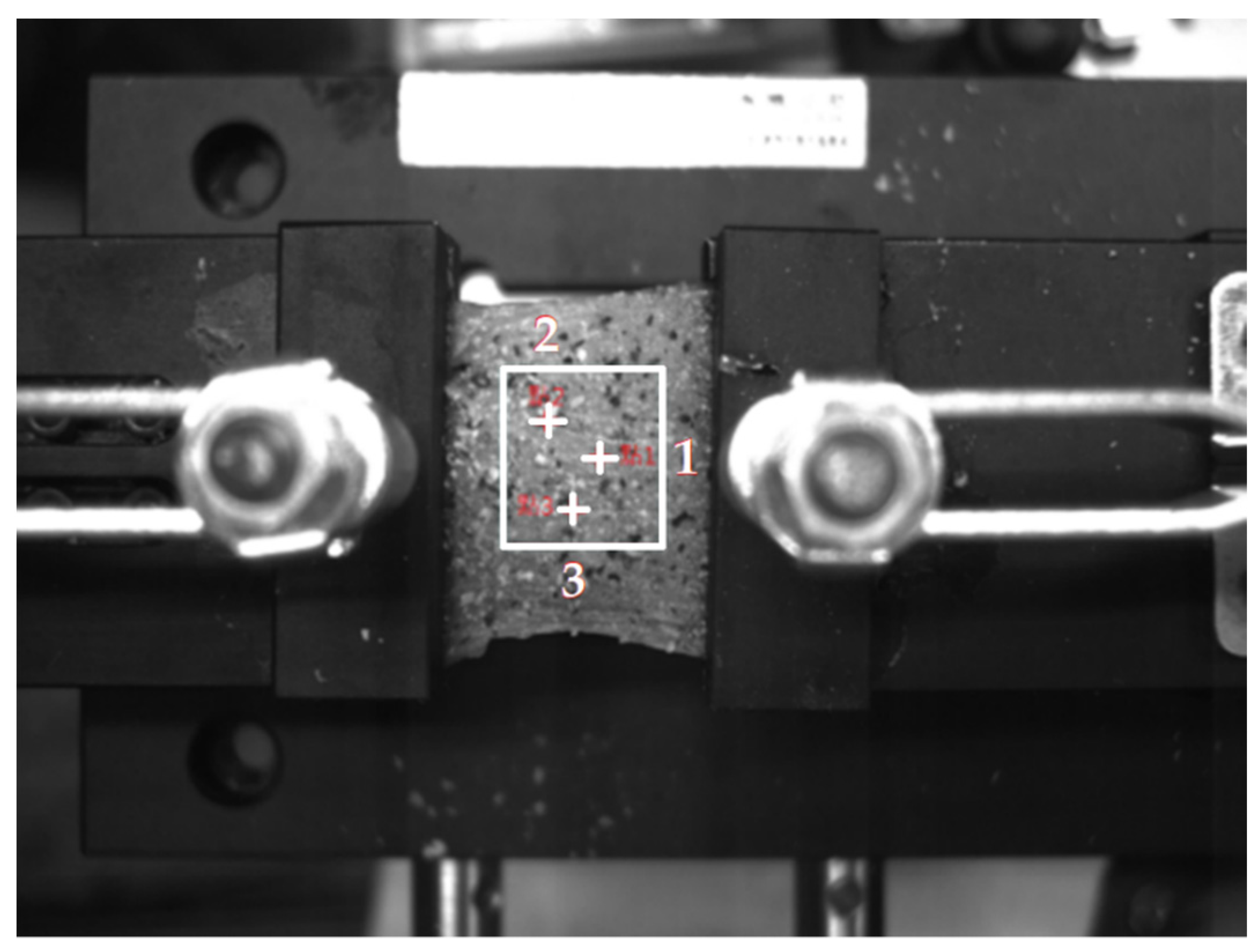
3.2. Test Specimen
3.3. Experimental Process
4. Results and Discussion
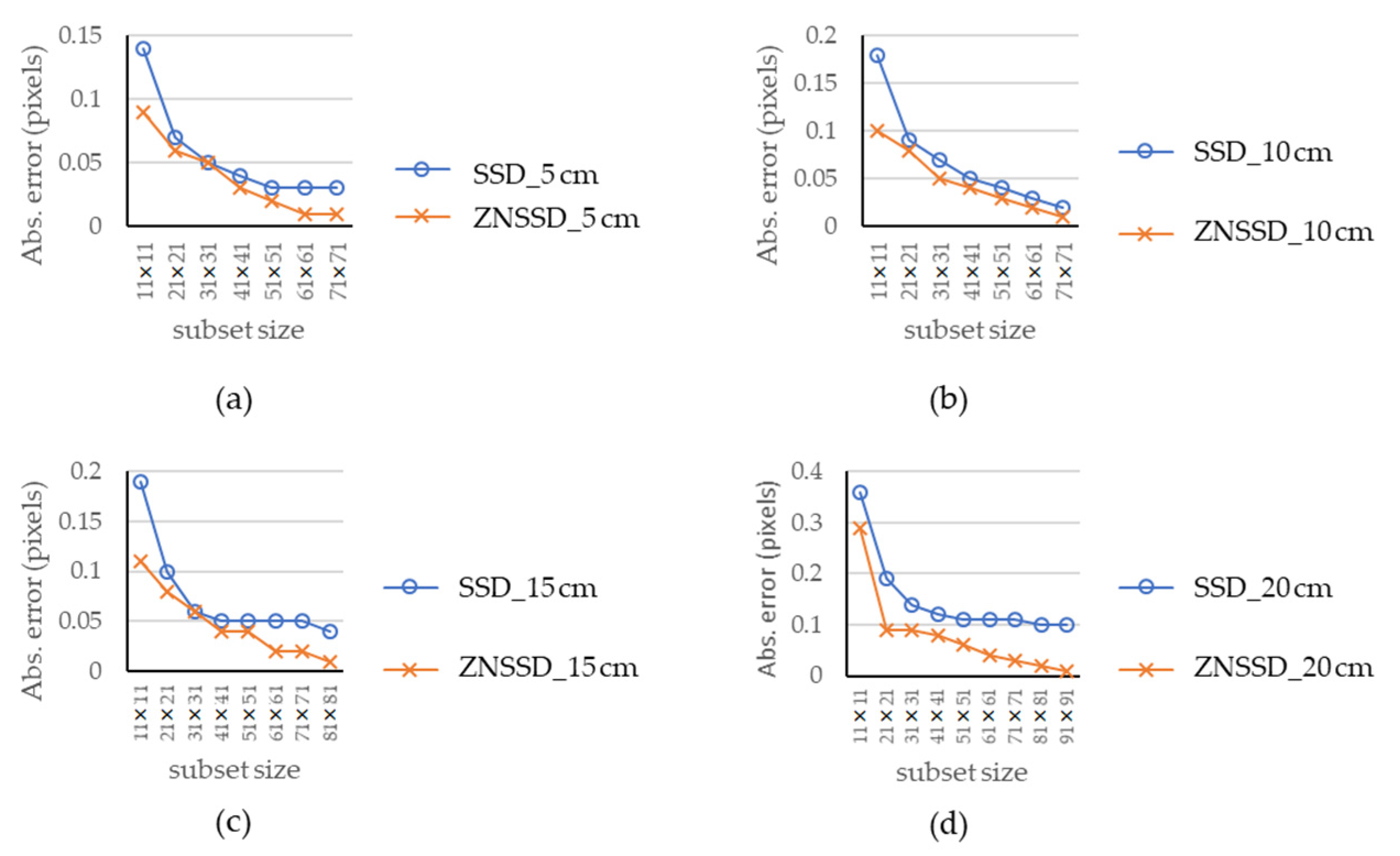
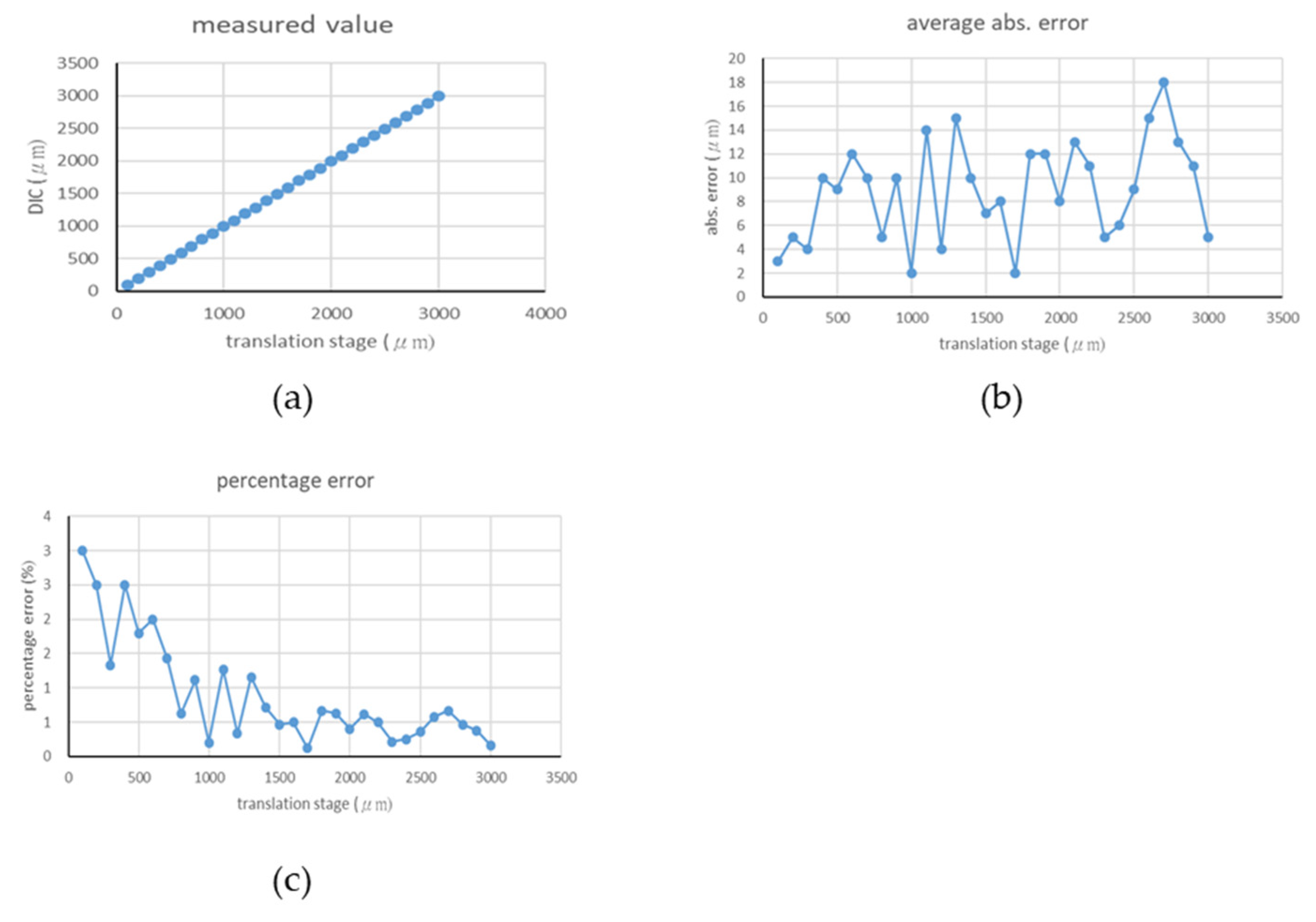
4.1. Rigid Body Translation Experiment
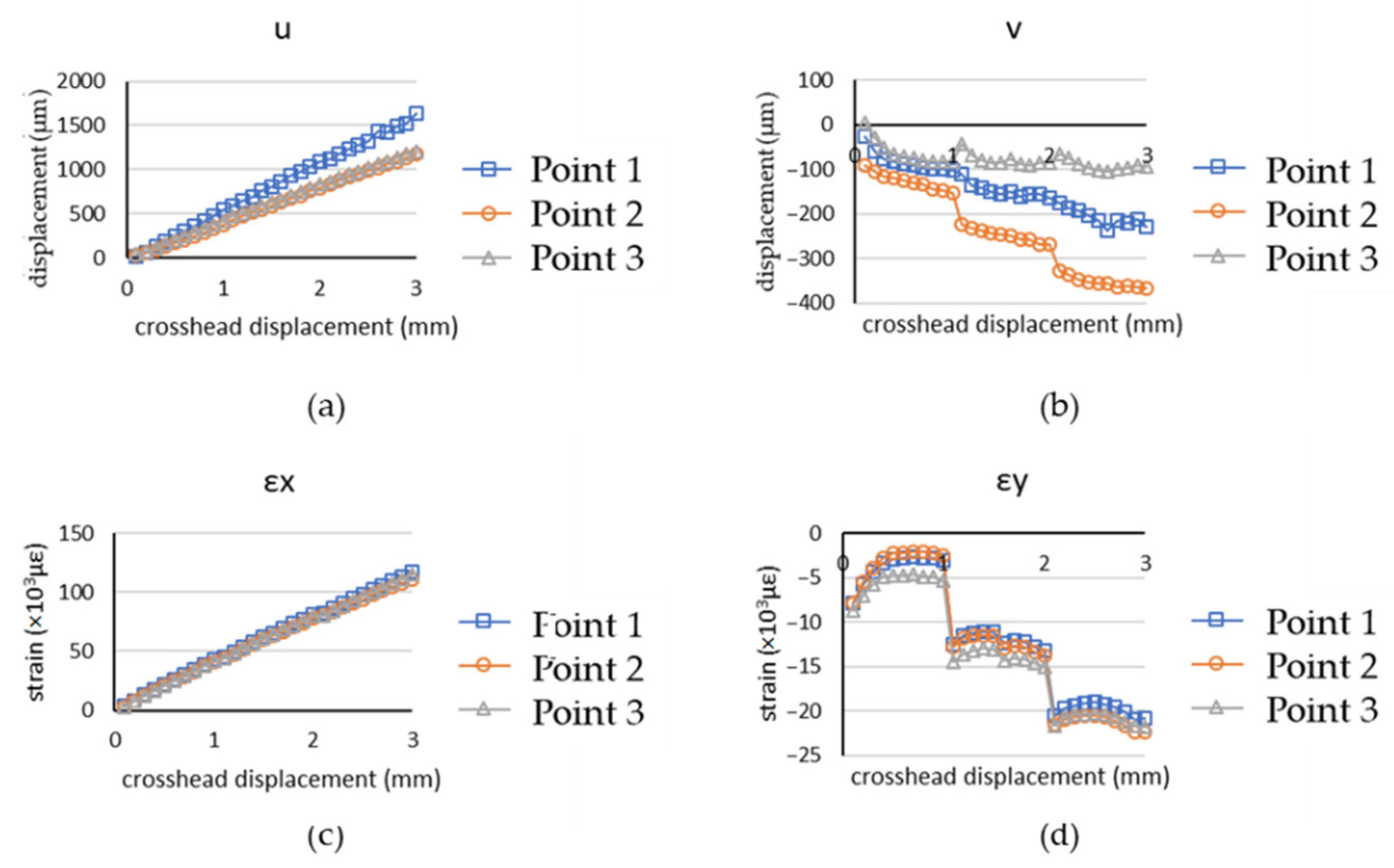
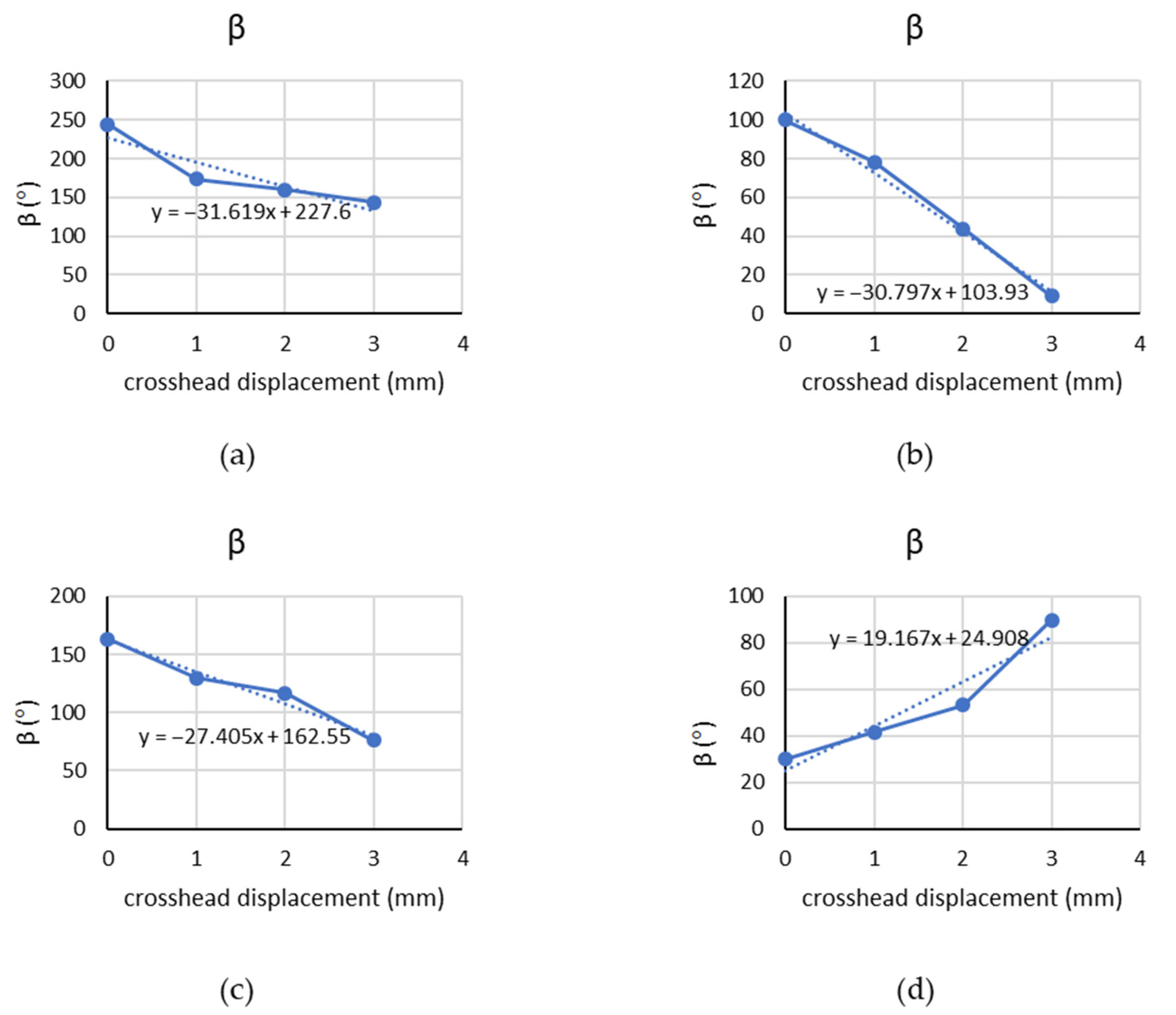
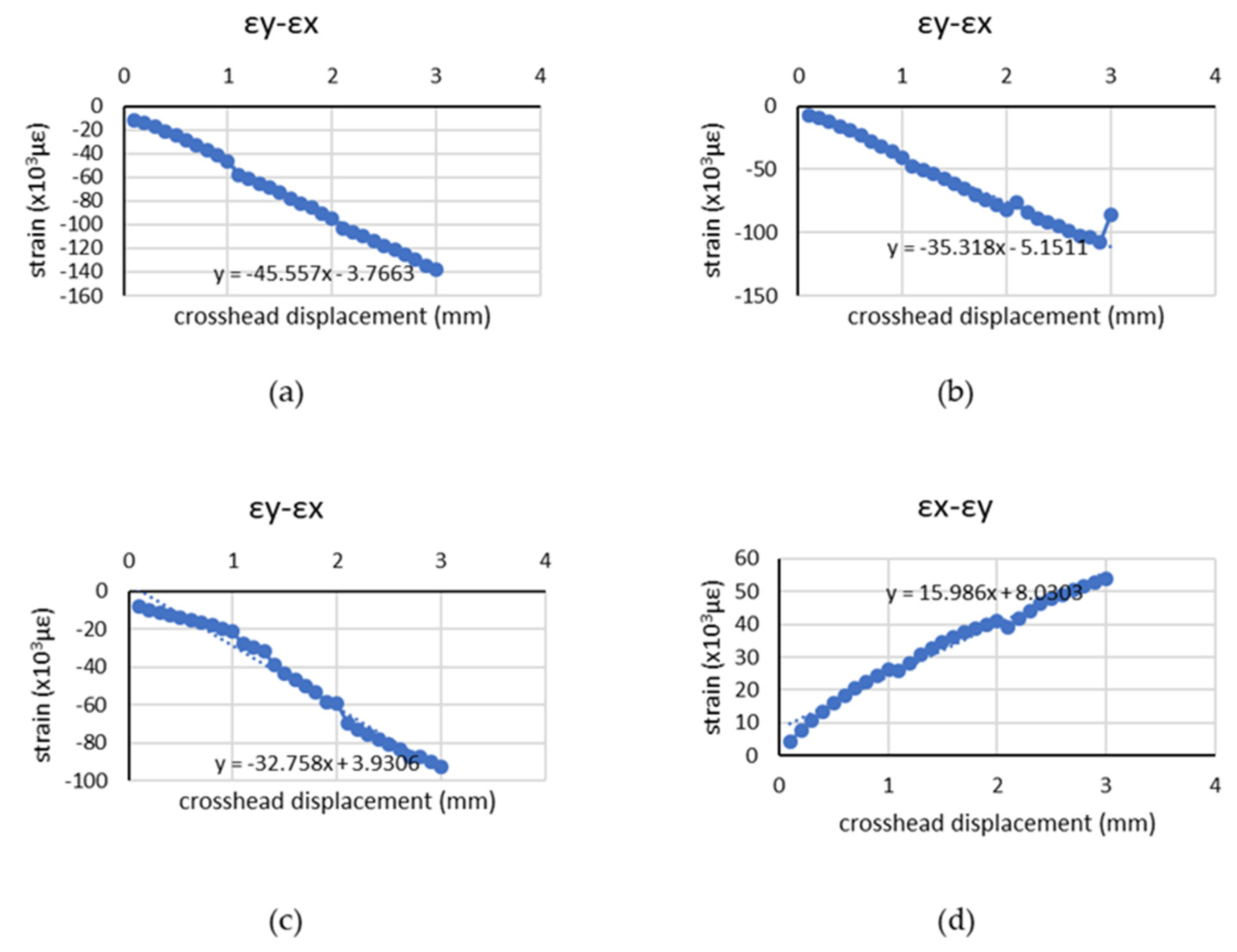
4.2. Chicken Breast Bio-Tissue Tensile Testing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Freddi, A.; Olmi, G.; Cristofolini, L. Experimental Stress Analysis for Materials and Structures: Stress Analysis Models for Developing Design Methodologies; Springer: Cham, Switzerland, 2015; pp. 172–175. [Google Scholar]
- Post, D.; Han, B. Handbook on Experimental Mechanics; Springer: Boston, MA, USA, 2008; Moiré Interferometry; pp. 627–654. [Google Scholar]
- Sutton, M.A.; Orteu, J.J.; Schreier, H.W. Image Correlation for Shape, Motion and Deformation Measurements; Springer Science + Business Media: New York, NY, USA, 2009; pp. 7–10. [Google Scholar]
- Sutton, M.A.; Wolters, W.J.; Peters, W.H.; Ranson, W.F.; McNeill, S.R. Determination of displacements using an improved digital correlation method. Image Vis. Comput. 1983, 1, 133–139. [Google Scholar] [CrossRef]
- Sutton, M.A.; Cheng, M.; Peters, W.H.; Chao, Y.J.; McNeill, S.R. Application of an optimized digital correlation method to planar deformation analysis. Image Vis. Comput. 1986, 4, 143–150. [Google Scholar] [CrossRef]
- Sutton, M.A.; McNeill, S.R.; Jang, J.; Babai, M. Effects of Subpixel Image Restoration On Digital Correlation Error Estimates. Opt. Eng. 1988, 27, 271070. [Google Scholar] [CrossRef]
- Tong, W. An Evaluation of Digital Image Correlation Criteria for Strain Mapping Applications. Strain 2005, 41, 167–175. [Google Scholar] [CrossRef]
- Pan, B.; Asundi, A.; Xie, H.; Gao, J. Digital image correlation using iterative least squares and pointwise least squares for displacement field and strain field measurements. Opt. Laser. Eng. 2009, 47, 865–874. [Google Scholar] [CrossRef]
- Pan, B.; Yu, L.; Wu, D.; Tang, L. Systematic errors in two-dimensional digital image correlation due to lens distortion. Opt. Laser. Eng. 2013, 51, 140–147. [Google Scholar] [CrossRef]
- Gao, Z.; Desai, J.P. Estimating zero-strain states of very soft tissue under gravity loading using digital image correlation. Med. Image Anal. 2010, 14, 126–137. [Google Scholar] [CrossRef] [Green Version]
- Luyckx, T.; Verstraete, M.; Roo, K.D.; Waele, W.D.; Bellemans, J.; Victor, J. Digital image correlation as a tool for three-dimensional strain analysis in human tendon tissue. J. Exp. Orthop. 2014, 1, 7. [Google Scholar] [CrossRef] [Green Version]
- Laura, C.S.; Darryl, G.T. The use of 2D ultrasound elastography for measuring tendon motion and strain. J. Biomech. 2014, 47, 750–754. [Google Scholar] [CrossRef] [Green Version]
- Luyckx, T.; Verstraete, M.; De Roo, K.; Dewaele, W.; Victor, J.; Bellemans, J. The effect of single radius TKA implantation and joint line proximalisation on the strain pattern in the sMCL of the knee. Orthop. Proc. 2013, 95, 401. [Google Scholar]
- Spencer, M.; Siegmund, T.; Mongeau, L. Determination of superior surface strains and stresses, and vocal fold contact pressure in a synthetic larynx model using digital image correlation. J. Acoust. Soc. Am. 2008, 123, 1089–1103. [Google Scholar] [CrossRef] [Green Version]
- Rizzuto, E.; Carosio, S.; Del Prete, Z. Characterization of a Digital Image Correlation System for Dynamic Strain Measurements of Small Biological Tissues. Exp. Tech. 2016, 40, 743–753. [Google Scholar] [CrossRef]
- Chuda-Kowalska, M.; Gajewski, T.; Garbowski, T. Mechanical characterization of orthotropic elastic parameters of a foam by the mixed experimental-numerical analysis. J. Theor. Appl. Mech. 2015, 53, 383–394. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.Y.F.; Chen, T.C.; Cheng, F.Y.; Tsai, A.T.; Lin, M.T. Digital image correlation of SEM images for surface deformation of CMOS IC. Microelectron. Eng. 2018, 201, 16–21. [Google Scholar] [CrossRef]
- Cheong, W.F.; Prahl, S.A.; Welch, A.J. A review of the optical properties of biological tissues. IEEE J. Quantum Electron. 1990, 26, 2166–2185. [Google Scholar] [CrossRef] [Green Version]
- Liao, R.; Zeng, N.; Jiang, X.; Li, D.; Yun, T.; He, Y.; Ma, H. Rotating linear polarization imaging technique for anisotropic tissues. J. Biomed. Opt. 2010, 15, 036014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood, M.F.; Ghosh, N.; Wallenburg, M.A.; Li, S.H.; Weisel, R.D.; Wilson, B.C.; Li, R.K.; Vitkin, I.A. Polarization birefrin-gence measurements for characterizing the myocardium, including healthy, infarcted, and stem-cell-regenerated tissues. J. Biomed. Opt. 2010, 15, 047009. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.W.; Huang, C.L.; Lo, Y.L.; Chang, Y.R. Analysis of optically anisotropic properties of biological tissues under stretching based on differential Mueller matrix formalism. J. Biomed. Opt. 2017, 22, 035006. [Google Scholar] [CrossRef]
- Chen, D.; Zeng, N.; Liu, C.; Ma, H. Characterization of muscle stretching and damage using polarization-sensitive optical coherence tomography (PS-OCT). In SPIE 8553, Optics in Health Care and Biomedical Optics V; Society of Photo-Optical Instrumentation Engineers: Bellingham, WA, USA, 2012. [Google Scholar] [CrossRef]
- Szczurowski, M.K.; Martynkien, T.; Statkiewicz-Barabach, G.; Urbanczyk, W.; Khan, L.; Webb, D.J. Measurements of stress-optic coefficient in polymer optical fibers. Opt. Lett. 2010, 35, 2013–2015. [Google Scholar] [CrossRef] [Green Version]
- Bertholds, A.; Dandliker, R. Determination of the individual strain-optic coefficients in single-mode optical fibres. J. Light. Technol. 1988, 6, 17–20. [Google Scholar] [CrossRef]
- Chen, T.Y.F.; Chou, Y.C.; Wang, Z.Y.; Lin, W.Y.; Lin, M.T. Using Digital Image Correlation on SEM Images of Strain Field after Ion Beam Milling for the Residual Stress Measurement of Thin Films. Materials 2020, 13, 1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, B.; Xie, H.; Guo, Z.; Hua, T. Full-field strain measurement using a two-dimensional Savitzky-Golay digital differentiator in digital image correlation. Opt. Eng. 2007, 46, 062001. [Google Scholar] [CrossRef]
- Farid, H.; Popescu, A.C. Blind Removal of Lens Distortion. J. Opt. Soc. Am. A 2001, 18, 2072–2078. [Google Scholar] [CrossRef] [Green Version]
- Dally, J.W.; Riley, W.F. Experimental Stress Analysis, 4th ed.; College House Enterprises LLC: Knoxville, TN, USA, 2005. [Google Scholar]
- Pham, T.T.H.; Lo, Y.L. Extraction of effective parameters of anisotropic optical materials using a decoupled analytical method. J. Biomed. Opt. 2012, 17, 025006. [Google Scholar] [CrossRef] [PubMed]
- Chenault, D.B.; Chipman, R.A. Measurements of linear diattenuation and linear retardance spectra with a rotating sample spectropolarimeter. Appl. Opt. 1993, 32, 3513–3519. [Google Scholar] [CrossRef] [PubMed]
- Chenault, D.B.; Chipman, R.A. Infrared birefringence spectra for cadmium-sulfide and cadmium selenide. Appl. Opt. 1993, 32, 4223–4227. [Google Scholar] [CrossRef]
- Chenault, D.B.; Chipman, R.A.; Lu, S.Y. Electro-optic coefficient spectrum of cadmium telluride. Appl. Opt. 1994, 33, 7382–7389. [Google Scholar] [CrossRef]
- Sornsin, E.A.; Chipman, R.A. Visible Mueller matrix spectropolarimetry. In SPIE 3121, Polarization: Measurement, Analysis, and Remote Sensing; Society of Photo-Optical Instrumentation Engineers: Bellingham, WA, USA, 1997. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.Y.-F.; Dang, N.M.; Wang, Z.-Y.; Chang, L.-W.; Ku, W.-Y.; Lo, Y.-L.; Lin, M.-T. Use of Digital Image Correlation Method to Measure Bio-Tissue Deformation. Coatings 2021, 11, 924. https://doi.org/10.3390/coatings11080924
Chen TY-F, Dang NM, Wang Z-Y, Chang L-W, Ku W-Y, Lo Y-L, Lin M-T. Use of Digital Image Correlation Method to Measure Bio-Tissue Deformation. Coatings. 2021; 11(8):924. https://doi.org/10.3390/coatings11080924
Chicago/Turabian StyleChen, Terry Yuan-Fang, Nhat Minh Dang, Zhao-Ying Wang, Liang-Wei Chang, Wei-Yu Ku, Yu-Lung Lo, and Ming-Tzer Lin. 2021. "Use of Digital Image Correlation Method to Measure Bio-Tissue Deformation" Coatings 11, no. 8: 924. https://doi.org/10.3390/coatings11080924
APA StyleChen, T. Y.-F., Dang, N. M., Wang, Z.-Y., Chang, L.-W., Ku, W.-Y., Lo, Y.-L., & Lin, M.-T. (2021). Use of Digital Image Correlation Method to Measure Bio-Tissue Deformation. Coatings, 11(8), 924. https://doi.org/10.3390/coatings11080924








